1. Introduction
The conservation of biocultural diversity in regions is fundamental for sustainable development and, over time, the necessary attention for the conservation of landscape heritage has been institutionalized (UNESCO, 1972) [
1]. UNESCO (2001) proposed the broad concept of cultural diversity, encompassing the characteristics of a given society’s dynamic material behavior, traditions, customs, and moral values [
2]. Cultural diversity is relevant when a variety of cultures foster the development of exchange and creativity of social groups, helping them to evolve and adapt to the environment.
Cultural diversity is indispensable for reproducing human society and ecosystems in the face of constant variations in their aesthetic, biophysical, climatic, and anthropogenic environments. It is also expressed in the body of knowledge and experiences generated by human groups in the enjoyment, creation, and transformation of landscapes [
3], which can be accumulated and preserved as a cultural, tangible, and intangible heritage. In this way, it is vital to understand the relationship between human social groups and the biomes they cohabit, in order to define how to conserve the landscape heritage of the biocultural diversity of a region or territory that explains the dynamics of Socioecological Systems (SES) embedded in a region.
Biocultural landscape heritage must be based on a clear and defined socio-biocultural proposal, which is useful for the development of a regional sustainable development strategy [
4]. In other words, the importance of cultural landscape heritage does not lie in the past, but in its use to explain the present and establish its intentionality in the future for the life of society and biodiversity.
The concept of a Socioecological System (SES) became relevant in sustainability science [
5] in the 21st century [
6]. It refers to the total system integrating social and ecological systems based on co-evolution [
7]. An SES comprises a set of sub-systems, relationships, and elements [
8] that interact under the conditions of heterogeneity, uncertainty, emergent properties, and self-regulation [
9], as well as under stochastic processes in different dimensions: organizational, scale, time, and space [
10]. An SES is a system of completely random behaviors in a non-linear dynamical system, through which a social capacity for resilience may be built [
11].
According to Levin et al., [
12], an SES is adaptive and complex as it brings together biotic resources and individual and social human agents with the capacity to act in response to changes in operating conditions. It also facilitates connectivity, allowing the system to regenerate after a radical change or collapse. In line with these authors, the main properties of an SES are: (a) integrality, which requires the continuous and discontinuous but complementary functioning of the set of sub-systems and endogenous and exogenous elements; (b) resilience, or the magnitude of perturbation that a system can withstand without changing to an alternative state with different structure and systemic functions; (c) diversity, or the variety of elements with which it operates; (d) redundancy, or the multiplicity of existing sub-systems, which, in case of failure, guarantees the vital functioning of the Socioecological System; (e) modularity, or the compartmentalization of sub-systems into modules such that their activity is as independent as possible, in order to isolate them from possible risks to the system.
Holling et al., (2001) have argued that SESs face contingent situations of structural problems, cyclical processes, or impacts of externalities that stimulate them to grow, contract, or stagnate. When these are generated, they act as weights and counterweights in dynamic equilibrium for the maintenance of the system in situations characterized by total or partial reversibility or irreversibility. They react, through non-linear feedback processes, to changes and adapt to the circumstances [
13]. Thus, the SES entails the existence of institutions or contractual social relations for the processes of systemic exchange, employing management actions, acceleration or braking, pressure, damping, biomimetic or not, inclusive or exclusive, and reproductive or destructive.
The SES concept has been linked to the landscape heritage of biocultural diversity since the first third of the 20th century, when Sauer’s Cultural Geography emerged [
14]. It considered the concept of the cultural landscape as the manifestation of the synthesis of the historical interactions of human society with nature, distinguishing it between regions or territories. Each region presents different historical trajectories of nature, society, culture, and forms of economic, social, and political life, which together form a culture characterized by its conception of the world and the interpretation of both natural phenomena and human historical and social facts.
Each human group is distinguished by its peculiar way of living and its capacity for perception and attitude towards the landscape of biocultural diversity and the specific human use of nature. Hanspach et al., [
15] have suggested that power games are reflected in the types of way of life, technological transformations, or trading styles that prevail. Thus, landscape heritage arises from the need for the social appropriation of nature, based on a synthetic and syncretic social identity located in a dynamic, living, felt, and perceived area subject to constant evolution, delimited as a territory or region [
16].
The object of this study is to analyze, among different cultural groups of a selected region, how the performance of a Socioecological System (SES) influences the conservation of biocultural diversity. The research question that guided this work was: how important is an SES’s performance for conserving biocultural diversity?
2. Materials of Study
2.1. Importance of the Biodiversity in the Study Region
The Huasteca Potosina is a region considered to be rich in biological and cultural diversity (Puig, 1991 [
17]; Rzedowski J., 1992 [
18]; Hudson, 2004 [
19]), derived from the geographical configuration that places it in the so-called Mexican transition zone between the Nearctic and Neotropical regions. It is located between the physiographic provinces of the Coastal Plain of the Gulf of Mexico and the Sierra Madre Oriental and is in the hydrological region of the Panuco River. These abiotic elements provide the necessary conditions for this region to possess a high diversity of vegetation types and rich endemism, which makes it one of Mexico’s priority inland regions [
20].
The region occupies part of the states of San Luis Potosí, Tamaulipas, Veracruz, Hidalgo, and portions of Querétaro and Puebla. Here, we deal only with the Huasteca Potosina. In terms of its relief, it has three areas: The first is the coastal plains (40–100 m above sea level, masl), distinguished by rivers, streams, and lagoons of the lower basin of the Panuco River. These plains cover a large part of the municipalities of Tamuín, Ébano, San Vicente Tancuayalab, and Tanquián, as well as portions of Aquismón, Tanlajás, and Tancanhuitz. The second area is the rolling hills (100–600 masl) found in the central and southern part of the region, in the municipalities of Tanlajas, Tanquián, San Antonio, Tampamolón, Huehuetlán, and Tancanhuitz. Finally, the third area is a portion of the Sierra Madre Oriental (100–1800 masl) with high slopes, from the northwest of the Sierra with the municipalities of Tamasopo and El Naranjo to the western end of the municipalities of Ciudad Valles and Aquismón and the south of the Sierra (municipalities of Xilitla, Huehuetlán, Coxcatlán, Tancanhuitz, Axtla de Terrazas, Matlapa, and Tamazunchale) [
21] (pp. 75–76).
The landscape heritage of the biocultural diversity of the Huasteca Potosina is appreciable (
Appendix A). It has been estimated that San Luis Potosí state has 226 floristic families, 1441 genera, and 5413 species [
22], of which 25% constitute species with a tropical affinity that inhabit the different variants of the seasonal forests of the Huasteca Potosina [
23]. However, during the last century, the land-use changes caused by the commercial expansion of agriculture and livestock farming have had a significant adverse environmental impact. In the critical areas of Huasteca, most of the primitive vegetation has disappeared and been replaced by other vegetation. The low deciduous forests of the plains have lost their original cover in the east of the region, due to the increase in pastureland for extensive livestock farming and large areas of sugar cane plantations, as well as other crops [
24]. The reduction and fragmentation of the tropical montane cloud forest and parts of the tropical semi-evergreen forest are due to increased citrus plantations, shade coffee orchards [
25], illegal forestall logging, and new agricultural and cattle ranching areas. [
26].
2.2. History and Socioecological Systems in the Huasteca Potosina
The SESs can be observed through the historical co-evolution of a social or ethnic group with the predominant ecosystem they inhabit. In this way, historical research and study of the narrative of the social actors can help to understand the main socioeconomic features prevailing in SES. The SES profile must be considered as the capacity of the social or ethnic group to have specific relationships with its corresponding predominant ecosystem, the variety of vegetation types, and the economic activities they were historically allowed to carry out in their space.
In this regard, we consider a brief overview of the formation of the social and historical identity of the Huasteca region before characterizing each SES. The ethnobiologist Ford [
27] has studied the relationship between the Maya civilization and the rainforest and found evidence that the use of nature by the Maya was based on work that implicitly or explicitly could be typified as “gardening” in the jungle. They adapted the land, utilizing the “slash and burn” technique to produce their food where there were good agricultural possibilities. This allowed them to cohabit with wildlife, facilitated the propagation of flora species in the forest and jungle to take advantage of them, and induced agroforestry circuits and spaces for the mobility of nature throughout the territory, to optimize their gathering, hunting, and fishing. In this sense, conserving the landscape heritage of biocultural diversity both in the Mayan jungles and in the Huasteca Potosina provided a means for their civilizational development and sustainable living culture.
Johansson [
28] has explained the regional history of the Teenek through the Nahua narrative. He states that the Teenek—a primary ethnic group of the Huasteca Potosina—represent a variant of the Mayan language since their appearance in the 11th century BC. They established Mayan cultural practices in the region, such as those related to the Mayan agriculture south of Mesoamerica. They also built two ceremonial centers before the coming of the Nahuas and the Europeans (13–15th century AD) and developed technology for managing water for urban and agricultural use in floodable zones, transported through the fluvial ways, and knew about coastal navigation. The Teenek developed commercial and cultural exchanges with the Olmecs, Toltecs, and Totonacs at different times until the 16th century AD [
29].
The Teenek also contributed, among others (e.g., the Nahua), to the propagation and consumption of Mesoamerican plants for food, medicine, tools, clothing, and domestic use. After the abandonment of the ceremonial centers (the current archaeological sites), their governance was based on a decentralized network of cacique governments in the principal localities of the Huasteca [
30]. Their cohabitation with nature was manifested in the dispersion of family hamlets spread out in the jungle and the forest. In the largest localities, they cohabited with people of other ethnic groups, and neighborhoods were integrated with several families conducting everyday activities, or there were ties of local social identity [
31]. These family and neighborhood groups had a system of loyalties to their cacique, to whom they paid tribute and defended themselves against aggressions from Chichimec tribes or other invaders [
32].
The former Nahua groups arrived and settled south of the Huasteca, in Hidalgo and Veracruz, without significant conflicts and adapted to the Huasteca region. The Nahua represent a confluence between the original Nahua tribes and those of Mexica origin, who came to incorporate the area into their empire in XV century AD. The main interest of the Mexica traders—the pochtecas—was to establish trade routes for the expansion of the Aztec empire. As they did in other parts of Mesoamerica, the Mexica established cohabitation with the dominated tribes, prioritizing the importance of trade for their empire [
33]. They occupied the old Nahua localities, and new ones of Mexica origin were also established next to the Teenek in agricultural areas, in order to trade products such as maize, beans, squash, cotton, and Chili, among others. The Nahua developed a sound knowledge of biocultural diversity through their home garden, milpa, and cultivated land. The Nahua have always distinguished themselves as having a greater organizational capacity and for leading agrarian conflicts [
34].
