Abstract
Despite advancements through satellite telemetry, knowledge of the behaviour and ecology of large raptors during natal dispersal is still poor, even though this transience phase is important in understanding the population dynamics and conservation of these keystone species. After this phase, which can take several years, the subsequent rapid transitional event on first territory settlement is less studied. It apparently occurs earlier without competition from existing territory occupants. The time of year when young large raptors settle on a territory has rarely been addressed empirically. Our study of seasonal timing of first settlement used data from 46 golden eagles GPS-tagged as nestlings in Scotland which were tracked to their first territory settlement, as adjudged by a robust algorithmic method. We show that when young golden eagles occupied their first territory, their settlements were uncommon in summer and most common in late winter/spring, but also occurred in autumn into early winter. The significant seasonal pattern was consistent, regardless of the probable prior occupancy status (vacant/occupied) of the settled territory (respectively, the likely absence or presence of defending territorial birds). This showed that seasonal territory settlement was unlikely to be related to any response from territory occupants. It infers further that seasonality in first territory settlement was underpinned by innate behaviours in dispersing eagles. Seasonal distribution of settlement dates was significantly different between sexes, with males more likely to settle in autumn, predominantly in prior vacant territories. We speculate on potential causative drivers behind our results and conclude that more research is clearly needed in this field of study.
1. Introduction
The time between the end of the Post Fledging Dependence Period (PFDP) [1,2] and first territory settlement [3] is poorly studied in large raptors. This is the phase of natal or juvenile dispersal [1,4,5,6,7] or transience [8,9,10,11,12] hereafter termed natal dispersal.
GPS-based satellite telemetric research has informed many prior knowledge gaps during natal dispersal for large raptors. Several studies have focussed on movements during early natal dispersal and, while novel and valuable, have often involved few individuals [13,14,15,16,17,18,19]. This reflects the early exploitation of GPS-telemetry as an investigative tool and the beginnings of its promissory utility [20,21,22].
Research surrounding the end of natal dispersal (or the immigration to a breeding site stage: [12]) is relatively scant, but GPS-telemetry has a potential role in gaining further information as technology advances and sample sizes improve [3,21,23,24,25]. This juncture is when young dispersing birds and/or floaters [23,26,27,28] shift to a population’s component as territory occupants with the prospect or realisation of reproduction. This territorial component, involving the number of occupants, their survival and their reproductive output is important in population dynamics [29,30,31,32,33].
The ecology and behaviour of birds during natal dispersal—transient between leaving the natal site and settling into the prospective breeding population—can nevertheless also be influential in population dynamics through survival [23,26,27,28,34]. The factors affecting how (typically younger) birds shift between being non-territorial during dispersal and settled territorial should therefore enhance understanding of large raptor populations [23].
Despite limitations on knowledge of the factors affecting this shift, results consistently indicate that dispersing large raptors can take several years to settle [23,35], but settle when younger when territories are unlikely to be occupied [3,36]. What is not known, however, is whether there is any seasonality in such settlements, and, if it exists, how it may happen.
Seasonality may be expected from indirect evidence. Intrusions by floaters into territories of Spanish imperial eagles Aquila adalberti were not recorded in summer through to early autumn [37]. In a close relative, the golden eagle Aquila chrysaetos, aggressive encounters (implying intrusions from non-territorial birds) were most frequent in late winter until egg-laying and were uncommon later [38,39]. Scottish golden eagles GPS-tracked during dispersal were less likely to intrude in occupied territories during summer when they were more likely to use temporary settlement areas (TSAs) [7].
Undulating flight displays of golden eagles may have a dual function of territory defence and pair-bonding or courtship [40]. In western Norway, most undulating flight displays occurred in late winter and early spring [41]; this was also recorded in the Outer Hebrides of Scotland [40], but here there was only a limited drop-off in displays during the main breeding season. This sub-population, largely discrete from the rest of Scotland [42], is at high density [43]. Hence, there is likely more pressure from non-territorial floaters on territory occupants in the Outer Hebrides. Coupled with a lower breeding success rate and early breeding failures [43,44], the territorial occupants may have greater energetic capacity and need to display longer.
Prior research on the indicators of non-territorial birds’ devotional efforts to settle consequently suggests that there may be seasonality in dispersing eagles’ first territory settlement. This has not been studied explicitly, however, nor has any relationship it may have with defensive reactions from territorial incumbents [37,38,39].
Informed primarily by an algorithmic method that estimates the date of first territory settlement by GPS-tagged nestlings [3], we used telemetric data from 46 golden eagles GPS-tagged in Scotland to address three questions:
Is there seasonality in the timing of first territory settlement? Based on indications from previous research, hypothetically, we expected that settlement would be less in summer.
If this expectation is supported, is defensive behaviour from territory occupants implicated? Insights from seasonal display behaviour and recorded interactions with intruding non-territorial birds [38,39,40,41] may not be too revelatory when, for example, flight displays may also function beyond territorial defence. In our study population, however, first territory settlement can occur in territories that beforehand were either vacant or occupied [3]. Our second expectation (null hypothesis) was that if the first expectation (less settlement in summer) was supported then this would be regardless of the likely prior occupancy status of the settled territory (and, hence, any defensive reaction from incumbents). This followed [7], given the propensity of vacant territories in our study population [3]. Support for this hypothesis would indicate that any seasonality in young eagles’ settlement may not be influenced by defensive occupants, and so was effectively due to the innate behaviours of dispersing eagles.
Is there a difference between sexes in any settlement seasonality? Birds’ behaviour during natal dispersal is replete with expectations and empirical differences based on sex [6,21,25,45,46,47,48,49].
