Estimation of Respiratory States Based on a Measurement Model of Airflow Characteristics in Powered Air-Purifying Respirators Using Differential Pressure and Pulse Width Modulation Control Signals—In the Development of a Public-Oriented Powered Air-Purifying Respirator as an Alternative to Lockdown Measures
Abstract
Highlights
- A method was developed to estimate respiratory states (exhalation/inhalation) in a public-oriented PAPR based on differential pressure and PWM duty value;
- The estimation model, derived under static conditions, was shown to remain valid even under dynamic conditions where the pump is actively controlled.
- The ability to infer respiratory states enables pressure control that assists breathing by reducing pressure during exhalation and increasing it during inhalation;
- This respiratory-assist control has the potential to enhance wearing comfort, contributing to the feasibility of PAPRs as alternatives to lockdowns for airborne infection control.
Abstract
1. Introduction
2. System Configuration and Measurement Method
3. Modeling of Airflow Characteristics and Its Evaluations
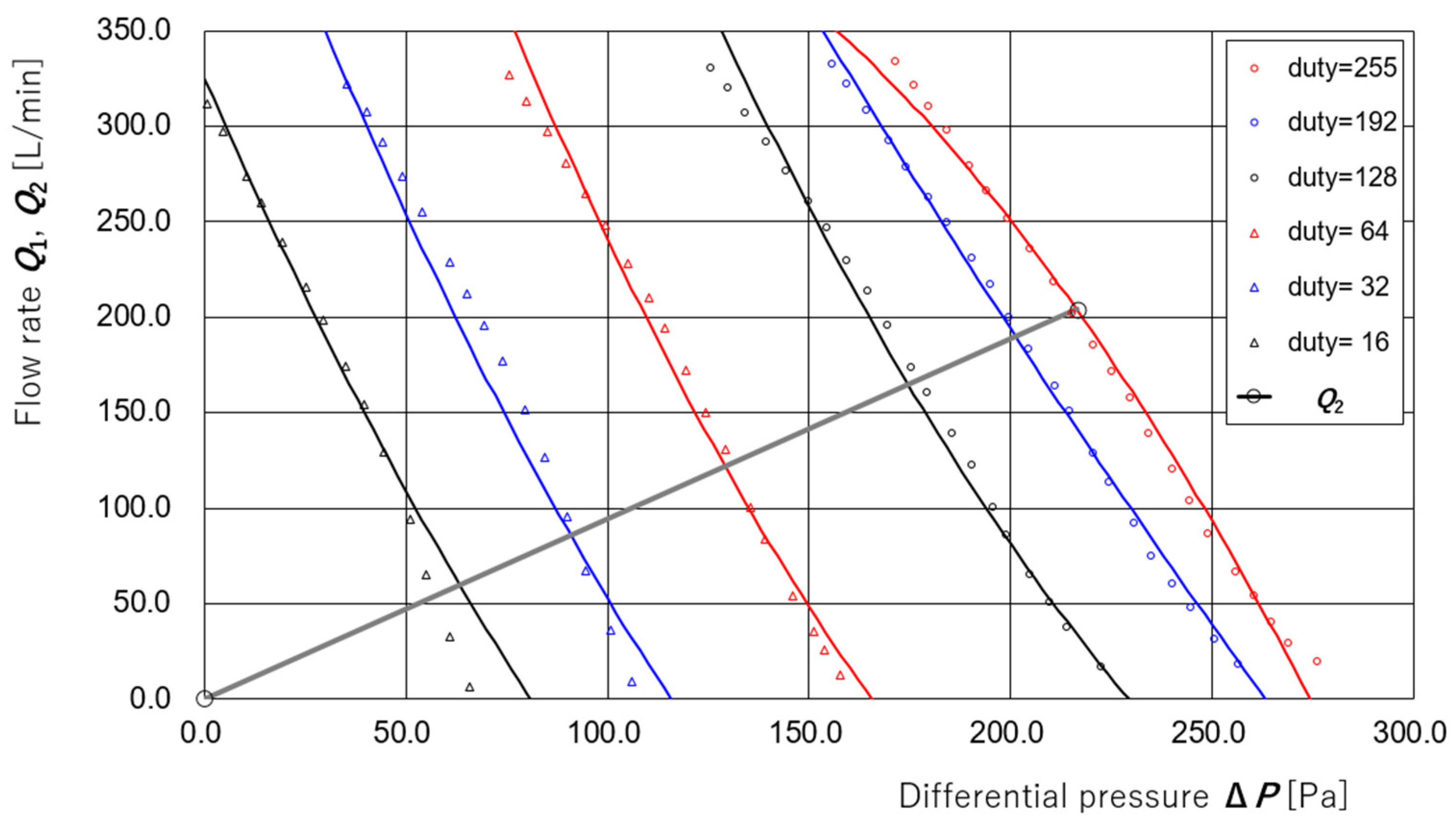