The Pame and the Xi’iuy language came from the Chichimec tribes, who were nomadic but often seasonally occupied the northern border with Mesoamerica. They were former neighbors of the Teenek and traded, among other things, turquoise, bird feathers (eagles), and bows and arrows in exchange for food. Later, their development of rain-fed agriculture for maize and beans allowed them to settle in the region. Due to their social nature, they had difficulty integrating into the Novo-Hispanic society, given the absence of permanent settlements. The Spanish could only dominate some groups of Pame through violence; their adhesion to catholic missions allowed them access to lands later taken from them by the landowners [
35]. Thus, the Pame were an ethnic group that became sedentary, mainly in the 18th and 19th century, and developed small family agricultural units in the lands at the western limits of the Huasteca Potosina, in areas of sub-montane scrubland, oak forests, and semi-arid transition zones [
36].
The mestizos formed a majority social group in the region from the nineteenth century onwards. They tended to acculturate and faced severe limitations in their knowledge of biocultural diversity. Today, openly or veiledly, some show contempt for the indigenous people but, if possible, they try to take advantage of them for their own benefit and interests. Likewise, they offer the capacity to adapt to changes in their economic, social, and political environment, which allows them to assume the generic peasant identity of the region, regardless of whether or not they include indigenous people. They are the primary beneficiaries of the current process of “gentrification” of indigenous areas caused by tourism dynamics [
37] and tend to represent a possible risk for conserving the authentic indigenous culture in Huasteca Potosina. Wood and fiber materials for building houses and handicrafts are becoming scarce and more expensive. The price of food has risen due to the demand for new mestizo employees in services and tourist attentions; therefore, the ejido lands owned by the ethnic groups are once again the object of greed. The speculative interest of tourist property developers seeks to dispossess ethnic groups from their places of natural beauty and landscape heritage. However, the most severe aspect is that the knowledge of biocultural diversity, including its meaning and usefulness, tends to be lost with changes in land use and the abandonment of wild plants, as their dissemination in the region is limited and their reproduction is complicated.
2.3. Profiles of SES in Huasteca
Alcorn J. [
38], an expert in ethnobiology, has researched Huasteca Potosina and formulated a hypothesis on the symbiosis of indigenous social systems and ecological systems in Huasteca. The use of the tropical semi-evergreen forest and low deciduous forest by the Teenek or Huastec indigenous groups, along with her study of their habitat in the Huasteca Potosina, have contributed to the understanding of sustainable agroforestry and the conservation of indigenous biodiversity, accompanied by family and community organizational forms and free individual and shared access to natural resources [
39].
The three ethnic groups that cohabit the Huasteca Potosina have generated an accelerated process of cultural syncretism in the region, resulting in the adoption of widespread cultural practices among the different SES. At a first level, they have a joint cultural base, practice economic pluriactivity, and have other common traits that are now being lost; among others, these include respect for traditional medicine; knowledge of biocultural diversity; handicraft making; appreciation of stews made from local agricultural, wild, and fish products; the use of the milpa as an instrument of family food security. The second level of cultural linkage of the three ethnic groups references the biocultural knowledge of the Teenek, who are currently disappearing and undergoing a fast cultural syncretism process, including festivities (Xantolo, liturgical celebrations of the localities), home remedies, and craft activities. An example is that the three ethnic groups learned about the management of the forest, jungle, sub-montane scrubland, and water flows for the continuous provision of food and medicines throughout the seasons [
40]. At present, it is commonly observed that such knowledge and practices have been lost over time. The role of complementary foods (i.e., seeds, fruits, roots) that were once useful to ensure good nutrition for their families in the face of low agricultural yields of maize and beans of the Huasteca has also declined sharply.
The profiles of the SES were constructed based on documentary and bibliographic reviews, as well as interviews with members of the ethnic and mestizo groups who participated in the research carried out in the localities visited and on field trips. The profiles of the four SES of the Huasteca region can be organized in terms of the main properties of the SES established by Levin et al., (2013), as detailed in
Table 1.
As can be seen from the considered set of properties, the landscape heritage of biocultural diversity tends to be at risk in the cases of the SES of the Pame and the mestizos. At the same time, the issues of the SES of the Teenek and the Nahua also present vulnerability under weak conditions. However, in the case of the Teenek, there is an uncertain future. At the same time, the Nahua have a greater capacity to adapt, even though this may imply more significant deterioration of the landscape heritage. Even now, the Nahua have a larger indigenous population than the Teenek (i.e., the ancient native people of the region).
Additional aspects contributing to confirming the conclusions on integrality analysis of SES are the main predominant economic features observed in the case studies and cited by other authors defining the four SES. This information is provided in
Appendix B. Regarding the socioeconomic characteristics of the communities visited in the fieldwork, please see
Appendix C.
The landscape heritage of the biocultural diversity of the Huasteca Potosina is characterized by mixed social dynamics and crossed environmental tendencies under conditions of significant uncertainty in the future. On the other hand, for Vidal and Brusca [
41], the fate of indigenous languages and their possible extinction is correlated with threats to the existence of biodiversity, which is why they have pointed out the importance of preserving linguistic diversity to conserve the landscape heritage of biocultural diversity in the region. Alcorn (1995) has established the argument of how, for centuries, ethnic groups have preserved biocultural diversity based on their biocultural knowledge and the sustainable use of the forest and jungle by ethnic groups. Alcorn found that, in a te’lom area, there were possibly more than 300 species (including exotic species such as sugar cane, coffee, banana, mango, and/or citrus that the Teenek incorporated in te’lom centuries ago) at the beginning of the 1980s [
42] (p. 396).
In this sense, it is worth asking: how important is the performance of an SES for the conservation of biocultural diversity? To answer this, it is necessary to consider the profile of each of the SES in the region and relate it to: (1) the information that each one has on the ethnobotanical species of the region; (2) the use that each of the SES gives to the ethnobotanical species they know; (3) the diversity of ethnobotanical species that exist in the predominant biome. We decided to use three conventional ethnobotanical indicators, in order to measure: (a) consensus on the information among informants; (b) the importance of cultural knowledge; (c) the biodiversity of the territory.
If an SES is at risk or vulnerable, it may be reflected in the state of conservation of biocultural diversity, either in processes of acculturation, reduced use of species, or decreased ethnobotanical diversity in the biomes. Namely, knowing the state of biocultural diversity in the biomes inhabited by the SES is a basic premise for planning and establishing—in a participatory manner with the SES of the region—a strategy to conserve the landscape heritage of biocultural diversity. This may be helpful in stopping the exclusive tourist gentrification, which affects the natural and economic heritage of indigenous groups, and preventing the application of technocratic conservation, which only considers the rescue of biodiversity through reproducing ethnobotanical species with higher economic value.
At present, there are better social and cultural capacities to take advantage of the landscape heritage of biocultural diversity, beyond focusing on the historical appropriation of ethnobotanical wealth [
43] without retribution to the indigenous groups that have developed and conserved it.
2.4. Ethnobotanical Information
To prepare the analysis and to apply the indicators, high-quality direct information is required. The scholar Salvador Luna Vargas conducted this work, with the support of Project SIP 2021-1438 (CIIEMAD, IPN), for a study on forest products of domestic use managed by the SES in the Huasteca Potosina [
44].
Appendix D presents the ethnobotanical data obtained in the fieldwork, including case studies of representative biomes and SES localities (the complete database of List of Forest Species Found can be found at doi: 10.17632/fsz9zs6hm8.1). Although it is not a complete list of total Huasteca species, it partially approximates the state of the diversity of forest species with domestic use. Its value was derived from the members of the ethnic groups and the mestizo peasants who participated in the research.
A total of 76 plant species were found, categorized into seven primary services: species for cabinetmaking use (14%); for firewood or fuel (30%); for work tools (14%); for medicinal use (22%); for shade or shelter (18%); for food (26%); for construction (the main category, 86% of the species). Similarly, considering the number of species used by each ethnic group, the Nahua group made the most essential domestic use of these forest species (43 species), followed by the Teenek group (who made use of 31 of the 76 species available), while both the Pame and the mestizos made only minor use of the forest species (21 species each).
In
Table 2, we can observe that the Nahua ethnic group had the most varied effective use of the species, followed by the Teenek, Pame, and mestizo groups, respectively. With the last two SES, no species related to shade (refuge) or medicinal form were found or reported in this fieldwork (in this table, these data were not included as, in some cases, they were repeated in other uses).
3. Method and Techniques
For this study, we applied the ethnographic method [
45]. In 2021, typical localities of each group were observed, and communication was established to facilitate their participation in this research. A bibliographic review and information provided by the informants of each group were compiled to form a profile of the Socioecological System of each group (
Section 2.3). The ethnobotanical data collection of species in the localities selected for the case studies was critical for the biocultural analysis. The techniques for measuring biocultural diversity conservation consisted of applying three commonly used ethnobotanical indices [
46,
47]. We chose not to use a specific biocultural index as the combined use of a species information level index, a cultural importance index, and a biodiversity index could provide a better picture of the biocultural importance of each SES. These three indices were employed as follows:
The first was the Informants Consensus Factor Index (
ICF), which considers the consensus role of the information obtained by the informant groups, regarding the ethnobotanical species used and the types of use given to them. This index evaluates the distribution of species’ information by type of use among informants and determines the homogeneity of informants’ knowledge about the same species (Castañeda y Alban) [
48].
The Informant Consensus Factor Index (
ICF) is calculated as follows:
where
Nur is the number of plants reported as being used for a specific type of use and
Nt is the number of plant species reported by informants for the type of use indicated.
The Informant Consensus Factor ranges from 0 to 1 in value, with the highest value (1) indicating the highest consensus of the species used for a particular type of use and the lowest value (0) indicating total disagreement among informants regarding the kind of use.
The second index used was the Cultural Importance Index (CII). This index considers two aspects: the number of types of use that a plant has and the number of groups of informants that indicate the culturing of that species for use (in one or more types). There were four groups of informants: the Teenek, Pame, and Nahuatl ethnic groups, as well as a mestizo group. Concerning the uses, seven types of service were considered: woodwork (E), firewood or fuel (L), tools (H), medicinal or ritual (M), shade or shelter (S), food (A), and construction (C).
The Cultural Importance Index (
CII) is calculated as follows:
where
N is the total number of informants;
NC is the total number of plant categories used;
UR denotes the species’ use reports;
i is the index of the interviewee
u is the category of plants;
CII is the cultural importance index of the species.
