Species in Disguise: A New Species of Hornshark from Northern Australia (Heterodontiformes: Heterodontidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphology
2.2. Vertebral Meristics
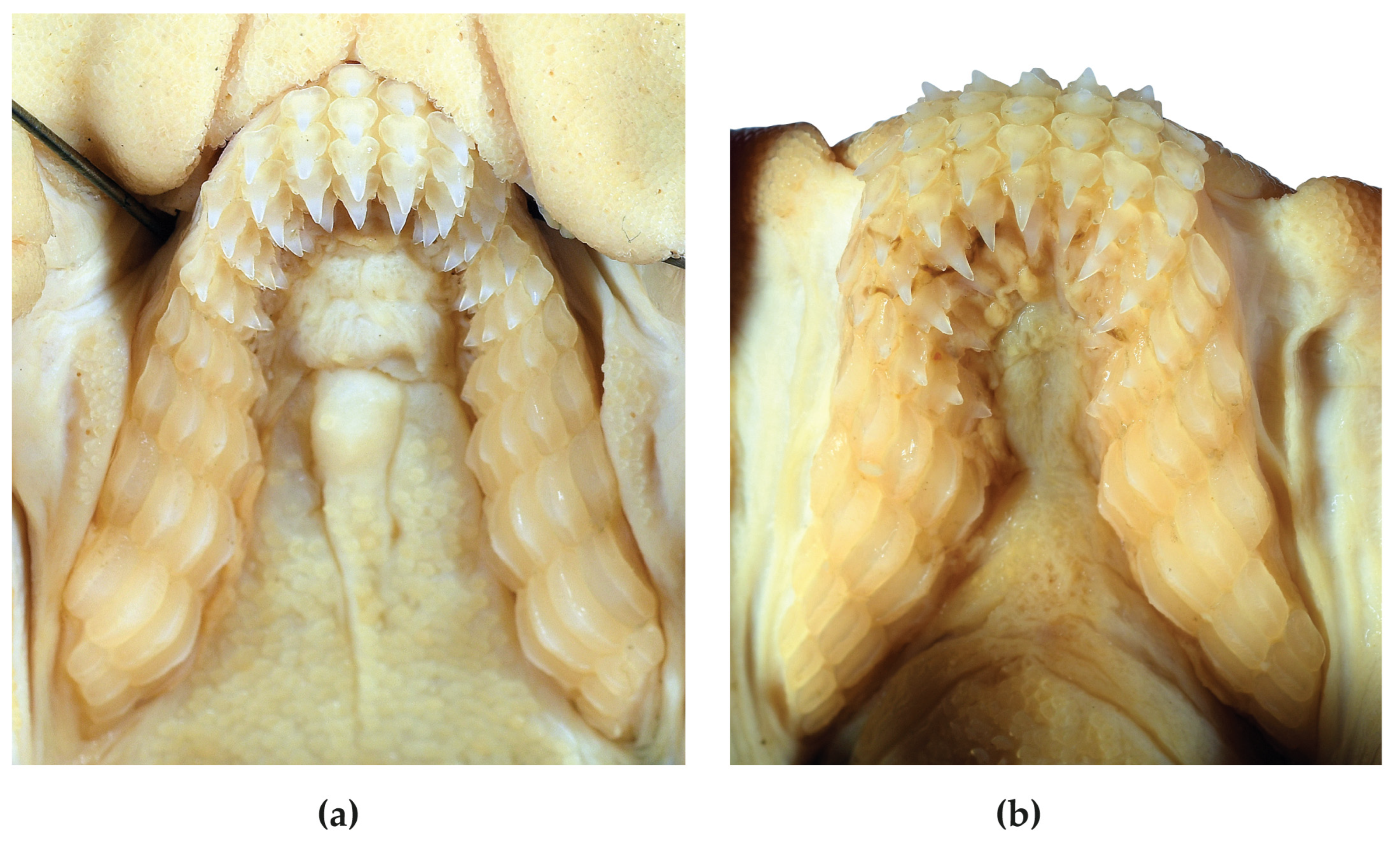
2.3. Dentition
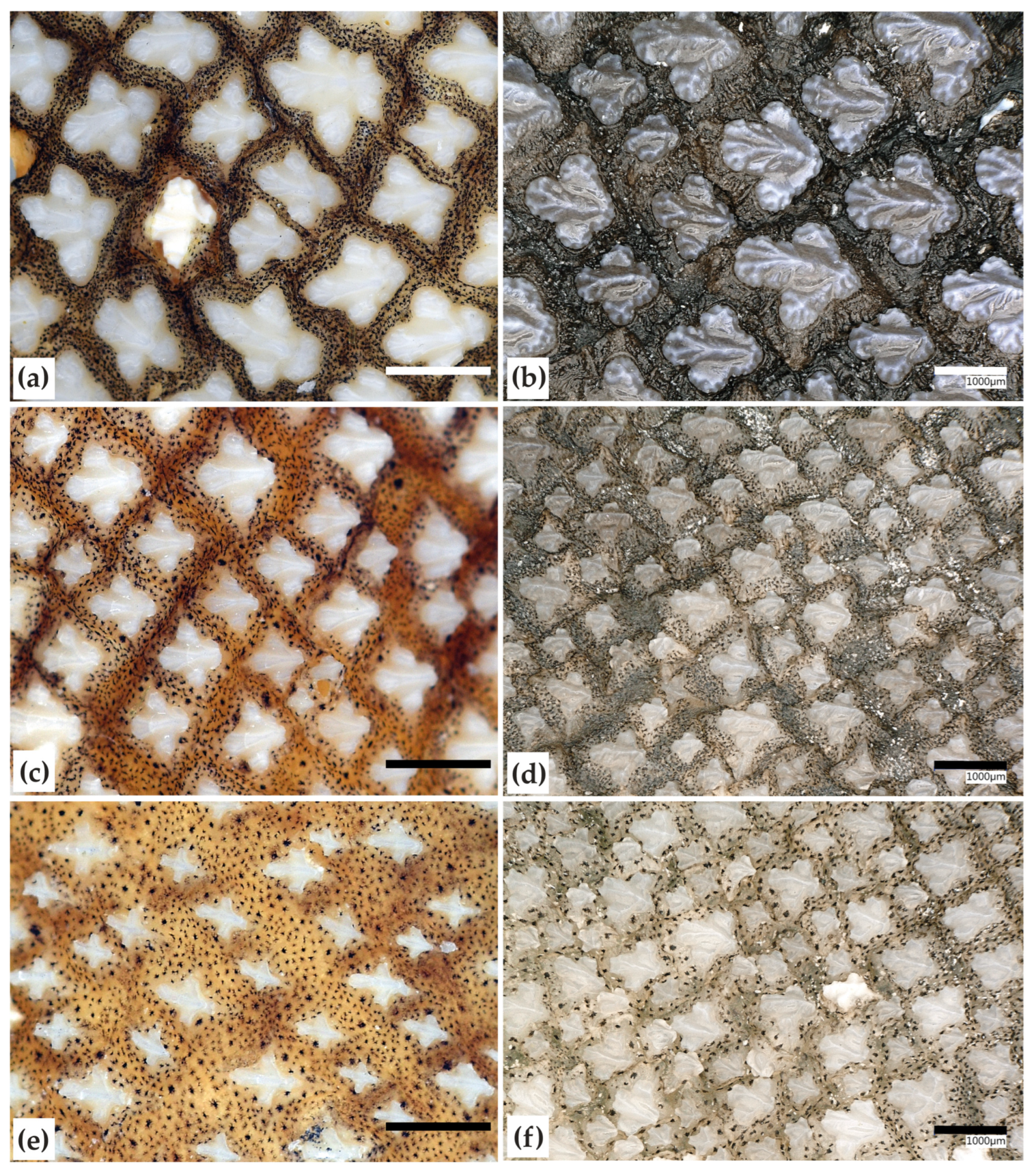
2.4. Denticles
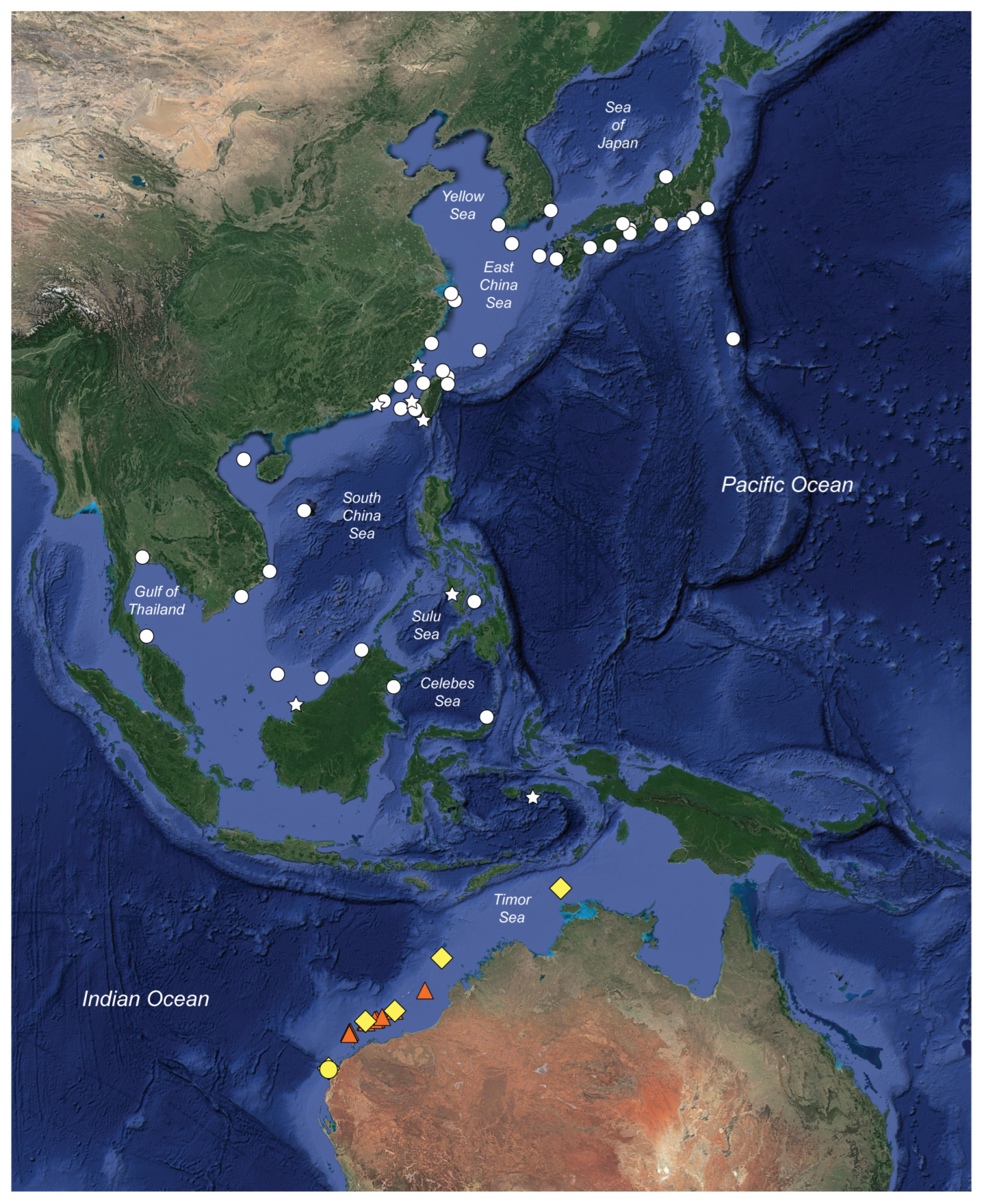
2.5. Distribution
2.6. Molecular Analyses
2.7. Institutional Acronyms
2.8. Comparative Material Examined
3. Results
3.1. Comparison with Congeners
3.2. Systematic Account
3.2.1. Synonymy
3.2.2. Type Material
3.2.3. Representative DNA Sequences
3.2.4. Etymology
3.2.5. Diagnosis
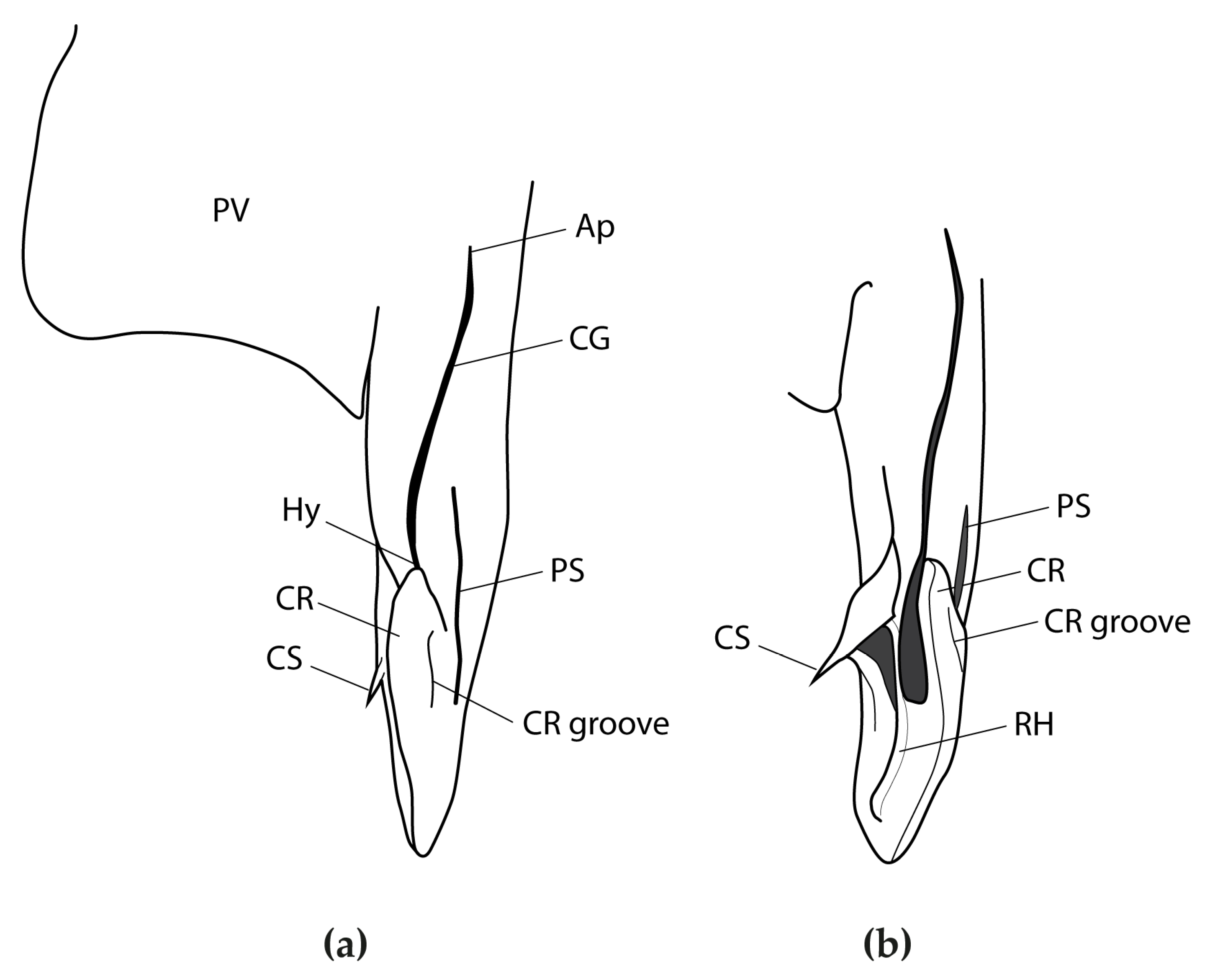
3.2.6. Description
3.2.7. Colour
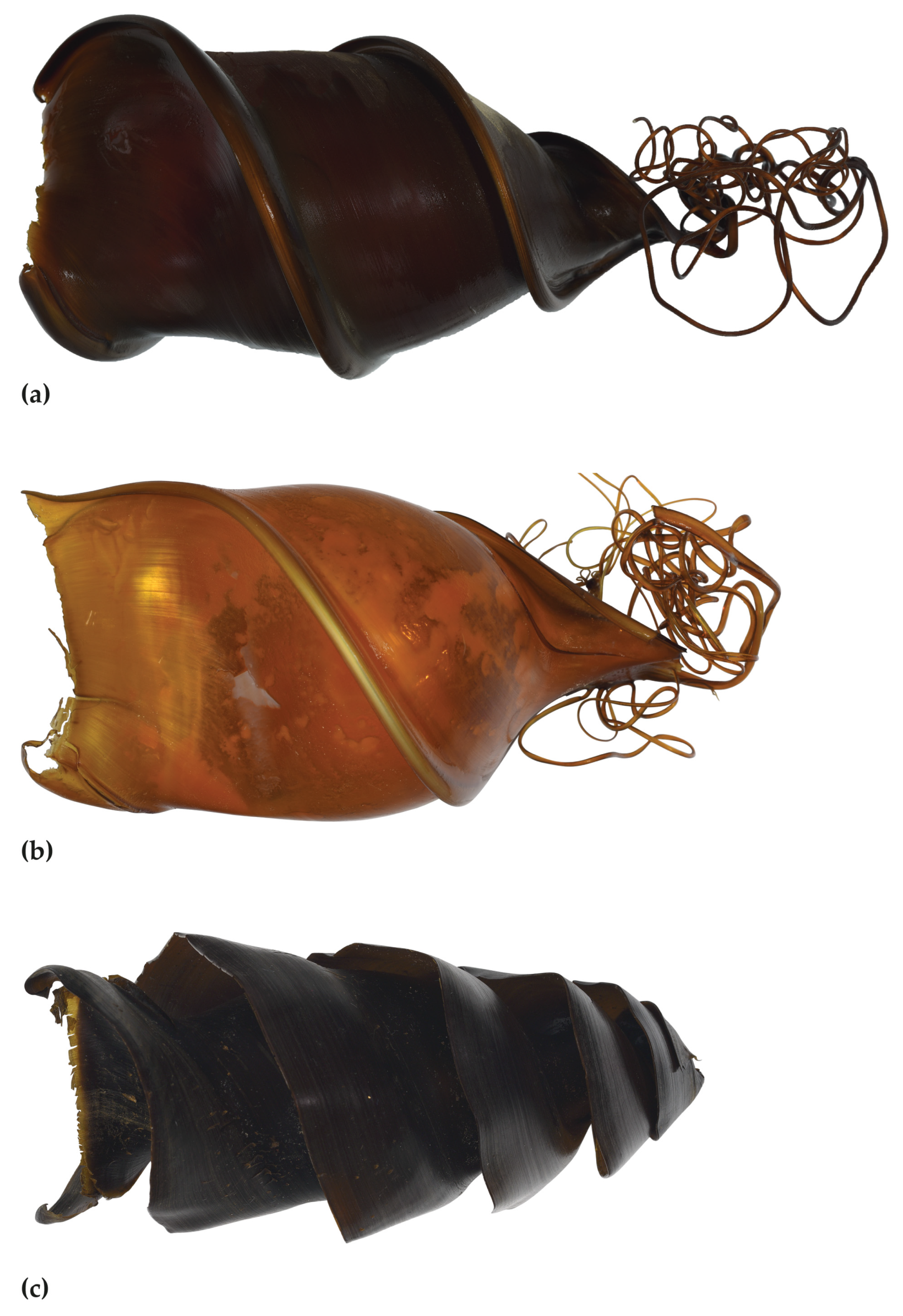
3.2.8. Egg Case
3.2.9. Size
3.2.10. Distribution
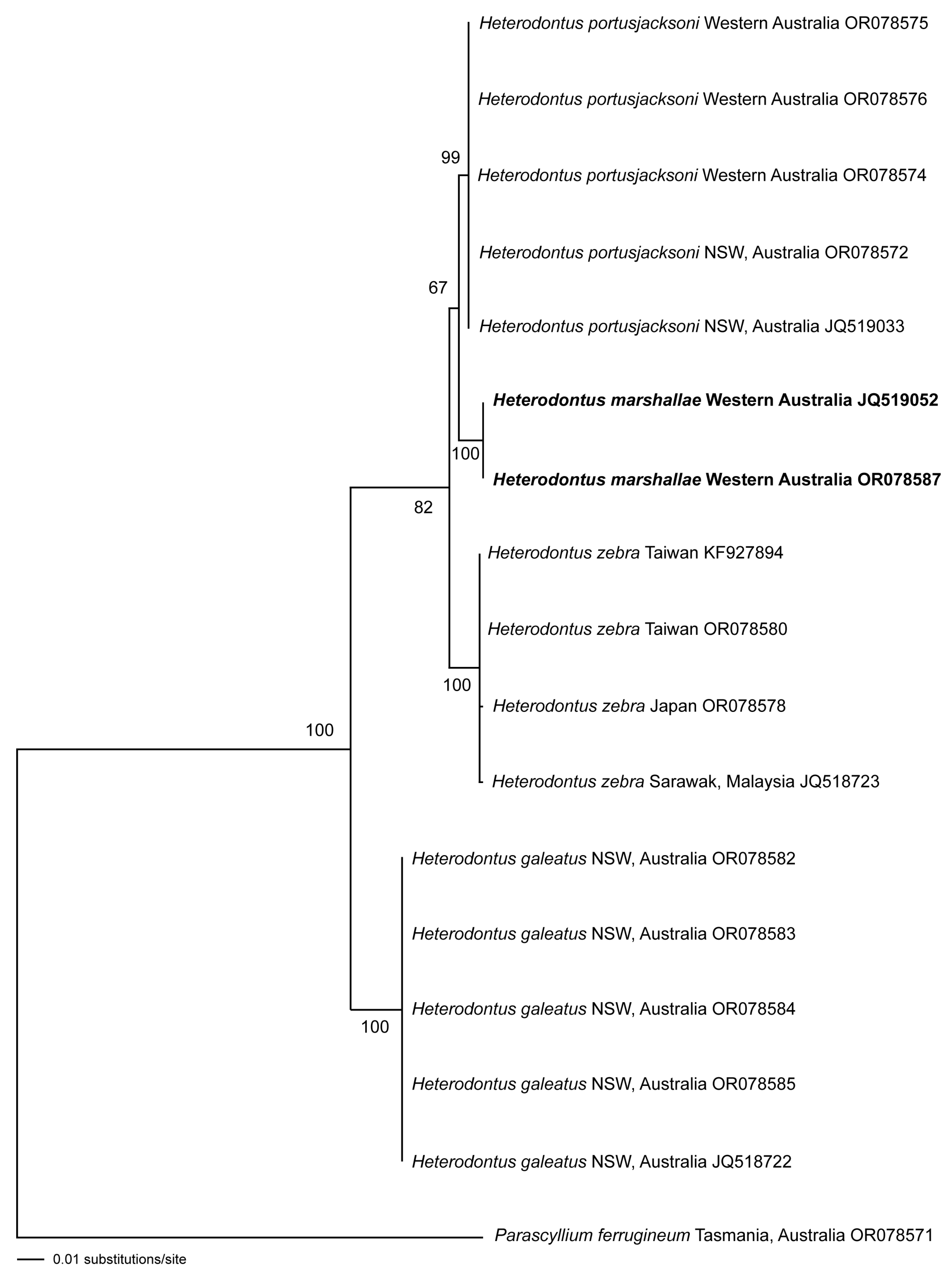
3.3. Molecular Analyses
3.4. Intraspecific Variation
3.5. Comparison with Congeners
4. Discussion
4.1. Comparison between Species
4.2. Dentition and Denticles
4.3. Distribution
4.4. Egg Cases
4.5. Key to Species of Heterodontus
- Body and fins: spotted ……………………………………………………………………….. 2Body and fins with stripes, bars or bands: not spotted ………………………………… 5
- Body and fins with white spots in adults and subadults; hatchlings with thin curved parallel lines on body ………………… Heterodontus ramalheira (Western Indian Ocean)Body and fins with dark spots and darker saddles (most evident in juveniles) ….… 3
- First dorsal fin origin behind pectoral fin bases… Heterodontus quoyi (Eastern Pacific)First dorsal fin origin over pectoral fin bases …………………………………………… 4
- Back and sides with small dark spots less than a third of eye diameter in size; no light-coloured bar on interorbital surface of head …………………………………….. ……………………………………………………… Heterodontus francisci (Eastern Pacific)Back and sides with larger dark spots at least half eye diameter in size; a light-coloured bar on interorbital surface of head.…… Heterodontus mexicanus (Eastern Pacific)
- Supraorbital crests: very high ……………… Heterodontus galeatus (eastern Australia)Supraorbital crests: moderate to low ……………………………………………………. 6
- Body with a distinctive harness-like pattern of dark stripes extending from in front of dorsal fin on the dorsal midline to the pectoral and pelvic fin bases …………………..……………………………………… Heterodontus portusjacksoni (southern Australia)Body with vertical dark bands or saddles not arranged in a harness pattern …….… 7
