Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors
Abstract
:1. Introduction
2. LSPR Biosensors for POC Molecule Detection and Monitoring
3. Current LSPR Biosensors for the Detection of Chemical and Biomolecules
3.1. LSPR Sensors Coupled with Solution Phase-Based Nanoparticles
3.2. LSPR Sensors Using Flat Substrate-Based Platforms
3.3. Nanoparticle-Coated Optical Fiber-Based LSPR Sensors
4. Conclusions and Future Perspectives
| Classification | Substrate | Receptor | Analyte | Linear Range, LOD | Assay Time | Real Sample | Features | Reference |
|---|---|---|---|---|---|---|---|---|
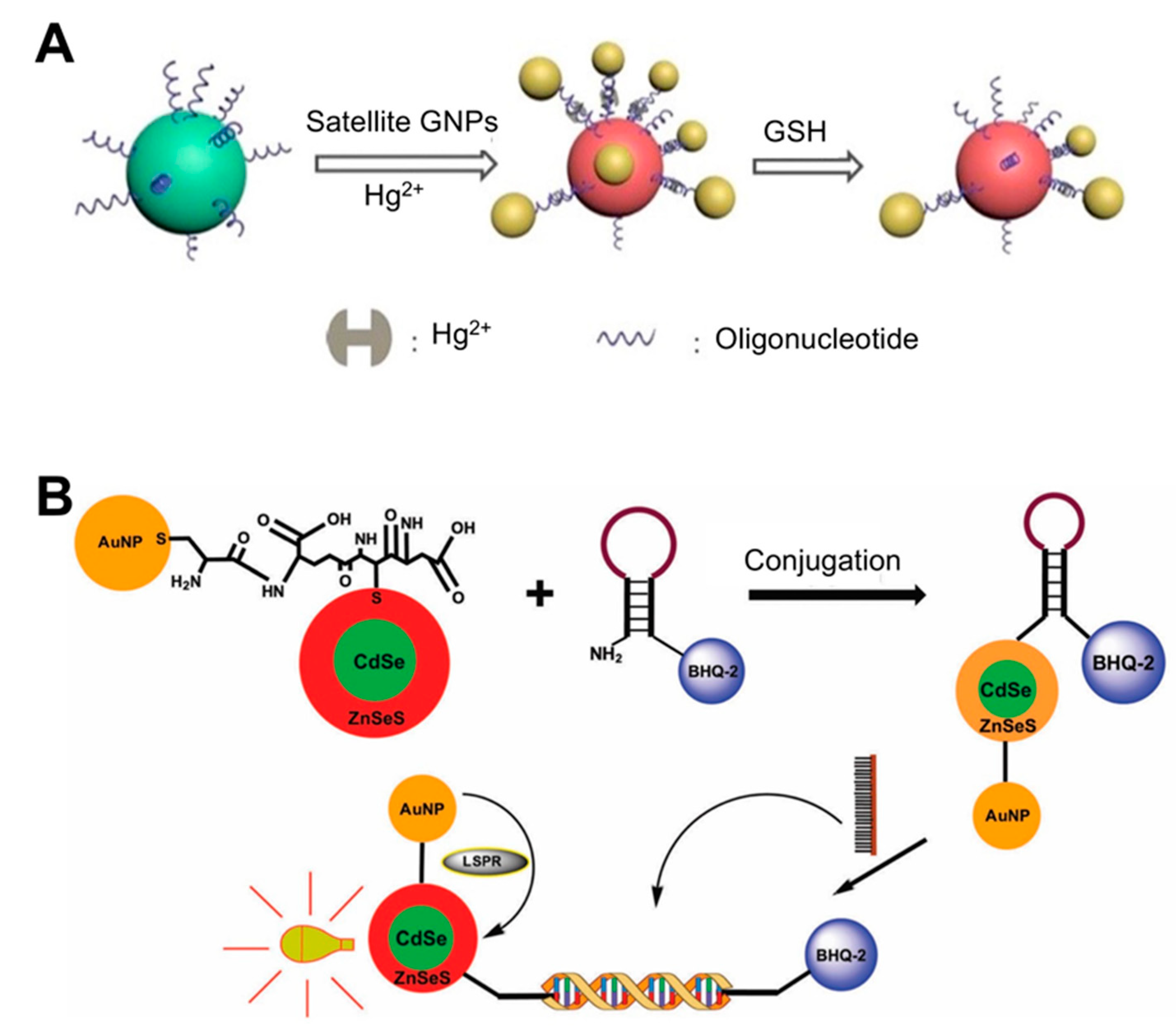
| Solution phase-based nanoparticle | AuNP-based core-satellite structure | Hg2+ incorporating DNA duplex | Glutathione (GSH) | 0.1 μM | 30 min | ND | Use of property of GSH with high affinity for Hg2+. Caused a blue shift in the LSPR peak by AuNP structural collapse upon exposure to GSH. | [36]; Figure 1A |
| CdSe/ZnSeS core/alloyed shell Quantum dot (Qdot) | DNA (molecular beacon) | Dengue virus | 20 copies per mL | ND | ND | Quencher use: Change in PL Qdot depending on the presence/absence of target DNA in the sample. Conjugation of Qdots and AuNPs: boosting PL of Qdots by LSPR from AuNPs. | [7]; Figure 1B | |
| AuNP | None | Melamine | 0 μM to 0.9 μM, 33 nM | ND | Liquid milk | Use of unmodified AuNPs without the need for a receptor due to the interaction of amine groups of melamine and AuNPs. Recovery rate of 99.2~111%. | [114] | |
| AuNP | Aptamer | Ochratoxin A (OTA) | 0.0316–316 ng/mL | >15 min | Spiked corn | Use of color change based on AuNPs aggregation caused by competition between aptamer-bound Au NPs and OTA. Use of double calibration curve method to widen the detection range. | [40] | |
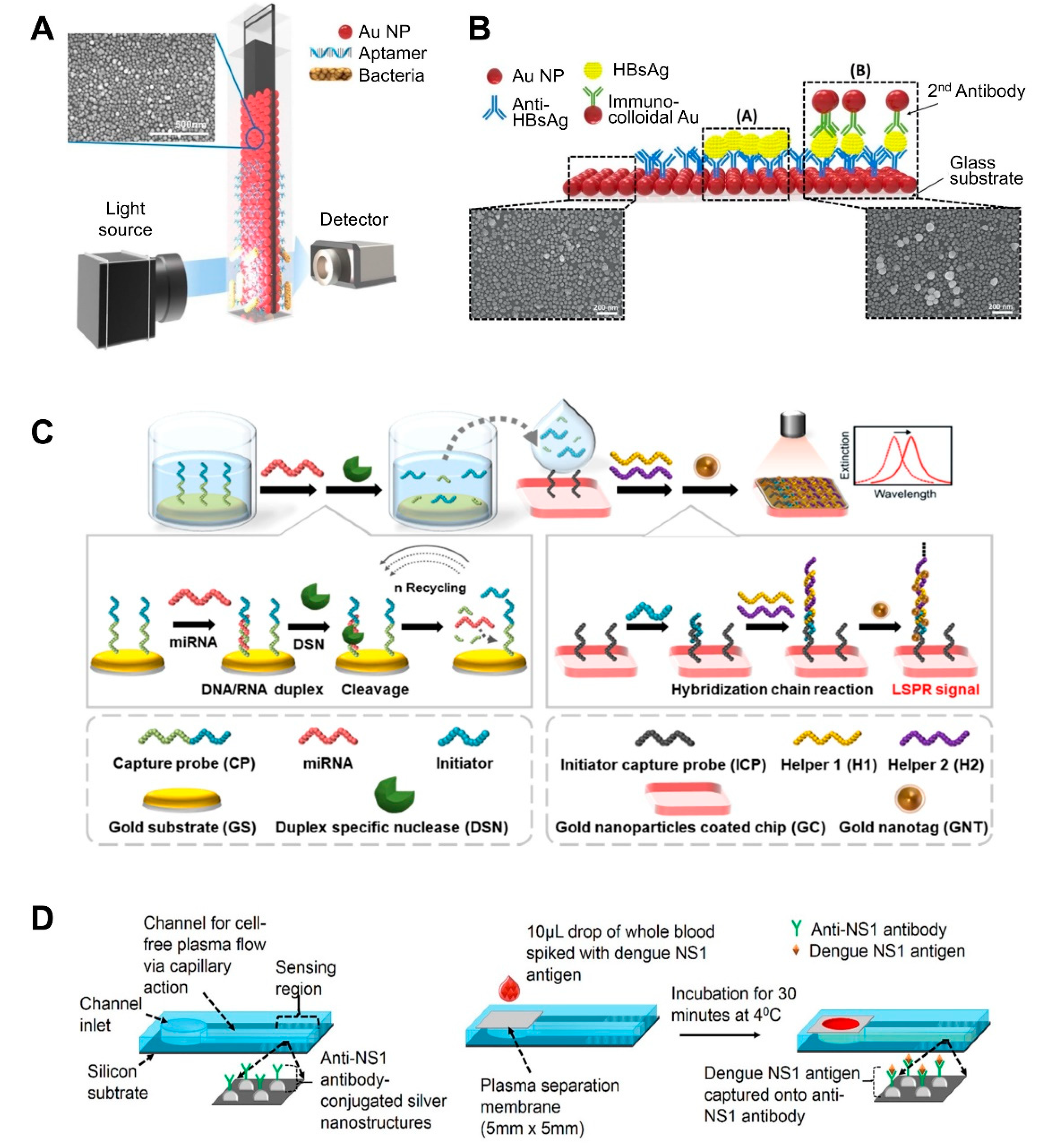
| NP-deposited flat substrate | AuNP on the glass slide | Aptamer | Salmonella typhimurium | 1.0 × 104 CFU/mL, 104 CFU/mL | >30–35 min | Pork meat | Fabrication of AuNP-coated transparent glass slide via a simple dipping adsorption method Use of APTES-immobilized glass slide to attach AuNPs | [57]; Figure 2A |
| Solid-based nanopatterned flatform | AuNP on the glass slide | Anti-CRP | C-reactive protein (CRP) | 0.01–10 μg/mL, 11.28 ng/mL | ND | ND | Fabrication of a plasmonically active strip by depositing AuNPs on an APTES-immobilized glass slide. Use of cysteine-protein G to attach a receptor. | [60] |
| Au nanorod (GNR) | Aptamer | 25-hydroxyvitamin D3 | 0.1–105 ng/mL, 0.1 ng/mL | ND | Human serum albumin sample | Use of citrate as a stabilizer of GNR: improving LSPR signal. | [62] | |
| Heteroassembled AuNPs | Antibody | Hepatitis B surface antigen | 100 fg/mL–10 ng/mL, 10 pg/mL | >10–15 min | Human serum | Use of a multi-layered plasmonic structure by linking different-sized AuNPs. | [59]; Figure 2B | |
| GNR on glass slide | Aptamer | OTA, AFB1, ATP, and K+ | 0.56 pM for OTA, 0.63 pM for AFB1, 0.87 pM for ATP, 1.05 pM for K+ | 30 min | Ground corn powder, Escherichia coli, human serum | Use of berberine as an LSPR signal enhancer, which incorporates into the G-quadruplex structure that forms when the aptamer binds to the analyte and undergoes a conformational change. | [61] | |
| GNR on glass slide | Aptamer | Saxitoxin | 5–10,000 μg/L, 2.46 μg/L | 30 min | Mussel sample | Use of newly developed aptamers by implementing the graphene oxide (GOx)-SELEX method. Recovery rate of 96.13~116.05%. | [115] | |
| AuNP on glass slide | Antibody | Alzheimer’s disease biomarkers | 4.9 fM for amyloid beta (Aβ)1–40,26 fM for Aβ1–42, and 23.6 fM for τ protein | ND | Human plasma | Multiplex detection using nanoparticles with different sizes and shapes, each of which was functionalized with various marker-specific antibodies. | [31] | |
| AuNP-coated glass slide | DNA | MicroRNAs (miRNAs) | 5 pM to 10 nM, 2.45 pM | ND | Mouse Sample (urine and plasma) | Incorporation of LSPR signal amplification strategy using a duplex-specific nuclease-mediated target recycling reaction. Use of Au NP coated with tannic acid (a hydrophilic polyphenol compound) that can interact with the phosphate backbone of DNA, thereby enhancing LSPR signal. | [63]; Figure 2C | |
| Ag nanoprism on glass | DNA probe | Bacterial DNA | 5 fg/μL of E. coli DNA, 300 cfu/mL | >15 min | ND | Fabrication of an LSPR platform by depositing Ag nanoprisms on poly-L-lysine-coated glass. Combined system consisting of microfluidic on-chip PCR and LSPRi using a digital micromirror device Real-time detection using a qPCR system. | [116] | |
| Ag nanocolumn on glass slide | Polymyxin B | Lipopolysaccharide endotoxin | 340 pg/mL | ND | ND | Use of 3-mercaptopropionic acid to stabilize the Ag nanocolumn against oxidation and nanoparticle detachment in aqueous environments. | [117] | |
| Ag nanocolumn on glass slide | Antibody | Prostate-Specific Antigen | 850 pg/mL | ND | ND | Use of 11-mercaptoundecanoic acid as a stabilizer of the Ag nanocolumn. | [118] | |
| Ag nanostructure on silicon substrate | NS1 antigen-specific antibody (IgG) | NS1 antigen of dengue virus | 0.06 μg/mL | >30 min | Whole blood | Fabrication of nanostructures by E-beam evaporation and thermal annealing of thin silver film. Integration of polyethersulfone membrane filter at the inlet of a biosensor for plasma separation. Small sample volume requirements (10 μL of whole blood sample). | [65]; Figure 2D | |