2. Methods
2.1. Study Area and Species
Scotland covers c. 80,000 km2 on the northwestern limit of Europe and hosts around 500 territorial golden eagle pairs occupying Scotland’s uplands [3,43], which are also used by non-territorial birds [44,50,51]. These uplands vary in geology, vegetation, topography and climatic influences.
Climatically, situated on the northeastern edge of the Atlantic Ocean, the west of Scotland is subjected more to the Atlantic Drift and is wetter and windier, with more equitable seasonal changes in weather and hence is more oceanic. The east is drier, with greater seasonal changes in weather and is more continental [39,44].
The contrasting oceanic/continental influences tend to produce upland vegetations which in the east are only found at higher altitudes, but which may occur at sea level in the west. The preferred open habitats are dry or wet heathland and peatland dominated by heather Calluna vulgaris and relatives in the east, with graminoids, sedges and deeper peatland more common in the west [39,44]. Irrespective of vegetation, a critical habitat factor is topography. A combination of altitude, slope and distance from ridges—largely surrogates for orographic wind energy availability—are highly influential in eagle movements and distribution [51].
The Scottish breeding population is resident and has been isolated from continental Europe for thousands of years [52]. In some areas of the eastern mainland associated with intensive management for shooting red grouse Lagopus lagopus scotica, illegal persecution of golden eagles (being predators of grouse) has created and in some places maintains many vacant [3,44,50,53,54]. This is in contrast with the west, where persecution appears minimal in recent history and there is a higher density of occupied territories, even though productivity here is substantially less than in the fewer eastern territories [39,43,44,54].
Hence, persistent and recent illegal practices in some parts of the east have created a variable competitive landscape across Scotland, with geographical differences in the availability of vacant territories and the persistence (turnover) of occupying birds. These illegal practices have therefore created an experimental environment that can elucidate several aspects of eagle population dynamics [3,44,45,50].
2.2. GPS Satellite Tagging
Tagging methods for Scottish golden eagles have been repeatedly described [1,2,3,51]. Nestlings were tagged when 50–70 days old [55,56] with a suitable transmitter and harness weights [24,57,58] involving a thoracic X-strap harness [59,60] comparable with [61,62]. This tagging method and others [60,63] were followed. The harness design did not affect the survival or physical injuries of golden eagles [62]. Tagging of Scottish eagles had no apparent adverse effects under these metrics and others, including breeding productivity [50].
Nestlings were primarily sexed on biometrics (e.g., [64,65]). For many birds, assignations were also supported by later observations of sexual role behaviour and camera-trapping records of pairs in the field [3]. Examination of telemetry records early in the breeding season when females spend more time at the nest than males [39] also allowed strong inference of sex assignations for all settled territorial birds. These additional sexing methods did not contradict the biometric method. Molecular techniques [42] from an opportunistic sample taken previously also confirmed biometric assignations of several birds [2], as did more recent molecular sexing of scores of birds translocated to the south of Scotland [66].
Several transmitter models were deployed, involving nestlings that subsequently settled on a territory [3,50]. Almost all deployed tags were manufactured by MTI (Microwave Telemetry Inc., Columbia, MD, USA) in the present study, however, and their specifications and transmission outputs are described elsewhere ([1,2,50,51]; notably [3]).
2.3. First Territory Settlement and Prior Occupancy Status
We used an algorithmic method to estimate the date of first territory settlement from birds GPS-tagged as nestlings [3]. This method has five stages, conceptually aligning terminology and research concerned with natal dispersal and broader population dynamics, notably, recruitment to a breeding population via territory occupancy through settlement. It accounts for potentially confounding factors, such as the use of TSAs and territorial excursion behaviour. When possible, its estimates were confirmed using field methods [3].
The algorithm is fully described in [3]. Briefly, it is based on three assumptions.
Assumption 1.
Once a bird is settled on a territorial home range, its movements should be relatively restricted spatially, but with temporal longevity; this assumption should be particularly evident for nocturnal records, when birds should more likely be within their prospective breeding territory when roosting, rather than records during excursive flights during daylight.
Assumption 2.
A settled bird will have constrained movements which can be measured using a threshold of distance moved since the last location using median locations.
Assumption 3.
Settled birds have excursive flights outside their range which are beyond the Assumption 2 distance threshold. However, these are of relatively short duration so distance travelled should be averaged over a period of days.
We cast the prior occupancy status of territories into two classes: vacant and occupied [3], such that, supported consistently by the most recent field assessments, deemed vacant areas have a long history of no, or at least very transitory, occupation and occupied areas have a long history of almost continuous occupancy. There is a rich historical database of known golden eagle territories in Scotland from several national censuses and prior records [43,67,68]. Periodic censuses are supplemented with annual efforts undertaken by experienced surveyors, typically members of the Scottish Raptor Study Group (SRSG) who contribute data to national censuses, and the Scottish Raptor Monitoring Scheme (SRMS: [69]). Both sources were consulted, with an emphasis on the local SRSG fieldworkers’ records (being more recent), who were blind to the reason for consultation [3].
This classification typically relied on survey efforts recording occupancy status up to four visits per year in known or prospective territories [70]. There was an inevitable difference between the timing of field surveys to document occupancy status and the greater temporal precision of the settlement algorithm [3], even though survey visits were seasonally concentrated during the summer (regarding several questions posed by our study: Section 1).