To identify the most culturally significant species, the Cultural Importance Index (CII), proposed by Tardío and Pardo-de-Santayana [
49] in 2008, is based on reports of use by species. It can also be seen as a redefinition of the Use Value of Phillips and Gentry [
50]. The CII index varies from 0 (when no one mentions the use of any plant) to NC (in the hypothetical case that all informants say that all species serve all use categories considered in the study).
The third considered index was the Shannon–Wiener Biodiversity Index (SWI), which is considered a good measure of diversity as it indicates that all communities that present the same index value are equivalent concerning their diversity; that is, equivalence classes can be created between communities that share the same Shannon Index. One of the advantages is that the index is not significantly affected by sample size. Several studies have sought to determine the effect of sample size and have concluded that sample size has a very slight impact on measures of species’ diversity.
The Shannon–Wiener Biodiversity Index (
SWI) is calculated as follows:
where
As the formula includes a logarithm, which signifies the inverse of the exponential function, there is no maximum value for the index. However, the minimum value is zero, indicating an absence of diversity. In our study, values less than two are interpreted as ecosystems with relatively low species’ diversity, while those greater than three indicate high diversity. One property of an ecosystem is the uncertainty regarding the species of a randomly selected individual in the community. This uncertainty can be calculated from the relative abundances of each species using information theory (Shannon, 1948); simply, Shannon’s entropy. Jost and biologists often call the index used here (
SWI) the
Shannon–Wiener Index or the Shannon–
Weaver Index [
51] (p. 40).
4. Results
The results for each calculated index were presented in this section, as well as tables of information, for better visualization of the processes. The
ICF indicated a greater consensus among the ethnic groups regarding certain species for the use of cabinetmaking; on the other hand, there was no agreement among the ethnic groups regarding the use of species for shade or shelter (
Table 3).
The results found for the Cultural Importance Index were low; the highest value was for the species
Cedrela odorata L., which all four ethnic groups used for cabinetmaking and three used for construction. Only one of them used it for shade or medicine. The maximum value in this combination would be that of a plant with all seven types of use for the three ethnic groups (7). On the other hand, a plant that had no use for any of the groups of informants would have a value of zero. In general, the plants did not show more than three types of use. For them to be used by the four groups of informants, it would be required that their distribution be homogeneous in the sites where the four groups are located (
Table 4).
Regarding the Shannon–Wiener Biodiversity Index (SWI), we can observe that both the low deciduous forest and the tropical semi-evergreen forest showed the highest values among the biomes (
Table 5), interpreted as adaptation that has allowed for the best possible diversification of species; thus, these predominant biomes in the Huasteca conserve more species than the others. Their richness in biodiversity has always been extraordinary and they are also the most extended biomes in the region. They have also been subject to intense interaction with the main indigenous groups inhabiting Huasteca.
However, the relative importance of species diversity in the agroforestry system (e.g., citrus orchards or sugar cane fields) is noteworthy, as these used to be areas of low deciduous forest and tropical semi-evergreen forest. Instead, the sub-montane scrubland and oak forest presented a deteriorating condition, possibly due to the degradation of the natural environment and the cultural loss suffered by the indigenous group that inhabits and uses these biomes. The areas categorized as “Others” had the lowest value (0.7); this was added as an additional control, including disturbed areas where mango, tamarind, ficus, and fruit trees had been abandoned and were not part of orchards or commercial plantations.
Table 6 shows the statistical information of the data used for calculation of the
SWI. Confidence intervals with an error of 5% are added. In the case of each of the biomes, their means fall within the values of the intervals, indicating that the results of
SWI are acceptable for the data selected for the study.
Both the low deciduous forest and tropical semi-evergreen forest had high variance and standard deviation. These two regions are the main biomes of the SES of the Teenek and the Nahuas ethnic groups, so it was to be expected that their values would be higher, as these are the SES that use the most species. Concerning the other biomes, their values were lower, due to the possible degradation of the natural resource and the abandonment of species caused by insufficient biocultural knowledge in the Pame and mestizo SESs. There was not much variability in their statistical results, but they still managed to enter within the 95% confidence level, therefore their results in the SWI were also accepted.
Table 7 shows that the results of the indices were within the range of normality. It is important to emphasize that the variance and standard deviation indicated how dispersed the data were. However, after analyzing the information, we observed a good normal distribution. The data selected for the study were not dispersed, allowing the indices to be calculated efficiently and accurately. Finally, we decided to perform an ANOVA statistical test on the differences in the average number of species in the biomes, using a significance level of 5%, and to apply the Tukey test to determine whether there was any statistical difference in the use of species (
Table 8).
ANOVA and Tukey statistical test
The null hypothesis and the alternative hypothesis were as follows:
H0: the average number of species used in each region presents 95% reliability.
H1: in at least one region, the average number of species used was different, with 95% reliability.
By performing the data analysis,
Table 7 and
Table 8 were obtained, including the statistical information for each region.
Our significance level was 5% (i.e., 0.05) and the p-value was less than this value (0.05 > 2.05738 × 10−17), so we rejected our null hypothesis (H0) and accepted the alternate hypothesis (H1), establishing that the average number of species used differed in at least one region.
Once H0 was rejected, we proceeded to verify which region was the one that had the difference, which was achieved through the Tukey test. For this statistical test, the value of the “honestly significant difference” (HSD) [
52] was taken. For this study, the HSD was equal to 0.16294.
Table 9 shows the Tukey values obtained by subtracting the region’s average minus the HSD. In the low deciduous forest versus the tropical semi-evergreen forest, the value was lower than the HSD (0.01316 < 0.016294), which indicated no difference between these two regions. On the other hand, with low forests versus the different regions, there was a difference between biomes.
Although rejecting H0 indicated that the average number of species used differed in at least one region, using Tukey’s test, the results of the Shannon–Wiener Biodiversity Index were acceptable. In the cases of oak forest, sub-montane scrubland, and the agroforestry system, where the Shannon Index had the lowest and most different values, the statistical results indicated that there was effectively no correlation between them in terms of species use, with each zone being different from the others in terms of its knowledge about each plant.
It can then be refuted that the low deciduous forest and the tropical semi-evergreen forest regions reached a value of 3.4. Statistically, these areas had the same diversification value, as there was no significant difference between them when the test was carried out.
Thus, our alternative hypothesis coincided with the results of the Shannon–Wiener Index, demonstrating that the biomes inhabited by the Teenek and the Nahua groups present a greater diversity of forest species for domestic use than the others (Pame and mestizos).
5. Discussion
The discussion of the results obtained in the previous section is based on the use of conventional ethnobotanical indicators to understand the relationships between biomes and SESs in the region. For this reason, we observe our results based on previous studies using ethnobotanical methodologies in this section.
According to Prance (2000) [
53], ethnobotany is an interdisciplinary science in which botany interacts with anthropology and has distinguished itself through the investigation of the existence of biological diversity. Despite recognizing the importance of the relationship between diversity and ethnic groups, Salick et al., (1999) [
54] have attempted to explain biological diversity by defining gradients, generally of a latitudinal or altitude type.
They have conducted studies on the richness and the knowledge of valuable plants and explained how the number of useful plants decreases as the height increases. Other studies have attributed the decrease in valuable species (especially timber) at higher altitudes to ecological reasons, as the number of large trees decreases with elevation. In addition, the use of tree species is more significant at medium elevations (Salick et al., 2004) [
55]. This approach has continued in recent studies by Heindorf et al., (2019 [
56]; 2021 [
57]) that stand out, in our case, due to being carried out in the Huasteca Potosina regarding edible plants in Teenek indigenous localities. In these works, again, the altitudinal gradient was shown to influence the richness of valuable plants as it was in the intermediate altitude where the most significant number of edible plants and cultivars were identified, mainly in the milpa and in family gardens.
According to the altitude range of the localities of the case studies used in this work, it was demonstrated that biodiversity acted as a function of the altitudinal gradient. However, authors such as Toledo (1994) [
58] have considered gradients to be only partially explanatory, as biomes such as the tropical montane cloud forest can occur at vast heights. Similarly, there are forest species (e.g.,
Cedrela Odorata) that, together with a community of species, coexist in various biomes located in different altitude gradients and are distinguished by having accompanied ethnic groups throughout their history, with a cultural trajectory and journey through different habitats. In the same sense, Weckerle et al., (2006) [
59] and Saqib et al., (2011) [
60] have paid more attention to the patterns of human settlements to explain the distribution of plants with ethnobotanical importance. They agreed with the premise that the more significant the diversity of species, the higher the possibility of relative abundance of valuable species, in addition to having free access to them by indigenous communities.
Bermúdez et al., (2005) [
61] considered the knowledge based on experience (habits and traditions) allows for the incorporation of ethnobotanical plant use as phenomena with dynamic natures, with unequal access within indigenous communities (e.g., deeper knowledge of the Shaman compared to the rest of the community, or differences in age or gender). Indigenous communities can lose or increase their biocultural diversity at different historical moments or, otherwise, evolve unevenly, both in their way of exploiting and conserving species and in representing the aspirations of their ethnic group regarding the use of species to generate possibilities for local development.
Zent S. and Zent E. (2004) [
62] have explained that changes in indigenous communities are related to factors such as the settlement pattern, land-use change, or subsistence strategies (labor migration), which tend to modify the biocultural diversity of ethnic groups. Moreover, they are susceptible to the acculturation process, which mainly affects the youngest, or problems arising from a more significant dependence on Western medicines to cure diseases, as medicines of ethnobotanical origin are unknown, mistrusted, or valuable species for their preparation have gone extinct.
Kessler (2006) [
63] has explained the importance of dynamic changes in biocultural diversity attributed to the behavior of ethnic groups. When he studied the location of a forest species (
Polylepis), its specimens can currently only be found on steep slopes and sites difficult to access in extreme environmental conditions. The author explained that these difficult circumstances were not always the case, but the rarity of the survivors is due to over-exploitation of its great ethnobotanical value.
The phylogenetic methodology emerges through the application of conventional ethnobotanical indices (
ICF,
CCI,
SWI), which enables us to understand: (a) sudden changes in species density that are not considered; (b) species’ site displacement shifts due to changing settlements of ethnic groups; (c) variations in biocultural diversity due to changes in the historical trajectory of ethnic groups and habitat changes (Chao et al., 2010 [
64]; Jost, 2018 [
65]; Zhang et al., 2018 [
66]; Feng and Squires, 2020 [
67]). In any case, these new methodological contributions tend to reflect the preoccupation of research on the dynamic systems influencing biocultural diversity regarding variations in SES behavior.