| Nickel-doped graphene (NDG) on self-assembled gold nanoislands (SAM AuNI) | GOx | 3-nitro-L-tyrosine (3-NT) | 0.1 pg/mL–10 ng/mL, 0.13 pg/mL | ND | Human serum | Fabrication of imprinted nanostructure by thermal annealing of Au, followed by spin coating and thermal annealing of graphene and nickel. Use of strong energy adsorption by π–π stacking interaction between NO2 site of 3-NT and NDG | [119] | |
| Poly(mPD-co-ASA) on SAM-AuNI | Poly(m-phenylenediamine-co-ani-line-2-sulfonic acid) (Poly(mPD-co-ASA)) | Pb2+ | 0.011 ppb–5 ppm, 0.011 ppb | ND | Drinking water | Use of poly(mPD-co-ASA) as a linker with AuNI and Pb2+ receptor. | [120] | |
| SAM-AuNIs | Anti-CD7 antibody | Exosome | 0.194–100 μg/mL, 0.194 μg/ml | ND | Serum, urine | Use of exosome properties with high affinity for AuNI due to its negative zeta potential value | [121] | |
| SAM-AuNIs | Anti-IgG | Human IgG antigen | 1 pM–100 pM, 1.188 pM | ND | Serum | [122] | ||
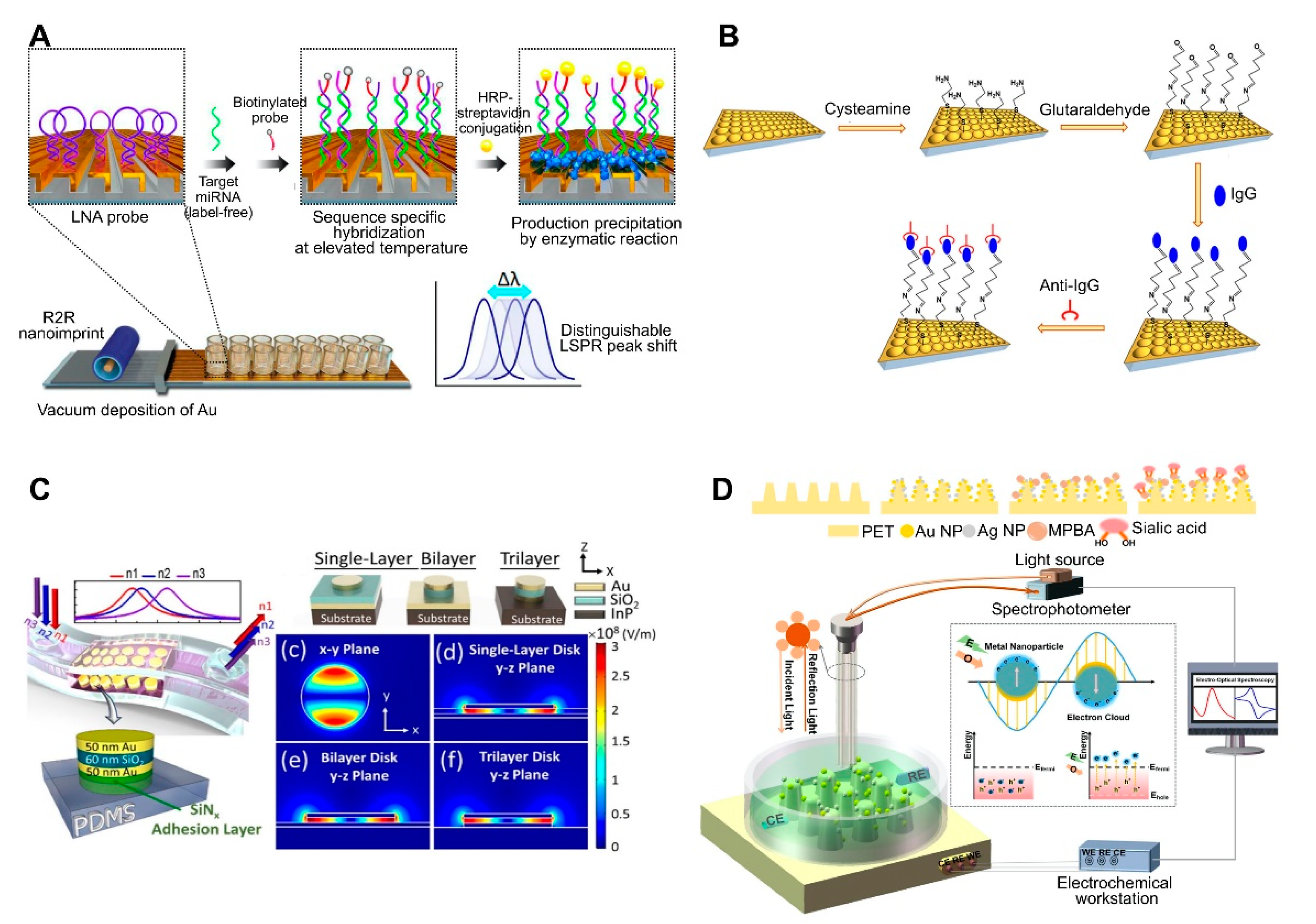
| 3D Au nanocups platform on polydimethylsiloxane (PDMS) surface | Antibody | Human IgG | 1.5 μg/mL | ND | ND | Fabrication of imprinted nanostructures by deposition of a polystyrene (PS) monolayer on glass, pouring PDMS on a PS layer, peeling off the PDMS film, and coating the PDMS substrate with an Au film. Fabrication of uniform and tunable platform by changing the PS size. | [50]; Figure 3B | |
| Metal–insulator–metal (MIM) nanodisks on PDMS | None | Cancer cell (adherent cell) | NA | ND | ND | Construction of a MIM nanodisk consisting of Au-SiO2-Au on an InP substrate. Fabrication of a flexible sensor by transferring a MIM nanodisk onto PDMS. | [51]; Figure 3C | |
| Au and AgNPs on PET cone array structure | Mercaptophenyl boronic acid | Sialic acid | 0.05–5 mM, 17 μM | ND | ND | Fabrication of core array nanostructures by depositing Au and AgNPs on (poly)ethylene terephthalate (PET). Combined system consisting of LSPR and an electrochemical sensing system. | [67]; Figure 3D | |
| Au-deposited 3D polyurethane acrylate (PUA) nanostructure | Locked nucleic acid | miRNAs | 13 fM (2.6 attomole in 200 μL) | ND | Primary cancer cell lines | Fabrication of 3D plasmonic nanostructure consisting of roll-to-roll nanoimprint lithography-used PUA nanograting pattern, followed by Au deposition. Detection of miRNA single-base mismatches down to the attomole level by incorporating a biotin-streptavidin-horseradish precipitation reaction. | [54]; Figure 3A | |
| Au nano-ellipsoid array on quartz substrate | Anti-CD63 antibody | Exosome | 1 ng/mL | <4 h | ND | Fabrication of nanostructures via AAO-templated Au deposition on a quartz substrate. Integration of LSPR and microfluidic systems. | [123] | |
| Au nanopillars on quartz coverslips | Anti-CD63 antibody | Exosome | ND | ND | MCF7 breastadenocarcinoma cells | Fabrication of Au nanopillar array by electron beam lithography. Enabled multiplexed measurement using LSPRi. | [52] | |
| Au nanopillar | Mercaptobenzoic acid | BSA | 234 pM | ND | ND | Working in the visible and infrared region by changing the patterned shapes and interpillar distances. | [53] | |
| Au film on glass wafer | Anti-IgG, anti-TNF-α, anti-CRP antibody | IgG, TNF-α, CRP | 10 ng/mL IgG, 10 ng/mL CRP | 3.5 h | ND | Fabrication of nanostructure using physical vapor evaporation followed by a rapid thermal annealing treatment. | [124] | |
| Polymethylmethacrylate (PMMA) on glass substrate | Aptamer | Staphylococcus aureus | 103 CFU/mL | 120 s | Milk | Fabrication of arrays of Au nanodisks on PMMA-treated glass substrate by using hole-mask colloidal lithography. Optimization of disk structure by varying diameter: improving LSPR signal. | [72] | |
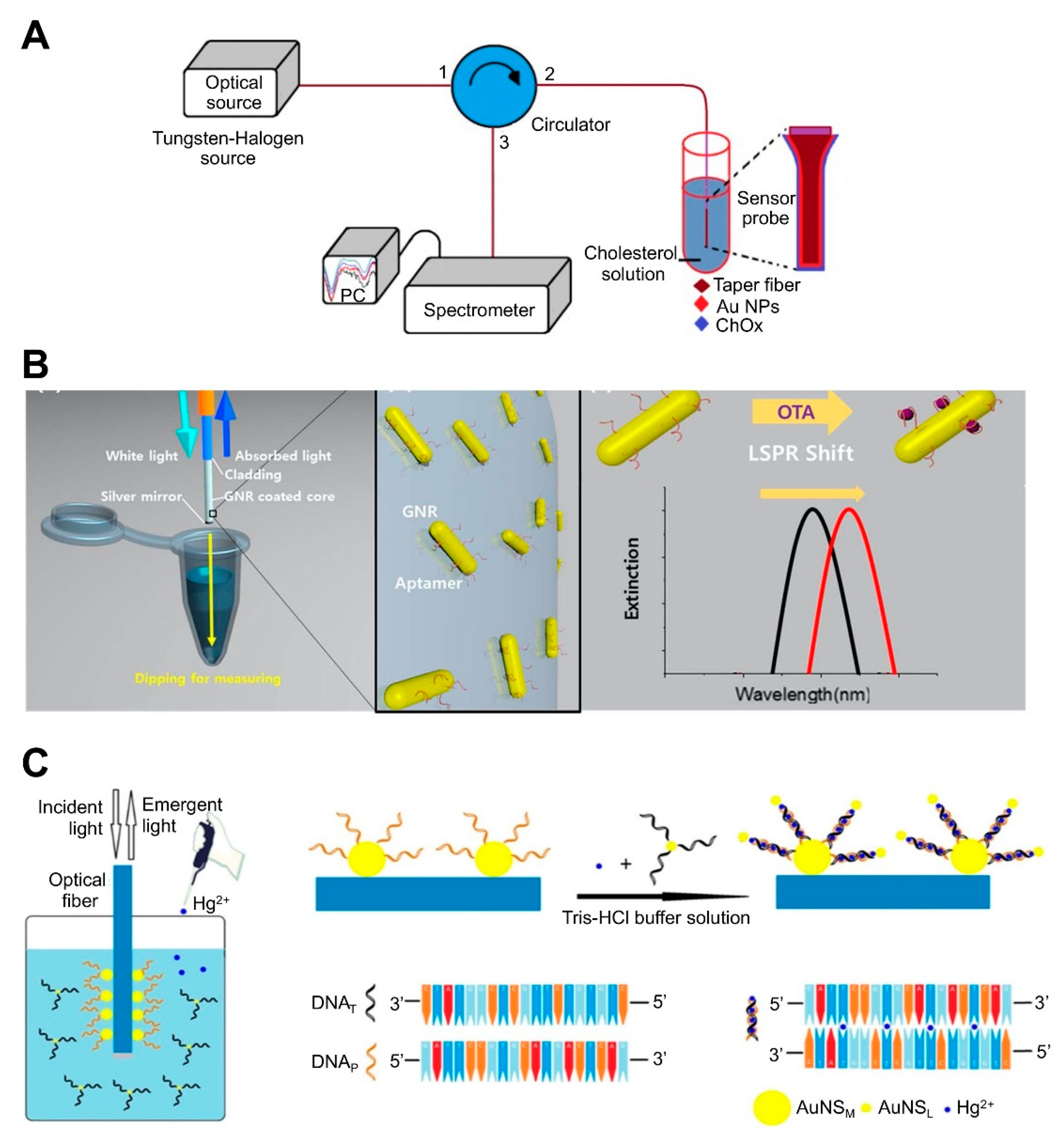
| NP-coated optic fiber-based platform | AuNPs-immobilized taperfiber | Cholesterol oxidase (ChOx) | Cholesterol | 10 nM–1 µM, 53.1 nM | ND | ND | Fabrication of sensing component by sequentially coating MUA-EDC/NHS-ChOx on AuNPs-immobilized fiber. | [85]; Figure 4A |
| GNRs immobilized on the optical fiber core surface | Aptamer | OTA | 10 pM to 100 nM, 12.0 pM | ND | Grape juice | Detection by simply dipping an optical fiber into a sample solution, allowing in situ detection. | [74]; Figure 4B | |
| AuNPs-coated optical fiber | Anti-transferrin, protein A | Transferrin, protein IgG | ND | ND | ND | Combined system consisting of capillary LSPR sensors and metal–oxide–semiconductor image sensors. Use of AuNPs-coated capillary as a microfluidic channel and sensing surface. Multiple detection for high throughput screening of biomolecular interactions | [96] | |
| Optical fiber with copper oxide nanoflower (CuO-NF) and Au NPs-coated GOx structure | 2-deoxy-D-glucose (2-DG) | Cancer cell | 1 × 102–1 × 106 cells/mL, 2–10 cells/mL | ND | ND | Use of multi-core fiber structure. Coating of optical fiber with GOx and CuO-NF: increasing surface area and adsorption capability. Discrimination of cancer cells using 2-DG that binds to GULP receptor: the presence of more GULP receptors on cancer cell, inducing a peak shift. Reusable through washing with PBS. | [79] | |
| AuNPs-coated optical fiber | Aptamer | Zearalenone (ZEN) | 1–480 ng/mL, 0.102 ng/mL | ND | Beer | Reusable by cutting and polishing a tip of optical fiber. | [80] | |
| Optical fiber | Anti-IgG antibody | IgG | 1 fg/mL to 100 fg/mL, 7 aM | 25–30 min | ND | Use of silver enhancer: amplifying the LSPR signal by catalytic reduction of silver around AuNPs. Use of U-bent optical fiber. | [90] | |