The classification method emphasised the most recent records of occupancy status [3], but there was repeated consistency, extending to decades in some cases, for many territories’ classified occupancy status [43,44,53,54,67,68]. On deemed vacancies, this was in large part because of persistent illegal persecution creating and maintaining vacant territories in eastern Scotland, associated with the management of intensive shooting of red grouse. By contrast, on deemed occupancy, away from these centres of persecution (mostly in western Scotland: [3,50]), there were also repeated records of territories being occupied persistently [3,43,44,54]. The classification method can only ever be a snapshot metric, as are many others in the study of large raptor biology (e.g., [71]). Unless all territorial birds are satellite-tagged or all potential golden eagle nest sites are continuously monitored there is no possibility of having an instantaneous record of the occupancy status of all current and future golden eagle ranges. Nonetheless, we can use the rich historical database, which stretches back to 1982 (and earlier), to make assumptions about the probable status of a range. Additionally, including the most recent records prior to estimated settlement dates, we are unaware of any instances in which our assumptions were incorrect based on many thousands of hours of field observations by the authors and other field workers (e.g., [3]). For simplicity, we assigned ranges probabilistically to one of two occupancy classes but recognise that there may be a small number of cases in which that assignment was possibly incorrect. On the other hand, as noted by [3], several territories classed as vacant were unequivocally new, despite decades of monitoring and longer-term historical records. This approach and definitional classification have provided previous insight into territory occupancy effects [3,25,49,50].
2.4. Statistical Analyses
Our full dataset was first evaluated as median and mean dates of first territory settlement. We cast data into monthly divisions for graphic illustration.
Analyses included the circular statistical package in R [72]. Data were treated as days of the year (1–365) and converted to radians; results were back-converted to dates for illustrative purposes (radians × 365)/2 × π). We used von Mises bootstrapping to derive 95% CLs directional (seasonal) dates around means [72,73].
Under our first question, we used the Hermans–Rasson test [74] in preference to the more usual Rayleigh’s Test of Uniformity to test whether the seasonal distribution of first territory settlement dates differed from a uniform alternative. A significant difference would indicate support for a seasonal difference in first territory settlement.
To address our subsequent questions, we contrasted data distributions (prior occupied vs. prior vacant territories, and male vs. female) using Watson’s U2 test [73]. These involve tests for the equality of polar vectors (dates of settlement).
We examined potential predictors (prior occupancy status and sex) singularly. Exploratory analyses considered GLM mixed effects models and Bayesian projected normal circular regression (package bpnreg: [72]) but were not used as they did not lead to any substantive differences to our single predictors’ analyses or provide further insight.
3. Results
Our 46 records on the seasonal timing of first territory settlement involved 19 in a prior occupied territory and 27 in a prior vacant territory, and 25 males and 21 females (Table 1). From all records, the median and mean settlement dates were 9 February and 8 February, respectively. Bootstrapping gave 95% CLs, around the mean, of 8 December to 15 March. Descriptively, therefore, the first territory settlement dates of GPS-tagged young eagles were clearly seasonal, with few or no records for several weeks during summer (Figure 1). Settlement tended to peak in late winter and spring, but also occurred in autumn through to early winter (Figure 1).

Table 1.
Raw data used in statistical analyses, by individually tagged eagle (Tag ID). M = Male, F = Female. Prior territory status: Y = occupied prior to settlement, N = vacant prior to settlement.

Figure 1.
Radial illustration of monthly distribution in estimated first territory settlement dates (n = 46).
The seasonal pattern (Figure 1) was significant (Hermans–Rasson test, p = 0.003), thereby supporting our first hypothesis. This significant seasonality was apparently driven by little settlement in the summer months.
Following from this support, on our second hypothesis there were few gross discrepancies between seasonal patterns in territory settlement according to prior occupancy status (vacant or occupied) (Figure 2). Watson’s U2 test showed no significant difference between settlement dates according to prior occupancy status on polar vectors (dates) (test statistic 0.0856, p > 0.10). There was no significant difference between the seasonal timing of settlement according to whether the settled territory was occupied or vacant.
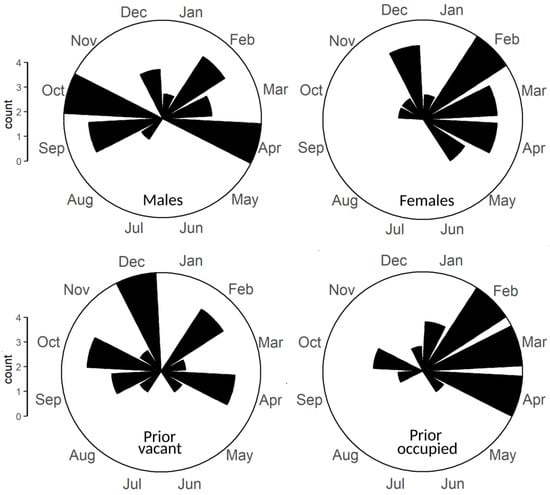
Figure 2.
Radial illustrations of monthly distribution in estimated first territory settlement dates according to: upper panels, prior occupancy status of the settled territory (occupied: n = 19; vacant: n = 27); and lower panels, sex (male: n = 25; female: n = 21).
On our third question, despite continued de facto support for limited settlement during summer, there were some sexual differences (Figure 2). Under Watson’s U2 tests, there was a significant difference in polar vectors (dates) (test statistic 0.2025, 0.01 < p < 0.05). The mean settlement dates were 1 November (95% CLs: 2 July–9 April) for males and 22 February (95% CLs: 24 January–18 March) for females. Males were more likely than females to settle in autumn, leading to a wider spread and an earlier mean settlement date; females’ settlement was concentrated in late winter and spring (Figure 2).
