Abstract
In Brazil, the Whitespotted Eagle Ray Aetobatus narinari is considered data-deficient due to the scarcity of basic information on its biology, ecology, and vulnerability to capture. Despite this, the species is caught by artisanal fishing along most of the coast, especially in the northeast of Brazil. This study analyzed mitochondrial DNA data in specimens of A. narinari caught by artisanal fishing in the northeast coast of Brazil to understand their population structure. For this, 42 individuals were sequenced at three mitochondrial genes: cytochrome oxidase 1 (COI), cytochrome b (Cytb), and NADH dehydrogenase subunit 4 (ND4). Concatenated COI-Cytb-ND4 sequences yielded 14 haplotypes, with moderate haplotype diversity (h = 0.646), low nucleotide diversity (π = 0.00087), and low fixation index ΦST values, indicating no population structure. Our results suggest that there is only one population of A. narinari in the study area. Genetic studies can contribute to improving management plans in these areas, avoiding the overexploitation of this and other species.
1. Introduction
The Whitespotted Eagle Ray Aetobatus narinari (Euphrasen, 1790) [1] was previously considered a circumglobal species [2]. However, phylogenetic studies—based on variations in color pattern, parasitology, and genetic evidence—have shown that this species only occurs in the Atlantic Ocean [3,4]. Due to this taxonomic redefinition, the conservation status of the species throughout its range is currently classified as endangered (EN) by the International Union for Conservation of Nature (IUCN) [5], while in Brazil, A. narinari is classified as data-deficient (DD) [6].
Studies of A. narinari in the Atlantic have encompassed topics such as reproduction [7,8,9,10,11], population genetic connectivity, and phylogeography based on mitochondrial (cytochrome oxidase 1 (COI) [4] and cytochrome b (Cytb) [4,12]) and nuclear markers [4,12,13,14], feeding ecology [15,16,17], age and growth [18], and variation in catch rate in artisanal fisheries [7,19]. Nevertheless, basic information on the biology of A. narinari is still scarce, and further studies are needed, especially in areas where this species is targeted or incidentally captured in fisheries. The fishing and commercialization of the A. narinari occurs in several countries of the Atlantic (Mexico, Puerto Rico, Venezuela, Cuba, Brazil) [7,9,19,20,21]; as such, data on population connectivity are critical in managing these populations.
Describing the molecular variation and genetic structure of populations is fundamental to understanding species health and long-term viability, as well as to better informing conservation directives [12,13,14]. This information is even more important in areas where populations are exploited by fisheries. Overexploitation of marine stocks can drastically reduce effective population size, resulting in a decrease in genetic diversity and possibly a concurrent increase in deleterious alleles and in inbreeding depression [22], preventing the replenishment of a species’ stocks and having unforeseen ecosystem consequences through trophic cascades [23]. This is especially true for elasmobranchs, since fishing pressure can deplete and possibly isolate populations of the Silky Shark Carcharhinus falciformis [24] and the Spotted Eagle Ray Aetobatus ocellatus [25].
Aetobatus narinari is caught by artisanal fisheries [20], which exploit individuals from nursery areas in the northeast coast of Brazil [26]. Despite the species’ vulnerability, there are still scarce studies on its biology in this region [11,26], hampering the assessment of its local conservation status. More information about A. narinari, such as its population structure, can direct more appropriate management measures for its conservation in the region. In this study, we aimed to (1) assess the genetic diversity of A. narinari specimens caught by artisanal fishing in different locations in northeastern Brazil and (2) test if mtDNA analysis was performed for a single Brazilian population or for other independent populations of this species in order to understand their population structure.
2. Materials and Methods
2.1. Study Area
The study was carried out in three artisanal fishing landing areas: on the southern coast of Paraiba in Acaú (07°30′51″ S; 34°49′11″ W) in the municipality of Pitimbu; on the northern coast of Pernambuco, in the localities of Barra de Catuama (07°40′31″ S; 34°49′48″ W); and in the municipality of Goiana, Jaguaribe (07°47′7″ S; 34°51′22″ W), in the municipality of Itamaracá (Figure 1). The distance from the capture and landing sites was approximately 10 km. Samples from landed individuals were collected monthly from April 2016 to August 2018.