| Optical fiber | IgG antibody | Staphylococcus aureus | 3.1 CFU/mL | ND | ND | Use of the tapered singlemode-no core-singlemode fiber coupler structure. | [78] | |
| MoS2/AuNPs-coated optical fiber | Aptamer | Shigella sonnei | 1 – 1×109, 1.56 CFU/mL | 5 min | ND | Use of single mode fiber-multi-core fiber structure. | [91] | |
| AuPd alloy-coated plastic optical fiber | Anti-cortisol | Cortisol | 1 pg/mL | ND | ND | Use of plastic optical fiber. | [92] | |
| Au film-coated optical fiber | Aptamer, HER2 antibody | Breast cancer HER2 protein | 9.3 ng/mL (77.4 pM) | 10 min | ND | HER2 biomarker detection using sandwich assay with anti-HER2 ssDNA aptamer and HER2 antibody. | [93] | |
| ZnO/AuNP-coated optical fiber | Ascorbate oxidase | Ascorbic acid | 1 µM to 200 µM, 12.56 µM | ND | ND | Use of tapered optical fiber structure immobilized with ZnO-AuNPs. | [81] | |
| AuNP-modified the bare core | Probe DNA | Hg2+ | 1–50 nM, 0.7 nM | ND | Pond water | Functionalization of DNA-attached Au NP monolayer on optical fibers, resulting in an increase in the refractive index at the nanometer length region and near field coupling enhancement produced by close proximity to another-attached Au NPs via DNA-DNA hybridization Use of PAH, yielding enhanced sensitivity due to the higher density and less aggregation of Au NPs Reusability by dipping optical fiber into 1% SDS solution for 5 min | [75]; Figure 4C |
| Challenge | Performance Improvement Strategies | Reference(s) |
|---|---|---|
| Sensitivity | Conjugation of Qdots | [7] |
| NP core-satellite structure | [36] | |
| Use of heteroassembled sandwich structure with multiple layers of Au NPs | [59] | |
| Use of LSPR signal enhancer molecule (e.g., berberine) | [61] | |
| Combining LSPR and electrochemical sensing | [67] | |
| Incorporation of enzyme reaction-assisted signal amplification | [63] | |
| Construction of a 3D nanocup platform | [50] | |
| Implementation of a split aptamer | [124] | |
| Integration of microfluidics Incorporation of silver enhancement using catalytic reduction of silver around AuNPs. | [78,116,125] [90] | |
| Low cost, Large scale fabrication | Use of copper | [66] |
| Use of PDMS | [50] | |
| Use of silicon as a substrate Use of plastic optical fiber | [65] [92] | |
| Quantification | Use of double calibration curve method | [40] |
| Multiple detection | Use of NPs with different sizes and shapes Integration of microfluidic system containing multiple channels | [31] [78] |
| Real-time detection | Combined system consisting of microfluidic on-chip PCR and LSPRi Use of a microfluidic nanoplasmonic platform | [116] |
| [125] | ||
| Reproducibility | Fabrication of periodically ordered array using PS with different sizes via the imprinting method | [50] |
| Reusability | Washing with solution such as PBS or containing SDS Cutting and polishing a tip of optical fiber | [75,79,81] [80] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, S.M.; Lee, S.Y. Optical biosensors for the detection of pathogenic microorganisms. Trends Biotechnol. 2016, 34, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Olaru, A.; Bala, C.; Jaffrezic-Renault, N.; Aboul-Enein, H.Y. Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit. Rev. Anal. Chem. 2015, 45, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Paliwal, A.; Gupta, V.; Tomar, M. Refractive index tuning of SiO2 for long range surface plasmon resonance based biosensor. Biosens. Bioelectron. 2020, 168, 112508. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-enhanced Raman spectroscopy for bioanalysis: Reliability and challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, E.; Jimenez de Aberasturi, D.; Liz-Marzan, L.M. Surface-enhanced Raman scattering tags for three-dimensional bioimaging and biomarker detection. ACS Sens. 2019, 4, 1126–1137. [Google Scholar] [CrossRef]
- Qi, M.; Zhang, N.M.Y.; Li, K.; Tjin, S.C.; Wei, L. Hybrid plasmonic fiber-optic sensors. Sensors 2020, 20, 3266. [Google Scholar] [CrossRef]
- Adegoke, O.; Park, E.Y. Bright luminescent optically engineered core/alloyed shell quantum dots: An ultrasensitive signal transducer for dengue virus RNA via localized surface plasmon resonance-induced hairpin hybridization. J. Mater. Chem. B 2017, 5, 3047–3058. [Google Scholar] [CrossRef]
- Csáki, A.; Stranik, O.; Fritzsche, W. Localized surface plasmon resonance based biosensing. Expert Rev. Mol. Diagn. 2018, 18, 279–296. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Chen, Z.; Wang, X.; Choo, J.; Chen, L. Plasmonic colorimetric sensors based on etching and growth of noble metal nanoparticles: Strategies and applications. Biosens. Bioelectron. 2018, 114, 52–65. [Google Scholar] [CrossRef]
- Li, Z.; Leustean, L.; Inci, F.; Zheng, M.; Demirci, U.; Wang, S. Plasmonic-based platforms for diagnosis of infectious diseases at the point-of-care. Biotechnol. Adv. 2019, 37, 107440. [Google Scholar] [CrossRef] [PubMed]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized surface plasmon resonance biosensing: Current challenges and approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Chu, S.; Senthilnathan, K.; Babu, P.R.; Nakkeeran, K.; Li, Q. Recent advances in plasmonic sensor-based fiber optic probes for biological applications. Appl. Sci. 2019, 9, 949. [Google Scholar] [CrossRef] [Green Version]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape-and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Miller, M.M.; Lazarides, A.A. Sensitivity of metal nanoparticle surface plasmon resonance to the dielectric environment. J. Phys. Chem. B 2005, 109, 21556–21565. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [Green Version]
