Elasmobranch Diversity at Reunion Island (Western Indian Ocean) and Catches by Recreational Fishers and a Shark Control Program
Abstract
1. Introduction
2. Material and Methods
2.1. Species Richness and Status of Conservation
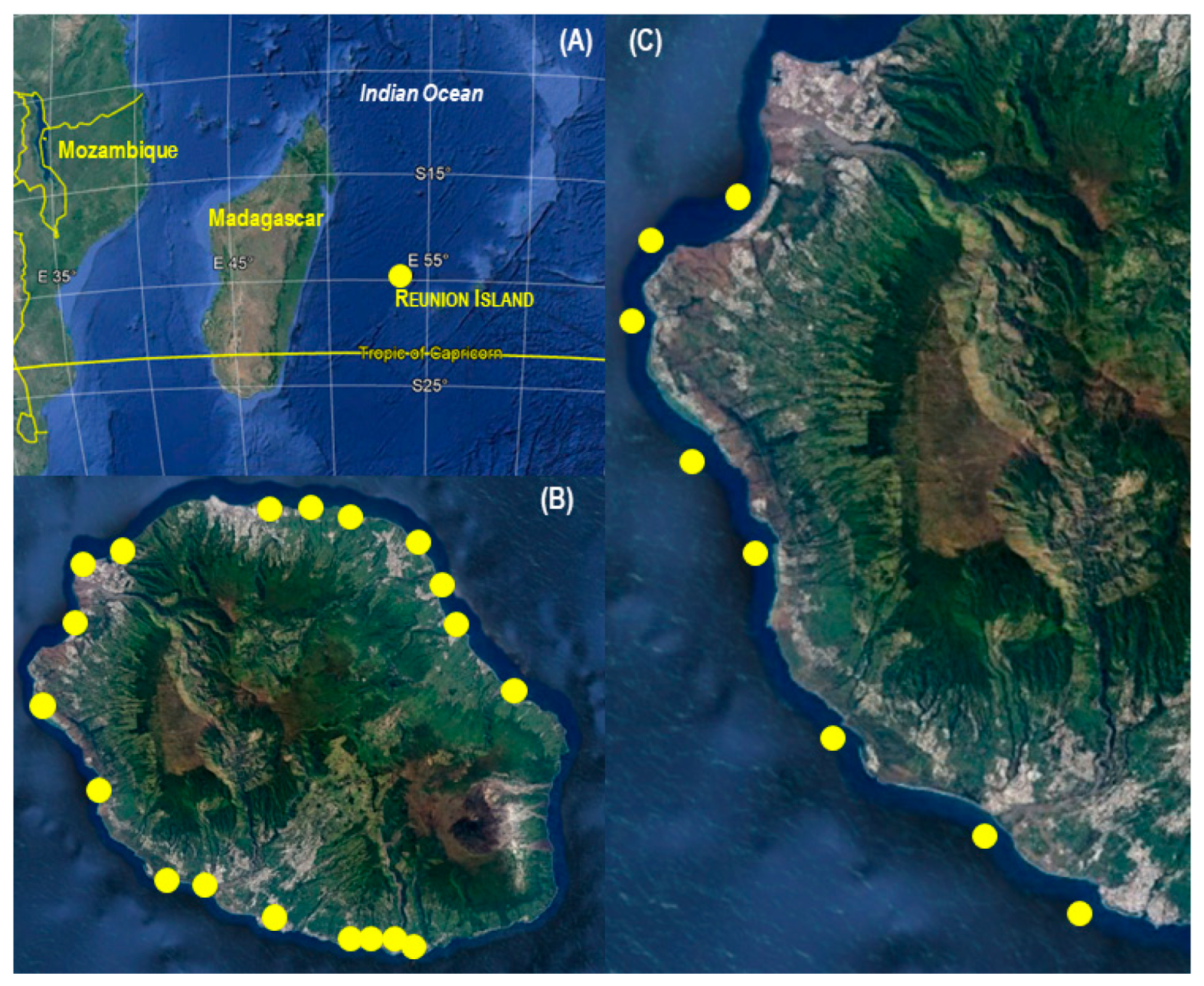
2.2. Shark Control Program
2.3. Recreational Fishing Activity
2.4. Species Molecular Identification of Specimens
2.5. Data Analyses
3. Results
3.1. Elasmobranch Diversity and Status of Conservation
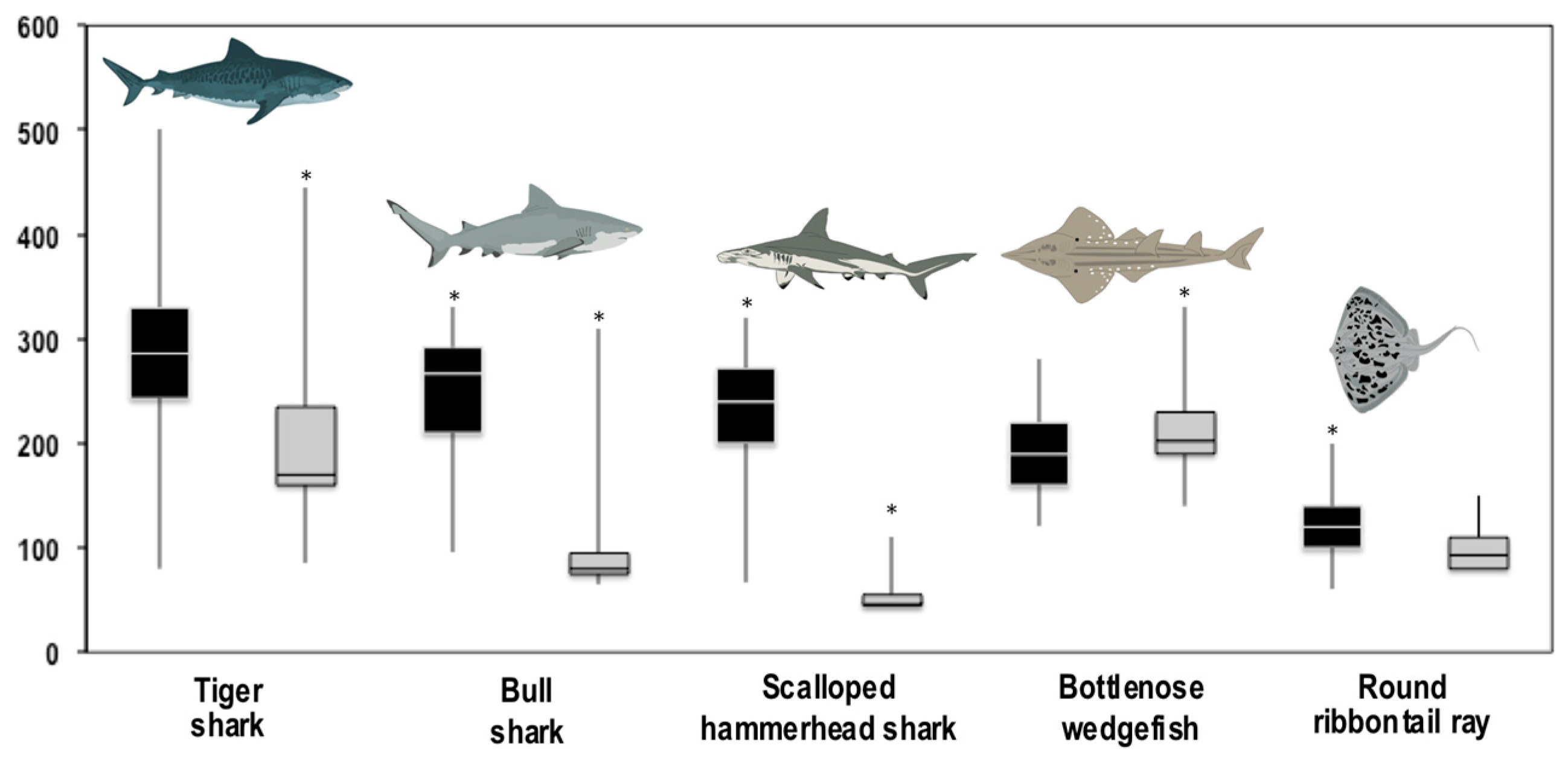
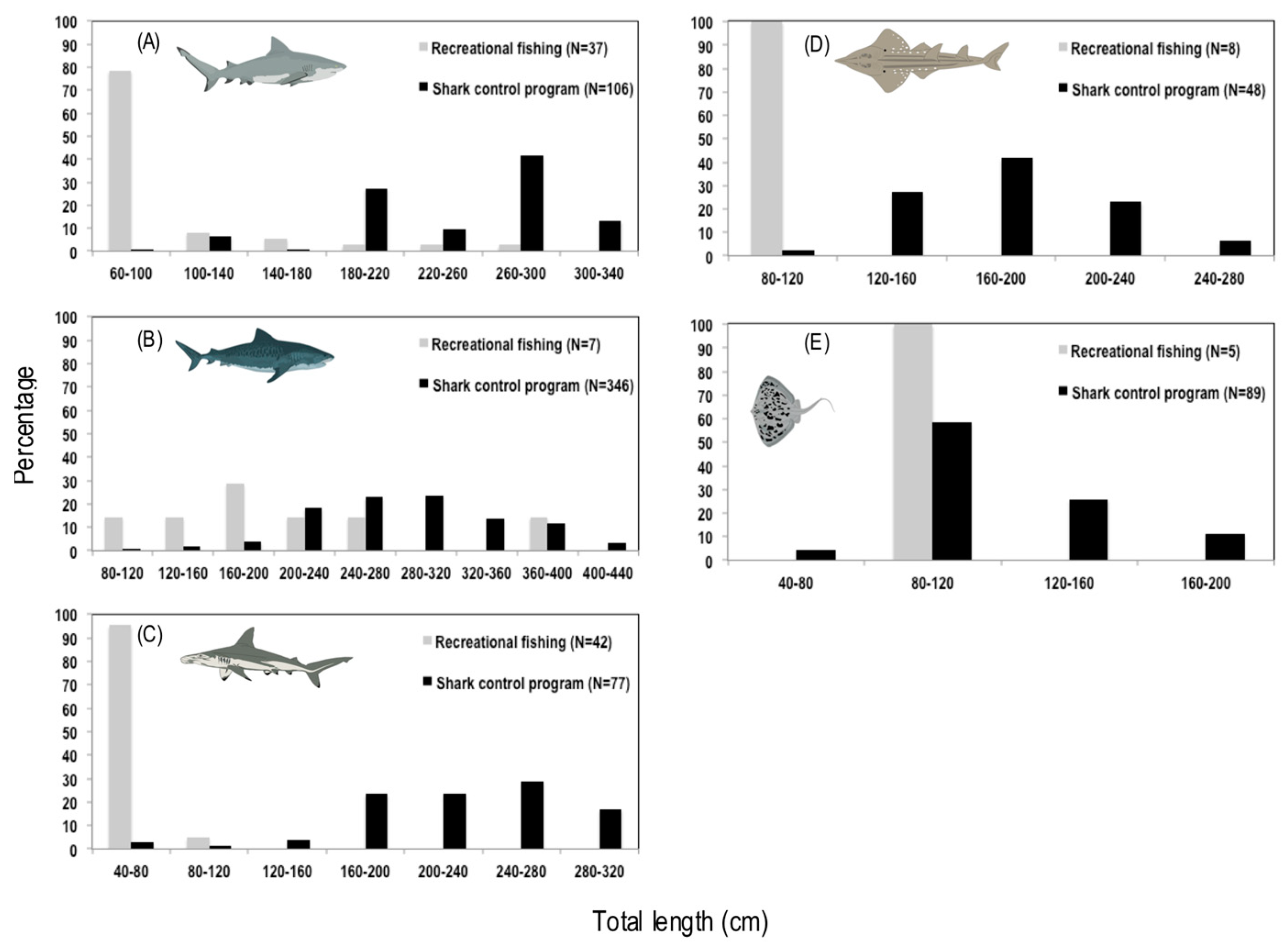
3.2. Composition and Size Distribution of the Catches
3.3. Seasonal Variations in the Catches
4. Discussion
4.1. Elasmobranch Richness in Reunion Island and Species Conservation Status
4.2. Spatial and Temporal Dynamics of the Catches of Elasmobranchs in Reunion Island
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of Planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef]
- Halpern, B.S.; Frazier, M.; Afflerbach, J.; Lowndes, J.S.; Micheli, F.; O’Hara, C.; Scarborough, C.E.; Selkoe, K.A. Recent pace of change in human impact on the world’s ocean. Sci. Rep. 2019, 9, 11609. [Google Scholar] [CrossRef]
- O’Hara, C.; Frazier, M.; Halpern, B.S. At-risk marine biodiversity faces extensive, expanding, and intensifying human impacts. Science 2021, 372, 84–87. [Google Scholar] [CrossRef]
- Crowder, L.B.; Hazen, E.L.; Avissar, N.; Bjorkland, R.K.; Latanich, C.; Ogburn, M.B. The Impacts of Fisheries on Marine Ecosystems and the Transition to Ecosystem-Based Management. Annu. Rev. Ecol. Evol. Sys. 2008, 39, 259–278. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Sherman, C.S.; Simpfendorfer, C.A.; Pacoureau, N.; Matsushiba, J.H.; Yan, H.F.; Walls, R.H.L.; Rigby, C.L.; VanderWright, W.J.; Jabado, R.W.; Pollom, R.A.; et al. Half a century of rising extinction risk of coral reef sharks and rays. Nat. Commun. 2023, 14, 15. [Google Scholar] [CrossRef]
- Lotze, H.K.; Worm, B. Historical baselines for large marine animals. TREE 2009, 24, 254–262. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H.; et al. Overfishing drives over o-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021, 31, 4773–4787. [Google Scholar] [CrossRef] [PubMed]
- Heithaus, M.R.; Frid, A.; Wirsing, A.J.; Worm, B. Predicting ecological consequences of marine top predator declines. TREE 2008, 23, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.A.; Heithaus, M.R.; McCauley, D.J.; Rasher, D.B.; Worm, B. Megafaunal impacts on structure and function of ocean ecosystems. Annu. Rev. Environ. Resour. 2016, 41, 83–116. [Google Scholar] [CrossRef]