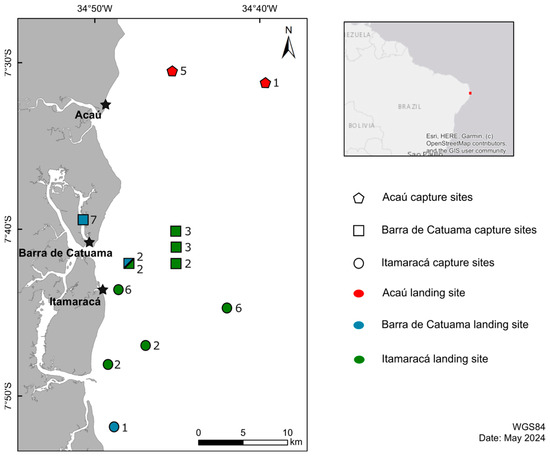
Figure 1.
Map of the study area indicating the capture sites (pentagone: Acaú; square: Barra de Catuama; circle: Itamaracá) and landing sites (red: Acaú; blue: Barra de Catuama; green: Itamaracá) of Aetobatus narinari samples on the coast of Paraíba and Pernambuco, Brazil. Number of individuals are indicated next to capture sites. For capture sites with several landing locations, the numbers of samples per landing sites are indicated vertically in alphabetical order. Map was generated with QGIS v 3.36.2 (https://www.qgis.org/ (accessed on 17 May 2024)) using polygons extracted from Google Earth (https://earth.google.com/web/ (accessed on 15 April 2024)).
2.2. Sampling
Tissue samples of 42 Aetobatus narinari [females: N = 24, 97–155 cm disc width (DW); males: N = 17, 49.6–141 cm DW; and one unidentified sex without DW information] were collected. Samples were taken at landing sites, and the catch locations were reported by fishermen. Individuals were caught in different fishing grounds throughout the study area, which were grouped into three larger areas and named according to their proximity to the landing sites (Figure 1). Since not all the individuals were caught and landed in the same area, we analyzed the data in two independent forms, considering both the capture sites and the landing sites (Table S1). For genetic analysis, a small piece of pelvic fin was collected from each specimen and stored in 95% ethanol in a freezer at −20 °C.
2.3. DNA Extraction, PCR, and Sequencing
Genomic DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). Three partial mitochondrial genes were amplified and sequenced: cytochrome oxidase 1 (COI), cytochrome b (Cytb), and NADH dehydrogenase subunit 4 (ND4). PCR amplifications were carried out in 25 µL reactions containing 12.5 µL of GoTaq®Green Master Mix (Promega, Madison, WI, USA), 7.9 µL sterile water, 1.5 µL each primer (concentration: 10 µM), and 1.6 µL total gDNA (concentration: 10 ng/µL). COI fragment was amplified using the primers developed by Meyer (2003) [27]: dgLCO-1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′—forward) and dgHCO-2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′—reverse). Cycling conditions for COI were 2 min at 95 °C, followed by 5 cycles of 30 s at 95 °C, 30 s at 46 °C, and 45 s at 72 °C and 30 cycles of 30 s at 95 °C, 30 s at 51 °C, 30 s at 72 °C, and 5 min at 72 °C for final extension. Cytb fragment was amplified and sequenced using the primers AnarCBF1 (5′-GAGGGGCAACTGTCATCACTAACC-3′—forward) and AnarCBR1 (5′-CGATTGGGAAAAGGAGGAGGAA-3′—reverse) from Richards et al. (2009) [28]. Cycling conditions were the same as in Sellas et al. (2015) [12]: 3 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 53 °C, and 45 s at 72 °C. ND4 fragment was amplified using the primers ND4 (5′-CACCTATGACTACCAAAAGCTCATGTAGAAGC-3′—forward) [29] and H12293-LEU (5′-TTGCACCAAGAGTTTTTGGTTCCTAAGACC-3′—reverse) [30]. ND4 cycling conditions were as follows: 2 min at 95 °C, followed by 35 cycles of 15 s at 94 °C, 30 s at 55 °C, 1 min at 72 °C, and 5 min at 72 °C. PCR products were purified with ExoSAP-IT (Thermo Fisher Scientific, Pittsburgh, PA, USA) and sequenced in both directions on an ABI 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA). Sequences were edited and aligned in BioEdit [31].
