Abstract
Parkinson’s disease (PD) is a neurodegenerative disease characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta, leading to alteration of the integrity of dopaminergic transporters (DATs). In recent years, some radiopharmaceuticals have been used in the clinic to evaluate the integrity of DATs. These include tropane derivatives such as radiolabeled β-CIT and FP-CIT with iodine-123 (123I), and TRODAT-1 with metastable technetium-99 (99mTc). Radiolabeling of β-CIT with radioactive iodine is based on electrophilic radioiodination using oxidizing agents, such as Chloramine T or Iodo-Gen®. For the first time, the present work performed a comparative study of the radiolabeling of β-CIT with iodine-131 (131I), using either Chloramine T or Iodo-Gen® as oxidizing agents, in order to improve the radiolabeling process of β-CIT and to choose the most advantageous oxidizing agent to be used in nuclear medicine. Both radiolabeling methods were similar and resulted in high radiochemical yield (> 95%), with suitable 131I-β-CIT stability up to 72 h. Although Chloramine T is a strong oxidizing agent, it was as effective as Iodo-Gen® for β-CIT radiolabeling with 131I, with the advantage of briefer reaction time and solubility in aqueous medium.
1. Introduction
Dopamine transporters (DATs) are transmembrane proteins present on the presynaptic membrane of dopaminergic neurons, playing an important role in the regulation of intensity and duration of dopaminergic transmission [1,2].
The pathophysiology of Parkinson’s disease (PD) is characterized by progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), leading to a denervation of the nigrostriatal tract that emits its terminal projections to the putamen and caudate nucleus with significant reduction of dopamine and, consequently, loss of DAT integrity [3,4,5].
Since it is a chronic and progressive disease, it is very important to perform an early diagnosis based on clinical evaluation then confirmed by imaging techniques. The clinical relevance of DAT integrity evaluation by diagnostic imaging techniques, such as single photon emission computed tomography (SPECT) and positron emission tomography (PET), are based on the differentiation between degenerative parkinsonism and other conditions not associated with dopamine loss, such as essential tremor, drug-induced parkinsonism, vascular parkinsonism, and psychogenic parkinsonism [6,7].
DAT-SPECT radiopharmaceuticals available for clinical use are all tropane derivatives: (i) β-CIT (2β-carbomethoxy-3β-(4-iodophenyl)tropane) and FP-CIT (N-3-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)nortropane) radiolabeled with iodine-123 (123I).; (ii) TRODAT-1 ([2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2 0.1]oct-2-yl]methyl](2-mercaptoethyl)amino]ethyl]amino]ethanethiolato(3-)-N2,N2’,S2,S2’]oxo-[1R-(exo-exo)]) radiolabeled with metastable technetium-99 (99mTc) [6,8,9].
β-CIT is a molecule that has been studied since the 90s [10]. Due to its binding affinity to presynaptic DATs, radioisotope-labeled β-CIT can differentiate PD from essential tremor with a sensibility of 95% and a specificity of 93% [8]. The proposed mechanism of β-CIT interaction with presynaptic DATs involves electrostatic interactions or hydrogen bonds [11]. In nuclear medicine, 123I-β-CIT is a diagnostic agent for SPECT imaging used both in the initial phase (in individuals with uncertain diagnosis) and in the follow-up of PD [12].
The radiolabeling of molecules, including β-CIT, with radioactive iodine (123I, 124I, 125I or 131I) is frequently based on electrophilic aromatic substitution using several oxidizing agents such as Chloramine T (N-chloro-p-toluenesulfonamide sodium salt), Iodo-Gen® (1,3,4,6-tetrachloro-3α,6β-diphenylglycouril), lactoperoxidase, and the solid-state variants as pre-coated Iodo-Gen® tubes, Iodo-Beads®, or Enzymobeads®. The most commonly used are Chloramine T and Iodo-Gen®, at room temperature [13,14,15,16].