- Roff, G.; Doropoulos, C.; Rogers, A.; Bozec, Y.-M.; Krueck, N.C.; Aurellado, E.; Priest, M.; Birrell, C.; Mumby, P.J. The Ecological Role of Sharks on Coral Reefs. TREE 2016, 31, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Flowers, K.I.; Heithaus, M.R.; Papastamatiou, Y.P. Buried in the sand: Uncovering the ecological roles and importance of rays. Fish Fish. 2020, 22, 106–127. [Google Scholar] [CrossRef]
- Bonfil, R. Overview of world elasmobranch fisheries. In FAO Technical Paper; FAO: Rome, Italy, 1994. [Google Scholar]
- Robinson, L.; Sauer, W.H.H. A first description of the artisanal shark fishery in northern Madagascar: Implications for management. Afr. J. Mar. Sci. 2013, 35, 9–15. [Google Scholar] [CrossRef]
- Oliver, S.; Braccini, M.; Newman, S.J.; Harvey, E.S. Global patterns in the bycatch of sharks and rays. Mar. Policy 2015, 54, 86–97. [Google Scholar] [CrossRef]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P. The effects of fishing on sharks, rays and chimaeras (Chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Musick, J.A.; Musick, S. Sharks (Special Topics C3). In Review of the State of the World Marine Fishery Resources; Food and Agriculture Organization of the United Nations: Rome, Italy, 2000; Volume 569, pp. 245–254. [Google Scholar]
- Dudley, S.F.J. A comparison of the shark control programs of New South Wales and Queensland (Australia) and KwaZulu-Natal (South Africa). Ocean Coast. 1997, 34, 1–27. [Google Scholar] [CrossRef]
- Roberts, C.M.; McClean, C.J.; Veron, J.E.; Hawkins, J.P.; Allen, G.R.; McAllister, D.E.; Mittermeier, C.G.; Schueler, F.W.; Spalding, M.; Wells, F. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 2002, 295, 1280–1284. [Google Scholar] [CrossRef]
- McDougall, I. The geochromology and evolution of the young volcanic island of Reunion, Indian Ocean. Geochim. Cosmochim. Acta 1970, 35, 261–288. [Google Scholar] [CrossRef]
- Bourmaud, C.A.F.; Abouidane, A.; Boissier, P.; Leclere, L.; Mirault, E.; Pennober, G. Coastal and marine biodiversity of La Reunion. Ind. J. Mar. Sci. 2005, 34, 98–103. [Google Scholar]
- Dulau-Drouot, V.; Boucaud, V.; Rota, B. Cetacean diversity off La Réunion Island (France). J. Mar. Biol. Ass. 2008, 88, 1263–1272. [Google Scholar] [CrossRef]
- Frick, R.; Mulochau, T.; Durville, P.; Chabanet, P.; Tessier, E.; Letourneur, Y. Annotated checklist of the fish species (Pisces) of La Réunion, including a Red list of threatened and declining species. Stutt. Beit. Natur. A 2009, 2, 1–168. [Google Scholar]