- Body with 22–36 narrow dark bars and saddles from snout to origin of caudal fin ……………………………………………………………………………….…………….… 8Body with 5–14 broad or narrow, diffuse-edged markings from snout to origin of caudal fin ……….…………..……………………………………………………………… 9
- Narrow triangular dark marking extending from snout to midline of head in front of eyes; dark bar extending onto pectoral fin from posterior gills…………………………..……………………………………………………. Heterodontus zebra (Northwest Pacific)Dark marking on snout semicircular, not narrowly triangular; no dark bar extending from onto pectoral fin from posterior gills …….…………………………………………..………………………………………. Heterodontus marshallae n. sp. (northern Australia)
- Dorsal, pectoral and caudal fins without abruptly black tips or white apical spots; body with 11–14 dark markings from snout to origin of caudal fin ……………………………………………………………………….. Heterodontus japonicus (Northwest Pacific)Dorsal and pectoral fins and ventral caudal fin lobe with abruptly black tips, dorsal fins with white apical spots; body with four or five broad dark saddles from snout tip to origin of caudal fin ………………… Heterodontus omanensis (Western Indian Ocean)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blainville, H.d. Prodrome d’une nouvelle distribution systématique du règne animal. J. Phys. Chim. Et D’histoire Nat. Paris 1816, 83, 244–267. [Google Scholar]
- Maisey, J.G. What is an ‘elasmobranch’? The impact of palaeontology in understanding elasmobranch phylogeny and evolution. J. Fish Biol. 2012, 80, 918–951. [Google Scholar] [CrossRef] [PubMed]
- Last, P.R.; Stevens, J.D. Sharks and Rays of Australia, 2nd ed.; CSIRO Publishing: Melbourne, Australia, 2009; pp. 1–644. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N. Eschmeyer’s Catalog of Fishes: Guide to Fish Collections. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/collections.asp (accessed on 8 May 2023).
- Girard, C.F. Characteristics of some cartilaginous fishes of the Pacific coast of North America. Proc. Acad. Nat. Sci. Philadelphia. 1855, 7, 196–197. [Google Scholar]
- Günther, A. Catalogue of the Physostomi, containing the families Gymnotidae, Symbranchidae, Muraenidae, Pegasidae, and of the Lophobranchii, Plectognathi, Dipnoi, Ganoidei, Chondropterygii, Cyclostomata, Leptocardii, in the British Museum. In Catalogue of the Fishes in the British Museum; Taylor and Francis: London, UK, 1870; Volume 8, pp. 1–549. [Google Scholar]
- Miklouho Maclay, N.d.; Macleay, W. Plagiostomata of the Pacific. Part II. Proc. Linn. Soc. N.S.W. 1884, 8, 426–431. [Google Scholar]