3.1. Derivation of Regression Equations for Supply and Exhaust Flow Rates
3.2. Estimation of Disturbance Flow Without Supply Pump Control
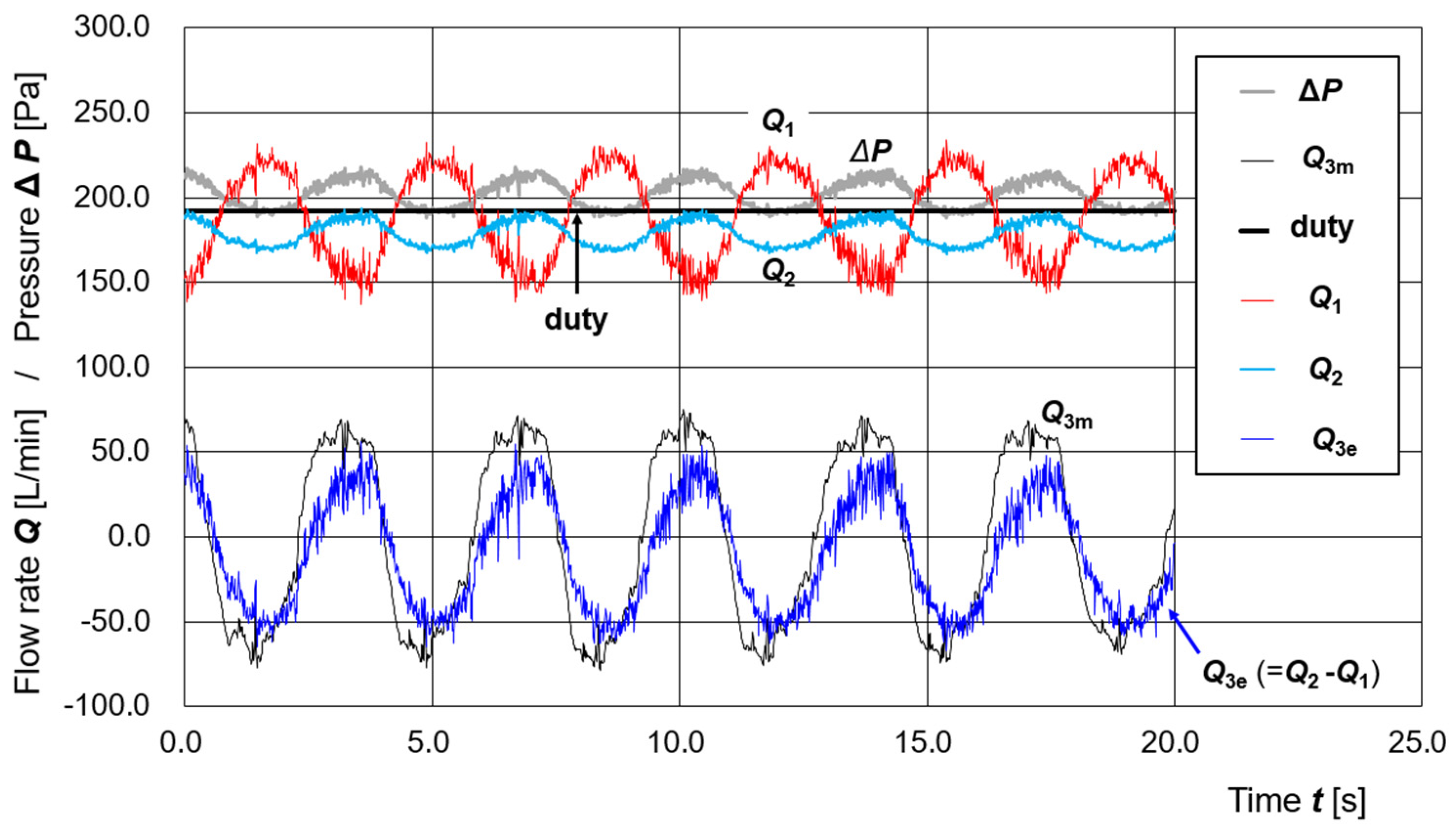
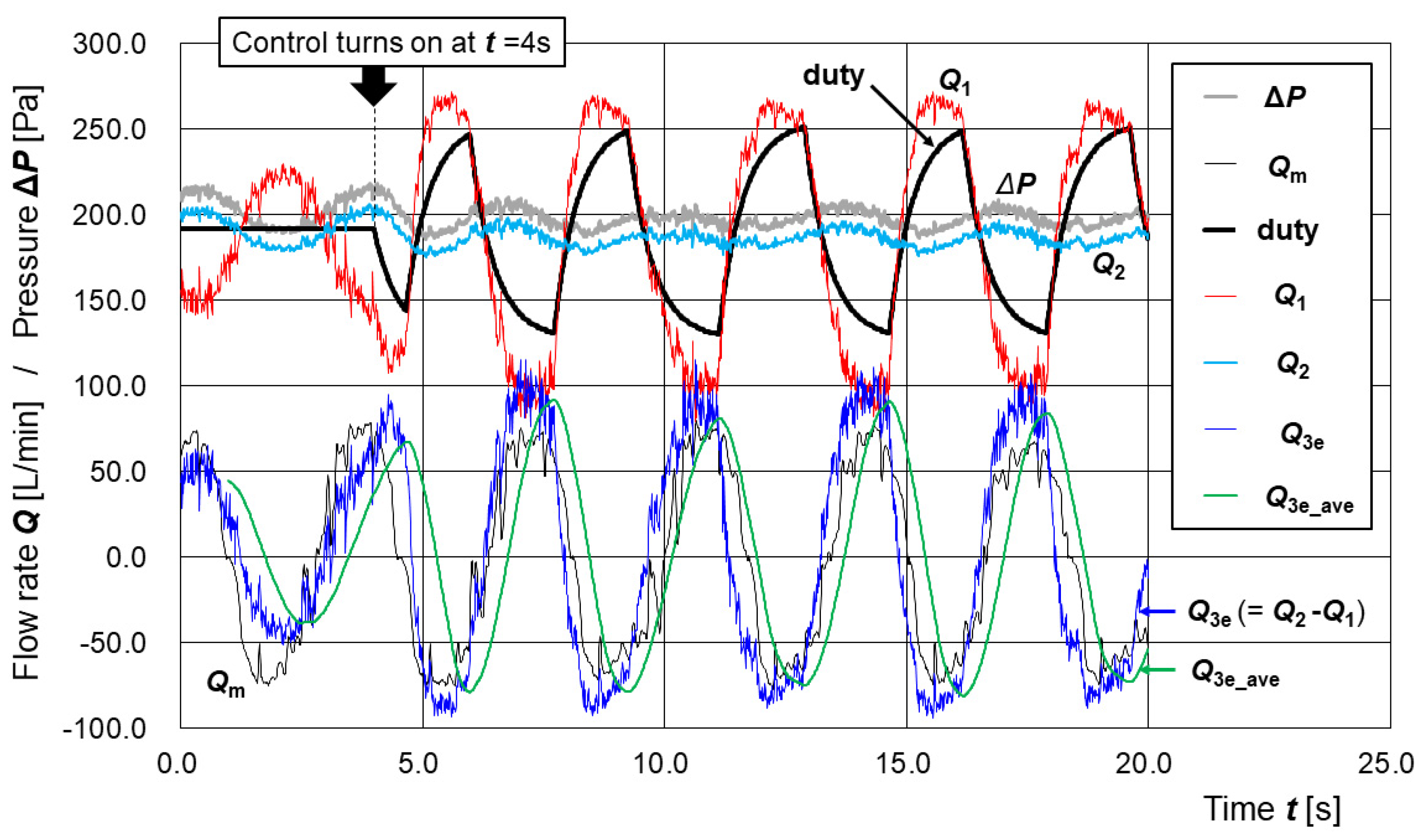
3.3. Estimation of Disturbance Flow Under Active Control of the Supply Pump
- (1)
- The moving average of the past 100 Q3e values, Q3e_ave, is calculated in every step in the time loop;
- (2)
- After the positive peak of Q3e_ave is detected, the duty value d is reduced by (dc − 128)/50 at every step, where dc is the current value of d in that step;
- (3)
- After the negative peak of Q3e_ave is detected, the duty value d is increased by (255 − dc)/50 at every step, where dc is the current value of d in that step.
4. Discussion
4.1. Validation of Regression Models Under Dynamic Conditions
4.2. Incorporation and Extension of Dynamic Characteristics into the Flow Rate Models
4.3. Preliminary Evaluation of the Control Algorithm and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAPR | Powered-Air Purifying Respirator |
| HEPA | High-Efficiency Particulate Air (filter) |
References
