Spatial–Temporal Pattern and Stability Analysis of Zooplankton Community Structure in the Lower Yellow River in China
Abstract
1. Introduction
2. Study Area and Research Methods
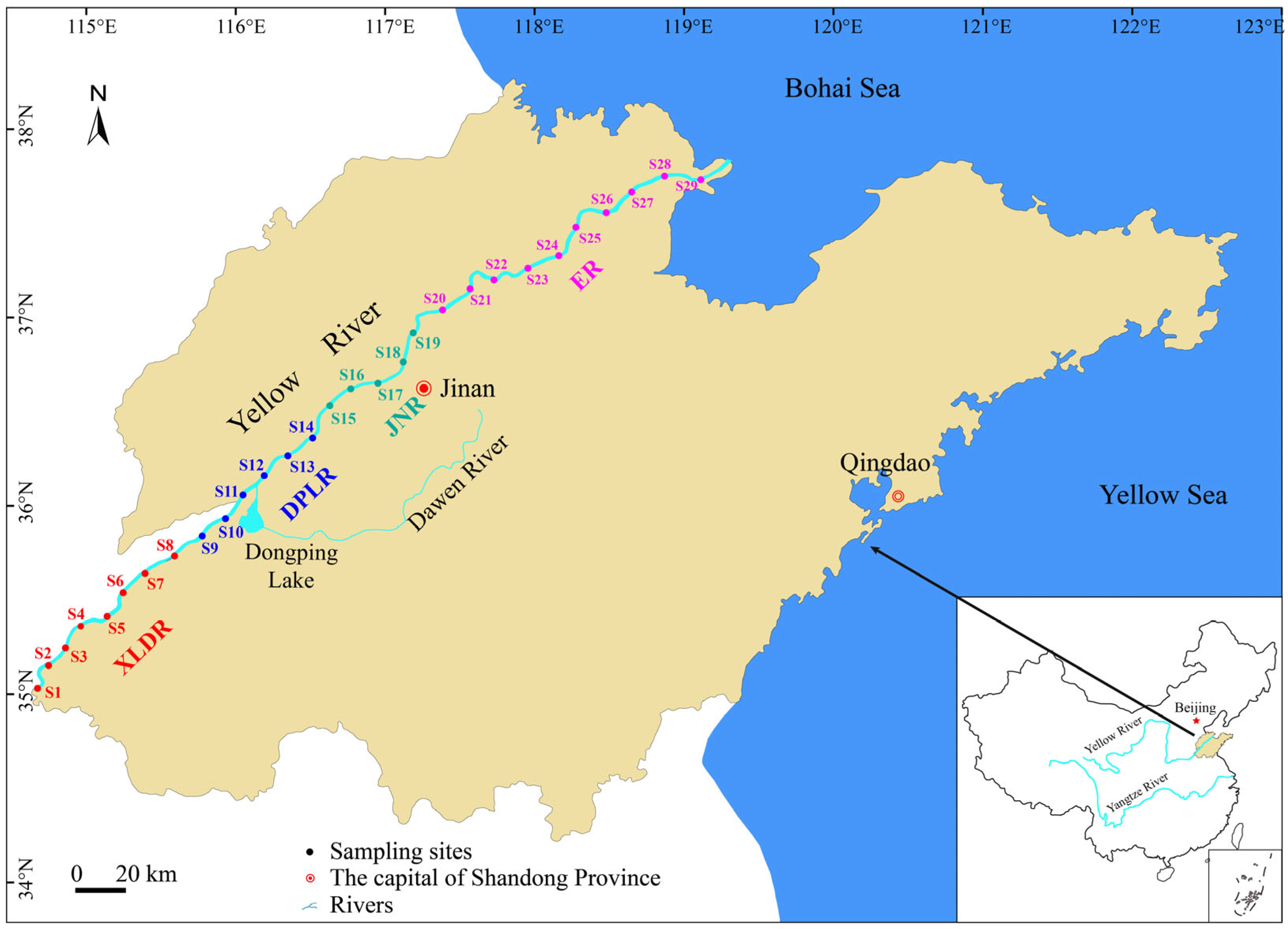
2.1. Study Area Description
2.2. Sample Designing
2.3. Sample Collection and Identification
2.4. Water Environmental Factor Determination
2.5. Data Statistical Analysis
3. Results
3.1. Species Composition of Zooplankton
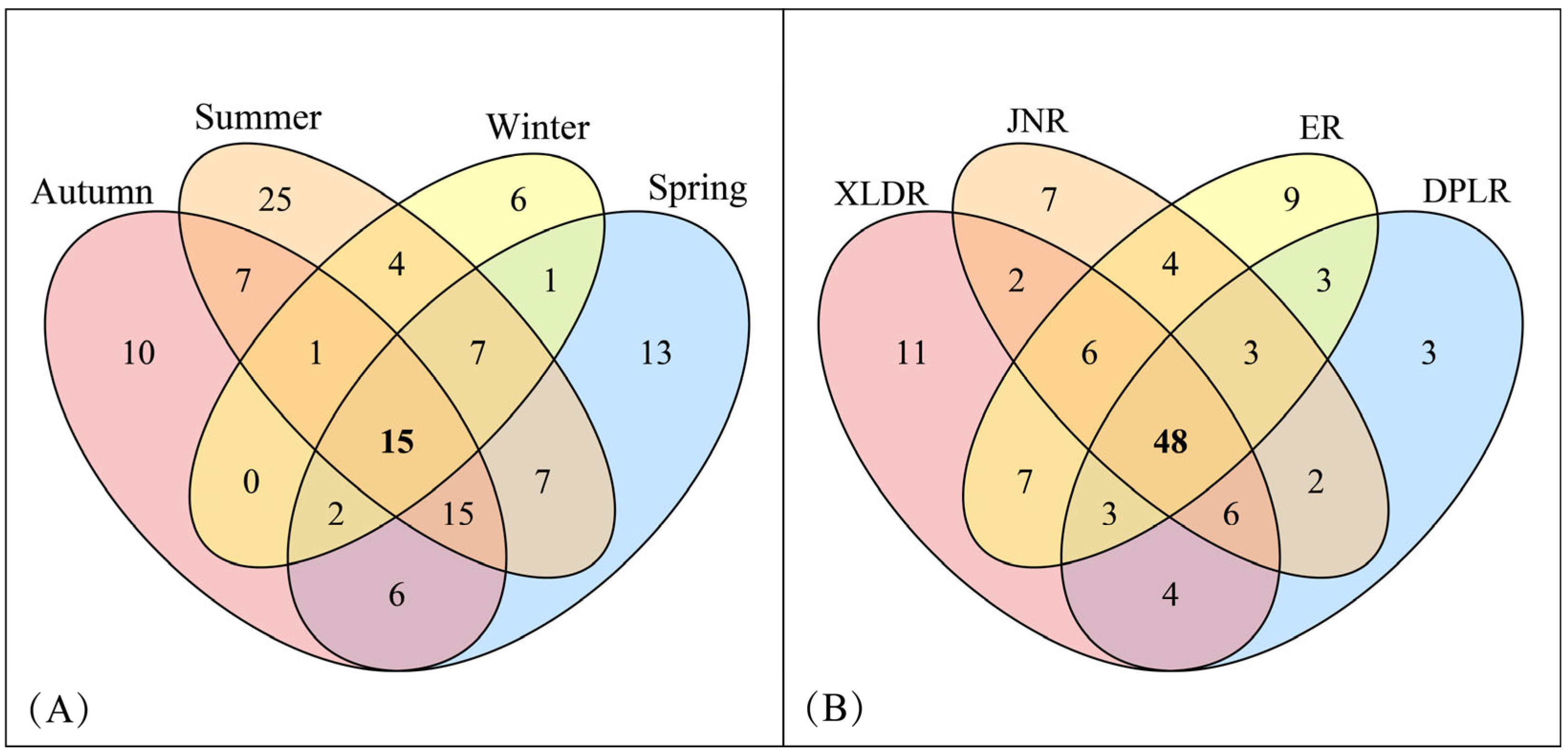
3.1.1. Species Richness
3.1.2. Dominant Species
3.2. Standing Stock of Zooplankton
3.2.1. Density
3.2.2. Biomass
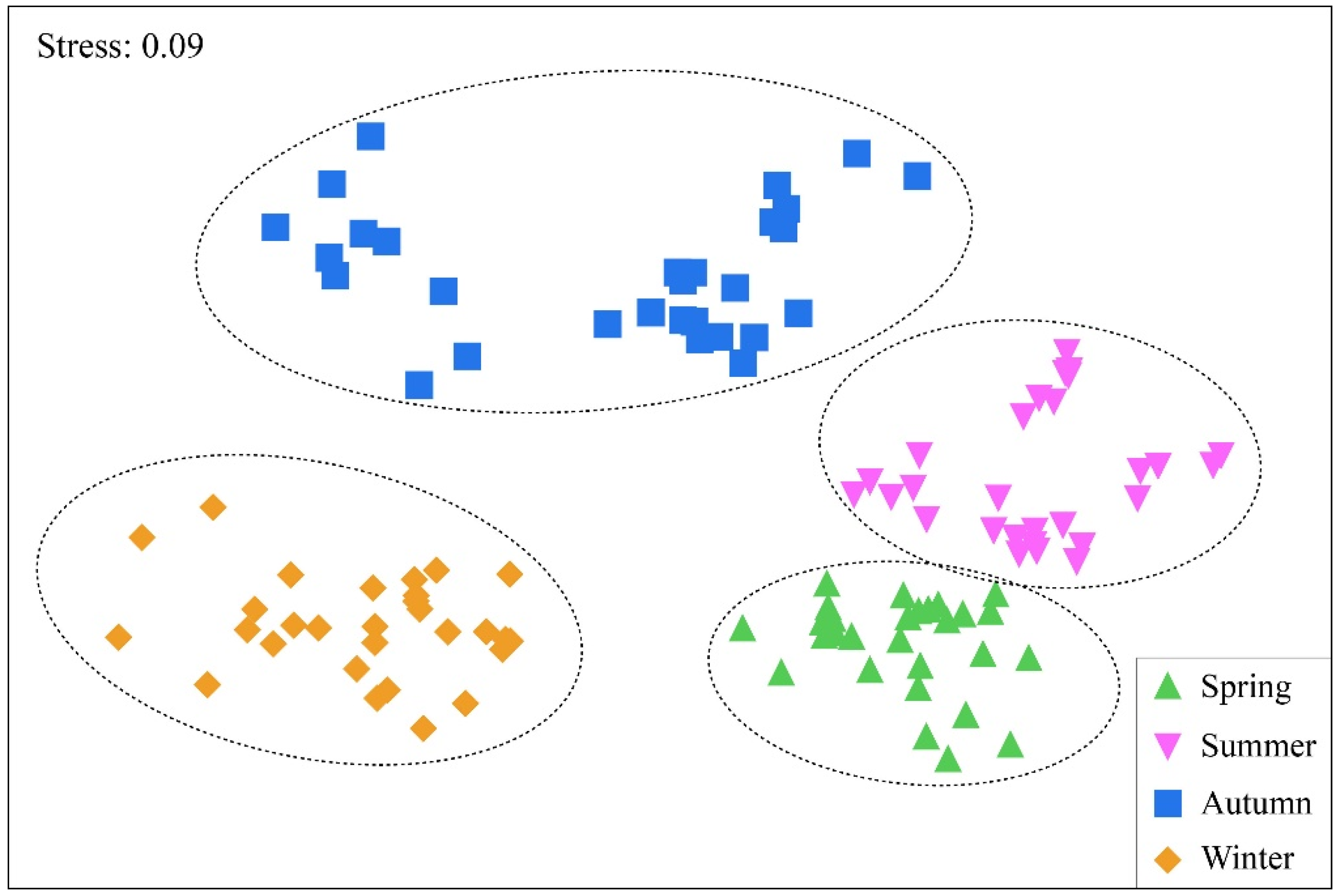
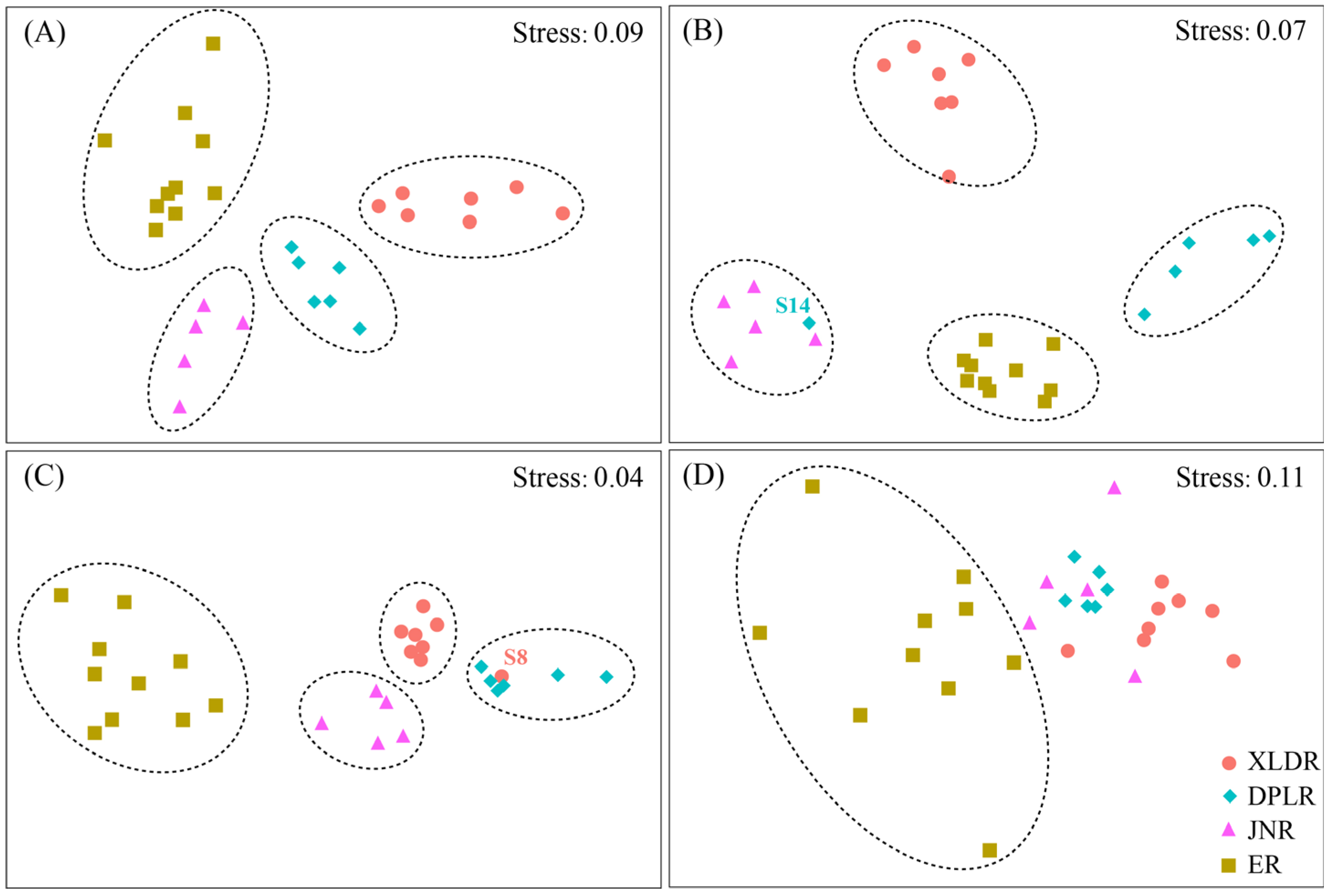
3.3. Characteristics of Zooplankton Community Structure
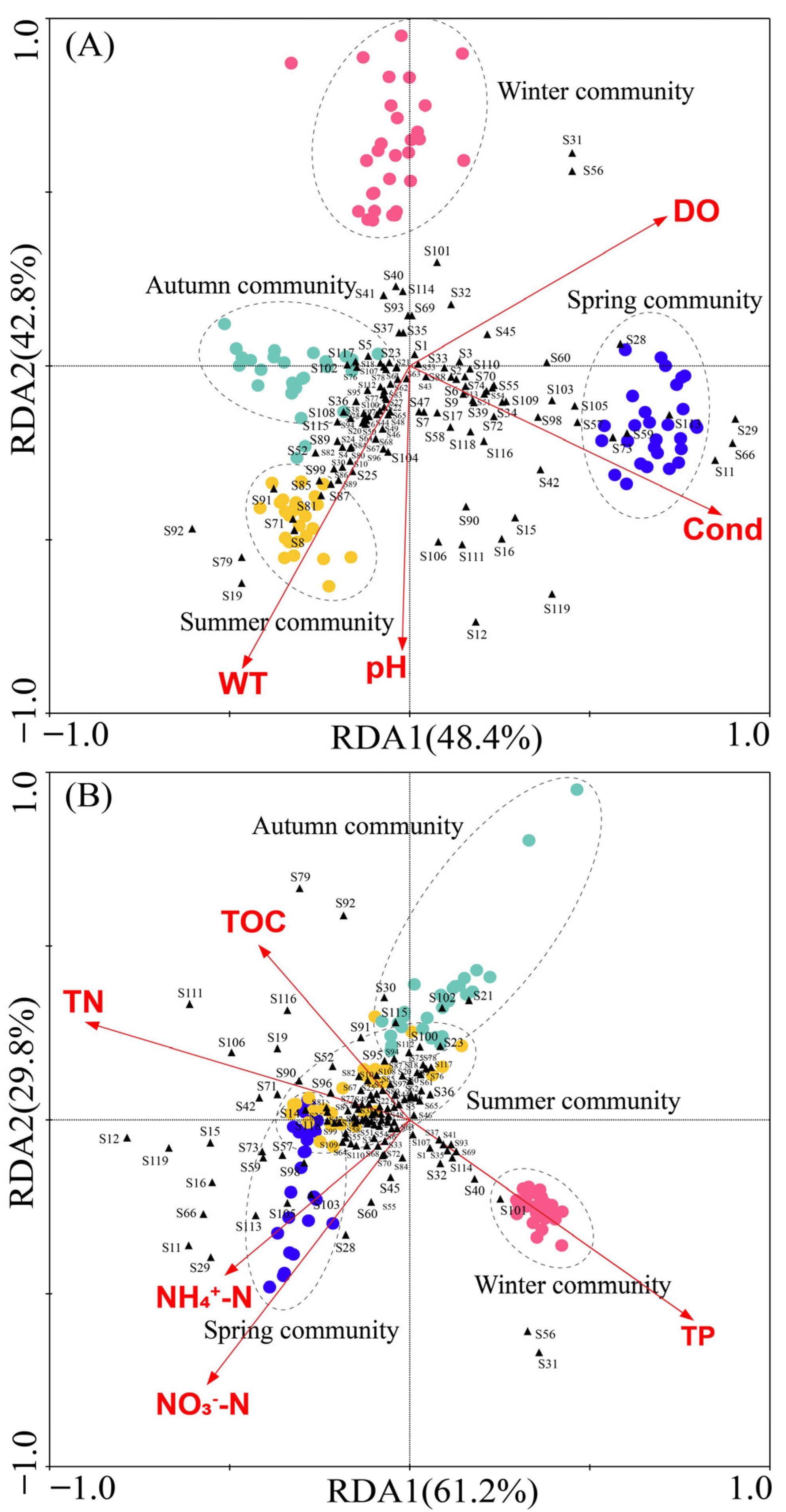
3.4. Relationship Between Zooplankton and Water Environmental Factors
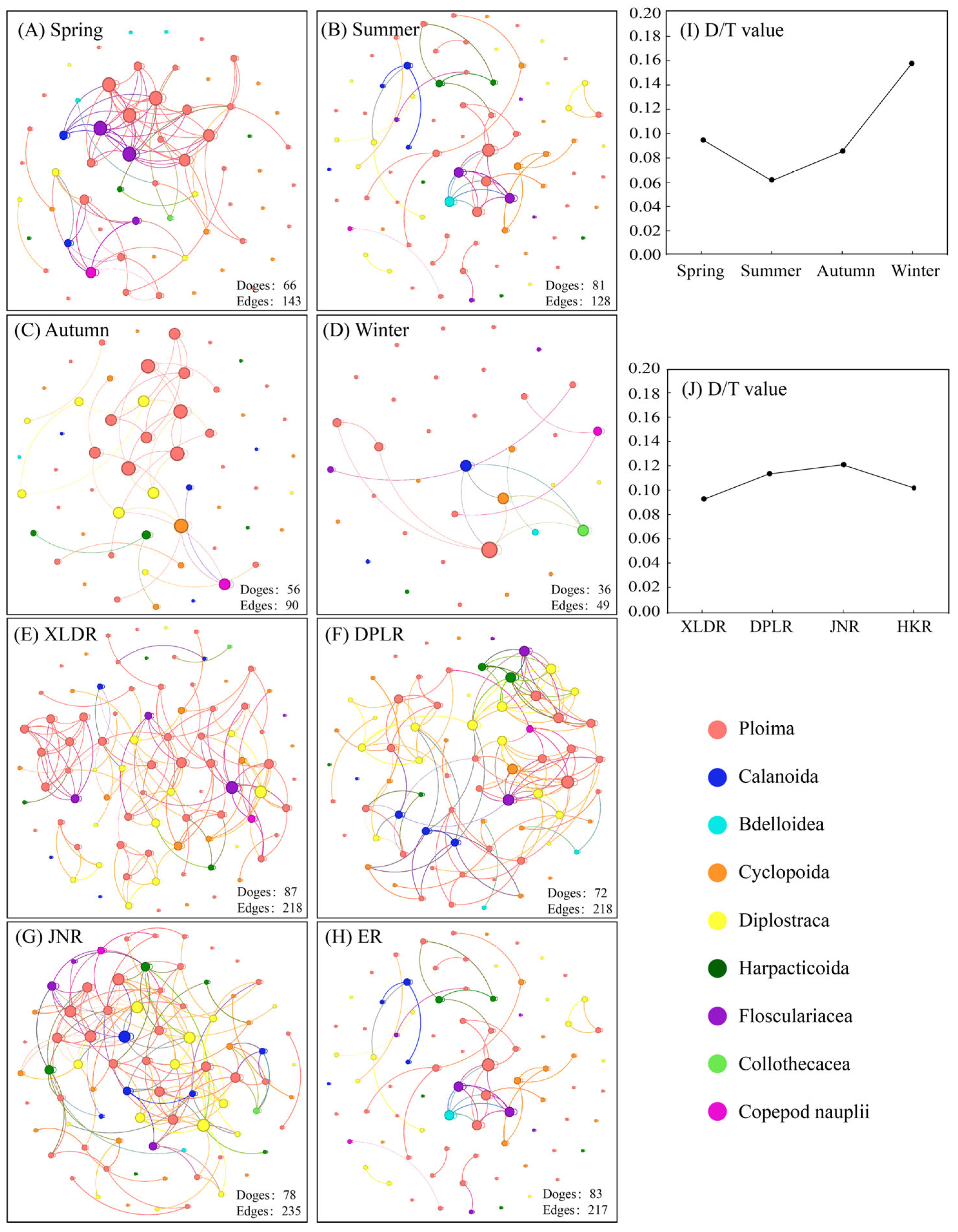
3.5. Stability of Zooplankton Community
4. Discussion
4.1. The Spatial–Temporal Pattern of Zooplankton Species Composition
4.2. The Spatial–Temporal Pattern of Zooplankton Standing Stock
4.3. The Spatial–Temporal Pattern of Zooplankton Community Structure
4.4. Zooplankton Community Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, M.W.; Qiu, Z.Q.; Li, R.R.; Li, K.Y.; Yu, S.J.; Liu, D. Monitoring Suspended Sediment Transport in the Lower Yellow River using Landsat Observations. Remote Sens. 2024, 16, 229. [Google Scholar] [CrossRef]
- Valentim, H.I.L.; Feio, M.J.; Almeida, S.F.P. Fluvial protected areas as a strategy to preserve riverine ecosystems—A review. Biodivers. Conserv. 2024, 33, 439–462. [Google Scholar] [CrossRef]
- Liu, L.N.; Zeng, J.J.; Wu, X.; Qu, J.S.; Li, X.M.; Zhang, J.; Han, J.Y. Review on Eco-Environment Research in the Yellow River Basin: A Bibliometric Perspective. Int. J. Environ. Res. Public Health 2022, 19, 11986. [Google Scholar] [CrossRef]
- Ma, B.S.; Xu, B.; Wei, K.J.; Zhu, X.Y.; Xu, J. Phytoplankton community structure and its relation to environmental conditions in the middle. Anning River, China. Chin. J. Ecol. 2022, 39, 3332–3341. [Google Scholar] [CrossRef]
- Tian, S.M.; Han, B.; Zhao, G.L.; Liang, S.; Jing, Y.C.; Jia, J.; Zhao, L.D. Analysis of the Characteristics of Water Ecological Environment Changes in the Yellow River Basin. People’s Yellow River 2024, 09, 112–119. [Google Scholar]
- Xin, Y.; Liu, X.Y. Coupling driving factors of eco-environmental protection and high-quality development in the Yellow River Basin. Front. Environ. Sci. 2022, 10, 951218. [Google Scholar] [CrossRef]