- Conand, C.; Ribes-Beaudemoulin, S.; Trentin, F.; Mulochau, T.; Boissin, E. Marine Biodiversity of La Reunion Island: Echinoderms. WIO J. Mar. Sci. 2018, 17, 111–124. [Google Scholar]
- Wickel, J.; Fricke, R.; Durville, P.; Chabanet, P.; Dumestre, M.; Mulochau, T.; Pinault, M.; Tessier, E. Updated checklist of the fish species of Reunion Island. In Évaluation de Statuts de Conservation Pour les Poisons Récifaux à la Réunion; Dumestre, M., Wickel, J., Eds.; Marex/Deal 974: Saint-Denis, France, 2020. [Google Scholar]
- Letourneur, Y.; Chabanet, P.; Durville, P.; Taquet, M.; Tessier, E.; Parmentier, M.; Quéro, J.-C.; Pothin, K. An updated checklist of the marine fish fauna of Reunion Island, South-Western Indian Ocean. Cybium 2004, 28, 199–216. [Google Scholar]
- Kiszka, J.J.; Jamon, A.; Wickel, J. Les Requins dans les îles de l’océan Indien Occidental: Biodiversité, Distribution et Interactions Avec les Activités Humaines; MAYSHARK: Mayotte, France, 2009. [Google Scholar]
- Mariani, S.; Fernandez, C.; Baillie, C.; Magalon, H.; Jaquemet, S. Shark and ray diversity, abundance and temporal variation around an Indian Ocean Island, inferred by eDNA metabarcoding. Conserv. Sci. Pract. 2021, 3, e407. [Google Scholar] [CrossRef]
- Lagabrielle, E.; Allibert, A.; Kiszka, J.J.; Loiseau, N.; Kilfoil, J.P.; Lemahieu, A. Environmental and anthropogenic factors affecting the increasing occurrence of shark-human interactions around a fast-developing Indian Ocean island. Sci. Rep. 2018, 8, 3676. [Google Scholar] [CrossRef] [PubMed]
- Guyomard, D.; Perry, C.; Tournoux, P.-U.; Cliff, G.; Peddemors, V.; Jaquemet, S. An innovative fishing gear to enhance the release of non-target species in coastal shark-control programs: The SMART (shark management alert in real-time) drumline. Fish. Res. 2019, 216, 6–17. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Oury, N.; Jaquemet, S.; Simon, G.; Casalot, L.; Vangrevelynghe, G.; Landron, F.; Magalon, H. Forensic genetic identification of sharks involved in human attacks. Foren. Sci. Int. Genet. 2021, 54, 102558. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D. BOLD: The barcode of life data system. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Barbuto, M.; Galimberti, A.; Ferri, E.; Labra, M.; Malandra, R.; Galli, P.; Casiraghi, M. DNA barcoding reveals fraudulent substitutions in shark seafood products: The Italian case of “palombo” (Mustelus spp.). Food Res. Int. 2010, 43, 376–381. [Google Scholar] [CrossRef]