- Taylor, L.R.J.; Castro-Aguirre, J.L. Heterodontus mexicanus, a new horn shark from the Golfo de California. An. Esc. Nac. Cien. Biol. 1972, 19, 123–143. [Google Scholar]
- Baldwin, Z.H. A new species of bullhead shark, genus Heterodontus (Heterodontiformes: Heterodontidae), from Oman. Copeia 2005, 2005, 262–264. [Google Scholar] [CrossRef]
- Meyer, F.A.A. Systematisch-Summarische Uebersicht der Neuesten Zoologischen Entdeckungen in Neuholland und Afrika; Dykische Buchhandlung: Leipzig, Germany, 1793; pp. 1–178. [Google Scholar]
- Fréminville, C.P.P. Notice sur une nouvelle espèce de poisson appartenant au genre Cestracion de Cuvier. Mag. Zool. Anat. Comp. Palæontol. (Sér. 2) 1840, 2, 1–3. [Google Scholar]
- Smith, J.L.B. Interesting fishes of three genera new to South Africa. Ann. Mag. Nat. Hist. (Ser. 12) 1949, 2, 367–374. [Google Scholar] [CrossRef]
- Gray, J.E. Description of three new species of fish, including two undescribed genera, discovered by John Reeves, Esq., in China. Zool. Misc. 1831, 1, 4–5. [Google Scholar]
- Naylor, G.J.P.; Caira, J.N.; Jensen, K.; Rosana, K.A.M.; White, W.T.; Last, P.R. A DNA sequence-based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bull. Am. Mus. Nat. Hist. 2012, 367, 1–263. [Google Scholar] [CrossRef]
- Compagno, L.J.V. Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date; FAO: Rome, Italy, 2001; pp. 1–269. [Google Scholar]
- Cuvier, G. Tome II. Les reptiles, les poissons, les mollusques et les annelids. In Le Règne Animal Distribué D’après Son Organisation, Pour Server de Base à L’histoire Naturelle des Animaux Et D’introduction à L’anatomie Compare; Deterville: Paris, France, 1817; pp. 1–532. [Google Scholar]
- Bleeker, P. Beschrijvingen van nieuwe en weinig bekende vischsoorten van Amboina, verzameld op eene reis door den Molukschen Archipel, gedaan in het gevolg van den Gouverneur Generaal Duymaer van Twist, in September en Oktober 1855. Acta Soc. Regiae Sci. Indo-Neêrlandicae 1856, 15, 1–76. [Google Scholar]
- Bloch, M.E.; Schneider, J.G. M. E. Blochii, Systema Ichthyologiae Iconibus cx Illustratum. Post Obitum Auctoris Opus Inchoatum Absolvit, Correxit, Interpolavit Jo. Gottlob Schneider, Saxo. Berolini. Sumtibus Auctoris Impressum et Bibliopolio Sanderiano Commissum; Sumtibus Auctoris Impressum et Bibliopolio Sanderiano Commissum: Berlin, Germany, 1801; pp. 1–584. [Google Scholar]
- Regan, C.T. Descriptions of some new sharks in the British Museum Collection. Ann. Mag. Nat. Hist. (Ser. 7) 1906, 18, 435–440. [Google Scholar] [CrossRef]