- Fujii, Y. An Engineering Alternative to Lockdown During COVID-19 and Other Airborne Infectious Disease Pandemics: Feasibility Study. JMIR Biomed. Eng. 2024, 9, e54666. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y. Examination of the requirements for powered air-purifying respirator (PAPR) utilization as an alternative to lockdown. Sci. Rep. 2025, 15, 1217. [Google Scholar] [CrossRef] [PubMed]
- Tilstra, A.M.; Polizzi, A.; Wagner, S.; Akimova, E.T. Projecting the long-term effects of the COVID-19 pandemic on U.S. population structure. Nat. Commun. 2024, 15, 2409. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, Y.; Myers, K.R.; Lakhani, K.R.; Wang, D. Potentially long-lasting effects of the pandemic on scientists. Nat. Commun. 2021, 12, 6188. [Google Scholar] [CrossRef]
- Rothwell, J.T.; Cojocaru, A.; Srinivasan, R.; Kim, Y.S. Global evidence on the economic effects of disease suppression during COVID-19. Humanit. Soc. Sci. Commun. 2024, 11, 78. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Nogrady, B. WHO redefines airborne transmission: What does that mean for future pandemics? Nature 2024. Available online: https://www.nature.com/articles/d41586-024-01173-7 (accessed on 4 May 2025). [CrossRef]
- Kharroubi, S.; Saleh, F. Are Lockdown Measures Effective Against COVID-19? Front. Public Health 2020, 8, 549692. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: A Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef]
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef]
- Morawska, L.; Milton, D.K. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; El-Harakeh, A.; Bognanni, A.; Lotfi, T.; Loeb, M.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Iezadi, S.; Gholipour, K.; Azami-Aghdash, S.; Ghiasi, A.; Rezapour, A.; Pourasghari, H.; Pashazadeh, F. Effectiveness of non-pharmaceutical public health interventions against COVID-19: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0260371. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (EPA). What Is a HEPA Filter? Available online: https://www.epa.gov/indoor-air-quality-iaq/what-hepa-filter (accessed on 19 February 2025).
- DHHS (NIOSH). Understanding Filtration Efficiency Testing and Fit Testing in Filtering Facepiece Respirators (FFRs). DHHS (NIOSH) Publication No. 2021-123. Available online: https://www.cdc.gov/niosh/docs/2021-123/pdfs/2021-123revised112021.pdf (accessed on 4 May 2025).