- Ma, L.M.; Bian, Y.L.; Lin, D. Analysis of the Causes of Ice Disasters and Research on Defense Measures in the Ning-Meng River Section of the Yellow River. People’s Yellow River 2024, 46, 62–67+85. [Google Scholar]
- Lu, N.; Zhou, H. Analysis of Ice Conditions in the Ordos Section of the Yellow River over the Past Decade. China Flood Drought Manag. 2024, 34, 121–124. [Google Scholar] [CrossRef]
- Xu, Q.Q. Cultural Connotations, Spatial Distribution, and General Characteristics of the Yellow River Basin. Water Econ. 2024, 42, 87–93. [Google Scholar]
- Song, J.; Hou, C.; Liu, Q.; Wu, X.; Wang, Y.; Yi, Y. Spatial and Temporal Variations in the Plankton Community Due to Water and Sediment Regulation in the Lower Reaches of the Yellow River. J. Clean. Prod. 2020, 261, 120972. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, J.; Zhang, Y.; Zhang, S. Emergy-Based Evaluation of Xiaolangdi Reservoir’s Impact on the Ecosystem Health and Services of the Lower Yellow River. Sustainability 2024, 20, 8857. [Google Scholar] [CrossRef]
- Liu, X.H.; Song, J.J.; Ren, Y.P.; Zhan, D.M.; Liu, T.; Liu, K.K.; Wu, H.; Xu, B.D. Spatio-temporal Patterns of Zooplankton Community in the Yellow River Estuary: Effects of Seasonal Variability and Water-Sediment Regulation. Mar. Environ. Res. 2023, 189, 106060. [Google Scholar] [CrossRef]
- He, M.T.; Yan, M.T.; Chen, X.F.; Wang, X.K.; Gong, H.; Wang, W.J.; Wang, J. Bioavailability and Toxicity of Microplastics to Zooplankton. Gondwana Res. 2022, 108, 120–126. [Google Scholar] [CrossRef]
- Pardianto, D.K.; Lee, B.R.; Choi, K.H.; Park, W. Seasonal Changes of Zooplankton Community around Dokdo in the East Sea. J. Environ. Biol. 2019, 40, 884–895. [Google Scholar] [CrossRef]
- Giering, S.L.C.; Wells, S.R.; Mayers, K.M.J.; Schuster, H.; Cornwell, L.; Fileman, E.S.; Atkinson, A.; Cook, K.B.; Preece, C.; Mayor, D.J. Seasonal Variation of Zooplankton Community Structure and Trophic Position in the Celtic Sea: A Stable Isotope and Biovolume Spectrum Approach. Prog. Oceanogr. 2019, 177, 101943. [Google Scholar] [CrossRef]
- Macarthur, R. Fluctuations of animal populations and a measure of community stability. Ecology 1955, 36, 533. [Google Scholar] [CrossRef]
- Bai, H.F.; Kong, F.H.; Wang, Y.R.; Song, J.X.; Cao, Y.L.; Jiang, X.H. Spatiotemporal Characteristics of Zooplankton Community Structure and Their Correlation with Environmental Factors in the Beiluo River Basin. J. Dalian Ocean. Univ. 2021, 05, 785–795. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, P.C.; Yang, Y.Y.; Li, F.; Li, S.W.; Xu, B.Q.; Zhang, X.M.; Wang, Y.H. Characteristics of Zooplankton Community Structure in the Yellow River Estuary during the 2020 Water and Sediment Regulation Project. Technol. Eng. 2022, 21, 9061–9070. [Google Scholar]