- Garman, S. The Plagiostomia (Shark, Skates and Rays). Mem. Mus. Comp. Zool. 1913, 36, 1–557. [Google Scholar] [CrossRef]
- Fowler, H.W. Contributions to the biology of the Philippine archipelago and adjacent regions. The fishes of the groups Elasmobranchii, Holocephali, Isospondyli, and Ostariophysi obtained by the United States Bureau of Fisheries Steamer “Albatross” in 1907 to 1910, chiefly in the Philippine Islands and adjacent seas. Bull. US Nat. Mus. 1941, 13, 1–879. [Google Scholar]
- Compagno, L.J.V. FAO Species Catalogue. Vol. 4, Sharks of the World. In An Annotated and Illustrated Catalogue of Shark Species Known to Date; FAO: Rome, Italy, 1984; pp. 1–655. [Google Scholar]
- Kyne, P.M.; Heupel, M.R.; White, W.T.; Simpfendorfer, C.A. The Action Plan for Australian Sharks and Rays 2021; National Environmental Science Program, Marine Biodiversity Hub: Hobart, Australia, 2021; pp. 1–436. [Google Scholar]
- Last, P.R.; White, W.T.; Pogonoski, J.J.; Gledhill, D.C.; Ward, B.; Yearsley, G.K. Part 1—Application of a rapid taxonomic approach to the genus Squalus. In Descriptions of New Dogfishes of the Genus Squalus (Squaloidea: Squalidae); CSIRO Marine and Atmospheric Research Paper 014; Last, P.R., White, W.T., Pogonoski, J.J., Eds.; CSIRO Australia: Hobart, Australia, 2007; pp. 1–10. [Google Scholar]
- Compagno, L.J.V. Sharks of the Order Carcharhiniformes; Princeton University Press: Princeton, USA, 1988; pp. 1–486. [Google Scholar]
- Casier, E. La faune ichthyologique de l’Yprésien de la Belgique. Mémoires Musée R. D’histoire Nat. Belg. 1946, 104, 3–267. [Google Scholar]
- Mollen, F.H. Making Louis Agassiz’s wish come true: Combining forces and a new protocol for collecting comparative skeletal material of sharks, skates and rays, as a comment and an addition to ‘The need of providing tooth morphology in descriptions of extant elasmobranch species’ by Guinot et al. (2018). Zootaxa 2019, 4571, 295–300. [Google Scholar] [CrossRef]
- Reif, W.-E. Squamation and ecology of sharks. Cour. Forschungsinst. Senckenberg 1985, 75, 1–101. [Google Scholar]
- Bleeker, P. Énumerátion des espèces de poissons actuellement connues du Japon et description de trois espèces inédites. Verh. K. Akad. Wet. 1879, 18, 1–33. [Google Scholar]
- Jordan, D.S.; Hubbs, C.L. Record of fishes obtained by David Starr Jordan in Japan, 1922. Mem. Carnegie Mus. 1925, 10, 93–346. [Google Scholar] [CrossRef]