Cross-tabulation of results for prior occupancy status and sex found few significant relationships, perhaps not surprising given sample sizes (Table 2: see also Figure 2). There were nonetheless some trends of interest. The mean settlement date in prior occupied territories was similar between the sexes (Table 2, Figure 2). In prior vacant territories, however, males settled about six months later in a year whereas in females it was weeks earlier, in a significant difference (Table 2). This suggested that the significant difference in the spread of settlement dates between the sexes (see above), wherein males were more likely to settle during the autumnal post-breeding season, was predominantly in prior vacant territories.

Table 2.
Cross tabulation of descriptive statistics for territory settlement dates according to prior occupancy status and sex (sample sizes in parentheses). Means and 95% CLs were derived by von Mises bootstrapping. Results of Watson’s U2 test are given for combinations on equality of polar vectors (NSD = no significant difference).
4. Discussion
We found significant seasonality in when dispersing golden eagles first settled on a territory, involving little settlement during the summer months. This confirmed previous indirect indicative evidence [7,37,38,39].
The lull in summer settlement was not due to the presence of territorial incumbents because the same summer lull occurred in all territories, irrespective of the assumed occupancy status. We could not ascertain the occupancy status precisely in every case immediately before our settlement dates, on a day-by-day basis. We acknowledge that classification may have been imprecise on such a short-term basis. Not all territories we classed as vacant may have been vacant (although that misclassification would have gone against all available data and would have required the sudden unexpected presence of a pair of eagles prior to the estimated settlement date—since a pair is required to deem occupancy). However, it seems highly unlikely that our classifications of territory occupancy involving decades of monitoring and more immediate records prior to settlement dates were always incorrect, and when several territories were new—and so vacant—and not previously recorded over decades of monitoring [3]. Our fundamental observation was of no or very few records of summer settlement. Despite some possible imprecision in our classification of territories’ occupancy status this possibility is inadequate to deny the universal finding and any role of territory occupancy status.
The South of Scotland Golden Eagle Project [66] has involved the translocation of several young golden eagles from the north, to reinforce the small number of territorial pairs and establish a more resilient regional sub-population through additional occupancy of new territories. SSGEP translocation methods are largely identical to those used by reintroduction projects involving the hacking method (e.g., [75,76]). To date, there have been 10 satellite-tagged translocated birds settled on a territory (Fielding et al. unpublished data). All these settlement events did not occur in the summer, consistent with our findings from wild-fledged birds. All these territories were previously unknown and novel (towards fulfilling SSGEP’s purpose) and were therefore vacant in our classification and not defended by any occupants.
Hence, it seems likely that in many cases settling birds were not responding to defensive reactions from occupants, so the seasonal feature was probably innate (i.e., part of dispersing birds’ inherent behavioural repertoire). How this innate settlement behaviour has evolved and persists in the absence of any territorial occupiers’ resistance is open to speculation. It may be an evolutionary ghost of adaptive behaviour in a fully occupied territorial environment when territory occupants in the summer may be more prone to be aggressively defensive of an active breeding attempt. During this period, however, territorial females are more likely concentrated on caring for their eggs/brood at the nest, and territorial males are more likely concerned with provisioning the breeding attempt [39]. Hence, extra time–energetic expenditure on territorial defence would appear to be difficult to maintain during summer and thereby provide an explanation for enhanced temporal defence in the evolutionary past.
Moreover, settlement frequently occurred when the breeding season was beginning in occupants’ preparation (notably, nest building) or in their egg-laying or early incubation stages [39]. If eagles’ undulating displays are even partially related to territory defence [41,77], then several studies have recorded peaks at this time of year [18,40,41]. Indeed, although it is difficult to separate cause and effect, it may be that seasonal display frequency is a response to greater intrusion intensity (and prospective settlement attempts: [7,37,38,39,40]). It does not appear to be a surrogate implicating a driver affecting seasonal settlement occurrence, since dispersing birds frequently settled during this period of display. This is also consistent with our results, showing that the absence of occupants, which would include any displaying, was not influential.
The spread of settlement dates differed between the sexes, with males more likely to settle in autumn, and females’ settlement concentrated in late winter and spring. The sexual difference in autumn settlement appeared to be because males were more likely to settle at this time in vacant territories. This seasonal difference between the sexes should not be due to males settling when younger because on this we previously found no sexual difference [3].
In other species, prospecting for territorial/breeding opportunities often occurs at the end of the breeding season, which is thought to be because at this time the quality of a territory or potential breeding opportunity can be adjudged through the presence and/or quantity of fledged young [20,78]. This may govern decisions on where to attempt settlement, which, in a long-lived species involving years of natal dispersal (such as the golden eagle), may trigger settlement attempts at the time of discovery [49]. In Scottish golden eagles, most fledged juveniles are still in their natal territory in the autumn [1,2,3] and will be readily identifiable [79] as a sign of breeding productivity.
Such a possible ‘immediately settle on prospection of breeding outputs’ scenario, however, appears to be a strawman argument (see also [49]). It does not explain our results, since they should apply equally to both sexes (not found) and the propensity for males to settle in autumn was in assumed vacant territories. Obviously, these were territories where their quality could not be adjudged on the presence or quantity of fledged juveniles.
Further research on this subject is clearly required, as there are several empirical features which elude thorough understanding. To date, on seasonal first territory settlement, our study and others have involved resident populations of large raptors. Future comparable research in migratory populations [16,34,80,81] could be illuminating when the seasonal window for first territory settlement is compressed because it is impossible for much of the non-breeding season.