- Camden, J.P.; Dieringer, J.A.; Wang, Y.; Masiello, D.J.; Marks, L.D.; Schatz, G.C.; Van Duyne, R.P. Probing the structure of single-molecule surface-enhanced Raman scattering hot spots. J. Am. Chem. Soc. 2008, 130, 12616–12617. [Google Scholar] [CrossRef]
- Cottat, M.; Thioune, N.; Gabudean, A.-M.; Lidgi-Guigui, N.; Focsan, M.; Astilean, S.; de la Chapelle, M.L. Localized surface plasmon resonance (LSPR) biosensor for the protein detection. Plasmonics 2013, 8, 699–704. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, B.-C.; Oh, B.-K.; Choi, J.-W. Highly sensitive localized surface plasmon resonance immunosensor for label-free detection of HIV-1. Nanomedicine 2013, 9, 1018–1026. [Google Scholar] [CrossRef]
- Haes, A.J.; Van Duyne, R.P. A unified view of propagating and localized surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2004, 379, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Pasricha, R.; Sastry, M. Ultra-low level optical detection of mercuric ions using biogenic gold nanotriangles. Analyst 2012, 137, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Shumaker-Parry, J.S.; Rochholz, H.; Kreiter, M. Fabrication of crescent-shaped optical antennas. Adv. Mater. 2005, 17, 2131–2134. [Google Scholar] [CrossRef]
- Yang, H.; Owiti, E.O.; Jiang, X.; Li, S.; Liu, P.; Sun, X. Localized surface plasmon resonance dependence on misaligned truncated Ag nanoprism dimer. Nanoscale Res. Lett. 2017, 12, 1–6. [Google Scholar] [CrossRef]
- Millstone, J.E.; Hurst, S.J.; Métraux, G.S.; Cutler, J.I.; Mirkin, C.A. Colloidal gold and silver triangular nanoprisms. Small 2009, 5, 646–664. [Google Scholar] [CrossRef]
- Li, W.; Camargo, P.H.; Au, L.; Zhang, Q.; Rycenga, M.; Xia, Y. Etching and dimerization: A simple and versatile route to dimers of silver nanospheres with a range of sizes. Angew Chem. Int. Ed. 2010, 122, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Kessentini, S.; Barchiesi, D.; D’Andrea, C.; Toma, A.; Guillot, N.; Di Fabrizio, E.; Fazio, B.; Marago, O.M.; Gucciardi, P.G.; Lamy de la Chapelle, M. Gold dimer nanoantenna with slanted gap for tunable LSPR and improved SERS. J. Phys. Chem. C 2014, 118, 3209–3219. [Google Scholar] [CrossRef]
- Ci, X.; Wu, B.; Song, M.; Liu, Y.; Chen, G.; Wu, E.; Zeng, H. Tunable Fano resonances in heterogenous Al–Ag nanorod dimers. Appl. Phys. A 2014, 117, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhu, J.; Li, J.; Zhao, J. Misalign-dependent double plasmon modes “switch” of gold triangular nanoplate dimers. J. Appl. Phys. 2015, 117, 063102. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.U.; Song, S.; Kim, S.; Sim, S.J. A shape-code nanoplasmonic biosensor for multiplex detection of Alzheimer’s disease biomarkers. Biosens. Bioelectron. 2018, 101, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, L.; Ma, W.; Kuang, H.; Wang, L.; Xu, C. Triple Raman label-encoded gold nanoparticle trimers for simultaneous heavy metal ion detection. Small 2015, 11, 3435–3439. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, S.; Lin, M.; Chen, X.; Lin, S.; Du, X.; Li, H.; Ye, H.; Qiu, B.; Lin, Z. Colorimetric detection of microcystin-LR based on disassembly of orient-aggregated gold nanoparticle dimers. Biosens. Bioelectron. 2015, 68, 475–480. [Google Scholar] [CrossRef]
- Yamazoe, S.; Takano, S.; Kurashige, W.; Yokoyama, T.; Nitta, K.; Negishi, Y.; Tsukuda, T. Hierarchy of bond stiffnesses within icosahedral-based gold clusters protected by thiolates. Nat. Commun. 2016, 7, 10414. [Google Scholar] [CrossRef] [PubMed]
- Wagener, P.; Jakobi, J.; Rehbock, C.; Chakravadhanula, V.S.K.; Thede, C.; Wiedwald, U.; Bartsch, M.; Kienle, L.; Barcikowski, S. Solvent-surface interactions control the phase structure in laser-generated iron-gold core-shell nanoparticles. Sci. Rep. 2016, 6, 23352. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-B.; Zhai, T.-T.; Liang, Y.-Y.; Wang, Y.-B.; Xia, X.-H. Gold core-satellite nanostructure linked by oligonucleotides for detection of glutathione with LSPR scattering spectrum. Talanta 2019, 193, 123–127. [Google Scholar] [CrossRef]
- Fang, Y.; Chang, W.-S.; Willingham, B.; Swanglap, P.; Dominguez-Medina, S.; Link, S. Plasmon emission quantum yield of single gold nanorods as a function of aspect ratio. ACS Nano 2012, 6, 7177–7184. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, T.; Li, W.; Hou, L.; Liu, J.; Gong, Q. Single-molecule spontaneous emission in the vicinity of an individual gold nanorod. J. Phys. Chem. C 2011, 115, 15822–15828. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, G.; Li, W.; Liu, J.; Hou, L.; Perriat, P.; Martini, M.; Tillement, O.; Gong, Q. Optimally designed nanoshell and matryoshka-nanoshell as a plasmonic-enhanced fluorescence probe. J. Phys. Chem. C 2012, 116, 8804–8812. [Google Scholar] [CrossRef]
- Liu, B.; Huang, R.; Yu, Y.; Su, R.; Qi, W.; He, Z. Gold nanoparticle-aptamer-based LSPR sensing of ochratoxin A at a widened detection range by double calibration curve method. Front. Chem. 2018, 6, 94. [Google Scholar] [CrossRef]