- Smith, B.G. The Heterodontid sharks: Their natural history, and the external development of Heterodontus (Cestracion) japonicus based on notes and drawings by Bashford Dean. In The Bashford Dean Memorial Volume. Archaic Fishes, Gudger, E.W., Ed.; The American Museum of Natural History: New York, USA, 1942; Volume 8, pp. 651–802. [Google Scholar]
- Kamohara, T. Description of the Fishes from the Provinces of Tosa and Kishu, Japan; Kochi Bunkyo Kyoukai: Kochi, Japan, 1950; p. 3+288+5+48+27. [Google Scholar]
- Chen, C.T.; Teshima, K.; Mizue, K. Studies on sharks-IV. Testes and spermatogeneses in selachians. Bull. Fac. Fish. Nagasaki Univ. 1973, 35, 53–65. [Google Scholar]
- Randall, J.E.; Ida, H.; Kato, K.; Pyle, R.L.; Earle, J.L. Annotated checklist of the inshore fishes of the Ogasawara Islands. Nat. Sci. Mus. Monogr. 1997, 11, 72–74. [Google Scholar]
- Yamashita, H.; Yoshida, T.; Motomura, H. Catalog of cartilaginous fish specimens from Kagoshima Prefecture. Nat. Kagoshima 2012, 38, 119–138. [Google Scholar]
- Nakabo, T. Fishes of Japan: With Pictorial Keys to the Species, 3rd ed.; Tokai Univerity Press: Kanagawa, Japan, 2013; pp. 1–2428. [Google Scholar]
- Mori, T. Check list of the fishes of Korea. Mem. Hyogo Univ. Agric. Biol. Ser. 1952, 1, 131. [Google Scholar]
- Peters, W.C.H. Über die von der chinesischen Regierung zu der internationalen Fischerei-Austellung gesandte Fischsammlung aus Ningpo. Ber. Akad. Wiss. Berlin 1881, 45, 921–927. [Google Scholar]
- Wu, X.W. Study of the fishes of Amoy. Part 1. Contrib. Biol. Lab. Sci. Soc. China (Zool. Ser.) 1929, 5, 1–90. [Google Scholar]
- Wang, K.F. Preliminary notes on the fishes of Chekiang (Elasmobranches). Contrib. Biol. Lab. Sci. Soc. China (Zool. Ser.) 1933, 9, 87–117. [Google Scholar]
- Chen, X.; Peng, X.; Huang, X.; Xiang, D. Complete mitochondrial genome of the Zebra bullhead shark Heterodontus zebra (Heterodontiformes: Heterodontidae). Mitochondrial DNA 2013, 25, 280–281. [Google Scholar] [CrossRef]
- Chen, J.T.F. A review of the sharks of Taiwan. Biol. Bull. Tunghai Univ. Dep. Biol. Coll. Sci. Ichthyol. Ser. 1963, 1, 1–102. [Google Scholar]
- Soto, J.M.R.; Mincarone, M.M. Collections of the Museu Oceanografico do vale do Itajai. I. Catalog of cartilaginous fishes (Myxini, Cephalaspidomorphi, Elasmobranchii, Holocephali). Mare Magnum 2004, 2, 1–125. [Google Scholar]
- Rigby, C.L.; Derrick, D.; Dharmadi; Fahmi; Ho, H.; Utzurrum, J.A.T. Heterodontus zebra. The IUCN Red List of Threatened Species 2020. 2020. Available online: https://www.iucnredlist.org/species/41825/68625931 (accessed on 14 April 2023).