2.4. Analysis of Genetic Diversity and Structure
For each individual, the three mitochondrial fragments were concatenated and trimmed to the same length, creating a single sequence per individual of COI-Cytb-ND4 of 1976 base pairs (the length of each gene was 695 base pairs for COI; 639 for Cytb; and 864 for ND4). Haplotype diversity (h), nucleotide diversity (π), and divergences of concatenated COI-Cytb-ND4 sequences within and between sites were estimated using average pairwise distances (p-distances) calculated in Arlequin 3.5 [32]. To examine the relationships between sampling sites, a haplotype network for concatenated sequences was constructed using the TCS algorithm [33] in PopART (http://popart.otago.ac.nz (accessed on 13 February 2024)). Additionally, to examine genetic differentiation, the fixation index (ΦST) and analyses of molecular variance (AMOVA) were calculated using Arlequin 3.5 [32]. AMOVAs were performed based on population pair differences divided into two geographical groups (1Itamaracá and Barra de Catuama, corresponding to the north coast of Pernambuco, and 2Acaú, corresponding to the south coast of Paraíba) to determine the amount of partitioned variation within and between geographical groupings of landing and capture sites. To test whether the populations evolved under neutrality, Tajima’s D was calculated in DnaSP v. 6 [34].
3. Results
The COI-Cytb-ND4 concatenated sequences yielded 14 haplotypes. The average value for haplotype diversity was moderate (h = 0.646) and low for nucleotide diversity (π = 0.00087). When considering capture sites as populations, the highest haplotype diversity was observed in Itamaracá (h = 0.745), and the lowest nucleotide diversity was in Acaú (π = 0.00044) (Table S1). When considering landing sites as populations, the highest haplotype diversity and lowest nucleotide diversity were observed in Acaú (h = 0.733/π = 0.00044) (Table S1). As a general pattern, there was not a large difference in haplotype and nucleotide diversity when comparing capture and landings sites. For the samples in general, Tajima’s D was significantly negative (TD = −1.99287, p < 0.05).
Fixation index values (ΦST) were not significantly different, considering either landing sites or sampling sites as populations. The values ranged from −0.007 (Acaú–Itamaracá) to 0.096 (Acaú–Barra de Catuama—this value seemed to show moderate differentiation but was not statistically significant) for landing sites and ranged from −0.010 (Barra de Catuama–Itamaracá) to 0.017 (Acaú–Itamaracá) for capture sites (Table 1).

Table 1.
Population analyses for Aetobatus narinari samples. Pairwise fixation index values (ΦST) of concatenated COI-Cytb-ND4 sequences between sampling regions.
Hierarchical AMOVAs attributed 99.31% of the overall variation within populations for landing sites and 99.90% within populations for capture sites (Table 2). There was no significant difference between the geographical groups for either landing or capture sites.

Table 2.
AMOVA measures of population subdivision in Aetobatus narinari for populations from Itamaracá, Barra de Catuama, and Acaú in northeast Brazil.
Only one haplotype (Hap_1) was found in all the sampled sites (either landing or sampling) according to the TCS haplotype network (Figure 2) and was shared by 25 individuals. Since some of the rays caught in Barra de Catuama were landed in Itamaracá, the diversity of haplotypes and the number of individuals collected on the north coast of Pernambuco varied. Consequently, Itamaracá represented 61.9% of the individuals and 71.4% of the haplotypes for the landing sites and 42.8% of the specimens and 57.1% of the haplotypes for the capture sites (Figure 2).
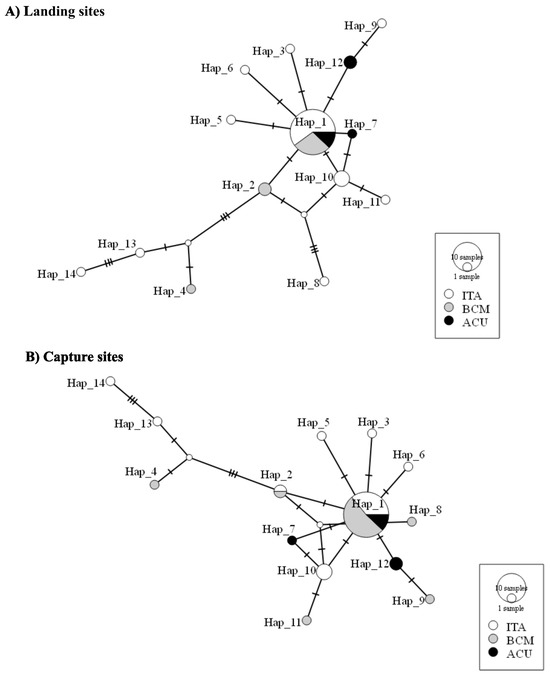
Figure 2.