Chloramine T has been used since 1962 [17]. It is a strong oxidizing agent, demanding shorter reaction periods, and is soluble in aqueous solutions [15,18]. On the other hand, Iodo-Gen® is a moderate oxidizing agent, requiring longer reaction times, and is insoluble under aqueous conditions [19,20].
To the best of our knowledge, until now there have been no comparative studies between these two oxidizing agents in β-CIT radiolabeling with radioactive iodine. Therefore, the present study aims to evaluate the electrophilic radioiodination of β-CIT with iodine-131 (131I), due to its 8.04 d half-life, using either Chloramine T trihydrate or Iodo-Gen®, in order to compare the radiolabeling efficiency and compound stability.
The importance of this work is based on the improvement of the radiolabeling process of β-CIT with radioactive iodine through the choice of the most advantageous oxidizing agent. This study may result in high quality radiopharmaceuticals labeled with 123I for use in nuclear medicine and, consequently, higher quality SPECT images of the DATs.
2. Results and Discussion
The electrophilic radioiodination of β-CIT, using the precursor trimethylstannyl-β-CIT (TMS-β-CIT), was performed using either Chloramine T or Iodo-Gen® as an oxidizing agent (Figure 1). The final product, 131I-β-CIT, was purified by solid-phase extraction (SPE) using Sep-Pak® C18. Radiochemical purity was evaluated by both ascendant chromatography and reversed phase-high performance liquid chromatography (RP-HPLC).
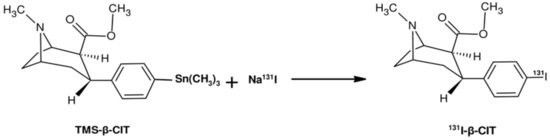
Figure 1.
Simplified scheme of the electrophilic radioiodination of β-CIT.
Ascendant chromatography was performed on thin-layer chromatography on silica gel (TLC-SG; Al) strips using 95% acetonitrile (ACN) as an eluent (Figure 2). Results are summarized in Table 1. In this chromatographic system, Na131I migrates with the solvent front (Rf = 0.9−1.0) and 131I-β-CIT remains in the origin (Rf = 0.1−0.3). Radiochemical yield was over 95% for both radiolabeling methods. SPE purification did not significantly improve the radiochemical purity; however, this procedure is recommended in order to remove iodate ions (IO3−), which are not detected by this chromatographic system. In general, radiopharmaceuticals must present high radiochemical purity level (>90%). Therefore, this comparative study showed that both radiolabeling procedures, using either Chloramine T or Iodo-Gen® as an oxidizing agent, yielded 131I-β-CIT with suitable radiochemical features, and that no significant differences were observed between radiolabeling methods (Table 1).
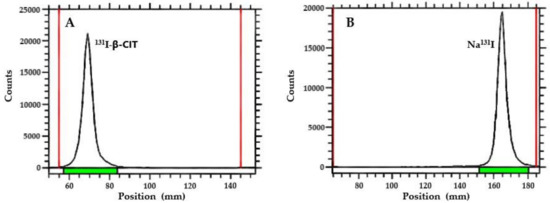
Figure 2.
TLC-chromatograms: (A) 131I-β-CIT (Rf = 0.1−0.3) and (B) Na131I (Rf = 0.9−1.0).

Table 1.
Radiolabeling yield and radiochemical purity of 131I-β-CIT.
The radiochemical purity of 131I-β-CIT was also evaluated by RP-HPLC analysis (Figure 3) in order to confirm ascendant chromatographic data. The unlabeled precursor TMS-β-CIT was analyzed and presented a retention time (RT) of 6.68 min (Figure 3A). Free Na131I was also evaluated, showing a different chromatographic profile (RT = 1.16 min) when compared to unlabeled precursor TMS-β-CIT (Figure 3B). The final product, 131I-β-CIT, obtained by both radiolabeling methods, using either Chloramine T or Iodo-Gen® as an oxidizing agent, showed high radiochemical purity (Figure 3C,D). Therefore, RP-HPLC analyses were in accordance with TLC-SG data, confirming that both radiolabeling methods yield 131I-β-CIT with high radiochemical purity and similar chromatographic profiles.