- Fourmanoir, P.; Nhu-Nhung, D.T. Liste complémentaire des poissons marins de Nha-Trang. Cah. ORSTOM. Série Océanographie 1965, 84, 1–114. [Google Scholar]
- Long, N. Data collection on shark fisheries in Viet Nam. In Report on the Study on Shark Production, Utilization, and Management in the ASEAN Region, 2003–2004; Secretariat, Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2006; pp. 131–163. [Google Scholar]
- Compagno, L.J.V.; Last, P.R.; Stevens, J.D.; Alava, M.N.M. Checklist of Philippine chondrichthyes; CSIRO: Hobart, Australia, 2005; pp. 1–103. [Google Scholar]
- Krajangdara, T. The Cartilaginous Fishes (Sharks, Rays, and Chimaeras) Found in Thai Waters and the Adjacent Areas; Department of Fisheries: Phuket, Thailand, 2017; pp. 1–231. [Google Scholar]
- Yano, K.; Ali, A.; Gambang, A.C.; Hamid, I.A.; Razak, S.A.; Zainal, A. Sharks and Rays of Malaysia and Brunei Darussalam; SEAFDEC/MFRDMD: Kuala Terengganu, Malaysia, 2005; pp. 1–557. [Google Scholar]
- Last, P.R.; White, W.T.; Caira, J.N.; Dharmadi; Fahmi; Jensen, K.; Lim, A.P.K.; Manjaji-Matsumoto, B.M.; Naylor, G.J.P.; Pogonoski, J.J.; et al. Sharks and Rays of Borneo; CSIRO Publishing: Melbourne, Australia, 2010; pp. 1–298. [Google Scholar]
- Bleeker, P. Beschrijvingen van nieuwe of weinig bekende vischsoorten van Manado en Makassar, grootendeels verzameld op eene reis naar den Molukschen Archipel in het gevolg van den Gouverneur Generaal Duymaer van Twist. Acta Soc. Regiae Sci. Indo-Neêrlandicae 1856, 16, 1–80. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 1989; Volume 1, pp. 1–1659. [Google Scholar]
- Stamatakis, A. RAxML–VI–HPC: Maximum Likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and postanalysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougement, J. A rapid bootstrap algorithm for the RAxML web-servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D. PAUP*. Phylogenetic Analysis Using Parsimony (* and other Methods). Version 4; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Berg, L.S. A classification of fish-like vertebrates. Izv. Akad. Nauk. USSR Seriya Biol. 1937, 4, 1277–1280. [Google Scholar]
- Gray, J.E. List of the Specimens of Fish in the Collection of the British Museum. Part I.—Chondropterygii; Order of the Trustees; British Museum: London, UK, 1851; pp. 1–160. [Google Scholar]
- Sainsbury, K.J.; Kailola, P.J.; Leyland, G.G. Continental Shelf Fishes of Northern and North-Western Australia: An Illustrated Guide; Clouston & Hall and Peter Pownall Fisheries Information Service: Canberra, Australia, 1985; pp. 1–375. [Google Scholar]
- Last, P.R.; Stevens, J.D. Sharks and Rays of Australia; CSIRO: Melbourne, Australia, 1994; pp. 1–513. [Google Scholar]
- Williams, A.; Last, P.R.; Gomon, M.F.; Paxton, J.R. Species composition and checklist of the demersal ichthyofauna of the continental slope off Western Australia (20–35° S). Rec. West. Aust. Mus. 1996, 18, 135–155. [Google Scholar]
- Ramm, D.C. Assessment of Groundfish Stocks in Northern Australian Waters between 127–137°E; Department of Primary Industry and Fisheries: Darwin, Australia, 1997; pp. 1–85. [Google Scholar]
- Hutchins, J.B. Checklist of the fishes of Western Australia. Rec. West. Aust. Mus. 2001, 63, 9–50. [Google Scholar] [CrossRef]
- McAuley, R.; Newbound, D.; Ashworth, R. Field Identification Guide to Western Australian Sharks and Shark-like Rays; Department of Fisheries, Western Australia: Perth, Australia, 2002; pp. 1–25. [Google Scholar]
- Compagno, L.J.V.; Dando, M.; Fowler, S. A Field Guide to the Sharks of the World; Harper Collins Publishing Ltd.: London, UK, 2004; pp. 1–368. [Google Scholar]
- Larson, H.K.; Williams, R.S.; Hammer, M.P. An annotated checklist of the fishes of the Northern Territory, Australia. Zootaxa 2013, 3696, 1–293. [Google Scholar] [CrossRef]
- Migom, F.; Hoedemakers, K. On the dentition of the extant species Heterodontus mexicanus and Heterodontus quoyi. Palaeo Ichthyol. 2023, 16, 1–32. [Google Scholar]
- Cappetta, H. Types dentaires adaptatifs chez les selaciens actuels et post-Paleozoiques. Palaeovertebrata 1986, 16, 57–76. [Google Scholar]
- Ebert, D.A.; Dando, M.; Fowler, S. Sharks of the World: A Complete Guide; Princeton University Press: Princeton, NJ, USA, 2021; pp. 1–607. [Google Scholar]
- Guinot, G.; Adnet, S.; Shimada, K.; Underwood, C.J.; Siversson, M.; Ward, D.J.; Kriwet, J.; Cappetta, H. On the need of providing tooth morphology in descriptions of extant elasmobranch species. Zootaxa 2018, 4461, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Powter, D.M.; Gladstone, W.; Platell, M. The influence of sex and maturity on the diet, mouth morphology and dentition of the Port Jackson shark, Heterodontus portusjacksoni. Mar. Freshwat. Res. 2010, 61, 74–85. [Google Scholar] [CrossRef]
- Hovestadt, D.C. Reassessment and revision of the fossil Heterodontidae (Chondrichthyes: Neoselachii) based on tooth morphology of extant taxa. Palaeontos 2018, 30, 1–73. [Google Scholar]
- Reif, W.-E. Morphogenesis, pattern formation and function of the dentition of Heterodontus (Selachii). Zoomorphologie 1976, 83, 1–47. [Google Scholar] [CrossRef]