The work presented here is comparable to the studies mentioned above. The relationships between the SESs of ethnic groups and biomes were found to be vital. Results of conventional quantitative methods from the ethnobotanical field were tested by ANOVA and Tukey tests. As a result, we found that the low deciduous forest and sub-evergreen rainforest biomes, which co-evolved mainly with the Nahua and the Teenek SESs, presented higher diversity and effective use of species. Thus, the profiles of these SESs offer better possibilities to conserve the landscape heritage of biocultural diversity, in contrast to the biomes linked to the Pame and mestizo SESs.
In addition, in any study, it can be said that it is essential to know the adequate methodological instruments for the examined study objective (Hoffman and Gallaher, 2007) [
68]. For this reason, we decided to utilize conventional ethnobotanical indicators to assess the state of conservation of biocultural diversity in the biomes linked to the SESs present in the region. In the future, it will be essential to collect a more appropriate statistical sample and to consider all ethnobotanical species (including food) and not just the forest species for domestic use by the four SES. Furthermore, a study should be carried out to assess the current use of te’lom by the SES of the Teenek, in order to determine whether they are transforming the spaces of collective action or not. Despite the above, it can be noted that consistent results were obtained, which are valuable for deepening the analysis of SESs and the conservation of the landscape heritage associated with the biocultural diversity of the Huasteca Potosina.
6. Conclusions
The main question guiding this work was: how important is the performance of an SES for conserving biocultural diversity? To answer this question, we sought to understand the relationship between the landscape heritage concepts of biocultural diversity and the SESs by analyzing the relationships between domestic forest species, biomes, and SESs using conventional ethnobotanical indicators, as well as checking their results through statistical testing.
In the analysis of the SESs, it was found that, in the case of the Pame and the mestizos, a situation critical to the deterioration of biocultural diversity has arisen. Regarding the Teenek and the Nahua, such a vulnerable situation can be overcome through participatory planning and by establishing a strategy for conserving the landscape heritage of biocultural diversity.
Assuming that the information provided was only related to species for domestic use, regarding the ICF, the highest-frequency species were related to cabinetmaking (i.e., to produce domestic furniture) and home construction. Regarding the CII, the most-used species was Cedrela odorata, which allows the production of high-quality and durable furniture, and which has a pleasant aroma due to the characteristic smell of its resin. In terms of the SWI, the low deciduous forest and the tropical semi-evergreen forest presented the highest values, indicating that the main biomes inhabited by the Teenek and the Nahua are those with the most remarkable diversity of forest species for domestic use. However, Cedrela odorata and its associated species’ community were prominent in both biomes, making it a species widely used by the most SESs.
The ANOVA and Tukey tests indicated that the low deciduous forest and tropical semi-evergreen forest biomes had the same diversification value, in agreement with the results of the Shannon–Wiener Index.
According to Ariel de Vidas and Vapnarsky (2016), “… Heritage is, in fact, a social relationship with collective time. Consequently, heritage objects or practices, considered inherited from the past, are closely related to a claimed collective identity. Heritagization is selecting what deserves to be labeled Heritage in the public sphere” [
69].
The conservation of landscape heritage is part of the cultural practices established in the SES. It is transformed with the co-evolution between the ethnic or social group and the biome that it inhabits. In this work, particular emphasis was placed on the importance of understanding the landscape heritage of biocultural diversity, in order to give direction and meaning to a sustainable development strategy for the Huasteca Potosina region.
Socioecological Systems allow for the development of ways of life, in terms of the economic, social, cultural, and political practices that are circumscribed and adapted to a given ecosystem. The ethnic groups are SESs that continue to co-evolve with nature and construct specific biocultural diversity, generating knowledge to select and propagate plants with various uses. However, an SES is characterized by growth, expansion, contraction, and collapse cycles that can affect the ethnic or social group, along with the biocultural diversity. The disappearance of a language, the extinction of an ethnic group, or a radical change in their way of life all impact the development of biological diversity that cannot be saved by greenhouses or by storing genes for industrial purposes. Thus, the importance of understanding the four SESs studied through their historical trajectory, in order to understand the main aspects of transformation in terms of its behavior and its relationship with conserving the landscape heritage of biocultural diversity, is clear.
According to their relationship with the conservation of landscape heritage of biocultural diversity, the SES of the Pame may approach a state of collapse, while the SES of the mestizo may continue with significant losses but may attempt to adapt to the circumstances. From this majority, a group of entrepreneurs who seek to gentrify regional tourism has emerged. The SES of the Teenek currently presents uncertainty under the dramatic changes that make it vulnerable and weak regarding the conservation of biocultural diversity and, finally, the SES of the Nahua also presents a condition of vulnerability. However, the Nahua SES demonstrated the most extraordinary biocultural knowledge and forms the most populous ethnic group. Still, given its trajectory, it can still adapt to circumstances and modify its cultural habits. Finally, the overall loss of biocultural diversity continues.
Thus, the conservation of landscape heritage of biocultural diversity can only be achieved through the ethnic groups continuing to practice their language, culture, and way of life, while improving their opportunities for well-being through sustainable use in the region.
Author Contributions
Conceptualization, M.d.R.P.-L.; methodology, H.A.B.-R., S.L.-V. and M.d.R.P.-L.; formal analysis: M.d.R.P.-L., S.L.-V. and H.A.B.-R.; research and data curation: S.L.-V.; writing—review and editing, M.d.R.P.-L., S.L.-V. and H.A.B.-R.; supervision: M.d.R.P.-L.; project administration, M.d.R.P.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Instituto Politécnico Nacional, IPN, Research Project SIP 20221646 granted by the Secretaría de Investigación y Posgrado, IPN.
Institutional Review Board Statement
Ethical review and approval was not necessary for this study due to the agreement and support of the ejido authorities during the transect walks in the local areas, as well as interviews with people in the communities.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Please refer to Pensado, Mario (2022), “Pensado-Diversity-10-Jul-2022”, Mendeley Data, V1, doi: 10.17632/fsz9zs6hm8.1.
Acknowledgments
The authors would like to thank the key informants in the four locations studied for sharing their knowledge and accompaniment in this field research: Gaspar Ortega Galvan, Lucio Pérez Rivera, and Taurino Martínez Domínguez from Ejido La Estribera; Mtro. Álvaro Hernández Castro and Leopoldo Castro González from Ejido La Palma; Gregorio Elías Martínez and Obispo Hernández Bautista from Ejido El Chuchupe; Ascensión Día Catarino, Bonifacio Rodríguez Pérez, and Gabriel Hernández Cabrera from ejido El Cristiano, Xilitla. We thank all ejido authorities and local people for their support in the transect walks in the production and extraction areas. Thanks, are also due to the Instituto Politécnico Nacional for the support granted to the research project SIP 2022-1646, as well as CONACYT for the financial support granted to Salvador Luna-Vargas, as a scholar of the DCEA’S Doctoral Programme at CIIEMAD, IPN, and to Mario del Roble Pensado-Leglise, for the support of the Sistema Nacional de Investigadores, SNI-CONACYT. H. A. Bustamante-Ramírez, graduate student from the School of Physics and Mathematics of the IPN, thanks the Institutional Social Service System for having participated in this project.
Conflicts of Interest
The authors declare no conflict of interest.
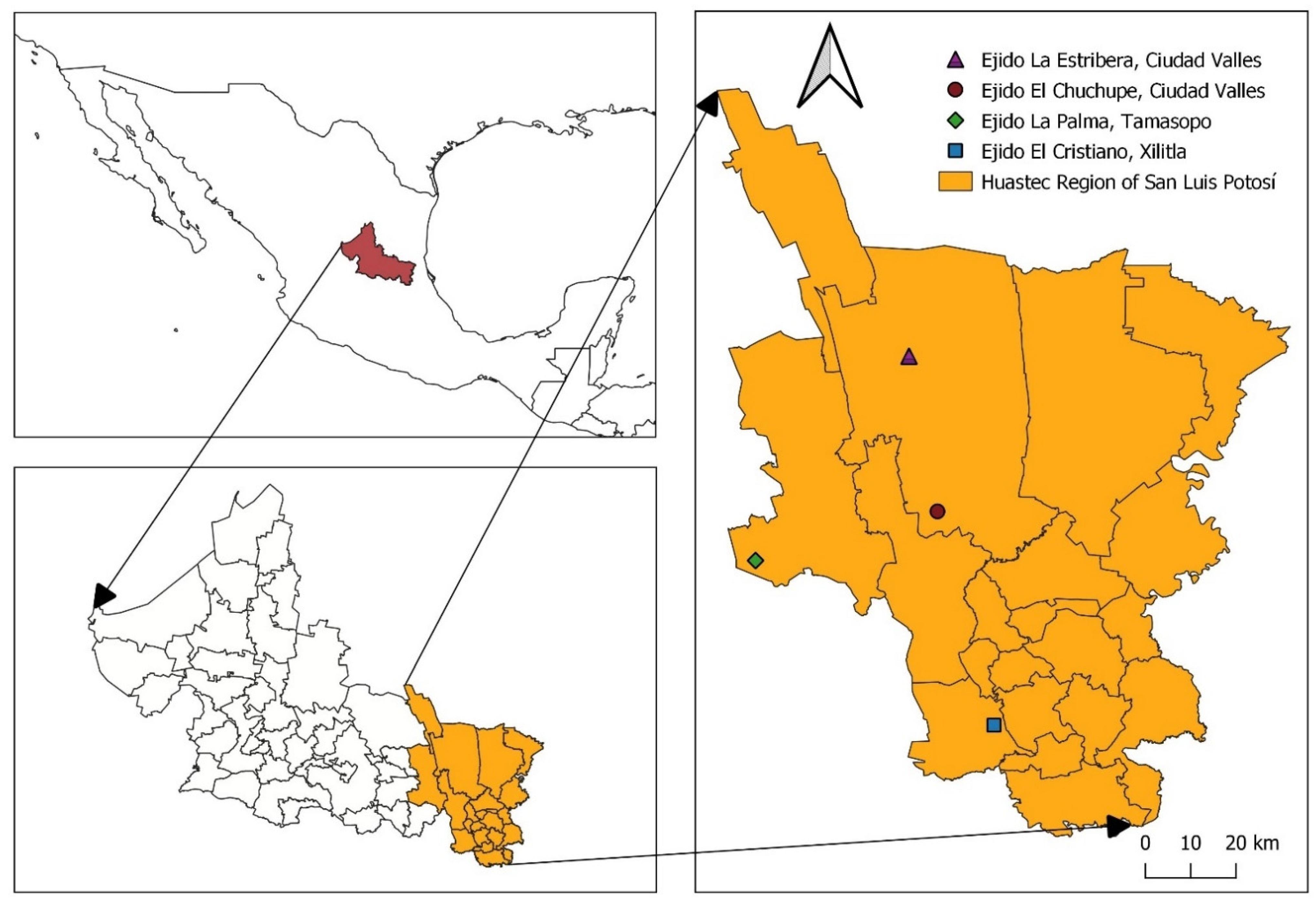
Appendix A
Figure A1.