Author Contributions
Conceptualization, D.P.W. and A.H.F.; methodology, all authors; validation, A.H.F. and D.P.W.; formal analysis, A.H.F. and D.P.W.; investigation, all authors; resources, D.P.W., D.A., S.B., R.R., R.T. and E.D.W.; data curation, all authors; writing—original draft, D.P.W. and A.H.F.; writing—review & editing, all authors; visualization, D.P.W. and A.H.F.; supervision, D.P.W.; project administration, D.P.W.; funding acquisition, D.P.W., D.A., S.B. and R.T. All authors have read and agreed to the published version of the manuscript.
Funding
Funding of tags and data download costs notably came from Natural Research, the Royal Society for the Protection of Birds, Roy Dennis Wildlife Foundation, Ruth Tingay, Forestry and Land Scotland, and SSE. Vattenfall assisted with funding for one tagged bird, and two tags were provided by Movetech. Chris Donald is thanked for a few tags funded by SNH. Manuscript production was financially supported by SSE under the research programme of the Regional Eagle Conservation Management Plan. For facilitating this continued support, we thank Nicki Small and Jenny Chambers most recently. Despite this support, SSE had no influence or commentary in the production of the manuscript. Natural Research funded the costs of open access publication.
International Review Board Statement
Not applicable.
Data Availability Statement
Raw data are presented in Table 1. We cannot publicly or responsibly provide grid references for natal nest sites or subsequent settled territorial nest sites through prohibition under UK legislative practice.
Acknowledgments
Tagging was undertaken by David Anderson, Roy Dennis, Brian Etheridge, Justin Grant, Duncan Orr-Ewing and Ewan Weston: all were appropriately licensed under disturbance, handling, ringing and tagging licences from SNH (Scottish Natural Heritage: latterly NatureScot) and British Trust for Ornithology (BTO). We are extremely grateful for the considerable supporting fieldwork from many members of the Scottish Raptor Study Group. Staff at MTI (tag manufacture and support) and BTO (licensing) were helpful. We are grateful to Emma Ahart, Thomas Plant, Nicki Small and Jenny Chambers (SSE) and Peter Robson (Scottish Power Renewables) for gaining permission to use data and to encourage tag funding. We thank SSGEP, the project board and the project’s manager, Cat Barlow, for providing data on satellite-tagged birds from South Scotland.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Weston, E.D.; Whitfield, D.P.; Travis, J.M.; Lambin, X. When do young birds disperse? Tests from studies of golden eagles in Scotland. BMC Ecol. 2013, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Weston, E.D.; Whitfield, D.P.; Travis, J.M.; Lambin, X. The contribution of flight capability to the post-fledging dependence period of golden eagles. J. Avian Biol. 2018, 49, e01265. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; Anderson, D.; Benn, S.; Dennis, R.; Grant, J.; Weston, E.D. Age of first territory settlement of Golden Eagles Aquila chrysaetos in a variable competitive landscape. Front. Ecol. Evol. 2022, 10, 743598. [Google Scholar] [CrossRef]
- Ferrer, M. Ontogeny of dispersal distances in young Spanish imperial eagles. Behav. Ecol. Sociobiol. 1993, 32, 259–263. [Google Scholar] [CrossRef][Green Version]
- Ferrer, M. Juvenile dispersal behavior and natal philopatry of a long-lived raptor, the Spanish Imperial Eagle Aquila adalberti. Ibis 1993, 135, 132–138. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Duffy, K.; McLeod, D.R.A.; Evans, R.J.; MacLennan, A.M.; Reid, R.; Sexton, D.; Wilson, J.D.; Douse, A. Juvenile dispersal of White-tailed Eagles in western Scotland. J. Raptor Res. 2009, 43, 110–120. [Google Scholar] [CrossRef]
- Weston, E. Juvenile Dispersal Behaviour in the Golden Eagle (Aquila chrysaetos). PhD Thesis, University of Aberdeen, Aberdeen, UK, 2014. [Google Scholar]
- Stenseth, N.C.; Lidicker, W.Z. (Eds.) The Study of Dispersal: A Conceptual Guide. In Animal Dispersal: Small Mammals as A Model; Chapman and Hall: London, UK, 1992; pp. 5–20. [Google Scholar]
- Clobert, J.; Le Galliard, J.F.; Cote, J.; Meylan, S.; Massot, M. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 2009, 12, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.M.; Penteriani, V. Behavioral states help translate dispersal movements into spatial distribution patterns of floaters. Am. Nat. 2008, 172, 475–485. [Google Scholar] [CrossRef]
- Delgado, M.M.; Penteriani, V.; Nams, V.O.; Campioni, L. Changes of movement patterns from early dispersal to settlement. Behav. Ecol. Sociobiol. 2009, 64, 35–43. [Google Scholar] [CrossRef]