- Jeon, H.C.; Heo, C.J.; Lee, S.Y.; Yang, S.M. Hierarchically ordered arrays of noncircular silicon nanowires featured by holographic lithography toward a high-fidelity sensing platform. Adv. Funct. Mater. 2012, 22, 4268–4274. [Google Scholar] [CrossRef]
- Li, W.; Xue, J.; Jiang, X.; Zhou, Z.; Ren, K.; Zhou, J. Low-cost replication of plasmonic gold nanomushroom arrays for transmission-mode and multichannel biosensing. RSC Adv. 2015, 5, 61270–61276. [Google Scholar] [CrossRef]
- Im, H.; Lee, S.H.; Wittenberg, N.J.; Johnson, T.W.; Lindquist, N.C.; Nagpal, P.; Norris, D.J.; Oh, S.-H. Template-stripped smooth Ag nanohole arrays with silica shells for surface plasmon resonance biosensing. ACS Nano 2011, 5, 6244–6253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-González, P.; Albella, P.; Golmar, F.; Arzubiaga, L.; Casanova, F.; Hueso, L.E.; Aizpurua, J.; Hillenbrand, R. Visualizing the near-field coupling and interference of bonding and anti-bonding modes in infrared dimer nanoantennas. Opt. Express 2013, 21, 1270–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muskens, O.L.; Giannini, V.; Sánchez-Gil, J.; Rivas, J.G. Optical scattering resonances of single and coupled dimer plasmonic nanoantennas. Opt. Express 2007, 15, 17736–17746. [Google Scholar] [CrossRef] [Green Version]
- Hao, F.; Nehl, C.L.; Hafner, J.H.; Nordlander, P. Plasmon resonances of a gold nanostar. Nano Lett. 2007, 7, 729–732. [Google Scholar] [CrossRef]
- Fischer, J.; Vogel, N.; Mohammadi, R.; Butt, H.-J.; Landfester, K.; Weiss, C.K.; Kreiter, M. Plasmon hybridization and strong near-field enhancements in opposing nanocrescent dimers with tunable resonances. Nanoscale 2011, 3, 4788–4797. [Google Scholar] [CrossRef]
- Shi, W.; Sahoo, Y.; Swihart, M.T.; Prasad, P. Gold nanoshells on polystyrene cores for control of surface plasmon resonance. Langmuir 2005, 21, 1610–1617. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Wang, Y.; Sun, K.; Chen, X.; Chen, H.; Zhou, J. Reproducible plasmonic nanopyramid array of various metals for highly sensitive refractometric and surface-enhanced Raman biosensing. ACS Omega 2018, 3, 14181–14187. [Google Scholar] [CrossRef]
- Focsan, M.; Craciun, A.; Potara, M.; Leordean, C.; Vulpoi, A.; Maniu, D.; Astilean, S. Flexible and tunable 3D gold nanocups platform as plasmonic biosensor for specific dual LSPR-SERS immuno-detection. Sci. Rep. 2017, 7, 14240. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-Y.; Lin, H.-T.; Lai, M.-S.; Shieh, T.-Y.; Peng, C.-C.; Shih, M.-H.; Tung, Y.-C. Flexible localized surface plasmon resonance sensor with metal–insulator–metal nanodisks on PDMS substrate. Sci. Rep. 2018, 8, 11812. [Google Scholar] [CrossRef]
- Raghu, D.; Christodoulides, J.A.; Christophersen, M.; Liu, J.L.; Anderson, G.P.; Robitaille, M.; Byers, J.M.; Raphael, M.P. Nanoplasmonic pillars engineered for single exosome detection. PLoS ONE 2018, 13, e0202773. [Google Scholar] [CrossRef] [Green Version]
- Rippa, M.; Castagna, R.; Tkachenko, V.; Zhou, J.; Petti, L. Engineered nanopatterned substrates for high-sensitive localized surface plasmon resonance: An assay on biomacromolecules. J. Mater. Chem. B 2017, 5, 5473–5478. [Google Scholar] [CrossRef]
- Na, H.-K.; Wi, J.-S.; Son, H.Y.; Ok, J.G.; Huh, Y.-M.; Lee, T.G. Discrimination of single nucleotide mismatches using a scalable, flexible, and transparent three-dimensional nanostructure-based plasmonic miRNA sensor with high sensitivity. Biosens. Bioelectron. 2018, 113, 39–45. [Google Scholar] [CrossRef]
- Acimovic, S.S.; Ortega, M.A.; Sanz, V.; Berthelot, J.; Garcia-Cordero, J.L.; Renger, J.; Maerkl, S.J.; Kreuzer, M.P.; Quidant, R. LSPR chip for parallel, rapid, and sensitive detection of cancer markers in serum. Nano Lett. 2014, 14, 2636–2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, G.A.; Estevez, M.-C.; Soler, M.; Lechuga, L.M. Recent advances in nanoplasmonic biosensors: Applications and lab-on-a-chip integration. Nanophotonics 2017, 6, 123–136. [Google Scholar] [CrossRef]
- Oh, S.Y.; Heo, N.S.; Shukla, S.; Cho, H.-J.; Vilian, A.E.; Kim, J.; Lee, S.Y.; Han, Y.-K.; Yoo, S.M.; Huh, Y.S. Development of gold nanoparticle-aptamer-based LSPR sensing chips for the rapid detection of Salmonella typhimurium in pork meat. Sci. Rep. 2017, 7, 10130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Q.; Li, W.; Tang, S.; Hu, Y.; Nie, Z.; Huang, Y.; Yao, S. A simple “clickable” biosensor for colorimetric detection of copper (II) ions based on unmodified gold nanoparticles. Biosens. Bioelectron. 2013, 41, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Oh, S.Y.; Shukla, S.; Hong, S.B.; Heo, N.S.; Bajpai, V.K.; Chun, H.S.; Jo, C.-H.; Choi, B.G.; Huh, Y.S. Heteroassembled gold nanoparticles with sandwich-immunoassay LSPR chip format for rapid and sensitive detection of hepatitis B virus surface antigen (HBsAg). Biosens. Bioelectron. 2018, 107, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Heo, N.S.; Bajpai, V.K.; Jang, S.-C.; Ok, G.; Cho, Y.; Huh, Y.S. Development of a cuvette-based LSPR sensor chip using a plasmonically active transparent strip. Front. Bioeng. Biotechnol. 2019, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Byun, J.-Y.; Jang, H.; Hong, D.; Kim, M.-G. A highly sensitive and widely adaptable plasmonic aptasensor using berberine for small-molecule detection. Biosens. Bioelectron. 2017, 97, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Lee, W.; Park, J.; Park, H.; Kim, M.; Kim, W.; Hong, J.; Park, J. Wide-range direct detection of 25-hydroxyvitamin D3 using polyethylene-glycol-free gold nanorod based on LSPR aptasensor. Biosens. Bioelectron. 2021, 181, 113118. [Google Scholar] [CrossRef] [PubMed]