Map of Huasteca Potosina Region and the Locations of the Four Case Studies. Source: prepared by author SLV, May, 2022.
Figure A1.
Map of Huasteca Potosina Region and the Locations of the Four Case Studies. Source: prepared by author SLV, May, 2022.
Appendix B
Table A1.
Predominant Economic Characteristics in the four SESs of Huasteca Potosina.
Table A1.
Predominant Economic Characteristics in the four SESs of Huasteca Potosina.
| | Teenek | Pame | Nahua | Mestizos |
|---|
| Type of small property | Ejidal and agrarian community | Ejidal | Ejido and private | Ejido and private |
| Size of crop area | Average of less than 5 ha | Average less than 2 ha | Average greater than 5 ha | Average greater than 5 ha |
| Type of crops or activities | Maize, beans, Chilli, squash, tomatoes, ornamental plants; sugar cane for piloncillo; honey; coffee; citrus and fruit trees at the family orchard level. Craft activities. | Maize, beans, Chilli, cactus, and other vegetables; sugar cane for piloncillo and craft activities. | Coffee, maize, beans, squash, Chilli, tomatoes; sugar cane for piloncillo; honey; commercial citrus plantation. Craft activities. | Cattle ranching; sugar cane for sugar mill; commercial citrus and elsewhere lychee plantations. |
| Common use areas | Te’lom from two to three ha and land for everyday use (fruit trees, bee honey, seeds, fruits, and roots). Non-timber forest products. | No everyday use of the land because the entire area is already parceled.
Non-timber forest products: (Brahea Dulcis) palm leaves, chamal seed (Dioon edule). | Common-use land: bee honey, seeds, fruits, and roots; timber forest products such as cedar (Cedrela Odorata), paradise Melia azedarach, and non-timber forest products. | No common-use land exists because the entire area has been parceled out. Timber products: Cedrela odorata, Tabebuia rosea, Piscidia piscipula. |
| Use of milpa | Yes | Yes | Yes | No |
| Home garden | Foodstuffs, any fruits, flowers, and medicinal plants. | Foodstuffs, any fruits, flowers, and medicinal plants. | Foodstuffs, any fruits, flowers, and medicinal plants). | Foodstuffs, any fruits, flowers. |
| Members affiliated with state, regional, and national producer organizations | Yes | Yes | Yes | Yes |
| Regional economic business associations | No | No | Yes | Yes |
| Women organized in local working groups | Yes | No | Few | No |
| Women in community decision-making bodies | Yes | No | No | Yes |
| Indigenous doctors and midwives | Yes | Yes | Yes | No |
| Income from the sale of livestock and livestock products predominantly | Pigs, poultry, and honey | Poultry and swine | Sheep, poultry, pigs, and a few head of cattle | Cattle, sheep and swine, dairy and cheese |
| Tianguis or street traders | Yes | Yes | Yes | Yes |
| Small intermediaries of agricultural, forestry, handicraft, and fishing products or grocery store owners | Few | Few | Few | Majority |
| Self-consumption and income from selling forest and forestry products (sawn wood, natural fiber, flowers, seeds, fruits, edible mushrooms, wild birds) | Yes | Few or none | Yes | No |
| Eventual consumption of aquaculture products | Yes | Yes | Yes | Yes |
| Family income from the sale of handicrafts | Yes | Yes | Yes | No |
| Household income from agricultural, construction, service, and urban informal economy salaried activities | Yes (general labor) | Yes (general labor) | Yes | Yes (specialist or team leader) |
| Management capacity to obtain subsidies and institutional support for private and social purposes | Lower | Lower | Higher | Higher |
Appendix C. Characterization of the Four Case Studies
In this work, four ejidos were selected, corresponding to four cultures (three indigenous and one mestizo) that cohabit the Huasteca Potosina region. These sites also represent at least one of the region’s plant formations with the most significant presence.
The first site corresponds to the ejido “La Estribera” (99°08′09.433″ W 22°10′49.198″ N), a mestizo community or group located northwest of the municipality of Ciudad Valles, parallel to the Sierra de la Colmena, with altitudinal variations from 125–745 m above sea level (masl). The average temperature of the coldest month is 17.7 °C and that of the warmest month is 31.4 °C. The average annual rainfall is 144.70 mm. The predominant vegetation is the low deciduous forest in the highlands, while, in the plains, the forest has been almost entirely replaced by pastureland for extensive cattle ranching and sugar cane monoculture. The dominant soils are lithosols in the highlands and chernozems in the plains. The inhabitants of this ejido are mestizos, and most economically active men are engaged in the agricultural production of sugar cane and small-scale cattle ranching. In 2010, the population of the ejido was 490 inhabitants and 130 houses; however, by 2020, it was reduced to 457 inhabitants and 130 houses (INEGI, 2020 [
70]).
The second site was the ejido “La Palma” (99°27′43.192″ W 21°44′46.125″ N), and a community was selected called “Sabinito de Tepehuajal,” located southwest of the municipality of Tamasopo. This locality is inhabited by the Xi’iuy, or Pame, ethnic group. The average altitude ranges from 782–1050 masl in the highest parts of the ejido. The average temperature in the coldest month is 17.3 °C and in the hottest month it is 30.6 °C. The average annual rainfall is 125.83 mm. The most representative vegetation in the community is the sub-montane scrubland to foothills and, in the higher areas of the common lands, oak forests are located in the transition zone between semi-arid formations and more humid groupings (Puig, 1991). The dominant soil is leptosol. The population is exclusively dedicated to the small-scale production of foodstuffs such as maize and nopal. This locality has only 15 inhabitants, of which 14 are over 60. The migratory phenomenon has caused the population to decrease from 27 inhabitants in 2010 living in eight houses to only 15 in the same eight private dwellings in 2020 (INEGI, 2020).
The third site was the ejido “El Chuchupe” (99°04′31.944″ W21°51′02.156″ N), and the community with the same name was visited. It is located in the area known as the Teenek mountain range, as it is located in a portion of the Sierra Madre Oriental and because it is inhabited by the Teenek or the Huastec ethnic group. This area is located southwest of the municipality of Ciudad Valles. The altitude ranges from 60–734 masl. The average temperature of the coldest month is 18.9 °C, while that of the warmest month is 32.2 °C. The average annual rainfall is 168.25 mm. The vegetation present in the plain is a low deciduous forest between 60–132 masl, almost entirely replaced by pastures induced for cattle grazing, which have become idle land and ruderal vegetation. On the other hand, in the higher portions of the Sierra Madre Oriental, the predominant vegetation is that of the tropical semi-evergreen forest, with some disturbed areas in the common-use lands ranging from 132–715 masl for rainfed agriculture through the slash and burn system. The dominant soil in the Sierra Madre Oriental portion is leptosol. In 2010, there was a population of 141 inhabitants with 39 dwellings. However, the population has decreased as of 2020, with only 128 inhabitants living in 44 private dwellings (an increase of 5 dwellings in ten years). Notably, eight out of ten inhabitants speak an indigenous language (INEGI, 2020). The economically active population is mainly engaged in rainfed agriculture, sugar cane cutting, and the construction industry in the municipal capital (as well as in other cities such as Guadalajara and Monterrey), while women are exclusively engaged in housework.
The fourth site was the ejido “El Cristiano”, where two (very close) Nahua localities called “La Tinaja” (98°57′15.236″ W 21°23′34.262″ N) and “Arroyo Seco” (98°57′20.044″ W 21°23′50.074″ N), belonging to the ejido located in the center-east of the municipality of Xilitla, were visited. The average altitude ranges between 235–492 masl. The average temperature of the coldest month is 12.6 °C, while that in the warmest month is 31.3 °C. The average annual precipitation is 195.54 mm. The most representative vegetation in the two localities is the medium sub-evergreen forest. The dominant soil is represented by a strip of luvisol in the middle of the leptosols. The main economic activity of the population is the family agriculture of coffee (
Coffea arabica) under the shade system of forest species such as
Gliricidia sepium,
Cojoba Arborea,
Inga vera, and
Melia azedarach; these species are almost always promoted for this purpose, replacing the natural vegetation of higher strata. Self-consumption crops such as maize (
Zea mays), beans (
Phaseolus Vulgaris and
Phaseolus coccineus), squash (
Cucurbita spp.), and chili (
Capsicum annuum) are also grown. These localities are ethnically predominant and are part of the Nahua group, which is distributed in the southeastern municipalities of San Luis Potosi. La Tinaja has a population of 438, divided into 249 women, 234 men, and 117 dwellings. The town of Arroyo Seco has a population of 259 inhabitants, divided into 125 women, 134 men, and 65 dwellings (INEGI, 2020). The degree of marginalization ranges from medium in Arroyo Seco to very high in La Tinaja, respectively (CONAPO, 2020 [
71]). This degree of marginalization has contributed to a decrease in the number of inhabitants, due to the phenomenon of migration to the town of Arroyo Seco as, unlike in La Tinaja, the population went from 368 inhabitants in 2010 to 259 in 2020. Meanwhile, in La Tinaja the population increased from 323 to 438 between 2010 and 2020 (115 inhabitants) from 2010 to 2020 (INEGI, 2020).
Sources:
Appendix D
Table A2.
List of Forestal Species Found (Complete Database of List of Forest Species Found Can be Found at doi: 10.17632/fsz9zs6hm8.1).
Table A2.