- Poessel, S.A.; Woodbridge, B.; Smith, B.W.; Murphy, R.K.; Bedrosian, B.E.; Bell, D.A.; Bittner, D.; Bloom, P.H.; Crandall, R.H.; Domenech, R.; et al. Interpreting long-distance movements of non-migratory golden eagles: Prospecting and nomadism? Ecosphere 2022, 13, e4072. [Google Scholar] [CrossRef]
- Soutullo, A.; Urios, V.; Ferrer, M.; Peñarrubia, S. Post-fledging behaviour in Golden Eagles Aquila chrysaetos: Onset of juvenile dispersal and progressive distancing from the nest. Ibis 2006, 148, 307–312. [Google Scholar] [CrossRef]
- Soutullo, A.; Urios, V.; Ferrer, M.; López-López, P. Habitat use by juvenile Golden Eagles Aquila chrysaetos in Spain. Bird Study 2008, 55, 236–240. [Google Scholar] [CrossRef]
- Urios, V.; Soutullo, A.; López-López, P.; Cadahía, L.; Limiñana, R.; Ferrer, M. The first case of successful breeding of a Golden Eagle Aquila chrysaetos tracked from birth by satellite telemetry. Acta Ornithol. 2007, 42, 205–209. [Google Scholar] [CrossRef]
- McIntyre, C.L.; Douglas, D.C.; Collopy, M.W. Movements of Golden Eagles (Aquila chrysaetos) from interior Alaska during their first year of independence. Auk 2008, 125, 214–224. [Google Scholar] [CrossRef]
- Cadahía, L.; López-López, P.; Urios, V.; Negro, J.J. Satellite telemetry reveals individual variation in juvenile Bonelli’s eagle dispersal areas. Eur. J. Wildl. Res. 2010, 56, 923–930. [Google Scholar] [CrossRef]
- Murphy, R.K.; Dunk, J.R.; Woodbridge, R.D.; Stahlecker, D.W.; LaPlante, D.W.; Millsap, B.A.; Jacobson, K.V. First-year dispersal of Golden Eagles from natal areas in the southwestern United States and implications for second-year settling. J. Raptor Res. 2017, 51, 216–233. [Google Scholar] [CrossRef]
- Cruz-Romo, J.L.; Sánchez-Vilchis, M.; Sánchez-Cordero, V.; Murphy, R.K.; Cruz-Molina, I.; Vargas-Velasco, J.J.; Valdés-Alarcón, M.; Millsap, B.A. First satellite telemetry study of movement behavior of juvenile golden eagles from Mexico. J. Raptor Res. 2022, 56, 28–36. [Google Scholar] [CrossRef]
- Ponchon, A.; Grémillet, D.; Doligez, B.; Chambert, T.; Tveraa, T.; González-Solís, J.; Boulinier, T. Tracking prospecting movements involved in breeding habitat selection: Insights, pitfalls and perspectives. Methods Ecol. Evol. 2013, 4, 143–150. [Google Scholar] [CrossRef]
- Millsap, B.A.; Harmata, A.R.; Stahlecker, D.W.; Mikesic, D.G. Natal dispersal distance of bald and golden eagles originating in the coterminous United States as inferred from band encounters. J. Raptor Res. 2014, 48, 13–23. [Google Scholar] [CrossRef]
- López-López, P. Individual-based tracking systems in ornithology: Welcome to the era of big data. Ardeola 2016, 63, 103–136. [Google Scholar] [CrossRef]
- Sergio, F.; Tavecchia, G.; Blas, J.; López, L.; Tanferna, A.; Hiraldo, F. Variation in age-structured vital rates of a long-lived raptor: Implications for population growth. Basic Appl. Ecol. 2011, 12, 107–115. [Google Scholar] [CrossRef]
- Sergio, F.; Tavecchia, G.; Tanferna, A.; López Jiménez, L.; Blas, J.; De Stephanis, R.; Marchant, T.A.; Kumar, N.; Hiraldo, F. No effect of satellite tagging on survival, recruitment, longevity, productivity and social dominance of a raptor, and the provisioning and condition of its offspring. J. Appl. Ecol. 2015, 52, 1665–1675. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; Anderson, D.; Benn, S.; Reid, R.; Tingay, R.; Weston, E. Sex difference in natal dispersal distances of Golden Eagles Aquila chrysaetos in Scotland. Ibis 2024, 166, 146–155. [Google Scholar] [CrossRef]
- Hunt, W.G. Raptor floaters at Moffat’s equilibrium. Oikos 1998, 82, 191–197. [Google Scholar] [CrossRef]
- Penteriani, V.; Otalora, F.; Sergio, F.; Ferrer, M. Environmental stochasticity in dispersal areas can explain the mysterious disappearance of breeding populations. Proc. R. Soc. Ser. B 2005, 272, 1265–1269. [Google Scholar] [CrossRef]
- Penteriani, V.; Otalora, F.; Ferrer, M. Floater survival affects population persistence. The role of prey availability and environmental stochasticity. Oikos 2005, 108, 523–534. [Google Scholar] [CrossRef]
- Ferrer, M.; Calderón, J. The Spanish Imperial Eagle Aquila adalberti C.L. Brehm 1861 in Doñana National Park (south west Spain): A study of population dynamics. Biol. Conserv. 1990, 51, 151–161. [Google Scholar] [CrossRef]
- Green, R.E.; Pienkowski, M.W.; Love, J.A. Long-term viability of the re-introduced population of the white-tailed eagle Haliaeetus albicilla in Scotland. J. Appl. Ecol. 1996, 33, 357–368. [Google Scholar] [CrossRef]
- Real, J.; Mañosa, S. Demography and conservation of western European Bonelli’s eagle populations. Biol. Conserv. 1997, 79, 59–66. [Google Scholar] [CrossRef]
- Saether, B.; Bakke, Ø. Avian life history variation and contribution of demographic traits to the population growth rate. Ecol. 2000, 81, 642–653. [Google Scholar] [CrossRef]