- Zuo, T.; Li, Y.T.; Zuo, M.; Cheng, Z.L.; Wang, J.; Wang, A.D. Seasonal Variations in the Structure of Planktonic Larval Communities in the Vicinity of the Yellow River Estuary. Acta Oceanol. Sin. 2022, 04, 47–56. [Google Scholar]
- Guo, W.; Li, M.; Yin, X.W.; Bai, H.F. Zooplankton Diversity and Water Quality Assessment in the Dawen River Wetland, Jinan. Henan Fish. 2024, 02, 37–40. [Google Scholar]
- Zhao, Z.K.; Li, H.W.; Song, X.Y.; Sun, W.Y. Dynamic Monitoring of Surface Water Bodies and Their Influencing Factors in the Yellow River Basin. Remote Sens. 2023, 15, 5157. [Google Scholar] [CrossRef]
- Xue, Z.; Li, R.X.; Li, C.H. Sustainability of Water Resources in Shandong Province Based on a System Dynamics Model of Water–Economy–Society for the Lower Yellow River. Sustainability 2022, 14, 3412. [Google Scholar] [CrossRef]
- Liu, A.B.; Yan, T.T.; Shi, S.X.; Zhao, W.J.; Ke, S.H.; Zhang, F.S. Trade-Off and Coordination between Development and Ecological Protection of Urban Agglomerations along Rivers: A Case Study of Urban Agglomerations in the Shandong Section of the Lower Yellow River. Land 2024, 13, 1368. [Google Scholar] [CrossRef]
- Ran, G.; Jian, S.Q.; Wu, Q.; Zhang, L.; Hu, C.H. Exploring the Dominant Runoff Processes in Two Typical Basins of the Yellow River, China. Water 2020, 12, 3055. [Google Scholar] [CrossRef]
- Yu, G.; Liu, T.; Wang, Q.; Li, T.; Li, X.; Song, G.; Feng, Y. Impact of Land Use/Land Cover Change on Ecological Quality during Urbanization in the Lower Yellow River Basin: A Case Study of Jinan City. Remote Sens. 2022, 14, 6273. [Google Scholar] [CrossRef]
- Yu, S.P. Research on Water Resources Management in Dongping Lake. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2018. (In Chinese). [Google Scholar]
- Li, Q.Y.; Li, B.; Xu, F.; Xu, L.H.; Li, D.J. Eastern gate operation issue to wear the Yellow River project operation management risk. Mech. Electr. Tech. Hydropower Stn. 2024, 47, 120–122. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, W.; Lu, Y.; Huang, J.; Xiao, Y. Quantitative Analysis of the Influence of the Xiaolangdi Reservoir on Water and Sediment in the Middle and Lower Reaches of the Yellow River. Int. J. Environ. Res. Public Health 2023, 20, 4351. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Y.J.; Li, H.D. Hydrochemical Characteristics and Controlling Factors of the Yellow River in Shandong Section. People’s Yellow River 2019, 40, 87–95. [Google Scholar]