List of Forestal Species Found (Complete Database of List of Forest Species Found Can be Found at doi: 10.17632/fsz9zs6hm8.1).
| No. | Family | Scientific Name | Common Name | Local Name |
|---|
| 1 | Anacardiaceae | Mangifera indica L. | Mango | Mango |
| 2 | Anacardiaceae | Spondias purpurea L. | Ciruela | Ciruela |
| 3 | Arecaceae | Brahea dulcis (Kunth) Mart.
| Bamel | Palma Loca |
| 4 | Arecaceae | Sabal mexicana Mart.
| Apachite | Palma |
| 5 | Asparagaceae | Yucca treculeana Carrière | Chocha | Samandoque |
| 6 | Asteraceae | Flourensia laurifolia DC.
| Hojancha | Hojancha |
| 7 | Bignoniaceae | Parmentiera aculeata (Kunth) Seem.
| Cuajilote | Chote |
| 8 | Bignoniaceae | Tabebuia rosea (Bertol.) A. DC.
| Macuili | Palo de Rosa |
| 9 | Boraginaceae | Cordia alliodora (Ruiz & Pav.) Oken | Bojón | Tabaquillo |
| 10 | Boraginaceae | Cordia boissieri A. DC.
| Anacahuita | Trompillo |
| 11 | Boraginaceae | Ehretia anacua (Terán & Berland.) I.M. Johnst.
| Anacua | Manzanillo |
| 12 | Burseraceae | Bursera simaruba (L.) Sarg. | Palo mulato | Chaca |
| 13 | Burseraceae | Protium copal (Schltdl. & Cham.) Engl.
| Copal | Copal |
| 14 | Euphorbiaceae | Cnidoscolus multilobus (Pax) I.M. Johnst.
| Mala mujer | Ortiga |
| 15 | Fabaceae | Enterolobium cyclocarpum (Jacq.) Griseb.
| Guanacaste | Orejón |
| 16 | Fabaceae | Erythrina americana Mill.
| Colorín | Pemoche |
| 17 | Fabaceae | Gliricidia sepium (Jacq.) Kunth ex Walp.
| Cocuite | Palo de sol |
| 18 | Fabaceae | Harpalyce arborescens A. Gray | Palo de Brasil | Chicharrillo |
| 19 | Fabaceae | Havardia pallens (Benth.) Britton & Rose | Tenaza | Tenaza |
| 20 | Fabaceae | Lysiloma acapulcense (Kunth) Benth.
| Tepehuaje | Tepeguaje |
| 21 | Fabaceae | Lysiloma divaricatum (Jacq.) J.F. Macbr.
| Palo blanco | Rajador |
| 22 | Fabaceae | Piscidia piscipula (L.) Sarg.
| Jabín | Chijol |
| 23 | Fabaceae | Prosopis glandulosa Torr.
| Mezquite | Mezquite |
| 24 | Fabaceae | Senna atomaria (L.) H.S. Irwin & Barneby | Palo zorrillo | Palo hediondo |
| 25 | Fabaceae | Tamarindus indica L. | Tamarindo | Tamarindo |
| 26 | Fagaceae | Quercus laeta Liebm.
| Encino blanco | Encino blanco |
| 27 | Fagaceae | Quercus polymorpha Schltdl. & Cham.
| Encino prieto | Encino prieto |
| 28 | Fagaceae | Quercus xalapensis * Bonpl.
| Encino colorado | Encino colorado |
| 29 | Lauraceae | Ocotea tampicensis (Meisn.) Hemsl.
| Laurelillo | Aguacatillo |
| 30 | Malvaceae | Ceiba pentandra (L.) Gaertn. | Ceiba | Ceiba |
| 31 | Malvaceae | Guazuma ulmifolia Lam.
| Guásima | Aquiche |
| 32 | Malvaceae | Heliocarpus donnellsmithii Rose | Jonote Blanco | Jonote |
| 33 | Meliaceae | Cedrela odorata * L. | Cedro | Cedro |
| 34 | Moraceae | Brosimum alicastrum Sw.
| Ramón | Ojite |
| 35 | Moraceae | Ficus benjamina L. | Ficus | Ficus |
| 36 | Moraceae | Maclura tinctoria (L.) D. Don ex Steud. | Mora | Mora |
| 37 | Poaceae | Guadua velutina Londoño & L.G. Clark | | Otate |
| 38 | Rhamnaceae | Colubrina elliptica (Sw.) Brizicky & W.L. Stern | Amole | Guayacán |
| 39 | Rhamnaceae | Karwinskia humboldtiana (Schult.) Zucc.
| Tullidora | Chanchanope |
| 40 | Rutaceae | Esenbeckia berlandieri Baill. Ex Hemsl.
| Hueso de tigre | Hueso de tigre |
| 41 | Salicaceae | Neopringlea integrifolia (Hemsl.) S. Watson | Palo estaca | Palo varilla |
| 42 | Salicaceae | Zuelania guidonia (Sw.) Britton & Millsp.
| Anona de Llano | Volantín |
| 43 | Sapotaceae | Manilkara zapota (L.) P. Royen | Chico Zapote | Chico |
| 44 | Sapotaceae | Pouteria sapota (Jacq.) H.E. Moore & Stearn | Mamey | Mamey |
| 45 | Ulmaceae | Phyllostylon rhamnoides (J. Poiss.) Taub.
| Cerón | Cerón |
| 46 | Fabaceae | Inga vera Willd.
| Chalahuite | Chalahuite |
| 47 | Fabaceae | Inga jinicuil Schltdl.
| Chalahuite | Chalahuite |
| 48 | Rubiaceae | Coffea arabica L. | Cafeto | Café |
| 49 | Meliaceae | Melia azedarach L. | Lila | Paraíso |
| 50 | Pending | Pending | Pending | Jarrilla |
| 51 | Malvaceae | Carpodiptera cubensis Griseb.
| Alzaprima | Rama de casa |
| 52 | Cannabaceae | Trema micrantha (L.) Blume | Capulín | Puam |
| 53 | Malvaceae | Heliocarpus appendiculatus Turcz.
| Jonote rojo | Jonote |
| 54 | Rutaceae | Citrus x aurantium L. | Naranja agria | Naranja agria |
| 55 | Rubiaceae | Exostema mexicanum A. Gray | Cascarillo | Hierbamaiz |
| 56 | Pending | Pending | Pending | Algodoncillo |
| 57 | Myrtaceae | Psidium guajava L. | Guayaba | Guayaba |
| 58 | Lauraceae | Persea americana Mill.
| Aguacate | Aguacate |
| 59 | Sapindaceae | Litchi chinensis Sonn.
| Lichi | Litche |
| 60 | Rutaceae | Citrus x limon (L.) Osbeck | Limón | Limón |
| 61 | Piperaceae | Piper auritum Kunth | Hierba Santa | Hierba Santa |
| 62 | Convolvulaceae | Ipomoea dumosa (Benth.) L.O. Williams | Quélite | Soyo |
| 63 | Lamiaceae | Hyptis verticillata Jacq.
| Huapazotl | Epazotillo |
| 64 | Rubiaceae | Hamelia patens Jacq.
| Coralillo | Madura plátano |
| 65 | Fabaceae | Cojoba arborea (L.) Britton & Rose | Aguacillo | Frijolillo |
| 66 | Poaceae | Saccharum officinarum L. | Caña | Caña |
| 67 | Pending | Pending | Pending | San Isidro |
| 68 | Asparagaceae | Cordyline fruticose (L.) A. Chev. | Banderilla | Banderilla |
| 69 | Rutaceae | Murraya paniculata (L.) Jack | Limonaria | Limonaria |
| 70 | Rosaceae | Eriobotrya japonica (Thunb.) Lindl.
| Níspero chino | Níspero |
| 71 | Sapindaceae | Cupania dentata DC.
| Cuisal | Cojolite |
| 72 | Euphorbiaceae | Croton draco Schltdl. & Cham.
| Drago | Sangre de grado |
| 73 | Pending | Pending | Pending | Cantarillo |
| 74 | Pending | Pending | Pending | Grevilia |
| 75 | Sapotaceae | Pouteria glomerata (Miq.) Radlk.
| Zapote prieto | Zocohuite |
| 76 | Pending | Pending | Bejuco de casa | Kakmekatl |
References
- Fowler, P.J. World Heritage Cultural Landscapes, 1992–2002. In World Heritage Papers 6; World Heritage Paper Series No. 6. World Heritage Cultural Landscapes; UNESCO: Paris, France, 2003; Available online: http://whc.unesco.org/documents/publi_wh_papers_06_en.pdf (accessed on 11 March 2022).
- UNESCO Universal Declaration on Cultural Diversity Adopted by the 31st Session of the General Conference of UNESCO on 2 November 2001. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000127162_spa (accessed on 10 March 2022).
- Rössler, M. Linking Forests and Cultural Diversity: The World Heritage Convention. In Forestry and Our Cultural Heritage, Proceedings of the Seminar, Sunne, Sweden, 13–15 June 2005; Ministerial Conference on the Protection of Forests in Europe: Warsaw, Poland, 2005; pp. 13–22. ISBN 108392239636. Available online: https://foresteurope.org/wp-content/uploads/2016/08/heritage.pdf (accessed on 10 March 2022).
- Eriksson, O. Coproduction of Food, Cultural Heritage and Biodiversity by Livestock Grazing in Swedish Semi-natural Grasslands. Front. Sustain. Food Syst. 2022, 6, 801327. [Google Scholar] [CrossRef]
- Pensado-Leglise, M.; Galván, C.; Marel, D. Chapter 4: El desarrollo y la sustentabilidad en el entorno multilateral de los problemas ambientales. In Apologías de la Sustentabilidad en el Siglo, 21st ed.; Carrasco, R., Cantú, R., Eds.; Altres Costa-Amic Editores: Puebla, México, 2018; pp. 103–127. [Google Scholar]
- Berkes, F.; Jolly, D. Adapting to climate change: Social-ecological resilience in a Canadian western Arctic community. Conserv. Ecol. 2001, 5, 18. Available online: https://www.ecologyandsociety.org/vol5/iss2/art18/ (accessed on 14 March 2022). [CrossRef]
- Peña-Puch, A.; Pérez-Jiménez, J.C.; Munguía-Gil, A.; Espinoza-Tenorio, A. Sistemas socio-ecológicos como unidad de manejo: El caso de las pesquerías de Campeche, México. Econ. Soc. Territ. 2021, 21, 113–145. [Google Scholar] [CrossRef]
- Collins, S.L.; Carpenter, S.R.; Swinton, S.M.; Orenstein, D.E.; Childers, D.L.; Gragson, T.L.; Grimm, N.B.; Grove, J.M.; Harlan, S.L.; Kaye, J.P.; et al. An integrated conceptual framework for long-term social-ecological research. Front. Ecol. Environ. 2010, 9, 351–357. Available online: https://www.fs.usda.gov/treesearch/pubs/39928 (accessed on 12 March 2022). [CrossRef]
- Folke, C. Resilience: The emergence of a perspective for social-ecological systems analyses. Glob. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]
- Challenger, A.; Córdova, A.; Chavero, E.L.; Equihua, M.; Maass, M. Opportunities and Obstacles to Socioecosystem-based Environmental Policy in Mexico: Expert Opinion at the Science-policy Interface. Ecol. Soc. 2018, 23, 31. Available online: www.ecologyandsociety.org/vol23/iss2/art31/ (accessed on 12 June 2021).