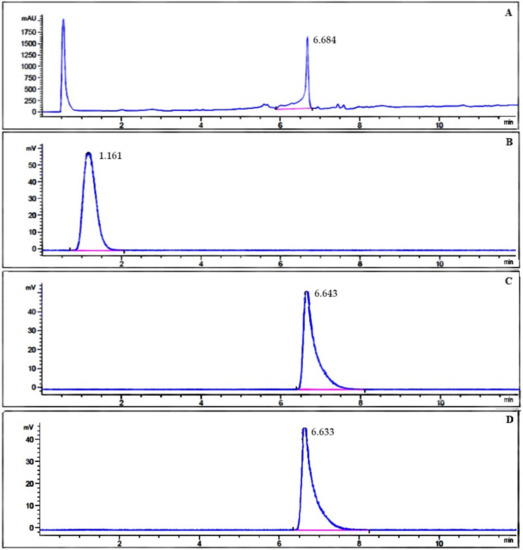
Figure 3.
RP-HPLC chromatograms of (A) unlabeled precursor TMS-β-CIT, (B) Na131I, and (C, D) 131I-β-CIT obtained by both radiolabeling methods.
The stability of 131I-β-CIT was analyzed by ascendant chromatography (Figure 4). The final product was stable up to 72 h. Although slight statistical differences in the stability were observed between both radiolabeling methods, radiochemical purity maintained over 94%, regardless of the storage way, at room temperature or at 2−8 °C (Figure 4A, B). It is important to consider that these small variations in stability in reaction medium should not affect the application of 131I-β-CIT. Moreover, 131I-β-CIT serum stability was also evaluated, and data showed high stability (>94%) up to 24 h, with no significant statistical differences between the radiolabeling methods (Figure 4C).
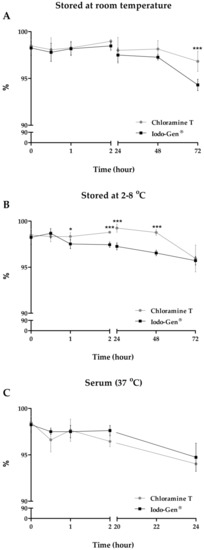
Figure 4.
Evaluation of 131I-β-CIT stability. Values are expressed as mean ± SD [(A) and (B): n = 9; (C): n = 3]. Asterisks indicate significant differences (*p < 0.05; ***p < 0.001).
The partition coefficient (P) of 131I-β-CIT was determined at room temperature by the ratio between n-octanol and 0.9% NaCl. For both radioiodination processes, data showed P tending to the hydrophobic range (Table 2). The hydrophobicity of 131I-β-CIT was also evaluated by the determination of the percentage of serum protein binding (SPB), incubating 131I-β-CIT with serum at 37 °C for 30 min. The results showed approximately 45% of SPB in both cases (Table 2).

Table 2.
Partition coefficient and serum protein binding of 131I-β-CIT.
The physicochemical features of the final product are in agreement, once P tending to the hydrophobic range relates to high SPB [21]. Furthermore, these data are consistent with the intended 131I-β-CIT clinical application, as a radiotracer for measuring presynaptic DAT density in order to provide information on the integrity of these terminals [12]. Therefore, 131I-β-CIT must cross the blood−brain barrier, which is easier for lipophilic molecules. In addition, it has been reported that 45−85% of SPB is related to higher uptake by the brain [22].
3. Materials and Methods
3.1. Electrophilic Radioiodination of β-CIT and SPE Purification
The electrophilic radioiodination of β-CIT was performed using either Chloramine T or Iodo-Gen® as an oxidizing agent followed by SPE purification according to previous methods [14,15,23,24,25], with some modifications. The precursor TMS-β-CIT was purchased from ABX Advanced Biochemical Compounds GmbH (Radeberg, Germany).