- Liu, Q.C.; Cai, J.; Xie, M.T.; Liu, X.P.; Tian, J.P.; He, W.J.; Zhang, D.J. Assessment of Ecosystem Service Values in the National Wetland Park of the Lower Yellow River in Shandong. Wetl. Sci. 2024, 05, 697–706. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Huang, X.F. Freshwater Plankton Research Methods; Science Press: Beijing, China, 1991. [Google Scholar]
- Wang, J.J. Fauna of Freshwater Rotifers in China; Science Press: Beijing, China, 1961. [Google Scholar]
- Crustacean Research Group, Institute of Zoology, Chinese Academy of Sciences. Fauna Sinica: Arthropoda: Crustacea: Freshwater Copepoda; Science Press: Beijing, China, 1979. [Google Scholar]
- Jiang, X.Z.; Du, N.S. Fauna Sinica: Arthropoda: Crustacea: Freshwater Cladocera; Science Press: Beijing, China, 1979. [Google Scholar]
- GB 11893-89; Water Quality—Determination of Total Phosphorus–Ammonium Molybdate Spectrophotometric Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 1990.
- HJ 199-2023; Water Quality—Determination of Total Nitrogen–Gas-Phase Molecular Absorption Spectrometry. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2024.
- HJ/T 346-2007; Water Quality—Determination of Nitrate-Nitrogen–Ultraviolet Spectrophotometry. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2007.
- HJ 195-2023; Water Quality—Determination of Ammonia Nitrogen–Gas-Phase Molecular Absorption Spectrometry. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2024.
- Zhang, Y.Z.; Zhang, L.L.; Yin, R.; Luan, H.N.; Liu, Z.J.; Chen, J.; Jiang, R.J. Spatial Niches of Dominant Zooplankton Species in Yueqing Bay, Zhejiang Province. J. Appl. Ecol. 2021, 1, 342–348. [Google Scholar]
- Lv, Q.; Hu, X.R.; Nie, X.; Ouyang, S.; Wang, C.; Qin, H.M. Impact of water level fluctuations on the succession of zooplankton in Poyang lake. Acta Ecol. Sinica 2020, 40, 1486–1495. [Google Scholar] [CrossRef]
- Xie, Q.M.; Li, C.C. Studies on the Composition and Seasonal Variations of Planktonic Copepoda in Poyang Lake. Jiangxi Sci. 1998, 16, 180–187. [Google Scholar]
- Leng, C.M.; Dong, G.C.; Liu, C.; Li, X.Q.; Zhu, S.W.; Ke, H. Investigation and Analysis of Plankton Community Characteristics in the Shandong Section of the Yellow River. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2016, 05, 668–673. [Google Scholar]
- Liang, J. Creating a New Situation for Ecological Protection and High-Quality Development in the Yellow River Basin. China Fisheries News, 9 December 2024; p. A04. [Google Scholar]