- Ki, J.; Lee, H.Y.; Son, H.Y.; Huh, Y.-M.; Haam, S. Sensitive plasmonic detection of miR-10b in biological samples using enzyme-assisted target recycling and developed LSPR probe. ACS Appl. Mater. Interfaces 2019, 11, 18923–18929. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Yoo, S.M. DNA-modifying enzyme reaction-based biosensors for disease diagnostics: Recent biotechnological advances and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Austin Suthanthiraraj, P.P.A.; Sen, A.K. Localized surface plasmon resonance (LSPR) biosensor based on thermally annealed silver nanostructures with on-chip blood-plasma separation for the detection of dengue non-structural protein NS1 antigen. Biosens. Bioelectron. 2019, 132, 38–46. [Google Scholar] [CrossRef]
- Kim, D.-K.; Yoo, S.M.; Park, T.J.; Yoshikawa, H.; Tamiya, E.; Park, J.Y.; Lee, S.Y. Plasmonic properties of the multispot copper-capped nanoparticle array chip and its application to optical biosensors for pathogen detection of multiplex DNAs. Anal. Chem. 2011, 83, 6215–6222. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Lu, Y.; Zhu, L.; Li, C.; Hu, L.; Li, J.; Jiang, J.; Low, S.; Liu, Q. Mutual promotion of electrochemical-localized surface plasmon resonance on nanochip for sensitive sialic acid detection. Biosens. Bioelectron. 2018, 117, 32–39. [Google Scholar] [CrossRef]
- Valsecchi, C.; Jones, T.; Wang, C.; Lochbihler, H.; Menezes, J.W.; Brolo, A.G. Low-cost leukemic serum marker screening using large area nanohole arrays on plastic substrates. ACS Sens. 2016, 1, 1103–1109. [Google Scholar] [CrossRef]
- Kahraman, M.; Daggumati, P.; Kurtulus, O.; Seker, E.; Wachsmann-Hogiu, S. Fabrication and characterization of flexible and tunable plasmonic nanostructures. Sci. Rep. 2013, 3, 3396. [Google Scholar] [CrossRef] [Green Version]
- Kahraman, M.; Wachsmann-Hogiu, S. Label-free and direct protein detection on 3D plasmonic nanovoid structures using surface-enhanced Raman scattering. Anal. Chim. Acta. 2015, 856, 74–81. [Google Scholar] [CrossRef]
- Lee, C.; Carney, R.P.; Hazari, S.; Smith, Z.J.; Knudson, A.; Robertson, C.S.; Lam, K.S.; Wachsmann-Hogiu, S. 3D plasmonic nanobowl platform for the study of exosomes in solution. Nanoscale 2015, 7, 9290–9297. [Google Scholar] [CrossRef] [PubMed]
- Khateb, H.; Klös, G.; Meyer, R.L.; Sutherland, D.S. Development of a label-free LSPR-apta sensor for Staphylococcus aureus detection. ACS Appl. Biol. Mater. 2020, 3, 3066–3077. [Google Scholar] [CrossRef]
- Kaye, S.; Zeng, Z.; Sanders, M.; Chittur, K.; Koelle, P.M.; Lindquist, R.; Manne, U.; Lin, Y.; Wei, J. Label-free detection of DNA hybridization with a compact LSPR-based fiber-optic sensor. Analyst 2017, 142, 1974–1981. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.-H.; Byun, J.-Y.; Kim, J.H.; Kim, M.-G. An optical fiber-based LSPR aptasensor for simple and rapid in-situ detection of ochratoxin A. Biosens. Bioelectron. 2018, 102, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Bian, C.; Sun, J.; Tong, J.; Xia, S. A wavelength-modulated localized surface plasmon resonance (LSPR) optical fiber sensor for sensitive detection of mercury (II) ion by gold nanoparticles-DNA conjugates. Biosens. Bioelectron. 2018, 114, 15–21. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Li, S.; Zhang, Y.; Sun, B.; Yu, Y.; Ren, H.; Jiang, S.; Yue, W. LSPR optical fiber biosensor based on a 3D composite structure of gold nanoparticles and multilayer graphene films. Opt. Express 2020, 28, 6071–6083. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Albert, J. Review of plasmonic fiber optic biochemical sensors: Improving the limit of detection. Anal. Bioanal. Chem. 2015, 407, 3883–3897. [Google Scholar] [CrossRef]
- Chen, L.; Leng, Y.-K.; Liu, B.; Liu, J.; Wan, S.-P.; Wu, T.; Yuan, J.; Shao, L.; Gu, G.; Fu, Y.Q.; et al. Ultrahigh-sensitivity label-free optical fiber biosensor based on a tapered singlemode- no core-singlemode coupler for Staphylococcus aureus detection. Sens. Actuators B Chem. 2020, 320, 128283. [Google Scholar]
- Singh, R.; Kumar, S.; Liu, F.Z.; Shuang, C.; Zhang, B.; Jha, R.; Kaushik, B.K. Etched multicore fiber sensor using copper oxide and gold nanoparticles decorated graphene oxide structure for cancer cells detection. Biosens. Bioelectron. 2020, 168, 112557. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiong, M.; Yan, H. A portable optical fiber biosensor for the detection of zearalenone based on the localized surface plasmon resonance. Sens. Actuators B Chem. 2021, 336, 129752. [Google Scholar] [CrossRef]
- Zhu, G.; Singh, L.; Wang, Y.; Singh, R.; Zhang, B.; Liu, F.; Kaushik, B.K.; Kumar, S. Tapered optical fiber-based LSPR biosensor for ascorbic acid detection. Photonic Sens. 2020. [Google Scholar] [CrossRef]
- Gao, S.; Qiu, H.; Zhang, C.; Jiang, S.; Li, Z.; Liu, X.; Yue, W.; Yang, C.; Huo, Y.; Feng, D. Absorbance response of a graphene oxide coated U-bent optical fiber sensor for aqueous ethanol detection. RSC Adv. 2016, 6, 15808–15815. [Google Scholar] [CrossRef]
- Zakaria, R.; Kam, W.; Ong, Y.; Yusoff, S.; Ahmad, H.; Mohammed, W.S. Fabrication and simulation studies on D-shaped optical fiber sensor via surface plasmon resonance. J. Mod. Opt. 2017, 64, 1443–1449. [Google Scholar] [CrossRef]