- Conand, F.; Marsac, F.; Tessier, E.; Conand, C. A ten-year period of daily sea surface temperature at a coastal station in Reunion Island, Indian Ocean (July 1993–April 2004): Patterns of variability and biological responses. WIO J. Mar. Sci. 2007, 6, 48222. [Google Scholar] [CrossRef]
- Jabado, R.W. Wedgefishes and Giant Guitarfishes: A Guide to Species Identification; Wildlife Conservation Society: New York, NY, USA, 2019. [Google Scholar]
- Bullock, R.; Ralph, G.; Stump, E.; Al Abdali, F.; Al Asfoor, J.; Al Buwaiqi, B.; Al Kindi, A.; Ambuali, A.; Birge, T.; Borsa, P.; et al. The Conservation Status of Marine Biodiversity of the Western Indian Ocean; IUCN: Gland, Switzerland, 2021; pp. vii + 32. [Google Scholar]
- Kiszka, J.J.; Van Der Elst, R.P. Elasmobranchs, p366–386. In Offshore Fisheries of the Southwest Indian Ocean: Their Status and the Impact on Vulnerable Species; Van Der Elst, R.P., Everett, B.I., Eds.; Oceanographic Research Institute: Pechardwip, Bangladesh, 2015; Special Publication; Volume 10, p. 448. [Google Scholar]
- McVean, A.R.; Walker, R.C.J.; Fanning, E. The traditional shark fisheries of southwest Madagascar: A study in the Toliara region. Fish. Res. 2006, 82, 280–289. [Google Scholar] [CrossRef]
- Nevill, J.; Robinson, J.; Giroux, F.; Isidore, M. Seychelles National Plan of Action for the Conservation and Management of Sharks; Seychelles Fishing Authority: Victoria, Seychelles, 2007; 59p. [Google Scholar]
- Poonian, C.N.S. A first assessment of elasmobranch catch in Mauritian artisanal fisheries using interview surveys. Phelsuma 2015, 23, 19–29. [Google Scholar]
- Kyne, P.M.; Mauvis, A.G.; Ebert, D.A.; Jabado, R.W.; Rigby, C.L.; Dharmadi; Pollock, C.M.; Herman, K.B.; Cheok, J.; Simpfendorfer, C.A.; et al. The thin edge of the wedge: Extremely high extinction risk in wedgefishes and giant guitarfishes. Aqua. Cons. Mar. Fresh. Ecos. 2020, 30, 1337–1361. [Google Scholar] [CrossRef]
- Humber, F.; Andrianmahaino, E.T.; Beriziny, T.; Botosoamananto, R.; Godley, B.J.; Gough, C.; Pedron, S.; Ramahery, V.; Broderick, A.C. Assessing the small-scale shark fishery of Madagascar through community-based monitoring and knowledge. Fish. Res. 2017, 186, 131–143. [Google Scholar] [CrossRef]
- Rigby, C.L.; Dulvy, N.K.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Herman, K.; Jabado, R.W.; Liu, K.M.; et al. Sphyrna lewini. In The IUCN Red List of Threatened Species; IUCN Red List: Cambridge, UK, 2019; p. eT3985A2918526. [Google Scholar]