- Kumar, A.; Dhawan, S.; Kumar, M.V.; Khare, M.; Nagendra, S.S.; Dubey, S.K.; Mehta, D.S. Detection and identification of shape, size, and concentration of particulate matter in ambient air using bright field microscopy-based system. Atmos. Pollut. Res. 2023, 14, 101913. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Tang, N.; Zhou, S.; Yu, J.; Ding, B. Spider-web-inspired PM0.3 filters based on self-sustained electrostatic nanostructured networks. Adv. Mater. 2020, 32, e2002361. [Google Scholar] [CrossRef]
- Essa, W.K.; Yasin, S.A.; Saeed, I.A.; Ali, G.A.M. Nanofiber-based face masks and respirators as COVID-19 protection: A review. Membranes 2021, 11, 250. [Google Scholar] [CrossRef]
- Occupational Safety and Health Administration (OSHA). Assigned Protection Factors for the Revised Respiratory Protection Standard. OSHA 3352-02. 2009. Available online: https://www.osha.gov/sites/default/files/publications/3352-APF-respirators.pdf (accessed on 4 May 2025).
- Occupational Safety and Health Administration (OSHA). Personal Protective Equipment. OSHA 1910.134. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134 (accessed on 4 May 2025).
- Johnson, B. OSHA infectious dose white paper. Appl. Biosaf. 2003, 8, 160–165. [Google Scholar] [CrossRef]
- Prentiss, M.; Chu, A.; Berggren, K.K. Finding the infectious dose for COVID-19 by applying an airborne-transmission model to superspreader events. PLoS ONE 2022, 17, e0265816. [Google Scholar] [CrossRef]
- M Corp. 3M Respiratory Protection. Available online: https://www.3mindia.in/3M/en_IN/p/c/ppe/respiratory-protection/ (accessed on 19 February 2025).
- Galindo, R.M.; Takita, A.; Carcasona, E.; Magalang, E.; Galindo, T.S.; Hashimoto, S.; Yamaguchi, T.; Tibay, E.U.; Shu, D.; Kobayashi, H.; et al. Very-Low-Cost Powered Air-Purifying Respirator (PAPR) ‘Distancing-Free Mask Industry (DFM-I) Prototype No.1’ and Proposal for a Lockdown-Free Industry. J. Technol. Soc. Sci. 2022, 6, 1–4. Available online: http://jtss.e-jikei.org/issue/archives/v06n02/JTSS_v06n02a001.pdf (accessed on 4 May 2025).
- M Corp. Assigned Protection Factors (APF) for 3M™ Hoods and Helmets. 2021. Available online: https://multimedia.3m.com/mws/media/418650O/assigned-protection-factors-for-hoods-and-helmets-technical-data-bulletin.pdf (accessed on 4 May 2025).
- Zhuang, E.; Chen, H.H.; Kolesnik, O.; Hines, S.E. Tolerability, user acceptance and preference for a novel reusable respirator among health care workers. Am. J. Infect. Control 2022, 51, 821–826. [Google Scholar] [CrossRef]
- Chong, L.; Bundele, A.; Sumner, J.; Mukhopadhyay, A. Advances in respiratory protective equipment: Practical experiences of CleanSpace® HALO™ by healthcare workers. J. Hosp. Infect. 2022, 124, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Nagel, J.; Gilbert, C.; Duchesne, J. Novel 3D printable powered air purifying respirator for emergency use during PPE shortage of the COVID-19 pandemic: A study protocol and device safety analysis. BMJ Open 2021, 11, e049605. [Google Scholar] [CrossRef] [PubMed]
- Pleil, J.D.; Wallace, M.A.G.; Davis, M.D.; Matty, C.M. The physics of human breathing: Flow, timing, volume, and pressure parameters for normal, on-demand, and ventilator respiration. J. Breath Res. 2021, 15, 042002. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8672270/ (accessed on 4 May 2025). [CrossRef] [PubMed]
- Fujii, Y.; Takita, A.; Yamaguchi, T.; Hashimoto, S. Japanese Patent No. 7616624. 8 January 2025. Available online: https://jglobal.jst.go.jp/detail?JGLOBAL_ID=202503001572519658 (accessed on 4 May 2025).