TCS haplotypes of Aetobatus narinari from northeast Brazil that were collected for this study. (A) Landing sites and (B) capture sites. Haplotype networks were constructed based on 1976 bp concatenated COI-Cytb-ND4 sequences. Each circle represents a different haplotype, with its size proportional to the number of individuals found with that haplotype; dashes on branches indicate base pair differences. The sampling sites are indicated by different colors referring to the region in which haplotypes were found. ITA—Itamaracá; BCM—Barra de Catuama; ACU—Acaú.
4. Discussion
We investigated the genetic structure and connectivity of A. narinari along part of the northeastern Brazilian coast using three fragments of the mitochondrial genome. Our results revealed that individuals sampled in this region originate from a single genetic stock, as neither capture nor landing sites could be differentiated genetically. Populations are not geographically structured throughout the study area. The absence of genetic differentiation, the moderate haplotype diversity, low nucleotide diversity, and significant negative Tajima’s D values suggest that the haplotypes are closely related, and also that the population has a greater number of closely related haplotypes and may have expanded recently.
In this study, samples were analyzed according to landing sites and capture sites independently, since not all individuals were captured and landed in the same area. Indeed, accounting for the potential mixing of independent genetic stocks in fisheries landing is key for successful management measures and policies (e.g., [35]). Information on catch sites is therefore essential for studies of specimens caught by fishing activities whose samples are obtained in landing areas, as conducted here. Based on the information provided by fishermen, some rays caught in Barra de Catuama were landed in Itamaracá, underlining the importance of this information. Based on our results, however, there are no significant differences in mitochondrial haplotype frequencies between populations, either considering capture or landing sites, indicating that all individuals of this study likely originate from a single genetic stock. This is not unexpected, as high gene flow between populations, moderate to high levels of genetic diversity, and high estimates of effective population size of A. narinari have been documented in the northwestern Atlantic, Gulf of Mexico, and Caribbean Sea based on mitochondrial and microsatellite data [12,14]. The long-distance movement capacities of A. narinari (thousands of kilometers along continental coasts and the open ocean; [12,14,36]) and its migratory behavior [9,19,36] may explain part of this general lack of genetic structuring at the ocean basin scale, as geographic distances do not represent a strong barrier to gene flow [14]. However, low genetic differentiation patterns on a local scale have also been documented, notably between populations of Florida and Mexico [12], which may be linked to site fidelity of some individuals [9,36,37].
Our results are consistent with this general pattern of low to undetectable levels of genetic differentiation between populations, pointing towards fisheries management policies that could cover large geographic regions of the Brazilian Atlantic coast. However, wider geographic coverage would be beneficial to confirm our results. Additionally, this first study is based only on mitochondrial data and therefore provides a useful, but incomplete, picture of the genetic diversity of A. narinari populations in the southwestern Atlantic. Additional genetic studies based on nuclear data, using microsatellite loci [38] or a genotyping-by-sequencing approach, would refine our results and provide estimates of extant effective population sizes and their historical variations [39]. These analyses will be performed in a future paper.