- Niella, Y.; Wiefels, A.; Almeida, U.; Jaquemet, S.; Lagabrielle, E.; Harcourt, R.; Peddemors, V.; Guyomard, D. Dynamics of marine predators off an oceanic island and implications for management of a preventative shark fishing program. Mar. Biol. 2021, 168, 42. [Google Scholar] [CrossRef]
- Pirog, A.; Magalon, H.; Poirout, T.; Jaquemet, S. Reproductive biology, multiple paternity and polyandry of the bull shark Carcharhinus leucas. J. Fish Biol. 2019, 95, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Paperno, R. Preliminary Assessment of a Nearshore Nursery Ground for the Scalloped Hammerhead off the Atlantic Coast of Florida. In Shark Nursery Grounds of the Gulf of Mexico and the East Coast Waters of the United States; McCandless, C.T., Kohler, N.E., Pratt, H.L., Eds.; Chapter: Preliminary Assessment of a Neashore Nursery Ground for the Scalloped Hammerhead off the Atlantic Coast of Florida; American Fisheries Society: New York, NY, USA, 2007; pp. 165–174. [Google Scholar]
- Cuevas-Gómez, G.A.; Pérez-Jiménez, J.C.; Méndez-Loeza, I.; Carrera-Fernández, M.; Castillo-Géniz, J.L. Identification of a nursery area for the critically endangered hammerhead shark (Sphyrna lewini) amid intense fisheries in the southern Gulf of Mexico. J. Fish Biol. 2020, 97, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Werry, J.M.; Lee, S.Y.; Lemckert, C.J.; Otway, N.M. Natural or artificial? Habitat-use by the bull shark, Carcharhinus leucas. PLoS ONE 2012, 7, e49796. [Google Scholar] [CrossRef]
- Zanella, I.; López-Garro, A.; Cure, K. Golfo Dulce: Critical habitat and nursery area for juvenile scalloped hammerhead sharks Sphyrna lewini in the Eastern Tropical Pacific Seascape. Environ. Biol. Fishes 2019, 102, 1291–1300. [Google Scholar] [CrossRef]

| Present Study (n = 65) | Letourneur et al. 2004 (n = 37) | Fricke et al. 2009 (n = 39) | Kizska et al. 2009 (n = 48) | Wickel et al. 2020 (n = 64) | IUCN Red List | Shark Control Program | Recreational Fishing | Mariani et al. 2021 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family (Number of Species) | Species | Habitat | Status of Conservation | N ind | Mean TL/DL (se) | Season | N ind | Mean TL/DL (se) | Season | Number of Reads | |||||
| Sharks (N = 50) | Alopiidae (n = 3) | Alopias pelagicus | x | Neritic/oceanic | EN | ||||||||||
| Alopias superciliosus | x | x | x | x | Neritic/oceanic | VU | 1 | Co | |||||||
| Alopias vulpinus | x | x | x | x | Neritic/oceanic | VU | |||||||||