- Hui, J.; Jie, Z.L.; He, H.Z.; Li, F.T. Ecological Characteristics and Spatiotemporal Distribution of Plankton in the Henan Section of the Yellow River. Hebei Fish. 2018, 05, 37–43. [Google Scholar]
- Xiang, H.; Lin, F.; Wu, Y.P.; Tian, B.; Cai, Z.Y.; Wu, Y.P.; Huang, P.J.; Liu, Q.F.; Zhou, Z.G. Analysis of the Community Structure Characteristics and Environmental Correlation of Zooplankton in the Middle Reaches of the Yangtze River at Yichang. Heilongjiang Fish. 2022, 05, 3–12. [Google Scholar]
- Qiao, S.W.; Yang, Y.Y.; Xu, B.C.; Yang, Y.; Zhu, M.M.; Li, F.; Yu, H.M. How the Water-Sediment Regulation Scheme in the Yellow River Affected the Estuary Ecosystem in the Last 10 Years? Sci. Total Environ. 2024, 927, 172002. [Google Scholar] [CrossRef]
- Natthida, J.; Sameer, M.P.; Supiyanit, M. Biodiversity and Species-Environment Relationships of Freshwater Zooplankton in Tropical Urban Ponds. Urban Ecosyst. 2023, 27, 827–840. [Google Scholar]
- Huang, L.; Xi, Y.L. Research Progress on Interspecific Competition of Brachionus plicatilis. Ecol. J. 2019, 10, 3177–3182. [Google Scholar] [CrossRef]
- Cui, W.H.; Jiang, W.L.; Zhang, D.P.; Li, S.F.; Dong, B.; Lv, Z.B.; Ren, Z.H. Study on the Ecological Niche of Dominant Zooplankton Species and Its Correlation with Environmental Factors in Spring and Autumn in the Coastal Area of Laizhou. Mar. Fish. 2024, 1–23. [Google Scholar] [CrossRef]
- Dong, A.; Yu, X.F.; Yin, Y.; Zhao, K. Seasonal variation characteristics and the factors affecting plankton community structure in the Yitong River, China. Int. J. Environ. Res. Public Health 2022, 19, 17030. [Google Scholar] [CrossRef]
- Zhang, S.M.; He, P.M.; Liu, W.; Liu, J.L.; Chen, S.W.; Han, Z.; Wu, M.Q. Seasonal Variations and the Relationship with Environmental Factors of Zooplankton in Urban Rivers of Shanghai. J. Aquat. Ecol. 2022, 05, 42–48. [Google Scholar] [CrossRef]
- Yin, C.J.; Yang, Y.L.; Ni, L.Y.; Chen, Y.S.; Wen, Z.H.; Su, H.J.; Guo, L.G. Temperature, nutrients, and planktivorous fish predation interact to drive crustacean zooplankton in a large plateau lake, southwest China. Aquat. Sci. 2022, 84, 22. [Google Scholar] [CrossRef]
- Chen, J.Q.; Zhao, K.; Cao, Y.; Wu, B.; Pang, W.T.; You, Q.M.; Wang, Q.X. The community structure of zooplankton and its relationship with environmental factors in Poyang Lake. Acta Ecol. Sin. 2020, 40, 6644–6658. [Google Scholar]