Despite the genetic parameters observed here for A. narinari populations (i.e., no genetic structuring and high genetic diversity), anthropogenic disturbance, such as targeted and incidental fishing, can threaten the species’ ability to persist at a resilient and stable genetic level [14]. Indeed, moderate and high levels of haplotype and nucleotide diversity have been found in other elasmobranch species undergoing fishing exploitation. Specimens of Aetobatus ocellatus (Vulnerable—VU) from the Indo-Pacific showed high haplotype diversity and nucleotide diversity statistically similar for Cytb (h = 0.80; π = 0.0126) and ND4 (h = 0.81; π = 0.0085) [40]. According to Cruz et al. (2021) [41], two species of Rhinobatidae on the southwest Atlantic coast showed considerable levels of haplotypic and nucleotide diversity in the mitochondrial control region, despite their conservation status, h = 0. 6518, and π = 0.0053 for Brazilian Guitarfish Pseudobatos horkelii (critically endangered—CR); h = 0.5185 and π = 0.0014 for Chola Guitarfish Pseudobatos percellens (endangered—EN). Specimens of Caribbean Sharpnose Shark Rhizoprionodon porosus (vulnerable—VU) from the Caribbean Sea and the Brazilian coast showed high haplotype diversity (h = 0.881), and their nucleotide diversity was π = 0.00278 [42], while on the northeast Pacific coast, Pacific Cownose Ray Rhinoptera steindachneri (near threatened—NE) showed low levels of genetic diversity (h = 0.077; π = 0.255%) for the mitochondrial NADH2 gene [43].
Since most genetic studies of A. narinari are based almost exclusively on areas where fishing for the species is prohibited, little is known about the genetic diversity of populations that occur in areas where the species is a fishing target and traded. According to Sellas et al. (2015) [12], in areas where A. narinari is fished, management units for the conservation of the species should be identified because unsustainable fishing practices can have significant effects on local populations. Thus, reliable management plans must consider avoiding fishing of pregnant females, newborns, and young, in addition to avoiding periods and areas of reproduction and parturition to mitigate the impacts of overexploitation on local populations. Therefore, in areas where A. narinari is caught by fishing, such as in northeastern Brazil, appropriate management measures must be taken to avoid depletion of this population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16070377/s1, Table S1: Specimen collection location, number of samples (N), sex, disc width (DW), number of haplotypes, haplotype diversity (h), and nucleotide diversity (π) of Aetobatus narinari in northeast Brazil.
Author Contributions
P.R.V.A. conceived and designed the study and carried out field work; P.R.V.A., B.D.P. and K.A.F. performed genetic analyses and interpretation of results; K.A.F. sequenced the PCR products at the Pritzker Laboratory for Molecular Systematics and Evolution (Fields Museum, Chicago); P.R.V.A., B.D.P., K.A.F., K.B.-H., R.L. and M.J.A. wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Capes—Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Foundation for the Improvement of Higher Education Personnel) (PhD scholarship granted to Priscila Rocha Vasconcelos Araújo; Scholarship/194—Doctoral Program Sandwich Abroad/Process nº {88881.189330/2018-01}) and CNPq (Rosangela Lessa Pq 1 Productivity Grant, nº 306672/2015-4).
Institutional Review Board Statement
The samples were collected under approval from the Chico Mendes Institute for Biodiversity Conservation (ICMBIO) authorization under Permanent License nº 49663-1.
Data Availability Statement
The 126 sequences (42 individuals, 3 loci) were submitted to NCBI on the 18 June 2024. The mitochondrial sequences produced for this study are available on GEnBank under the accession numbers BankIt2843312: PP951253 - PP951378.
Acknowledgments
The authors would like to thank Demian D. Chapman for laboratory space and the necessary structure for DNA extraction and PCR at the Predator Ecology & Conservation Lab (Department of Biological Sciences, Florida International University); the fishermen for cooperation in providing information and collaboration in obtaining samples; and to Morgane Dendoncker (Université du Québec en Outaouais) for producing the study area map.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Euphrasen, B.A. Raja (Narinari). Kongliga Vetensk. Akad. Nya Handl. 1790, 11, 217–219. [Google Scholar]
- Bigelow, H.B.; Schroeder, W.C. Sawfishes, Guitarfishes, Skates and Rays. In Fishes of the Western North Atlantic; Memoir Sears Foundation for Marine Research: New Haven, CT, USA, 1953; pp. 1–514. [Google Scholar]
- White, W.; Last, P.; Naylor, G.; Jensen, K.; Caira, J.N. Clarification of Aetobatus ocellatus (Kuhl, 1823) as a Valid Species, and a Comparison with Aetobatus narinari (Euphrasen, 1790) (Rajiformes: Myliobatidae). Descr. New Sharks Rays Borneo 2010, 32, 141–164. [Google Scholar]
- Sales, J.B.L.; De Oliveira, C.N.; Dos Santos, W.C.R.; Rotundo, M.M.; Ferreira, Y.; Ready, J.; Sampaio, I.; Oliveira, C.; Cruz, V.P.; Lara-Mendoza, R.E.; et al. Phylogeography of Eagle Rays of the Genus Aetobatus: Aetobatus narinari Is Restricted to the Continental Western Atlantic Ocean. Hydrobiologia 2019, 836, 169–183. [Google Scholar] [CrossRef]
- Dulvy, N.; Carlson, J.; Charvet, P.; Bassos-Hull, K.; Blanco-Parra, M.-D.-P.; Chartrain, E.; Derrick, D.; Dia, M.; Diop, M.; Doherty, P.; et al. Aetobatus narinari-Whitespotted Eagle Ray. The IUCN Red List of Threatened Species 2021. 2021. Available online: https://www.iucnredlist.org/species/42564343/2924463 (accessed on 12 December 2023).