| Carcharhinidae (n = 18) | Carcharhinus albimarginatus | x | x | x | x | Neritic/deep benthic | VU | 4 | 177 (47) | W, Wa | |||||
| Carcharhinus amblyrhynchos | C. wheeleri | x | x | x | Neritic | EN | 2 | 158 (19) | W | ||||||
| Carcharhinus brachyurus | x | Neritic/oceanic | VU | 9 | 245 (8) | W | |||||||||
| Carcharhinus brevipinna | x | x | x | x | Neritic | VU | |||||||||
| Carcharhinus falciformis | x | x | x | Neritic/oceanic/deep Benthic | DD | 15,679 | |||||||||
| Carcharhinus humani | x | Neritic | DD | ||||||||||||
| Carcharhinus leucas | x | x | x | Wetland/neritic/supratidal | VU | 107 | 247 (5) | S, Co, W, Wa | 73 | 102 (8) | S, Co, W, Wa | 228,385 | |||
| Carcharhinus limbatus | x | x | x | x | Neritic/oceanic | VU | |||||||||
| Carcharhinus longimanus | x | x | x | x | Neritic/oceanic | CR | |||||||||
| Carcharhinus melanopterus | x | x | x | x | Neritic/intertidal | VU | |||||||||
| Carcharhinus obscurus | Neritic/oceanic | 2 ? | W, Wa | 1 | W | ||||||||||
| Carcharhinus plumbeus | x | x | x | x | Neritic/deep benthic | EN | 4 | 150 (17) | S, W | 2 | Co | ||||
| Carcharhinus sorrah | x | x | x | x | Neritic | NT | |||||||||
| Galeocerdo cuvier | x | x | x | x | Neritic/oceanic | NT | 349 | 290 (3) | S, Co, W, Wa | 9 | 202 (34) | S, Co, W, Wa | 979,818 | ||
| Loxodon macrorhinus | x | x | x | Neritic | NT | 37 | 82 (2) | S, Co, W, Wa | 39 | S, Co, W, Wa | 22 | ||||
| Negaprion acutidens | x | x | Neritic/intertidal | EN | |||||||||||
| Prionace glauca | x | x | x | x | Neritic/oceanic | NT | |||||||||
| Triaenodon obesus | x | x | x | x | Neritic | VU | 1 | 55 | W | ||||||
| Centrophoridae (n = 3) | Centrophorus moluccensis | x | x | x | x | Deep benthic | VU | 2 | S, W | ||||||
| Deania profundorum | x | Deep benthic | NT | ||||||||||||
| Deania quadrispinosa | x | Deep benthic | VU | ||||||||||||
| Dalatiidae (n = 2) | Euprotomicrus bispinatus | x | x | x | x | Oceanic | LC | ||||||||
| Isistius brasiliensis | x | Oceanic | LC | ||||||||||||
| Ginglymostomatidae (n = 1) | Nebrius ferrugineus | x | x | x | x | Neritic | VU | 12 | 271 (11) | S, Co, W, Wa | 2 | S, W | |||
| Hexanchidae (n = 4) | Heptranchias perlo | x | x | x | x | Neritic/oceanic/deep Benthic | NT | ||||||||
| Hexanchus griseus | x | x | x | Deep benthic | NT | ||||||||||
| Hexanchus nakamurai | H. vitulus | x | H. vitulus | x | Oceanic/deep benthic | NT | |||||||||
| Notorynchus cepedianus | x | x | x | Neritic/deep benthic | VU | ||||||||||