- Fujii, Y.; Takita, A.; Hashimoto, S. Japanese Patent No. 7515044. 4 July 2024. Available online: https://jglobal.jst.go.jp/detail?JGLOBAL_ID=202403002056869269 (accessed on 4 May 2025).
- Teng, G.; Shi, G.; Zhu, J.; Zhao, C. Research on the air supply adjustment technology of breath-following powered air-purifying respirators. Sci. Rep. 2023, 13, 12219. [Google Scholar] [CrossRef]
- Galindo, R.M.; Takita, A.; Carcasona, E.; Magalang, E.; Galindo, T.S.; Hashimoto, S.; Yamaguchi, T.; Tibay, E.U.; Shu, D.W.; Kobayashi, H.; et al. Low-Cost Powered Air-Purifying Respirator (PAPR) ‘Distancing-Free Mask Frontline (DFM-F) Prototype No.1’ for the Operational Tests in Hospitals in Cebu City, Philippines. J. Mech. Electr. Intell. Syst. 2022, 5, 1–6. Available online: http://jmeis.e-jikei.org/ARCHIVES/v05n02/JMEIS_v05n02a001.pdf (accessed on 4 May 2025).
- Iizuka, A.; Galindo, R.M.; Carcasona, E.; Takita, A.; Fujii, Y. Evaluation of Air-supply Units of Powered Air-Purifying Respirators. In Proceedings of the ICTSS 2024, Cebu, Philippines, 15–19 December 2024; Available online: https://conf.e-jikei.org/ICTSS/2024/proceedings/materials/proc_files/IPS01/ICTSS2024_IPS01_02.pdf (accessed on 4 May 2025).