- ICMBIO. Avaliação Do Risco de Extinção Dos Elasmobrânquios e Quimeras No Brasil: 2010–2012; ICMBIO/MMA/CEPSUL: Itajaí, Brazil, 2016. [Google Scholar]
- Tagliafico, A.; Rago, N.; Rangel, M.; Mendoza, J. Exploitation and Reproduction of the Spotted Eagle Ray (Aetobatus narinari) in the Los Frailes Archipelago, Venezuela. Fish. Bull. 2012, 110, 307–316. [Google Scholar]
- Janse, M.; Kappe, A.; van Kuijk, B.L.M. Paternity Testing Using the Poisonous Sting in Captive White-Spotted Eagle Rays Aetobatus narinari: A Non-Invasive Tool for Captive Sustainability Programmes. J. Fish Biol. 2013, 82, 1082–1085. [Google Scholar] [CrossRef]
- Bassos-Hull, K.; Wilkinson, K.; Hull, P.; Dougherty, D.; Omori, K.; Ailloud, L.; Morris, J.; Hueter, R. Life History and Seasonal Occurrence of the Spotted Eagle Ray, Aetobatus narinari, in the Eastern Gulf of Mexico. Environ. Biol. Fishes 2014, 97, 1039–1056. [Google Scholar] [CrossRef]
- Harmon, T.S.; Kamerman, T.Y.; Corwin, A.L.; Sellas, A.B. Consecutive Parthenogenetic Births in a Spotted Eagle Ray Aetobatus narinari. J. Fish Biol. 2016, 88, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Araújo, P.; Oddone, M.C.; Evêncio-Neto, J.; LESSA, R. Reproductive Biology of the Whitespotted Eagle Ray Aetobatus narinari (Myliobatiformes) Captured in the Coast of Paraíba and Pernambuco, Brazil. J. Fish Biol. 2022, 100, 944–957. [Google Scholar] [CrossRef]
- Sellas, A.; Bassos-Hull, K.; Pérez-Jiménez, J.; Valdes, J.; Bernal, M.; Hueter, R. Population Structure and Seasonal Migration of the Spotted Eagle Ray, Aetobatus narinari. J. Hered. 2015, 106, 266–275. [Google Scholar] [CrossRef]
- Sellas, A.; Bassos-Hull, K.; Hueter, R.; Feldheim, K. Isolation and Characterization of Polymorphic Microsatellite Markers from the Spotted Eagle Ray (Aetobatus narinari). Conserv. Genet. Resour. 2011, 3, 609–611. [Google Scholar] [CrossRef]
- Newby, J.; Darden, T.; Shedlock, A. Population Genetic Structure of Spotted Eagle Rays, Aetobatus narinari, off Sarasota, Florida and the Southeastern United States. Copeia 2014, 2014, 503–512. [Google Scholar] [CrossRef]
- Ajemian, M.J.; Powers, S.P.; Murdoch, T.J.T. Estimating the Potential Impacts of Large Mesopredators on Benthic Resources: Integrative Assessment of Spotted Eagle Ray Foraging Ecology in Bermuda. PLoS ONE 2012, 7, e40227. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Flores, F.; Pérez-Jiménez, J.C.; Méndez-Loeza, I.; Bassos-Hull, K.; Ajemian, M.J. Comparison between the Feeding Habits of Spotted Eagle Ray (Aetobatus narinari) and Their Potential Prey in the Southern Gulf of Mexico. J. Mar. Biol. Assoc. United Kingd. 2019, 99, 661–672. [Google Scholar] [CrossRef]
- Cahill, B.V.; Eckert, R.J.; Bassos-Hull, K.; Ostendorf, T.J.; Voss, J.D.; DeGroot, B.C.; Ajemian, M.J. Diet and Feeding Ecology of the Whitespotted Eagle Ray (Aetobatus narinari) from Florida Coastal Waters Revealed via DNA Barcoding. Fishes 2023, 8, 388. [Google Scholar] [CrossRef]