- Batabyal, A.; Kourtit, K. An Analysis of Resilience in Complex Socioeconomic Systems; Munich Personal RePEc Archive, MPRA: Munich, Germany, 2021; p. 105197. Available online: https://mpra.ub.uni-muenchen.de/105197/ (accessed on 11 March 2022).
- Levin, S.; Xepapadeas, T.; Crépin, A.-S.; Norberg, J.; de Zeeuw, A.; Folke, C.; Hughes, T.; Arrow, K.; Barrett, S.; Daily, G.; et al. Social-ecological systems as complex adaptive systems: Modeling and policy implications. Environ. Dev. Econ. 2012, 18, 111–132. [Google Scholar] [CrossRef]
- Holling, C.S. Understanding the Complexity of Economic, Ecological, and Social Systems. Ecosystems 2001, 4, 390–405. [Google Scholar] [CrossRef]
- Sauer, C.O. The Morphology of Landscape; University of California Publications in Geography: Oakland, CA, USA, 1925; Volume 2, pp. 19–53, reprinted in Land and Life: A Selection from the Writings of Carl Ortwin Sauer; Leighley, J., Ed.; University of California Press: Berkeley, CA, USA, 1963. [Google Scholar]
- Hanspach, J.; Haider, L.J.; Oteros-Rozas, E.; Olafsson, A.S.; Gulsrud, N.M.; Raymond, C.M.; Torralba, M.; Martín-López, B.; Bieling, C.; García-Martín, M.; et al. Biocultural approaches to sustainability: A systematic review of the scientific literature. People Nat. 2020, 2, 643–659. [Google Scholar] [CrossRef]
- Ávila-Meléndez, L.; Pensado-Leglise, M.; Mendoza-Magallón, L. Aportes del Enfoque de la Geografía Cultural del Paisaje Para las Políticas de Desarrollo Territorial en México; Revista Controle Social e Desenvolvimento Territorial, Fundación Getulio Vargas, UFRRJ y Universidad Federal do Tocantins, Brasil Diciembre, DOSSIÊ TEMÁTICO: Direcionalidades emergentes das políticas de desenvolvimento territorial frente aos impactos da Pandemia na América Latina; 2020; Volume 6, pp. 108–131. ISBN 25271253. Available online: https://sistemas.uft.edu.br/periodicos/index.php/csdt/issue/view/514 (accessed on 12 March 2022).
- Puig, H. Vegetación de la Huasteca, México: Estudio Fitogeográfico y Ecológico; Institut de Recherche pour le Développement en Coopération (ORSTOM); Instituto de Ecología, A.C., Ed.; Centre d’Études Mexicaines et Centraméricaines (CEMCA): Mexico City, Mexico, 1991; Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers20-08/36275.pdf (accessed on 11 March 2022).
- Rzedowski, J. Diversidad y Origenes de la Flora Fanerogámica de México; Trueba, C., Ed.; Revista Ciencias UNAM: Mexico City, Mexico, 1992; Volume 6, pp. 3–21. [Google Scholar]
- Hudson, P. Geomorphic context of the prehistoric Huastec floodplain environments: Lower Panuco basin, Mexico. J. Archaeol. Sci. 2004, 31, 653–668. [Google Scholar] [CrossRef]
- Arriaga, L.; Espinoza, J.; Aguilar, C.; Martínez, E.L.G.; Loa, E. Regiones Terrestres Prioritarias de México; Comisión Nacional para el Conocimiento y uso de la Biodiversidad: Mexico City, Mexico, 2000; Available online: https://iefectividad.conanp.gob.mx/i-efectividad/FSIyPS/RB%20Volc%C3%A1n%20Tacan%C3%A1/1%20ATRIBUTOS%20DEL%20ANP/REGION%20TERRESTRE%20PRIORITARIA%20TACANA.pdf (accessed on 12 March 2022).
- Cendejas, G.H.; Avalos, A.; Urquijo, P. El te’lom ¿una alternativa a la deforestación en La Huasteca? Análisis de un sistema agroforestal entre los teenek potosinos. In Etnoagroforestería en México; Moreno-Calles, A., Casas, A., Toledo-Manzur, V.M., Vallejo-Ramos, M., Eds.; ENES-Morelia/IIES, UNAM: Morelia, México, 2016; pp. 71–91. [Google Scholar]
- Villaseñor, J.L. Checklist of the Native Vascular Plants of Mexico. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- De Nova-Vázquez, J.A. La diversidad florística potosina, un patrimonio que debemos conservar. Univ. Potos. 2018, 223, 4–10. [Google Scholar]
- Reyes, H.; Aguilar, M.A.; Trejo, V. Cambios en la cubierta vegetal y uso del suelo en el área del proyecto Pujal-Coy, San Luis Potosí, México 1973–2000. Investig. Geográficas Boletín Inst. Geogr. 2006, 59, 26–42. [Google Scholar]
- Barthas, B. De la selva al naranjal (transformaciones de la agricultura indígena en la Huasteca potosina). In El Campo Mexicano: Una Modernización a Marchas Forzadas D.F.; Bovin, P., Ed.; Centro Francés de Estudios Mexicanos y Centroamericanos (CEMCA): Mexico City, México, 1996; pp. 183–199. [Google Scholar]
- Leija, E.; Reyes, H.; Fortanelli, J.; Palacio, G. Situación actual del bosque de niebla en el estado de San Luis Potosí, México. Investig. Cienc. 2011, 53, 3–11. [Google Scholar]
- Ford, A. Dominant plants of the Maya Forest and gardens of El Pilar: Implications or paleoenvironmental reconstructions. J. Ethnobiol. 2008, 28, 179–199. [Google Scholar] [CrossRef]
- Johansson, P. La imagen del huasteco en el espejo de la cultura náhuatl prehispánica. Estud. Cult. Náhuatl 2012, 44, 65–133. [Google Scholar]
- Urquijo, P. Paisaje, Territorio y Paisaje Ritual: La Huasteca Potosina. Estudio de Geografía Histórica. Master’s Thesis, Universidad Michoacana, Morelia, México, 2008. [Google Scholar]
- Stresser-Péan, G. Viaje a la Huasteca con Guy Stresser-Péan. Distrito Federal: Fondo de Cultura Económica (FCE); Centro de Estudios Mexicanos y Centro Americanos (CEMCA): Mexico City, Mexcio, 2008. [Google Scholar]
- Aquiles, M. Teenek Huastecos de San Luis Potosí. Proyecto: Perfiles Indígenas de México, Documento de Trabajo; 2008; México; p. 48. Available online: https://www.aacademica.org/salomon.nahmad.sitton/50 (accessed on 14 March 2022).
- Cendejas, G.H. Tenek Lab Teje. Etnicidad y Transformaciones Agrarias en el Ejido de la Concepción, Tanlajas, San Luís Potosí. Master’s Thesis, El Colegio de San Luis, San Luis Potosí, México, 2007. [Google Scholar]
- Salinas, M.C. Rebelión Indígena en la Huasteca Potosina, 1879–1882. In Colección “Documentos de Investigación”; El Colegio Mexiquense: Toluca, México, 2003; p. 24. [Google Scholar]
- Jabardo Pereda, V. La Lucha por la tierra en la Huasteca Potosina (México): De Peones A Patrones. Rev. Investig. Geográf. Univ. Alicante 2016, 65, 153–168. [Google Scholar] [CrossRef][Green Version]
- Silva, J.A.R. Pames, franciscanos y estancieros en Rioverde, Valles y sur de Nuevo Santander, 1600–1800. Relaciones 2009, 30, 225–266. [Google Scholar]
- INPI (Instituto Nacional de los Pueblos Indígenas). Pames/Giomar Ordóñez Cabezas—México: CDI: PNUD. 2004; 31p, ISBN 970-753-027-8. Available online: https://www.gob.mx/inpi/documentos/monografia-de-los-pames (accessed on 16 March 2022).
- Luna, S.; Valderrábano, M.; Suárez, I.; Alcérreca, L. Methodological Proposal to Evaluate Touristic Activity with Local Sustainability Criteria in the Hydrographic Sub-Basins of the Huasteca Potosina, Mexico. In Sustainable Development Research and Practice in Mexico and Selected Latin American Countries; Leal, W., Noyola-Cherpitel, R., Medellín-Milán, P., Ruiz, V., Eds.; Springer: Manchester, UK, 2018; pp. 217–240. [Google Scholar] [CrossRef]
- Alcorn, J. Economic Botany, Conservation, and Development: What is the Connection. Ann. Mo. Bot. Gard. 1995, 82, 34–46. [Google Scholar] [CrossRef]
- Toledo, V.M.; Ortiz-Espejel, B.; Cortés, L.; Moguel, P.; Ordoñez, M.D.J. The multiple uses of tropical forests by indigenous peoples in Mexico: A case of adaptive management. Conserv. Ecol. 2003, 7, 9. Available online: http://www.consecol.org/vol7/iss3/art9/ (accessed on 12 March 2022). [CrossRef]
- Ávila-Uribe, M.; Suárez-Soto, M.L.; Díaz-Perea, J. Campesinos Tének en una comunidad campesina rural de la Huasteca Potosina complementan su dieta básica con plantas locales. Boletín Soc. Bot. Méx. 1994, 54, 3–15. [Google Scholar] [CrossRef]
- Vidal, O.; Brusca, R.C. Mexico’s Biocultural Diversity in Peril. Rev. Biol. Trop. 2020, 68, 669–691. [Google Scholar] [CrossRef]
- Alcorn, J. Development Policy, Forests, and Peasant Farms: Reflections on Huastec-Managed Forests’ Contributions to Commercial Production and Resource Conservation. Rev. Econ. Bot. 1984, 38, 396. [Google Scholar] [CrossRef]
- De Micheli, A.; Izaguirre-Ávila, R. De la herbolaria medicinal novohispana a los inicios de estudios botánico-farmacológicos sistematizados (bosquejo histórico). Arch. Cardiol. Méx. 2009, 79 (Suppl. S2), 95–101. [Google Scholar] [PubMed]
- Luna-Vargas, S.; Pensado-Leglise, M.R.; Godínez-Vizuet, M.R.O. Aprovechamiento Forestal Sostenible y Conservación del Patrimonio Paisajístico en la Huasteca Potosina. In Proceedings of the 4° Congreso Internacional de la Red de Medio Ambiente, Cuernavaca, Morelos, Mexico, 7 October 2021. [Google Scholar]
- Guber, R. La Etnografía, Método, Campo y Reflexividad; Grupo Editorial Norma: Bogotá, Colombia, 2001. [Google Scholar]
- Begossi, A. Use of Ecological Methods Diversity Indices. Econ. Bot. 1996, 50, 280–289. [Google Scholar] [CrossRef]
- Reyes-Garcia, V.; Huanca, T.S.; Vadez, V.; Leonard, W.; Wilkie, D. Cultural—Practical—And Economic Value Of Wild Plants: A Quantitative Study in The Bolivian Amazon. Econ. Bot. 2006, 60, 62–74. [Google Scholar] [CrossRef]
- Castañeda, R.; Albán, J. Importancia cultural de la flora silvestre del distrito de Pamparomás, Ancash, Perú. In Revista Ecología Aplicada; Universidad Nacional Agraria La Molina: Lima, Perú, 2016; Volume 15, p. 2. [Google Scholar]
- Tardio, J.; Pardo-de-Santayana, M. Cultural importance indices: A comparative analysis of the useful wild plants of southern Cantabria (northern Spain). Econ. Bot. 2008, 62, 24–39. [Google Scholar] [CrossRef]
- Phillips, O.; Gentry, A.H. The Useful Plants of Tambopata, Peru, I. Statistical Hypotheses Tests with a New Quantitative Technique. Econ. Bot. 1993, 47, 15–32. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Tao, Y.; Jun, Y.; Qiu, Z.-C.; Ding, X.-Y.; Wang, Y.-H.; Wang, Y.-H. Wild plants are used by the Lhoba people in Douyu Village, characterized by high mountains and valleys, in southeastern Tibet, China. J. Ethnobiol. Ethnomed. 2021, 17, 46. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Tukey’s Honestly Significant Difference (HSD) Test. In Encyclopedia of Research Design; Salkind, N., Ed.; SAGE: Thousand Oaks, CA, USA, 2010; Available online: https://personal.utdallas.edu/~Herve/abdi-HSD2010-pretty.pdf (accessed on 16 March 2022).