- Stahl, J.T.; Oli, M.K. Relative importance of life history variables to population growth rate. Ecol. Model. 2006, 198, 23–39. [Google Scholar] [CrossRef]
- Katzner, T.E.; Bragin, E.A.; Milner-Gulland, E.J. Modelling populations of long-lived birds of prey for conservation: A study of Imperial eagles (Aquila heliaca) in Kazakhstan. Biol. Conserv. 2006, 132, 322–335. [Google Scholar] [CrossRef]
- Newton, I. Population Ecology of Raptors; Poyser: Berkhamstead, UK, 1979. [Google Scholar]
- Morandini, V.; Dietz, S.; Newton, I.; Ferrer, M. The role of age of first breeding in modeling raptor reintroductions. Ecol. Evol. 2019, 9, 2978–2985. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Morandini, V.; Newton, I. Floater interference reflects territory quality in the Spanish imperial eagle Aquila adalberti: A test of a density dependent mechanism. Ibis 2015, 157, 849–859. [Google Scholar] [CrossRef]
- Bahat, O. Aspects in the Ecology and Biodynamics of the Golden Eagle (Aquila chrysaetos homeyeri) in the Arid Regions of Israel. Master’s Thesis, Tel Aviv University, Tel Aviv, Israel, 1989. [Google Scholar]
- Watson, J. The Golden Eagle, 2nd ed.; Poyser: London, UK, 2010. [Google Scholar]
- Reid, R.; Haworth, P.F.; Fielding, A.H.; Whitfield, D.P. Spatial distribution of undulating flight displays of territorial Golden Eagles Aquila chrysaetos in Lewis, Scotland. Bird Study 2019, 66, 407–412. [Google Scholar] [CrossRef]
- Bergo, G. Territorial behaviour of golden eagles in western Norway. Brit. Birds 1987, 80, 361–376. [Google Scholar]
- Ogden, R.E.; Heap, E.; McEwing, R.; Tingay, R.; Whitfield, D.P. Population structure and dispersal history in Scottish Golden Eagles Aquila chrysaetos revealed by molecular genetic analysis of territorial birds. Ibis 2015, 157, 834–848. [Google Scholar] [CrossRef]
- Hayhow, D.B.; Benn, S.; Stevenson, A.; Stirling-Aird, P.K.; Eaton, M.A. Status of Golden Eagle Aquila chrysaetos in Britain in 2015. Bird Study 2017, 64, 281–294. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; McLeod, D.R.A.; Haworth, P.F. A Conservation Framework for Golden Eagles: Implications for Their Conservation And Management in Scotland; Scottish Natural Heritage Commissioned Report No.193; SNH: Battleby, Scotland, 2008. [Google Scholar]
- Greenwood, P.J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 1980, 28, 1140–1162. [Google Scholar] [CrossRef]
- Greenwood, P.; Harvey, P.H. The natal and breeding dispersal of birds. Annu. Rev. Ecol. Syst. 1982, 13, 1–21. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Douse, A.; Evans, R.J.; Grant, J.; Love, J.; McLeod, D.R.A.; Reid, R.; Wilson, J.D. Natal and breeding dispersal in a reintroduced population of White-tailed Eagles Haliaeetus albicilla. Bird Study 2009, 56, 177–186. [Google Scholar] [CrossRef]
- Murphy, R.K.; Stahlecker, D.W.; Millsap, B.A.; Jacobson, K.V.; Johnson, A.; Smith, C.S.; Tator, K.J.; Kruse, K.L. Natal dispersal distance of Golden Eagles in the southwestern United States. J. Fish Wildl. Manag. 2019, 10, 213–218. [Google Scholar] [CrossRef]
- Fielding, A.H.; Anderson, D.; Benn, S.; Reid, R.; Tingay, R.; Weston, E.D.; Whitfield, D.P. Substantial variation in prospecting behaviour of young Golden Eagles defies expectations from potential predictors. Diversity 2023, 15, 506. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H. Analyses of The Fates of Satellite Tracked Golden Eagles in Scotland; SNH Commissioned Report No. 982; SNH: Battleby, Scotland, 2017. [Google Scholar]
- Fielding, A.H.; Haworth, P.; Anderson, D.; Benn, S.; Dennis, R.; Weston, E.; Whitfield, D.P. A simple topographical model to predict Golden Eagle Aquila chrysaetos space use during dispersal. Ibis 2020, 162, 400–415. [Google Scholar] [CrossRef]
- Sato, Y.; Ogden, R.; Kishida, T.; Nakajima, N.; Maeda, T.; Inoue-Murayama, M. Population history of the golden eagle inferred from whole-genome sequencing of three of its subspecies. Biol. J. Linn. Soc. 2020, 120, 826–838. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; McLeod, D.R.A.; Haworth, P.F.; Watson, J. A conservation framework for the golden eagle in Scotland: Refining condition targets and assessment of constraint influence. Biol. Conserv. 2006, 130, 465–480. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; McLeod, D.R.A.; Morton, K.; Stirling-Aird, P.; Eaton, M.A. Factors constraining the distribution of Golden Eagles Aquila chrysaetos in Scotland. Bird Study 2007, 54, 199–211. [Google Scholar] [CrossRef]
- Hoechlin, D.R. Development of golden eaglets in southern California. West. Birds 1976, 7, 137–152. [Google Scholar]
- Peterson, D. Möt Kungsörnen; ICA Bokförlag: Västerås, Sweden, 1997. [Google Scholar]
- Phillips, R.A.; Xavier, J.C.; Croxall, J.P. Effects of satellite transmitters on albatrosses and petrels. Auk 2003, 120, 1082–1090. [Google Scholar] [CrossRef]