For the Chloramine T method, an aliquot of Na131I solution (7.4−14.8 MBq) was added to a vial containing the precursor TMS-β-CIT (0.12 µmol/50 µL EtOH). Next, 10 µL of Chloramine T trihydrate solution (1.5 mg.mL−1) and 4 µL of HCl solution (0.1 mol.L−1) were added. The mixture (pH = 3.0−3.5) was kept at room temperature for 3 min and the reaction was quenched with 40 µL of NaOH solution (0.01 mol.L−1). The final pH was 6.0−6.5.
For the Iodo-Gen® method, an aliquot of Na131I solution (37.0−44.4 MBq) was added to a vial containing the precursor TMS-β-CIT (0.12 µmol/50 µL EtOH). Next, 37.5 µL of Iodo-Gen® solution (6.2 mg.mL−1) and 75 µL of H3PO4 solution (0.1 mol.L−) were added. The mixture (pH = 3.0−3.5) was kept at room temperature for 15 min and the reaction was quenched with 20 µL of NaOH solution (0.1 mol.L−1). The final pH was 6.0−6.5.
After each radiolabeling method, the final product (131I-β-CIT) was purified by SPE using Sep-Pak® C18, preconditioned with EtOH (5 mL) and 0.9% NaCl (5 mL). Free 131I was removed in 0.9% NaCl (5 mL) and 131I-β-CIT was eluted by EtOH (2 mL).
3.2. Radiochemical Purity Analysis
Radiochemical purity was evaluated by ascendant chromatography (n = 9) and RP-HPLC.
Ascendant chromatography was performed by TLC-SG (Al) strips (Merck) using 95% ACN as an eluent. The radioactivity was determined with an AR-2000 radio-TLC Imaging Scanner (Eckert & Ziegler, Germany).
RP-HPLC analyses were performed on a 1290 Infinity II UHPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a radioactivity detector (Eckert & Ziegler, Germany) and Open Lab ECM data system (Agilent Technologies, Santa Clara, CA, USA). The analytical column was a Phenomenex Kinetex® Reversed Phase C18 (100 mm × 3 mm; 2.6 µm) maintained at 30 °C. Mobile phase A was 0.1% (v:v) TFA in water. Mobile phase B was 0.1% (v:v) TFA in MeOH. The gradient of mobile phase B was: 10% (0.0−1.0 min); 10−90% (1.0−8.0 min); 90% (8.0−10.5 min); and 90−10% (10.5−12.0 min). The flow rate was 0.8 mL.min−1 and the UV detector was set at 284 nm.
3.3. Stability Studies
The stability of 131I-β-CIT, stored at room temperature and at 2−8 °C, was evaluated at 0.5, 1, 2, 24, 48, and 72 h (n = 9). Beyond that, the serum stability of 131I-β-CIT, incubated at 37 °C under slight agitation (500 rpm), was evaluated at 1, 2, and 24 h (n = 3). In both cases, the stability of 131I-β-CIT was analyzed by ascendant chromatography, as described in the previous section.
3.4. Partition Coefficient
An aliquot of purified 131I-β-CIT (50 µL) was added in a mixture of n-octanol and 0.9% NaCl (1:1) and submitted to agitation (n = 5). The mixture was centrifuged (825 g; 3 min). Aliquots of 100 µL of both aqueous and organic phases were collected, and their radioactivities were measured with a 2480 automatic gamma counter Wizard2™ 3” (PerkinElmer, Waltham, MA, USA).
3.5. Serum Protein Binding
An aliquot of 131I-β-CIT (25 µL) was added to 475 µL of serum and incubated at 37 °C for 30 min (n = 5). Post-incubation, serum proteins were precipitated with 500 µL of 10% trichloroacetic acid and the content was centrifuged (825 g; 10 min; 3x). Pellets’ and supernatants’ radioactivities were measured with a 2480 automatic gamma counter Wizard2™ 3” (PerkinElmer, Waltham, MA, USA).