- Reif, W.-E. Ontogenese des Hautskelettes von Heterodontus falcifer (Selachii) aus dem Undertithon. Vergleichende morphologie der Hautzänchen der Haie. Stutt. Beitr. Naturkd. B 1973, 7, 1–16. [Google Scholar]
- Reif, W.-E. Morphogenese und musterbildung des hautzähnchen-skelettes von Heterodontus. Lethaia 1974, 7, 25–42. [Google Scholar] [CrossRef]
- Keesing, J. Benthic Habitats and Biodiversity of the Dampier and Montebello Australian Marine Parks; CSIRO: Perth, Australia, 2019; pp. 1–236. [Google Scholar]
- Last, P.R.; Pogonoski, J.J.; Gledhill, D.C.; White, W.T.; Walker, C.J. The deepwater demersal ichthyofauna of the western Coral Sea. Zootaxa 2014, 3887, 191–224. [Google Scholar] [CrossRef]
- Ebert, D.A. Sharks, Rays, and Chimaeras of California; University of California Press: Berkeley, CA, USA, 2003; pp. 1–284. [Google Scholar]
- Powter, D.M.; Gladstone, W. The reproductive biology and ecology of the Port Jackson shark Heterodontus portusjacksoni in the coastal waters of eastern Australia. J. Fish Biol. 2008, 72, 2615–2633. [Google Scholar] [CrossRef]







| H. marshallae n. sp. | H. zebra | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Paratypes | Mean | sd. | |||||||
| Character | Holo | Min | Max | Min | Max | Mean | sd. | ||
| Total length (mm) | 541 | 355 | 601 | 514.0 | 98.4 | 212 | 1005 | 479.9 | 271.1 |
| Precaudal length | 73.9 | 73.2 | 78.4 | 75.8 | 2.0 | 67.3 | 81.1 | 73.7 | 4.6 |
| Pre-second dorsal length | 48.5 | 46.1 | 53.1 | 50.0 | 2.6 | 46.6 | 57.1 | 50.7 | 3.7 |
| Pre-first dorsal length | 26.0 | 23.9 | 29.5 | 26.9 | 1.9 | 23.4 | 28.6 | 25.1 | 1.8 |
| Pre-first dorsal length (horizontal) | 23.1 | 22.2 | 26.6 | 24.7 | 1.9 | 21.2 | 21.9 | 21.5 | 0.5 |
| Head length | 22.3 | 20.3 | 24.1 | 22.7 | 1.5 | 19.5 | 23.1 | 20.6 | 1.3 |
| Head length (horizontal) | 21.6 | 20.3 | 23.5 | 21.9 | 1.1 | 18.2 | 22.1 | 19.5 | 1.4 |
| Prebranchial length | 17.5 | 15.6 | 19.2 | 17.7 | 1.3 | 15.1 | 17.6 | 16.2 | 1.0 |
| Prespiracular length | 12.0 | 10.5 | 13.3 | 12.1 | 1.0 | 10.5 | 13.3 | 11.5 | 0.9 |
| Preorbital length | 9.6 | 8.1 | 10.5 | 9.4 | 0.9 | 7.6 | 11.1 | 8.5 | 1.2 |
| Preorbital length (horizontal) | 7.9 | 6.5 | 8.9 | 8.1 | 0.9 | 5.4 | 9.6 | 6.8 | 1.4 |
| Prenarial length | 3.8 | 3.1 | 4.4 | 3.9 | 0.5 | 3.3 | 3.7 | 3.5 | 0.2 |
| Prenarial length (horizontal) | 2.1 | 0.9 | 2.7 | 2.0 | 0.6 | 1.8 | 2.7 | 2.1 | 0.3 |
| Preoral length | 3.5 | 2.6 | 3.3 | 3.1 | 0.4 | 2.1 | 3.9 | 3.2 | 0.7 |
| Prepectoral length | 21.1 | 21.2 | 24.1 | 22.3 | 1.2 | 17.5 | 21.1 | 19.2 | 1.4 |
| Prepelvic length | 39.0 | 36.3 | 41.9 | 40.1 | 2.2 | 34.6 | 44.4 | 38.1 | 3.9 |
| Pre-cloacal length | 39.9 | 40.8 | 44.3 | 42.3 | 1.7 | 38.2 | 48.8 | 41.9 | 4.2 |
| Preanal length | 58.2 | 55.2 | 62.2 | 59.6 | 2.6 | 57.6 | 58.9 | 58.3 | 0.9 |
| Interdorsal space | 17.3 | 15.3 | 17.8 | 16.8 | 0.9 | 15.6 | 20.9 | 18.5 | 2.0 |
| Dorsal–caudal space | 17.6 | 16.4 | 18.7 | 17.8 | 0.8 | 13.4 | 16.7 | 15.6 | 1.2 |
| Pectoral–pelvic space | 12.4 | 11.6 | 14.7 | 13.4 | 1.2 | 11.3 | 16.6 | 13.7 | 1.8 |
| Pelvic–anal space | 14.7 | 11.7 | 15.5 | 14.4 | 1.4 | 15.2 | 17.6 | 16.4 | 1.7 |
| Anal–caudal space | 13.2 | 11.0 | 13.5 | 12.5 | 1.0 | 9.4 | 10.5 | 10.0 | 0.8 |
| Interorbital space | 7.8 | 7.4 | 7.9 | 7.7 | 0.2 | 6.2 | 8.0 | 6.9 | 0.7 |
| Eye length | 3.7 | 3.7 | 3.8 | 3.7 | 0.0 | 2.6 | 3.7 | 3.3 | 0.3 |
| Eye height | 2.2 | 2.1 | 2.5 | 2.3 | 0.2 | 1.6 | 2.6 | 2.0 | 0.4 |
| Spiracle length | 0.7 | 0.4 | 0.7 | 0.6 | 0.1 | 0.2 | 0.6 | 0.4 | 0.2 |
| Nostril width | 1.9 | 1.4 | 2.1 | 1.9 | 0.2 | 1.1 | 1.6 | 1.2 | 0.2 |
| Internarial space | 4.9 | 4.9 | 5.9 | 5.4 | 0.4 | 3.9 | 5.8 | 5.2 | 0.8 |
| Mouth width | 9.1 | 8.1 | 10.2 | 9.3 | 0.8 | 7.2 | 9.1 | 8.3 | 0.7 |
| Upper labial furrow length | 1.5 | 1.3 | 1.7 | 1.5 | 0.2 | 0.8 | 1.4 | 1.1 | 0.2 |
| Lower labial furrow length | 3.0 | 3.2 | 3.9 | 3.5 | 0.3 | 3.5 | 3.9 | 3.7 | 0.2 |
| Inner nostril to upper labial furrow | 1.9 | 1.8 | 1.9 | 1.9 | 0.1 | 1.3 | 2.5 | 1.9 | 0.5 |
| First gill slit height | 4.4 | 4.1 | 5.3 | 4.6 | 0.5 | 3.2 | 5.0 | 3.9 | 0.6 |
| Fifth gill slit height | 2.6 | 2.0 | 3.3 | 2.6 | 0.4 | 1.8 | 3.4 | 2.4 | 0.6 |
| Intergill length | 4.9 | 4.7 | 5.8 | 5.3 | 0.4 | 4.2 | 5.2 | 4.7 | 0.4 |
| Pectoral fin—anterior margin | 20.1 | 18.5 | 22.4 | 20.5 | 1.7 | 18.8 | 26.1 | 22.1 | 2.7 |
| Pectoral fin—height | 19.0 | 15.1 | 18.3 | 17.4 | 1.4 | 17.2 | 19.3 | 18.3 | 1.5 |
| Pectoral fin—inner margin | 6.5 | 5.7 | 7.0 | 6.3 | 0.5 | 5.5 | 9.6 | 7.6 | 1.4 |
| Pectoral fin—base | 7.7 | 7.2 | 8.0 | 7.6 | 0.2 | 7.0 | 8.9 | 8.0 | 0.7 |
| Pectoral fin—posterior margin | 15.2 | 14.0 | 17.6 | 15.4 | 1.5 | 13.6 | 17.7 | 15.7 | 1.4 |
| Dorsal caudal margin | 25.2 | 21.4 | 25.6 | 23.5 | 1.8 | 17.9 | 28.0 | 24.4 | 3.3 |
| Preventral caudal margin | 14.9 | 13.1 | 14.5 | 13.9 | 0.7 | 12.1 | 14.5 | 13.2 | 1.0 |
| Upper postventral caudal margin | 5.2 | 3.8 | 5.3 | 4.7 | 0.6 | 4.2 | 6.5 | 5.4 | 1.6 |
| Lower postventral caudal margin | 5.2 | 4.7 | 6.1 | 5.2 | 0.5 | 3.2 | 3.5 | 3.3 | 0.3 |
| Caudal fork width | 8.5 | 7.5 | 9.3 | 8.1 | 0.7 | 8.8 | 8.9 | 8.8 | 0.1 |