- Al-Qazwini, Y.; Noor, A.; Yaacob, M.H.; Harun, S.W.; Mahdi, M. Experimental realization and performance evaluation of refractive index SPR sensor based on unmasked short tapered multimode-fiber operating in aqueous environments. Sens. Actuators A Phys. 2015, 236, 38–43. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, B.K.; Singh, R.; Chen, N.-K.; Yang, Q.S.; Zhang, X.; Wang, W.; Zhang, B. LSPR-based cholesterol biosensor using a tapered optical fiber structure. Biomed. Opt. Express 2019, 10, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Mukherji, S.; Mukherji, S. Probing the localized surface plasmon field of a gold nanoparticle-based fibre optic biosensor. Plasmonics 2016, 11, 753–761. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, S.; Zheng, X.; Wang, Y.; Xu, W. Optical fiber LSPR biosensor prepared by gold nanoparticle assembly on polyelectrolyte multilayer. Sensors 2010, 10, 3585–3596. [Google Scholar] [CrossRef] [Green Version]
- Hall, W.P.; Modica, J.; Anker, J.; Lin, Y.; Mrksich, M.; Van Duyne, R.P. A conformation-and ion-sensitive plasmonic biosensor. Nano Lett. 2011, 11, 1098–1105. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Das, A.; Zhang, X.; Schatz, G.C.; Sligar, S.G.; Van Duyne, R.P. Resonance surface plasmon spectroscopy: Low molecular weight substrate binding to cytochrome P450. J. Am. Chem. Soc. 2006, 128, 11004–11005. [Google Scholar] [CrossRef]
- Bandaru, R.; Divagar, M.; Khanna, S.; Danny, C.G.; Gupta, S.; Janakiraman, V.; Sai, V.V.R. U-bent fiber optic plasmonic biosensor platform for ultrasensitive analyte detection. Sens. Actuators B Chem. 2020, 321, 128463. [Google Scholar] [CrossRef]
- Kumar, S.; Guo, Z.; Singh, R.; Wang, Q. MoS2 functionalized multicore fiber probes for selective detection of shigella bacteria based on localized plasmon. J. Lightw. Technol. 2020. [Google Scholar] [CrossRef]
- Leitão, C.; Leal-Junior, A.; Almeida, A.R.; Pereira, S.O.; Costa, F.M.; Pinto, J.L.; Marques, C. Cortisol AuPd plasmonic unclad POF biosensor. Biotechnol. Rep. 2021, 29, e00587. [Google Scholar] [CrossRef] [PubMed]
- Loyez, M.; Lobry, M.; Hassan, E.M.; De Rosa, M.C.; Caucheteur, C.; Wattiez, R. HER2 breast cancer biomarker detection using a sandwich optical fiber assay. Talanta 2021, 221, 121452. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, S.S.; Šípová, H.; Emilsson, G.; Dahlin, A.B.; Antosiewicz, T.J.; Käll, M. Superior LSPR substrates based on electromagnetic decoupling for on-a-chip high-throughput label-free biosensing. Light Sci. Appl. 2017, 6, e17042. [Google Scholar] [CrossRef] [PubMed]
- Augel, L.; Berkmann, F.; Latta, D.; Fischer, I.A.; Bechler, S.; Elogail, Y.; Kostecki, K.; Potje-Kamloth, K.; Schulze, J. Optofluidic sensor system with Ge PIN photodetector for CMOS-compatible sensing. Microfluid. Nanofluid. 2017, 21, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, N.; Li, P.; Yu, L.; Chen, S.; Zhang, Y.; Jing, Z.; Peng, W. Low-cost localized surface plasmon resonance biosensing platform with a response enhancement for protein detection. Nanomaterials 2019, 9, 1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakoor, A.; Cheah, B.C.; Hao, D.; Al-Rawhani, M.; Nagy, B.; Grant, J.; Dale, C.; Keegan, N.; McNeil, C.; Cumming, D.R. Plasmonic sensor monolithically integrated with a CMOS photodiode. ACS Photonics 2016, 3, 1926–1933. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Kwon, Y.S.; Kim, J.H.; Gu, M.B. Multiple GO-SELEX for efficient screening of flexible aptamers. Chem. Commun. 2014, 50, 10513–10516. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, H.P.; Smiley, R.D.; Jaykus, L.-A. Selection of DNA aptamers for capture and detection of Salmonella typhimurium using a whole-cell SELEX approach in conjunction with cell sorting. Appl. Microbiol. Biotechnol. 2013, 97, 3677–3686. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Raston, N.H.A.; Gu, M.B. An ultra-sensitive colorimetric detection of tetracyclines using the shortest aptamer with highly enhanced affinity. Chem. Commun. 2014, 50, 40–42. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Wu, J.; Yin, B.; Jiang, X. Click chemistry-mediated nanosensors for biochemical assays. Theranostics 2016, 6, 969. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.-Y.; Yuan, F.; Zhang, X.-H.; Zhou, Y.-L.; Zhang, X.-X. Simultaneous multiple single nucleotide polymorphism detection based on click chemistry combined with DNA-encoded probes. Chem. Sci. 2018, 9, 3335–3340. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Zhou, N.; Yuan, M.; Li, D.; Yang, D. Tunable surface plasmon resonance frequencies of monodisperse indium tin oxide nanoparticles by controlling composition, size, and morphology. Nanoscale Res. Lett. 2014, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Manthiram, K.; Alivisatos, A.P. Tunable localized surface plasmon resonances in tungsten oxide nanocrystals. J. Am. Chem. Soc. 2012, 134, 3995–3998. [Google Scholar] [CrossRef]
- Wieneke, R.; Raulf, A.; Kollmannsperger, A.; Heilemann, M.; Tampé, R. SLAP: Small labeling pair for single-molecule super-resolution imaging. Angew Chem. Int. Ed. 2015, 54, 10216–10219. [Google Scholar] [CrossRef]