- Boggio-Pasqua, A.; Bassos-Hull, K.; Aeberhard, W.H.; Hoopes, L.A.; Swider, D.A.; Wilkinson, K.A.; Dureuil, M. Whitespotted Eagle Ray (Aetobatus narinari) Age and Growth in Wild (in Situ) versus Aquarium-Housed (Ex Situ) Individuals: Implications for Conservation and Management. Front. Mar. Sci. 2022, 9, 960822. [Google Scholar] [CrossRef]
- Cuevas-Zimbrón, E.; Pérez-Jiménez, J.C.; Méndez-Loeza, I. Spatial and Seasonal Variation in a Target Fishery for Spotted Eagle Ray Aetobatus narinari in the Southern Gulf of Mexico. Fish. Sci. 2011, 77, 723–730. [Google Scholar] [CrossRef]
- Lessa, R.P.; Santana, F.M.; Rincón, G.; Gadig, O.B.F.; El-Deir, A.C.A. Biodiversidade de Elasmobrânquios Do Brasil; Ministério do Meio Ambiente (MMA), Projeto de Conservação e Utilização Sustentável da Diversidade Biológica Brasileira (PROBIO): Recife, Brazil, 1999. [Google Scholar]
- Dubick, J.D. Age and Growth of the Spotted Eagle Ray, Aetobatus narinari (Euphrasen, 1790), from Southwest Puerto Rico with Notes on Its Biology and Life History. Master’s Thesis, Universidade de Porto Rico, Mayaguez, Puerto Rico, 2000. [Google Scholar]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A.; McInness, K.H. A Primer of Conservation Genetics; Reprinted; Cambridge University Press: Cambridge, UK, 2005; ISBN 978-0-521-83110-9. [Google Scholar]
- Heithaus, M.R.; Frid, A.; Wirsing, A.J.; Worm, B. Predicting Ecological Consequences of Marine Top Predator Declines. Trends Ecol. Evol. 2008, 23, 202–210. [Google Scholar] [CrossRef]
- Clarke, C.R.; Karl, S.A.; Horn, R.L.; Bernard, A.M.; Lea, J.S.; Hazin, F.H.; Prodöhl, P.A.; Shivji, M.S. Global Mitochondrial DNA Phylogeography and Population Structure of the Silky Shark, Carcharhinus falciformis. Mar. Biol. 2015, 162, 945–955. [Google Scholar] [CrossRef]
- Schluessel, V.; Bennett, M.B.; Collin, S.P. Diet and Reproduction in the White-Spotted Eagle Ray Aetobatus narinari from Queensland, Australia and the Penghu Islands, Taiwan. Mar. Freshw. Res. 2010, 61, 1278–1289. [Google Scholar] [CrossRef]
- Yokota, L.; Lessa, R.P. A Nursery Area for Sharks and Rays in Northeastern Brazil. Environ. Biol. Fishes 2006, 75, 349–360. [Google Scholar] [CrossRef]
- Meyer, C.P. Molecular Systematics of Cowries (Gastropoda: Cypraeidae) and Diversification Patterns in the Tropics. Biol. J. Linn. Soc. 2003, 79, 401–459. [Google Scholar] [CrossRef]
- Richards, V.P.; Henning, M.; Witzell, W.; Shivji, M.S. Species Delineation and Evolutionary History of the Globally Distributed Spotted Eagle Ray (Aetobatus narinari). J. Hered. 2009, 100, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Arèvalo, E.; Davis, S.K.; Sites, J.W., Jr. Mitochondrial DNA Sequence Divergence and Phylogenetic Relationships among Eight Chromosome Races of the Sceloporus Grammicus Complex (Phrynosomatidae) in Central Mexico. Syst. Biol. 1994, 43, 387–418. [Google Scholar] [CrossRef]