4. Conclusions
In summary, the data demonstrated that both radiolabeling methods, using either Chloramine T or Iodo-Gen® as an oxidizing agent, yield 131I-β-CIT with similar radiochemical parameters. Although Chloramine T induces harder reaction conditions when compared to Iodo-Gen®, the results showed that it is possible to use Chloramine T instead of Iodo-Gen® without compromising the radiolabeling result and the final product stability, provided that the physicochemical parameters of each radiolabeling process are respected (pH, reaction time, temperature). Furthermore, it is important to highlight that Chloramine T is a water-soluble reagent with strong oxidizing properties requiring a briefer reaction time, simplifying the process in nuclear medicine. Therefore, this comparative study presents the possibility of alternating between Iodo-Gen® and Chloramine T in the radiolabeling process of β-CIT with 123/131I, depending on the availability and costs of the oxidizing agents, without changing the integrity of the final product, used for SPECT imaging diagnosis of PD in nuclear medicine.
Author Contributions
Conceptualization, M.R.F.F.d.B.; methodology, A.C.R.D., D.V.S., A.C.C.M. and E.V.d.A.; data analysis and statistics, A.C.R.D. and L.L.F.; writing—original draft preparation, A.C.R.D. and L.L.F.; writing—review and editing, all authors; supervision, L.M.; project administration, M.R.F.F.d.B.
Funding
This research received no external funding.
Acknowledgements
The authors would like to thank Hospital Israelita Albert Einstein (HIAE), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for their grants and fellowships. Thanks are also due to Centro de Radiofarmácia (CR) of Instituto de Pesquisas Energéticas e Nucleares of Comissão Nacional de Energia Nuclear (IPEN-CNEN/SP) for the supply of Na131I, Centro de Experimentação e Treinamento em Cirurgia (CETEC) for the infrastructure, and Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS) for the donation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, E. A new era of clinical dopamine transporter imaging using 123I-FP-CIT. J. Nucl. Med. Technol. 2012, 40, 222–228. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23160562 (accessed on 28 March 2017). [CrossRef] [PubMed]
- Ba, F.; Martin, W.R.W. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Parkinsonism Relat. Disord. 2015, 21, 87–94. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25487733 (accessed on 14 April 2017). [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25904081 (accessed on 22 February 2018). [CrossRef]
- Williams-Gray, C.H.; Worth, P.F. Parkinson’s disease. Medicine 2016, 44, 542–546. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative stress in Parkinson’s disease: A systematic review and meta-analysis. Front. Mol. Neurosci. 2018, 11, 1–7. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30026688 (accessed on 10 December 2018). [CrossRef] [PubMed]
- Varrone, A.; Halldin, C. Molecular imaging of the dopamine transporter. J. Nucl. Med. 2010, 51, 1331–1334. Available online: https://www.ncbi.nlm.nih.gov/pubmed/20720060 (accessed on 5 March 2017). [CrossRef] [PubMed]
- Cummings, J.L.; Henchcliffe, C.; Schaier, S.; Simuni, T.; Waxman, A.; Kemp, P. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain 2011, 134, 3146–3166. [Google Scholar] [CrossRef]
- Shen, L.; Liao, M.; Tseng, Y. Recent advances in imaging of dopaminergic neurons for evaluation of neuropsychiatric disorders. J. Biomed. Biotechnol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Li, H.; Zhang, H. SPECT molecular imaging in Parkinson’s disease. J. Biomed. Biotechnol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Kägi, G.; Bhatia, K.P.; Tolosa, E. The role of DAT-SPECT in movement disorders. J. Neurol. Neurosurg. Psychiatry 2010, 81, 5–12. Available online: https://www.ncbi.nlm.nih.gov/pubmed/20019219 (accessed on 9 March 2017). [CrossRef]