- Hoffmann, J.E.; Plass, T.; Nikić, I.; Aramburu, I.V.; Koehler, C.; Gillandt, H.; Lemke, E.A.; Schultz, C. Highly stable trans-cyclooctene amino Acids for live-cell labeling. Chem. Eur. J. 2015, 21, 12266–12270. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, S.; Gao, D.; Hu, D.; Gong, P.; Sheng, Z.; Deng, J.; Ma, Y.; Cai, L. Click-functionalized compact quantum dots protected by multidentate-imidazole ligands: Conjugation-ready nanotags for living-virus labeling and imaging. J. Am. Chem. Soc. 2012, 134, 8388–8391. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, P.; Gao, D.; Zhang, Y.; Li, P.; Liu, L.; Wang, C.; Wang, H.; Ma, Y.; Cai, L. Noninvasive visualization of respiratory viral infection using bioorthogonal conjugated near-infrared-emitting quantum dots. ACS Nano 2014, 8, 5468–5477. [Google Scholar] [CrossRef]
- Seker, U.O.S.; Demir, H.V. Material binding peptides for nanotechnology. Molecules 2011, 16, 1426–1451. [Google Scholar] [CrossRef] [Green Version]
- Wright, L.B.; Palafox-Hernandez, J.P.; Rodger, P.M.; Corni, S.; Walsh, T.R. Facet selectivity in gold binding peptides: Exploiting interfacial water structure. Chem. Sci. 2015, 6, 5204–5214. [Google Scholar] [CrossRef] [Green Version]
- Kodama, T.; Yoshihara, A.; Goel, I.; Sekino, M.; Kuwahata, A.; Yoshimori, A.; Murayama, Y.; Ishihara, K.; Ekdahl, K.N.; Nilsson, B. Identification of metal-binding peptides and their conjugation onto nanoparticles of superparamagnetic iron oxides and liposomes. ACS Appl. Mater. Interfaces 2020, 12, 24623–24634. [Google Scholar] [CrossRef] [PubMed]
- Akcapinar, G.B.; Sezerman, O.U. Computational approaches for de novo design and redesign of metal-binding sites on proteins. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haberal, I.; Oğul, H. Prediction of protein metal binding sites using deep neural networks. Mol. Inform. 2019, 38, 1800169. [Google Scholar] [CrossRef]
- Chang, K.; Wang, S.; Zhang, H.; Guo, Q.; Hu, X.; Lin, Z.; Sun, H.; Jiang, M.; Hu, J. Colorimetric detection of melamine in milk by using gold nanoparticles-based LSPR via optical fibers. PLoS ONE 2017, 12, e0177131. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-J.; Park, J.-H.; Lee, B.; Kim, M.-G. Label-free direct detection of saxitoxin based on a localized surface plasmon resonance aptasensor. Toxins 2019, 11, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haber, J.; Gascoyne, P.; Sokolov, K. Rapid real-time recirculating PCR using localized surface plasmon resonance (LSPR) and piezo-electric pumping. Lab. Chip. 2017, 17, 2821–2830. [Google Scholar] [CrossRef]
- Zandieh, M.; Hosseini, S.N.; Vossoughi, M.; Khatami, M.; Abbasian, S.; Moshaii, A. Label-free and simple detection of endotoxins using a sensitive LSPR biosensor based on silver nanocolumns. Anal. Biochem. 2018, 548, 96–101. [Google Scholar] [CrossRef]
- Taghavi, A.; Rahbarizadeh, F.; Abbasian, S.; Moshaii, A. Label-free LSPR prostate-specific antigen immune-sensor based on GLAD-fabricated silver nano-columns. Plasmonics 2019, 1–8. [Google Scholar] [CrossRef]
- Ng, S.P.; Qiu, G.; Ding, N.; Lu, X.; Wu, C.-M.L. Label-free detection of 3-nitro-l-tyrosine with nickel-doped graphene localized surface plasmon resonance biosensor. Biosens. Bioelectron. 2017, 89, 468–476. [Google Scholar] [CrossRef]
- Qiu, G.; Ng, S.P.; Liang, X.; Ding, N.; Chen, X.; Wu, C.-M.L. Label-free LSPR detection of trace lead (II) ions in drinking water by synthetic poly (mPD-co-ASA) nanoparticles on gold nanoislands. Anal. Chem. 2017, 89, 1985–1993. [Google Scholar] [CrossRef]
- Thakur, A.; Qiu, G.; Siu-Pang, N.; Guan, J.; Yue, J.; Lee, Y.; Wu, C.-M.L. Direct detection of two different tumor-derived extracellular vesicles by SAM-AuNIs LSPR biosensor. Biosens. Bioelectron. 2017, 94, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Ng, S.P.; Wu, L.C.-M. Dielectric functionalization for differential phase detecting localized surface plasmon resonance biosensor. Sens. Actuators B Chem. 2016, 234, 247–254. [Google Scholar] [CrossRef]
- Lv, X.; Geng, Z.; Su, Y.; Fan, Z.; Wang, S.; Fang, W.; Chen, H. Label-Free Exosome detection based on a low-cost plasmonic biosensor array integrated with microfluidics. Langmuir 2019, 35, 9816–9824. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-S.; Chen, P.-P.; Lin, T.-H.; Huang, N.-T. A Localized surface plasmon resonance (LSPR) sensor integrated automated microfluidic system for multiplex inflammatory biomarker detection. Analyst 2020, 145, 7654. [Google Scholar] [CrossRef]
- Roether, J.; Chu, K.-Y.; Willenbacher, N.; Shen, A.Q.; Bhalla, N. Real-time monitoring of DNA immobilization and detection of DNA polymerase activity by a microfluidic nanoplasmonic platform. Biosens. Bioelectron. 2019, 142, 111528. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.M.; Park, J.S.; Jung, S.-W.; Yeom, J.; Yoo, S.M. Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors. Sensors 2021, 21, 3191. https://doi.org/10.3390/s21093191
Kim DM, Park JS, Jung S-W, Yeom J, Yoo SM. Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors. Sensors. 2021; 21(9):3191. https://doi.org/10.3390/s21093191
Chicago/Turabian StyleKim, Dong Min, Jong Seong Park, Seung-Woon Jung, Jinho Yeom, and Seung Min Yoo. 2021. "Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors" Sensors 21, no. 9: 3191. https://doi.org/10.3390/s21093191
APA StyleKim, D. M., Park, J. S., Jung, S.-W., Yeom, J., & Yoo, S. M. (2021). Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors. Sensors, 21(9), 3191. https://doi.org/10.3390/s21093191