- Wang, S.Q.; Pan, B.Z.; Yang, Z.J.; Hu, E.; Zhang, X.; Li, G.; Hu, J.X. Characteristics of the plankton community and interspecific relationships of dominant species in the Jing River Basin. J. Appl. Ecol. 2025, 36, 587–595. [Google Scholar]
- Liang, D.; Xia, J.; Song, J.X.; Chang, J.B.; Wu, Q.; Cheng, D.N.; Ren, Y.X. Diversity of plankton and key species ecological niche characteristics in the Wei River based on eDNA technology. Environ. Sci. 2021, 42, 4708–4716. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, J.; Kong, D.F.; Chen, T. Survey and evaluation of aquatic biota communities in typical mountain rivers of the Yellow River Basin: A case study of Dayu River. Environ. Sci. Technol. 2024, 39, 52–57. [Google Scholar] [CrossRef]
- Luan, L.L.; Gao, L.M.; Qiu, Y.H.; Zhang, Z.; Ge, J.; Zhao, X.L. Levels of nutrient enrichment determine the emergence of zooplankton from resting egg banks. Hydrobiologia 2023, 851, 1259–1273. [Google Scholar] [CrossRef]
- Sandlund, O.T. The drift of zooplankton and micro benthos in the river Strandaelva, westem Norwway. Hydrobiologia 1982, 94, 33–48. [Google Scholar] [CrossRef]
- Lao, S.C.; Chen, Z.W.; Yan, R.W.; Li, D.Y. Technical study on draining water from Dongping Lake to the Nan Si Lake. Shandong Water Sci. Technol. 1997, 4, 36–39. [Google Scholar]
- Kippen, N.K.; Zhang, C.; Mleczek, M.; Špoljar, M. Rotifers as indicators of trophic state in small water bodies with different catchments (field vs. forest). Hydrobiologia 2024. prepublish. [Google Scholar] [CrossRef]
- Qin, H.M.; Cao, X.Y.; Cui, L.Y.; Lv, Q.; Chen, T.T. The Influence of Human Interference on Zooplankton and Fungal Diversity in Poyang Lake Watershed in China. Diversity 2022, 12, 296. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, K.; Zhang, Q.J.; Cai, Y.J.; Zhang, Y.Z.; Gong, Z.J.; Xiang, X.L. Spatio-temporal distribution characteristics and driving factors of zooplankton in Hongze Lake. Environ. Sci. 2021, 40, 3753–3762. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhang, X.W. eDNA metabarcoding in zooplankton improves the ecological status assessment of aquatic ecosystems. Environ. Int. 2020, 134, 105230. [Google Scholar] [CrossRef]
- Lígia, A.P.; Carlos, C.; Filipe, M.; Alexandra, G.M.; Manuel, J.R.; Miguel, P. Climate forcing on estuarine zooplanktonic production. Mar. Pollut. Bull. 2023, 194, 115287. [Google Scholar] [CrossRef]
- Geng, Z.Y.; Zhao, L.L.; Jia, X.; Hu, Y.F.; Liu, S.Z.; Liu, Q.; Song, J. Seasonal differences and functional group characteristics of plankton community structure in Cetian Reservoir, Shanxi Province. J. Dalian Ocean. Univ. 2024, 45, 101–113. [Google Scholar] [CrossRef]