- Deepa, M.; Vidhyapriya, R.; Raagul, A.S. A novel breath pattern model and analysis to minimize patient discomfort in medical ventilators. Sci. Rep. 2025, 15, 2075. [Google Scholar] [CrossRef]
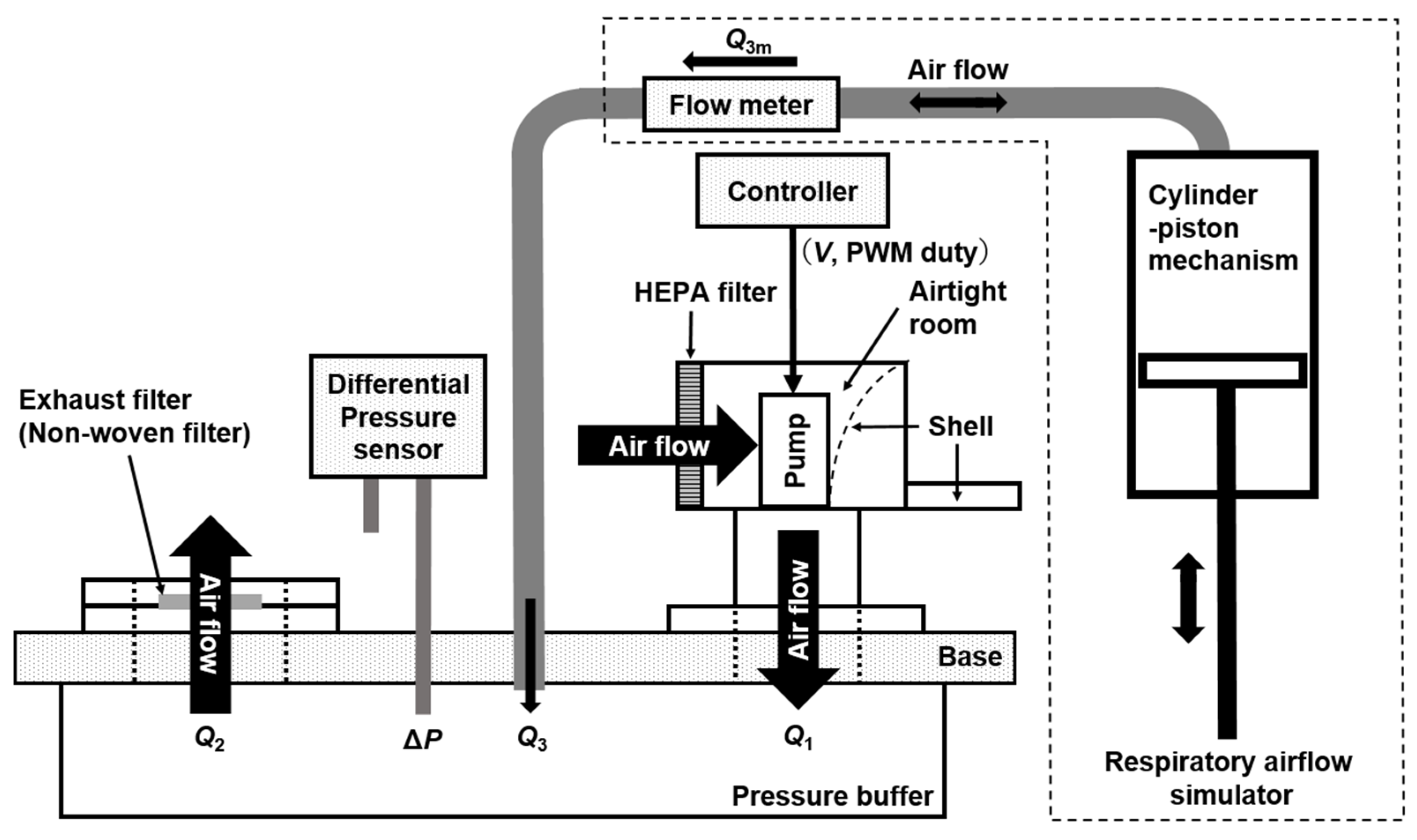
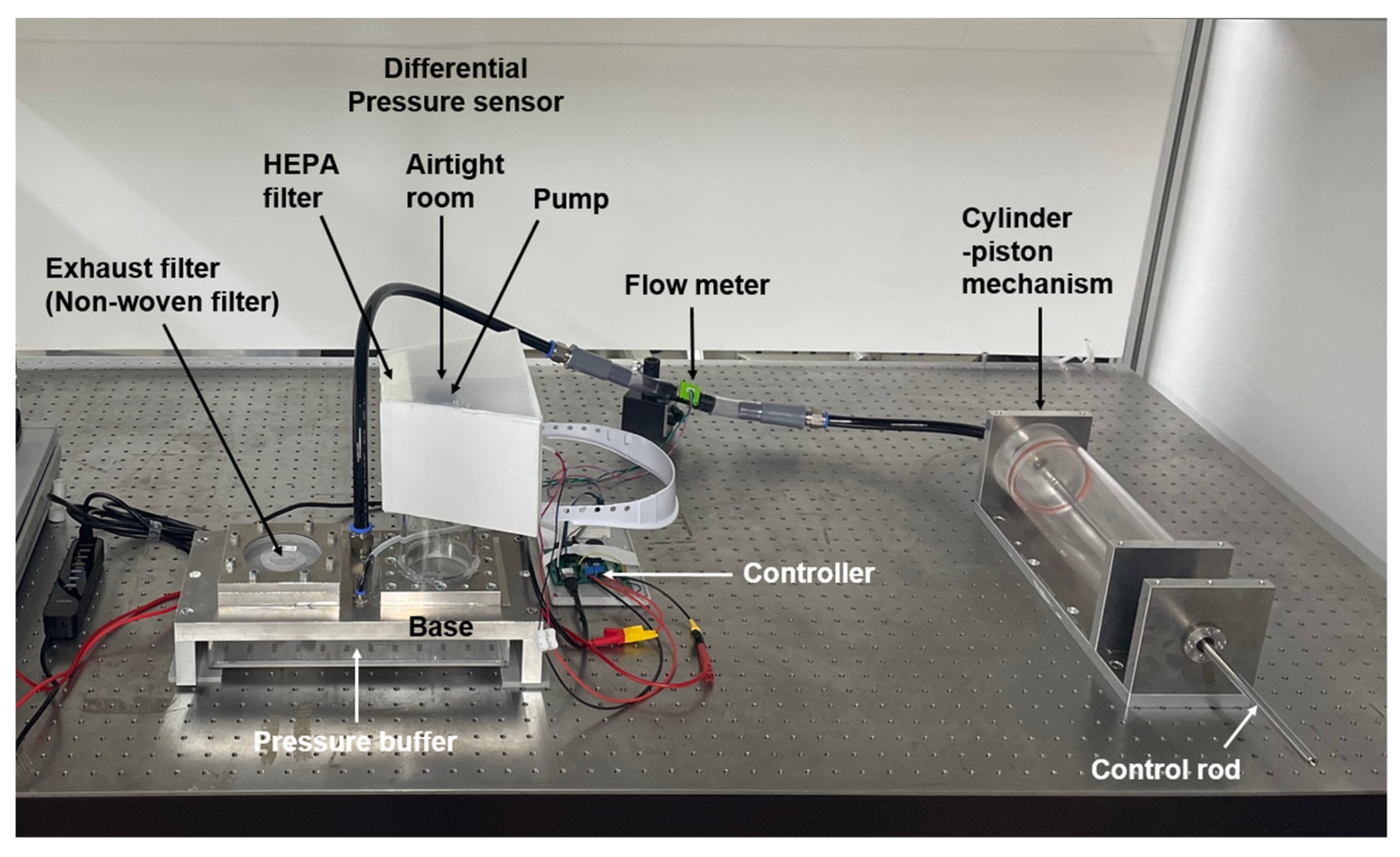


| Model | [A] Versaflo TR-300+ (3M) | [B] A Prototype PAPR |
|---|---|---|
| Photo |  |  |
| Filter | Nonwoven filter | HEPA filter |
| Internal Pressure | positive | positive |
| Flow rate [L/min] | 180 (Low-mode) or 200 (High-mode) | 400 |
| Cost | Price: USD 1000 | Parts cost: USD 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, Y.; Takita, A.; Hashimoto, S.; Amagai, K. Estimation of Respiratory States Based on a Measurement Model of Airflow Characteristics in Powered Air-Purifying Respirators Using Differential Pressure and Pulse Width Modulation Control Signals—In the Development of a Public-Oriented Powered Air-Purifying Respirator as an Alternative to Lockdown Measures. Sensors 2025, 25, 2939. https://doi.org/10.3390/s25092939