- Kenward, R.E. A Manual for Wildlife Radio Tagging; Academic Press: London, UK, 2001. [Google Scholar]
- Davies, M.; Green, R. A classification of methods used to attach devices to vultures and condors. Vulture News 2020, 78, 12–17. [Google Scholar] [CrossRef]
- Orr-Ewing, D.; Anderson, D.; Weston, E. Thoracic X-Strap Harness. Design and Method 2. Vulture News 2020, 78, 27–30. [Google Scholar] [CrossRef]
- Thaxter, C.B.; Ross-Smith, V.H.; Clark, J.A.; Clark, N.A.; Conway, G.J.; Masden, E.; Wade, H.M.; Leat, E.H.K.; Gear, S.C.; Marsh, M.; et al. Contrasting effects of GPS device and harness attachment on adult survival of Lesser Black-backed Gulls Larus fuscus and Great Skuas Stercorarius skua. Ibis 2016, 158, 279–290. [Google Scholar] [CrossRef]
- García, V.; Iglesias-Lebrija, J.J.; Moreno-Opo, R. Null effects of Garcelon harnessing method and transmitter type on soaring raptors. Ibis 2021, 163, 899–912. [Google Scholar] [CrossRef]
- Anderson, D.; Arkumarev, V.; Bildstein, K.; Botha, A.; Bowden, C.G.R.; Davies, M.; Duriez, O.; Forbes, N.A.; Godino, A.; Green, R.E.; et al. A practical guide to methods for attaching research devices to vultures and condors. IUCN Species Survival Commission Vulture Specialist Group Technical Publication No. 1. Vulture News 2020, 78, 1–72. [Google Scholar] [CrossRef]
- Bortolotti, G.R. Age and sex variation in Golden Eagles. J. Field Ornithol. 1984, 55, 54–66. [Google Scholar]
- Harmata, A.; Montopoli, G. Morphometric sex determination of North American Golden Eagles. J. Raptor Res. 2013, 47, 108–116. [Google Scholar] [CrossRef]
- SSGEP. The South of Scotland Golden Eagle Project. 2023. Available online: https://www.goldeneaglessouthofscotland.co.uk/about/the-project (accessed on 19 September 2023).
- Dennis, R.H.; Ellis, P.M.; Broad, R.A.; Langslow, D.R. The status of the Golden Eagle in Britain. Br. Birds 1984, 77, 592–607. [Google Scholar]
- Green, R.E. The status of the Golden Eagle in Britain in 1992. Bird Study 1996, 43, 20–27. [Google Scholar] [CrossRef]
- Challis, A.; Edwards, C.; Heavisides, A.; Holling, M.; Kortland, K.; Mattingley, W.; Riddle, G.; Roos, S.; Stevenson, A.; Stirling-Aird, P.K.; et al. The Scottish Raptor Monitoring Scheme: Recent developments in good practice monitoring. Bird Study 2018, 65, S21–S34. [Google Scholar] [CrossRef]
- Hardey, J.; Crick, H.Q.P.; Wernham, C.V.; Riley, H.; Etheridge, B.; Thompson, D.B.A. Raptors. A Field Guide for Surveys and Monitoring, 3rd ed.; The Stationery Office: Edinburgh, UK, 2013. [Google Scholar]
- Hunt, W.G.; Law, P.R. Commentary: Subadult nest occupancy rates and floater-to-breeder ratios in raptor population assessment. J. Raptor Res. 2023, 57, 1–5. [Google Scholar] [CrossRef]
- Agostinelli, C.; Lund, U. R Package ‘Circular’: Circular Statistics (Version 0.4–93). 2017. Available online: https://r-forge.r-project.org/projects/circular (accessed on 5 December 2023).
- Landler, L.; Ruxton, G.D.; Malkemper, E.P. Advice on comparing two independent samples of circular data in biology. Sci. Rep. 2021, 11, 20337. [Google Scholar] [CrossRef]
- Landler, L.; Ruxton, G.D.; Malkemper, E.P. The Hermans–Rasson test as a powerful alternative to the Rayleigh test for circular statistics in biology. BMC Ecol. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Ferrer, M.; Morandini, V.; Baguena, G.; Newton, I. Reintroducing endangered raptors: A case study of supplementary feeding and removal of nestlings from wild populations. J. Appl. Ecol. 2018, 55, 1360–1367. [Google Scholar] [CrossRef]
- Ferrer, M.; Evans, R.; Hedley, J.; Hollamby, S.; Meredith, A.; Morandini, V.; Selly, O.; Smith, C.; Whitfield, D.P. Hacking techniques improve health and nutritional status of nestling White-tailed Eagles. Ecol. Evol. 2023, 13, e9776. [Google Scholar] [CrossRef] [PubMed]
- Harmata, A.R. What is the function of undulating flight display in golden eagles? Raptor Res. 1982, 16, 103–109. [Google Scholar]
- Reed, J.M.; Boulinier, T.; Danchin, E.; Oring, L.W. Informed Dispersal. In Current Ornithology; Nolan, V., Ketterson, E.D., Thompson, C.F., Eds.; Springer: Boston, MA, USA, 1999; Volume 15. [Google Scholar] [CrossRef]
- Bloom, P.H.; Clark, W.S. Molt and sequence of plumages of golden eagles and a technique for in-hand ageing. N. Am. Bird Bander 2001, 26, 97–116. [Google Scholar]
- McIntyre, C.L.; Schmidt, J.H. Ecological and environmental correlates of territory occupancy and breeding performance of migratory Golden Eagles Aquila chrysaetos in interior Alaska. Ibis 2012, 154, 124–135. [Google Scholar] [CrossRef]
- Nygård, T.; Jacobsen, K.-O.; Johnsen, T.V.; Systad, G.H. Dispersal and survival of juvenile golden eagles (Aquila chrysaetos) from Finnmark, northern Norway. J. Raptor Res. 2016, 50, 144–160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).