- Ni, L.X.; Li, H.Y.; Zhou, L.; Shi, J.H.; Nie, Y.; Zhao, F.; Li, S.Y. Structural characteristics of zooplankton communities in Hongze Lake driven by water environmental factors from 2016 to 2020. Environ. Monit. Assess. 2023, 195, 1503. [Google Scholar] [CrossRef]
- Mondal, S.; Palit, D.; Hazra, N. Spatial pattern analysis of zooplankton and surface water of pit lakes (Raniganj coal field, India). Water Sci. 2023, 37, 98–116. [Google Scholar] [CrossRef]
- Song, J.; Yi, Y.J.; Hou, C.Y.; Yang, Y.F. The impact of water regulation and sediment control at the Xiaolangdi Reservoir on downstream river plankton. People’s Yellow River 2019, 41, 38–43+75. [Google Scholar]
- Chen, X.L. Study on the Spatiotemporal Dynamics and Driving Factors of Plankton Community in the Regulatory Lakes of the Eastern Route of the South-to-North Water Diversion Project. Master’s Thesis, Qufu Normal University, Qufu, China, 2024. [Google Scholar] [CrossRef]
- Barros, A.; Hartman, R.; Bashevkin, S.M.; Burdi, C.E. Years of drought and salt: Decreasing flows determine the distribution of zooplankton resources in the San Francisco Estuary. San Franc. Estuary Watershed Sci. 2024, 22, 3. [Google Scholar] [CrossRef]
- Dong, W.J.; Xu, Q.J.; Dong, J.; Pang, Y.; Tao, Y.R. Ecological health assessment of lakes based on the coordination of “three waters”: A case study of Li Lake in Tai Lake. J. Environ. Eng. Technol. 2025, 15, 100–110. [Google Scholar]
- Zhang, S.Y.; Xu, Z.W.; Gong, L.Q.; Luo, H.; Yu, D.K.; Sun, M.F.; Shang, J.W.; Qin, H.M. Seasonal succession characteristics of zooplankton community in shallow dished sub-lakes of Poyang Lake and influencing factors during early Fish Banning period. J. Lake Sci. 2025, 37, 1–14. Available online: http://www.hnsti.ac.cn/Xcl/Article/Detail?id=2031569220845 (accessed on 25 December 2024).
- Lv, M.R.; Ding, Y.F.; Zhang, R.; Liu, Y.; Kan, D.Q.; Zhang, Z.; Yang, J.X. Community structure of metameric zooplankton and evaluation of water quality and trophic state in Jinshuitan Reservoir. Resour. Environ. Yangtze River Basin 2023, 32, 354–364. [Google Scholar]
- Zhang, H.; Zou, H.Y.; Zhao, L.; Li, X.W. Seasonal distribution and dynamic evolution of antibiotics and evaluation of their resistance selection potential and ecotoxicological risk at a wastewater treatment plant in Jinan, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 44505–44517. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Dai, S.; Xie, Q.M. Preliminary study on the toxic effects of two quinolone antibiotics on Daphnia magna. Chin. J. Antibiot. 2024, 49, 832–840. [Google Scholar] [CrossRef]