| Lamnidae (n = 3) | Carcharodon carcharias | x | x | x | x | Neritic/oceanic | VU | ||||||||
| Isurus oxyrinchus | x | x | x | x | Oceanic | EN | 1 | 313 | W | 1 | 200 | Co | |||
| Isurus paucus | x | x | Oceanic | EN | |||||||||||
| Odontaspididae (n = 1) | Carcharias taurus | x | x | Neritic/deep benthic | CR | ||||||||||
| Pseudocarchariidae (n = 1) | Pseudcarcharias kamoharai | x | x | Oceanic | LC | ||||||||||
| Rhincodontidae (n = 1) | Rhincodon typus | x | x | x | x | Neritic/oceanic | EN | ||||||||
| Somniosidae (n = 3) | Centroscymnus owstonii | x | Oceanic/deep benthic | VU | |||||||||||
| Centroselachus crepidater | Centroscymnus crepidater | x | x | x | Deep benthic | NT | |||||||||
| Zameus squamulosus | x | Oceanic/deep benthic | LC | ||||||||||||
| Sphyrnidae (n = 3) | Sphyrna lewini | ? | x | x | Neritic/oceanic | CR | 81 | 233 (6) | S, Co, W, Wa | 120 | 51 (2) | S, Co, W, Wa | 1,220,474 | ||
| Sphyrna mokarran | x | x | x | x | Neritic/oceanic | CR | |||||||||
| Sphyrna zygaena | ? | x | Neritic/oceanic | VU | 5 | 225 (26) | W | 1 | W | 5058 | |||||
| Squalidae (n = 4) | Cirrhigaleus asper | Squalus asper | x | x | x | Deep benthic | DD | ||||||||
| Squalus blainville | x | Neritic/deep benthic | DD | ||||||||||||
| Squalus megalops | x | x | x | x | Neritic/deep benthic | LC | 4 | S | |||||||
| Squalus mitsikurii | x | x | Deep benthic | EN | |||||||||||
| Stegostomatidae (n = 1) | Stegostoma tigrinum | x | x | Neritic/oceanic | EN | 213,431 | |||||||||
| Triakidae (n = 2) | Mustelus mosis | x | x | x | Neritic/deep benthic | NT | 3 | 100 (15) | S, W | 35 | 88 (10) | S, Co, W | |||
| Mustelus palumbes | x | Neritic/deep benthic | LC | ||||||||||||
| Batoids (N = 15) | Dasyatidae (n = 6) | Bathytoshia lata | Dasyatis thetidis | Dasyatis thetidis | Dasyatis thetidis | x | Neritic/deep benthic | VU | 33 | 151 (27) | S, Co, W, Wa | 20 | S, Co, W, Wa | ||
| Dasyatis chrysonota | Dasyatis pastinaca | x | Neritic | NT | |||||||||||
| Neotrygon caeruleopunctata | x | Neritic/intertidal | LC | ||||||||||||
| Pateobatis fai | x | Neritic | VU | 9 | S, Co, W | ||||||||||
| Pteroplatytrygon violacea | Dasyatis violacea | x | Oceanic | LC | 225,925 | ||||||||||
| Taeniurops meyeni | Taeniura meyeni | x | x | x | Neritic/deep benthic | VU | 98 | 119 (4) | S, Co, W, Wa | 44 | 97 (6) | S, Co, W, Wa | 347,215 | ||
| Mobulidae (n = 1) | Mobula birostris | Manta birostris | x | Manta birostris | x | Neritic/oceanic | EN | ||||||||
| Mobulidae (n = 1) | Mobula tarapacana | x | x | Neritic/oceanic | EN | ||||||||||