Fujii Y, Takita A, Hashimoto S, Amagai K. Estimation of Respiratory States Based on a Measurement Model of Airflow Characteristics in Powered Air-Purifying Respirators Using Differential Pressure and Pulse Width Modulation Control Signals—In the Development of a Public-Oriented Powered Air-Purifying Respirator as an Alternative to Lockdown Measures. Sensors. 2025; 25(9):2939. https://doi.org/10.3390/s25092939
Chicago/Turabian StyleFujii, Yusaku, Akihiro Takita, Seiji Hashimoto, and Kenji Amagai. 2025. "Estimation of Respiratory States Based on a Measurement Model of Airflow Characteristics in Powered Air-Purifying Respirators Using Differential Pressure and Pulse Width Modulation Control Signals—In the Development of a Public-Oriented Powered Air-Purifying Respirator as an Alternative to Lockdown Measures" Sensors 25, no. 9: 2939. https://doi.org/10.3390/s25092939
APA StyleFujii, Y., Takita, A., Hashimoto, S., & Amagai, K. (2025). Estimation of Respiratory States Based on a Measurement Model of Airflow Characteristics in Powered Air-Purifying Respirators Using Differential Pressure and Pulse Width Modulation Control Signals—In the Development of a Public-Oriented Powered Air-Purifying Respirator as an Alternative to Lockdown Measures. Sensors, 25(9), 2939. https://doi.org/10.3390/s25092939