- Wang, W.; Dang, Y.C.; Duan, W.S. Overview of the ecological and environmental impact of the Xiaolangdi Water Conservancy Hub Project on the Yellow River. In Proceedings of the 2021 Annual Conference of the Chinese Water Resources Association, Beijing, China, 25 October 2021. [Google Scholar] [CrossRef]

| Dominant Species | Dominance (Y) | |||
|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |
| Brachionus angularis | 0.094 | |||
| Brachionus calyciflorus | 0.257 | 0.476 | 0.083 | 0.115 |
| Brachionus diversicornis | 0.034 | |||
| Keratella quadrala | 0.094 | |||
| Notholca labis | 0.219 | |||
| Polyarthra dolichoptera | 0.146 | |||
| Filinia maior | 0.066 | |||
| Diaphanosoma dubium | 0.116 | |||
| Bosmina longirostris | 0.049 | 0.582 | ||
| Sinocalanus dorrii | 0.021 | 0.049 | ||
| Schmackeria forbesi | 0.031 | |||
| Microcyclops varicans | 0.037 | |||
| Mesocyclops leuckarti | 0.066 | |||
| Dominant Species | Dominance (Y) | |||
|---|---|---|---|---|
| XLDR | DPLR | JNR | ER | |
| Brachionus angularis | 0.028 | 0.029 | ||
| Brachionus calyciflorus | 0.151 | 0.499 | 0.398 | 0.350 |
| Keratella quadrata | 0.035 | 0.020 | ||
| Diaphanosoma dubium | 0.077 | |||
| Bosmina longirostris | 0.086 | 0.119 | 0.031 | 0.021 |
| Sinocalanus dorrii | 0.022 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, S.; Sun, M.; Han, J.; Wang, Z.; Chen, X.; Chen, Z.; Qin, H. Spatial–Temporal Pattern and Stability Analysis of Zooplankton Community Structure in the Lower Yellow River in China. Diversity 2025, 17, 162. https://doi.org/10.3390/d17030162
Wang Y, Zhang S, Sun M, Han J, Wang Z, Chen X, Chen Z, Qin H. Spatial–Temporal Pattern and Stability Analysis of Zooplankton Community Structure in the Lower Yellow River in China. Diversity. 2025; 17(3):162. https://doi.org/10.3390/d17030162
Chicago/Turabian StyleWang, Yaowei, Shiyuan Zhang, Minfang Sun, Jiamin Han, Ziyue Wang, Xinlei Chen, Zengfei Chen, and Haiming Qin. 2025. "Spatial–Temporal Pattern and Stability Analysis of Zooplankton Community Structure in the Lower Yellow River in China" Diversity 17, no. 3: 162. https://doi.org/10.3390/d17030162
APA StyleWang, Y., Zhang, S., Sun, M., Han, J., Wang, Z., Chen, X., Chen, Z., & Qin, H. (2025). Spatial–Temporal Pattern and Stability Analysis of Zooplankton Community Structure in the Lower Yellow River in China. Diversity, 17(3), 162. https://doi.org/10.3390/d17030162