- Inoue, J.G.; Miya, M.; Tsukamoto, K.; Nishida, M. A Mitogenomic Perspective on the Basal Teleostean Phylogeny: Resolving Higher-Level Relationships with Longer DNA Sequences. Mol. Phylogenet. Evol. 2001, 20, 275–285. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Excoffier, L.; Lischer, H.E.L. Arlequin Suite Ver 3.5: A New Series of Programs to Perform Population Genetics Analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Clement, M.; Snell, Q.; Walke, P.; Posada, D.; Crandall, K. TCS: Estimating Gene Genealogies. In Proceedings of the 16th International Parallel and Distributed Processing Symposium, Fort Lauderdale, FL, USA, 15–19 April 2002; p. 7. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Reiss, H.; Hoarau, G.; Dickey-Collas, M.; Wolff, W.J. Genetic Population Structure of Marine Fish: Mismatch between Biological and Fisheries Management Units. Fish Fish. 2009, 10, 361–395. [Google Scholar] [CrossRef]
- DeGroot, B.C.; Bassos-Hull, K.; Wilkinson, K.A.; Lowerre-Barbieri, S.; Poulakis, G.R.; Ajemian, M.J. Variable Migration Patterns of Whitespotted Eagle Rays Aetobatus narinari along Florida’s Coastlines. Mar. Biol. 2021, 168, 18. [Google Scholar] [CrossRef]
- Flowers, K.I.; Henderson, A.C.; Lupton, J.L.; Chapman, D.D. Site Affinity of Whitespotted Eagle Rays Aetobatus narinari Assessed Using Photographic Identification. J. Fish Biol. 2017, 91, 1337–1349. [Google Scholar] [CrossRef]
- Ensing, D.; Crozier, W.W.; Boylan, P.; O’Maoiléidigh, N.; McGinnity, P. An Analysis of Genetic Stock Identification on a Small Geographical Scale Using Microsatellite Markers, and Its Application in the Management of a Mixed-Stock Fishery for Atlantic Salmon Salmo salar in Ireland. J. Fish Biol. 2013, 82, 2080–2094. [Google Scholar] [CrossRef] [PubMed]
- Postaire, B.D.; Devloo-Delva, F.; Brunnschweiler, J.M.; Charvet, P.; Chen, X.; Cliff, G.; Daly, R.; Drymon, J.M.; Espinoza, M.; Fernando, D.; et al. Global Genetic Diversity and Historical Demography of the Bull Shark. J. Biogeogr. 2024, 51, 632–648. [Google Scholar] [CrossRef]
- Schluessel, V.; Broderick, D.; Collin, S.P.; Ovenden, J.R. Evidence for Extensive Population Structure in the White-Spotted Eagle Ray within the Indo-Pacific Inferred from Mitochondrial Gene Sequences. J. Zool. 2010, 281, 46–55. [Google Scholar] [CrossRef]
- Cruz, V.P.; Adachi, A.M.C.L.; Oliveira, P.H.; Ribeiro, G.S.; Paim, F.G.; Souza, B.C.; Rodrigues, A.S.F.; Vianna, M.; Delpiani, S.M.; Díaz de Astarloa, J.M.; et al. Genetic Diversity in Two Threatened Species of Guitarfish (Elasmobranchii: Rhinobatidae) from the Brazilian and Argentinian Coasts: An Alert for Conservation. Neotropical Ichthyol. 2021, 19, e210012. [Google Scholar] [CrossRef]
- Mendonça, F.F.; Oliveira, C.; Gadig, O.B.F.; Foresti, F. Phylogeography and Genetic Population Structure of Caribbean Sharpnose Shark Rhizoprionodon porosus. Rev. Fish Biol. Fish. 2011, 21, 799–814. [Google Scholar] [CrossRef]
- Sandoval-Castillo, J.; Rocha-Olivares, A. Deep Mitochondrial Divergence in Baja California Populations of an Aquilopelagic Elasmobranch: The Golden Cownose Ray. J. Hered. 2011, 102, 269–274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).