| Myliobatidae (n = 2) | Aetobatus ocellatus | x | Neritic/oceanic | VU | 10 | 116 (5) | S, W, Wa | 5 | S, Co, W, Wa | ||||||
| Myliobatis aquila | x | x | x | x | Neritic/oceanic | CR | |||||||||
| Pristidae (n = 3) | Pristis pectinata | x | x | x | x | Neritic/intertidal | CR | ||||||||
| Pristis pristis | Pristis microdon | x | Pristis microdon | x | Wetland/neritic/intertidal | CR | |||||||||
| Pristis zijsron | x | Neritic/intertidal | CR | ||||||||||||
| Rhinidae (n = 1) | Rhynchobatus australiae | R. djiddensis | R. djiddensis | R. djiddensis | R. djiddensis | Neritic | CR | 51 | 191 (5) | S, Co, W, Wa | 30 | 182 (8) | S, Co, W, Wa | 476,221 | |
| Torpedinidae n = 1) | Torpedo fuscomaculata | x | x | x | Neritic/intertidal/deep benthic | DD | 3 | S, Co, Wa | |||||||
| Unidentified specimens | 61 | 18 | |||||||||||||
| Total number of individuals | 869 | 420 | |||||||||||||
| Total number of individuals in summer (S) | 302 | 201 | |||||||||||||
| Total number of individuals in winter (W) | 249 | 110 | |||||||||||||
| Total number of individuals during transition periods (C, Wa) | 218 | 109 | |||||||||||||
| Species richness | 17 | 20 | |||||||||||||
| Species richness in summer (S) | 11 | 14 | |||||||||||||
| Species richness in winter (W) | 17 | 15 | |||||||||||||
| Species richness during transition periods (C, Wa) | 11 | 14 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaquemet, S.; Oury, N.; Poirout, T.; Gadenne, J.; Magalon, H.; Gauthier, A. Elasmobranch Diversity at Reunion Island (Western Indian Ocean) and Catches by Recreational Fishers and a Shark Control Program. Diversity 2023, 15, 768. https://doi.org/10.3390/d15060768
Jaquemet S, Oury N, Poirout T, Gadenne J, Magalon H, Gauthier A. Elasmobranch Diversity at Reunion Island (Western Indian Ocean) and Catches by Recreational Fishers and a Shark Control Program. Diversity. 2023; 15(6):768. https://doi.org/10.3390/d15060768
Chicago/Turabian StyleJaquemet, S., N. Oury, T. Poirout, J. Gadenne, H. Magalon, and A. Gauthier. 2023. "Elasmobranch Diversity at Reunion Island (Western Indian Ocean) and Catches by Recreational Fishers and a Shark Control Program" Diversity 15, no. 6: 768. https://doi.org/10.3390/d15060768
APA StyleJaquemet, S., Oury, N., Poirout, T., Gadenne, J., Magalon, H., & Gauthier, A. (2023). Elasmobranch Diversity at Reunion Island (Western Indian Ocean) and Catches by Recreational Fishers and a Shark Control Program. Diversity, 15(6), 768. https://doi.org/10.3390/d15060768