| Caudal fork length | 12.0 | 10.6 | 13.3 | 11.5 | 1.0 | 12.4 | 12.5 | 12.5 | 0.1 |
| Caudal terminal lobe length | 11.5 | 10.2 | 13.5 | 11.1 | 1.3 | 11.9 | 12.6 | 12.2 | 0.5 |
| Caudal terminal margin length | 9.8 | 9.1 | 10.9 | 9.8 | 0.7 | 6.9 | 10.6 | 8.7 | 1.4 |
| Caudal subterminal margin | 4.5 | 4.2 | 6.2 | 4.9 | 0.7 | 5.2 | 6.6 | 5.9 | 1.0 |
| First dorsal fin—length | 13.4 | 13.7 | 14.6 | 14.1 | 0.5 | 12.0 | 15.4 | 13.5 | 1.1 |
| First dorsal fin—anterior margin | 16.9 | 14.0 | 20.0 | 16.3 | 2.2 | 11.7 | 29.5 | 19.5 | 5.8 |
| First dorsal fin—base | 9.0 | 9.1 | 9.8 | 9.4 | 0.3 | 8.2 | 9.6 | 8.8 | 0.5 |
| First dorsal fin—height | 12.5 | 9.2 | 15.6 | 12.0 | 2.3 | 8.8 | 22.3 | 15.1 | 5.2 |
| First dorsal fin—inner margin | 4.6 | 4.3 | 5.7 | 4.8 | 0.5 | 4.0 | 6.2 | 4.9 | 0.8 |
| First dorsal fin—posterior margin | 12.4 | 9.0 | 13.4 | 11.5 | 1.6 | 9.2 | 20.0 | 14.0 | 4.3 |
| First dorsal fin—soft fin length | 9.4 | 8.3 | 10.4 | 9.5 | 0.8 | 9.3 | 10.3 | 9.8 | 0.7 |
| Exposed first dorsal spine length | 4.5 | 3.9 | 4.3 | 4.2 | 0.3 | 1.0 | 3.7 | 2.3 | 1.0 |
| Base first dorsal spine | 1.1 | 1.2 | 1.4 | 1.3 | 0.1 | 0.8 | 1.6 | 1.2 | 0.3 |
| Second dorsal fin—length | 12.0 | 11.1 | 12.8 | 12.2 | 0.6 | 9.6 | 12.7 | 11.2 | 1.0 |
| Second dorsal fin—anterior margin | 12.8 | 10.1 | 14.5 | 12.4 | 1.5 | 8.9 | 20.1 | 14.3 | 3.8 |
| Second dorsal fin—base | 8.3 | 6.8 | 8.6 | 7.8 | 0.7 | 6.4 | 8.1 | 7.4 | 0.6 |
| Second dorsal fin—height | 8.8 | 6.7 | 11.1 | 8.5 | 1.5 | 6.8 | 16.4 | 10.4 | 3.8 |
| Second dorsal fin—inner margin | 4.0 | 3.8 | 5.3 | 4.4 | 0.5 | 3.4 | 4.3 | 3.9 | 0.4 |
| Second dorsal fin—posterior margin | 8.7 | 6.8 | 9.2 | 8.2 | 0.8 | 5.7 | 11.8 | 8.5 | 2.1 |
| Second dorsal fin—soft fin length | 6.8 | 6.5 | 7.3 | 6.9 | 0.3 | 6.2 | 6.8 | 6.5 | 0.5 |
| Exposed second dorsal spine length | 4.2 | 3.9 | 4.7 | 4.2 | 0.3 | 2.0 | 4.0 | 2.8 | 0.7 |
| Base second dorsal spine | 1.1 | 1.1 | 1.2 | 1.1 | 0.0 | 0.8 | 1.4 | 1.0 | 0.2 |
| Pelvic fin—length | 10.9 | 10.6 | 12.2 | 11.3 | 0.6 | 10.4 | 13.2 | 11.9 | 0.9 |
| Pelvic fin—height | 8.2 | 6.5 | 8.0 | 7.6 | 0.6 | 6.6 | 8.1 | 7.2 | 0.5 |
| Pelvic fin—inner margin length | 6.3 | 5.9 | 6.5 | 6.2 | 0.2 | 4.4 | 6.8 | 5.8 | 0.9 |
| Clasper outer length | 8.3 | 3.1 | 9.9 | 7.6 | 3.1 | 2.5 | 2.7 | 2.6 | 0.2 |
| Clasper inner length | 11.7 | 5.9 | 13.6 | 10.8 | 3.4 | 5.0 | 6.1 | 5.6 | 0.8 |
| Clasper base width | 2.4 | 1.1 | 3.0 | 2.3 | 0.9 | 1.1 | 1.3 | 1.2 | 0.1 |
| Anal fin—length | 8.2 | 7.3 | 7.8 | 7.6 | 0.3 | 7.5 | 10.8 | 8.7 | 1.0 |
| Anal fin—base | 4.9 | 4.2 | 5.0 | 4.7 | 0.3 | 5.2 | 7.2 | 5.8 | 0.7 |
| Anal fin—height | 4.7 | 3.8 | 5.0 | 4.4 | 0.5 | 4.1 | 5.3 | 4.6 | 0.4 |
| Anal fin—anterior margin | 9.4 | 8.4 | 9.2 | 8.9 | 0.4 | 8.8 | 10.8 | 9.9 | 0.8 |
| Anal fin—posterior margin | 4.0 | 3.2 | 4.5 | 4.1 | 0.5 | 3.6 | 4.8 | 4.1 | 0.5 |
| Anal fin—inner margin | 3.1 | 2.2 | 4.0 | 3.0 | 0.6 | 2.6 | 3.7 | 3.2 | 0.5 |
| Head height | 12.9 | 12.0 | 13.8 | 13.0 | 0.6 | 11.1 | 12.0 | 11.6 | 0.3 |
| Trunk height | 13.5 | 12.8 | 15.0 | 13.9 | 0.8 | 10.6 | 13.6 | 12.4 | 1.1 |
| Abdomen height | 14.0 | 11.2 | 13.8 | 13.0 | 1.1 | 11.2 | 13.1 | 11.9 | 0.8 |
| Tail height | 7.6 | 7.1 | 8.5 | 7.8 | 0.5 | 6.5 | 8.6 | 7.6 | 0.7 |
| Caudal peduncle height | 3.1 | 3.2 | 3.5 | 3.3 | 0.2 | 2.7 | 3.7 | 3.4 | 0.4 |
| Head width | 12.2 | 11.1 | 13.8 | 12.7 | 0.9 | 11.8 | 14.1 | 12.8 | 0.9 |
| Head width at anterior nostrils | 7.8 | 6.5 | 7.8 | 7.2 | 0.6 | 7.3 | 9.2 | 8.2 | 0.8 |
| Head width at anterior mouth | 8.9 | 7.5 | 9.4 | 8.7 | 0.7 | 9.4 | 10.6 | 10.0 | 0.5 |
| Trunk width | 12.1 | 9.7 | 13.0 | 12.1 | 1.2 | 9.5 | 13.5 | 11.4 | 1.5 |
| Abdomen width | 11.5 | 9.5 | 13.1 | 11.6 | 1.2 | 8.3 | 12.6 | 10.8 | 1.5 |
| Tail width | 7.3 | 6.3 | 8.2 | 7.4 | 0.6 | 4.7 | 7.6 | 6.3 | 1.1 |
| Caudal peduncle width | 3.5 | 2.6 | 4.1 | 3.3 | 0.5 | 2.4 | 3.5 | 2.9 | 0.4 |
| Second dorsal fin insertion to anal fin origin | 2.2 | 1.5 | 2.9 | 2.2 | 0.5 | 0.5 | 2.7 | 1.3 | 0.8 |
| Anal fin insertion to ventral caudal fin origin | 12.4 | 10.4 | 14.0 | 12.4 | 1.2 | 10.3 | 11.9 | 11.0 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, W.T.; Mollen, F.H.; O’Neill, H.L.; Yang, L.; Naylor, G.J.P. Species in Disguise: A New Species of Hornshark from Northern Australia (Heterodontiformes: Heterodontidae). Diversity 2023, 15, 849. https://doi.org/10.3390/d15070849
White WT, Mollen FH, O’Neill HL, Yang L, Naylor GJP. Species in Disguise: A New Species of Hornshark from Northern Australia (Heterodontiformes: Heterodontidae). Diversity. 2023; 15(7):849. https://doi.org/10.3390/d15070849
Chicago/Turabian StyleWhite, William T., Frederik H. Mollen, Helen L. O’Neill, Lei Yang, and Gavin J. P. Naylor. 2023. "Species in Disguise: A New Species of Hornshark from Northern Australia (Heterodontiformes: Heterodontidae)" Diversity 15, no. 7: 849. https://doi.org/10.3390/d15070849
APA StyleWhite, W. T., Mollen, F. H., O’Neill, H. L., Yang, L., & Naylor, G. J. P. (2023). Species in Disguise: A New Species of Hornshark from Northern Australia (Heterodontiformes: Heterodontidae). Diversity, 15(7), 849. https://doi.org/10.3390/d15070849