- Bois, F.; Baldwin, R.M.; Kula, N.S.; Baldessarini, R.J.; Innis, R.B.; Tamagnan, G. Synthesis and monoamine transporter affinity of 3’-analogs of 2β-carbomethoxy-3-β-(4’-iodophenyl)tropane (β-CIT). Bioorg. Med. Chem. Lett. 2004, 14, 2117–2120. [Google Scholar] [CrossRef] [PubMed]
- Eerola, J.; Tienari, P.J.; Kaakkola, S.; Nikkinen, P.; Launes, J. How useful is [123I]β-CIT SPECT in clinical practice? J. Neurol. Neurosurg. Psychiatry 2005, 76, 1211–1216. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1739796/ (accessed on 13 December 2016). [CrossRef]
- Markwell, M.A.K. A new solid-state reagent to iodinate proteins: I. Conditions for the efficient labeling of antiserum. Anal. Biochem. 1982, 125, 427–432. [Google Scholar] [CrossRef]
- Carpinelli, A.; Matarrese, M.; Moresco, R.M.; Simonelli, P.; Todde, S.; Magni, F.; Galli Kienle, M.; Fazio, F. Radiosynthesis of [123I]βCIT, a selective ligand for the study of the dopaminergic and serotoninergic systems in human brain. Appl. Radiat. Isotopes 2001, 54, 93–95. [Google Scholar] [CrossRef]
- Sihver, W.; Drewes, B.; Schulze, A.; Olsson, R.A.; Coenen, H.H. Evaluation of novel tropane analogues in comparison with the binding characteristics of [18F]FP-CIT and [131I]β-CIT. Nucl. Med. Biol. 2007, 34, 211–219. [Google Scholar] [CrossRef]
- Blois, E.; Chan, H.S.; Breeman, W.A.P. Iodination and stability of somatostatin analogues: Comparison of iodination techniques. A practical overview. Curr. Top. Med. Chem. 2012, 12, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.M.; Greenwood, F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature 1962, 194, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Tashtoush, B.M.; Traboulsi, A.A.; Dittert, L.; Hussain, A.A. Chloramine-T in radiolabeling techniques. IV. Penta-O-acetyl-N-chloro-N-methylglucamine as an oxidizing agent in radiolabeling techniques. Anal. Biochem. 2001, 288, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Fraker, P.J.; Speck, J.C., Jr. Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem. Biophys. Res. Commun. 1978, 80, 849–857. [Google Scholar] [CrossRef]
- Amin, A.M.; Gouda, A.A.; El-Sheikh, R.; Seddik, U.; Hussien, H. Radioiodination, purification and bioevaluation of Piroxicam in comparison with Meloxicam for imaging of inflammation. J. Radioanal. Nucl. Chem. 2009, 280, 589–598. [Google Scholar] [CrossRef]
- Lexa, K.W.; Dolghih, E.; Jacobson, M.P. A structure-based model for predicting serum albumin binding. PLoS ONE 2014, 9, 1–12. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24691448 (accessed on 20 June 2018). [CrossRef]
- Tavares, A.A.; Lewsey, J.; Dewar, D.; Pimlott, S.L. Radiotracer properties determined by high performance liquid chromatography: A potential tool for brain radiotracer discovery. Nucl. Med. Biol. 2012, 39, 127–135. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21958855 (accessed on 29 July 2018). [CrossRef]
- Katsifis, A.; Papazian, V.; Jackson, T.; Loc’h, C. A rapid and efficient preparation of [123I]radiopharmaceuticals using a small HPLC (Rocket) column. Appl. Radiat. Isotopes 2006, 64, 27–31. [Google Scholar] [CrossRef]
- Saji, H.; Iida, Y.; Kawashima, H.; Ogawa, M.; Kitamura, Y.; Mukai, T.; Shimazu, S.; Yoneda, F. In vivo imaging of brain dopaminergic neurotransmission system in small animals with high-resolution single photon emission computed tomography. Anal. Sci. 2003, 19, 67–71. [Google Scholar] [CrossRef]
- Fuchigami, T.; Mizoguchi, T.; Ishikawa, N.; Haratake, M.; Yoshida, S.; Magata, Y.; Nakayama, M. Synthesis and evaluation of a radioiodinated 4,6-diaryl-3-cyano-2-pyridinone derivative as a survivin targeting SPECT probe for tumor imaging. Bioorg. Med. Chem. Lett. 2016, 26, 999–1004. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26733475 (accessed on 21 January 2018). [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).