- Prance, G.T. Ethnobotany and the future of conservation. Biologist 2000, 47, 65–68. Available online: https://pubmed.ncbi.nlm.nih.gov/11190230/ (accessed on 17 March 2022). [PubMed]
- Salick, J.; Biun, A.; Martin, G.; Apin, L.; Beaman, R. Whence useful plants? A direct relationship between biodiversity and useful plants among the Dusun of Mt. Kinabalu. Biodivers. Conserv. 1999, 8, 797–818. [Google Scholar] [CrossRef]
- Salick, J.; Anderson, D.; Woo, J.; Sherman, R.; Cili, N.; Ana; Dorje, S. Tibetan ethnobotany and gradient analyses: Menri (Medicine mountains), Eastern Himalayas. In Bridging Scales and Epistemologies: Linking Local Knowledge and Global Science in Multi-Scale Assessments; Millennium Ecosystem Assessment, Ed.; Millennium Ecosystem Assessment: Alexandria, Egypt, 2004; Available online: https://www.millenniumassessment.org/documents/bridging/papers/salick.jan.pdf (accessed on 15 March 2022).
- Heindorf, C.; Reyes-Agüero, J.A.; Fortanelli-Martínez, J.; Van Hooft, A. More than Maize, Bananas, and Coffee: The Inter– and Intraspecific Diversity of Edible Plants of the Huastec Mayan Landscape Mosaics in Mexico. Econ. Bot. 2021, 75, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Heindorf, C.; Reyes-Agüero, J.; van Hooft, A.; Fortanelli-Martínez, J. Inter and Intraspecific Edible Plant Diversity of the Tének Milpa Fields in Mexico. Econ. Bot. 2019, 73, 158–174. [Google Scholar] [CrossRef]
- Manuel, T.V. La diversidad biológica de México. Nuevos retos para la investigación en los noventas. Ciencias 1994, 34, 42–59. [Google Scholar]
- Weckerle, C.S.; Huber, F.K.; Yongping, Y.; Weibang, S. Plan Knowledge of the Shuhi in the Hengduan Mountains, Southwest China. Econ. Bot. 2006, 60, 3–23. [Google Scholar] [CrossRef]
- Saqib, Z.; Malik, R.N.; Shinwari, M.I.; Shinwari, Z.K. Species richness, ethnobotanical species richness and human settlements along a Himalayan altitudinal gradient: Prioritizing plant conservation in Palas Valley, Pakistan. Pak. J. Bot. 2011, 43, 129–133. [Google Scholar]
- Alexis, B.; María, A.O.-M.; Dilia, V. La Investigación etnobotánica sobre plantas medicinales: Una revisión de sus objetivos y enfoques actuales. Interciencia 2005, 30, 453–459. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0378-18442005000800005&lng=es (accessed on 15 March 2022).
- Zent, S.; Zent, E. Ethnobotanical Convergence, Divergence, and Change among the Hotï of the Venezuelan Guayana. Adv. Econ. Bot. 2004, 15, 37–78. [Google Scholar]
- Kessler, M. Bosques de Polylepis. In Botánica Económica de los Andes Centrales; Moraes, M., Ollgaard, B., Kvist, L.P., Borchsenius, F., Balslev, H., Eds.; Universidad Mayor de San Andres: La Paz, Bolivia, 2006; pp. 110–120. [Google Scholar]
- Chao, A.; Chiu, C.H.; Jost, L. Phylogenetic diversity measures based on Hill numbers. Philos. Trans. R. Soc. Biol. Sci. 2010, 365, 3599–3609. [Google Scholar] [CrossRef] [PubMed]
- Jost, L. ¿Qué entendemos por diversidad? El camino hacia la cuantificación. Mètode Sci. Stud. J. 2018, 98, 39–45. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Ding, Y. Phylogeny of the Stipa and implications for grassland evolution in China: Based on biogeographic evidence. Biogeosciences 2018, 1–19. [Google Scholar] [CrossRef]
- Feng, H.; Squires, V.R. Socio-Environmental Dynamics of Alpine Grasslands, Steppes and Meadows of the Qinghai–Tibetan Plateau, China: A Commentary. Appl. Sci. 2020, 10, 6488. [Google Scholar] [CrossRef]
- Hoffman, B.; Gallaher, T. Importance Indices in Ethnobotany. Ethnobot. Res. Appl. 2007, 5, 201–218. [Google Scholar] [CrossRef]
- de Vidas, A.A.; Vapnarsky, V. Culture: Modes d’emploi. La patrimonialisation à l’épreuve du terrain. Colloque Final du Projet ANR FABRIQ’AM-La Fabrique des Patrimoines Dans les Amériques Indiennes Aujourd’hui, 30 May–1 June 2016. [CrossRef]
- INEGI. Censo de Población y Vivienda 2020. Obtenido de Conjunto de Datos ITER. 2020. Available online: https://www.inegi.org.mx/programas/ccpv/2020/?ps=microdatos#:~:text=Los%20productos%20Principales%20resultados%20por%20localidad%20%28ITER%29%20y,las%20cabeceras%20municipales%2C%20independientemente%20del%20n%C3%BAmero%20de%20habitantes (accessed on 18 March 2022).
- CONAPO. Consejo Nacional de Población. Obtenido de Índice de Marginación por Localidad. 2020. Available online: https://www.gob.mx/conapo/documentos/indices-de-marginacion-2020-284372 (accessed on 18 March 2022).
Table 1.
Profiles of four SESs of Huasteca Potosina.
Table 1.
Profiles of four SESs of Huasteca Potosina.
| | Teenek | Nahua | Pame | Mestizo |
|---|
| Case Studies | Ejido El Chuchupe (60–734 masl) | Ejido El Cristiano, La Tinaja y El Arroyo Seco (235–492 masl) | Ejido La Palma, El Sabinito de Tepehuajal (782–1050 masl) | Ejido la Estribera (125–745 masl) |
| Type of Biome | Low deciduous forest and tropical semi-evergreen forest | Low deciduous forest and tropical semi-evergreen forest; small areas of oak forest. | Sub-montane scrubland (450–900 masl); small areas of oak forest. | Agro-industrial systems (sugar cane, citric); others (grasslands). |
| Continuous Operation | Families/ejidos/agrarian communities | Families/ejidos/agrarian communities | Families/ejidos | Ejidos |
| Discontinuous Operation | Crisis | Conflicts | Permanent family out-migration/land leasing | Economic crisis/social decomposition (−) and/or social mobility (+) |
| Complementarity Endogenous Subsystems | Milpa, te’lom, home garden, cultivated land | Milpa, orchard, home garden, cultivated land, services | Milpa, Cultivated land, home garden | Cultivated land, home garden, Services/gentrification of indigenous areas (tourism) |
| Complementarity Exogenous Subsystems | Intra- and extra-regional wage works | Control of teams of wage workers, agricultural, and service activities | Intra- and extra-regional wage works | Management of groups of wage workers, agricultural, and service activities |
| Street commerce | Small and street commerce | Street trade | All levels of trade |
| Cultural Resilience | Family language and culture | Language, social-local culture | The absolute decrease in families | Individuality peri-urban society |
| Biological Diversity | Rich, but weak, in transition | Rich, but weak, in transition | Reduced, eroded lands | Disappearing biodiversity: commercial monocultures, fruit trees, and grazing land |
| Redundancy | Family/indigenous/agrarian identity | Identity/indigenous/agrarian-familial | Family identity | Social identity, local |
| Modularity | Different sociocultural dynamics in each locality | Different sociocultural dynamics in each locality | Housing defection | Social peri-urban living |
| Integrality: Landscape Heritage of Biocultural Diversity | Vulnerable/greater weakness/uncertainty | Vulnerable/weakness/adaptation | At risk (population decline) | At risk (acculturation) |
Table 2.
Number of species for each use by SES of Huasteca Potosina.
Table 2.
Number of species for each use by SES of Huasteca Potosina.
| | SES
Teenek | SES
Nahua | SES
Pame | SES
Mestizo |
|---|
| Cabinetmaking (CM) | 3 | 3 | 4 | 1 |
| Firewood or fuel (F) | 6 | 7 | 4 | 6 |
| Tools (T) | 3 | 3 | 2 | 2 |
| Medicinal or ritual (R) | 3 | 14 | 0 | 0 |
| Shade or shelter (S) | 5 | 9 | 0 | 0 |
| Food (Fo) | 4 | 11 | 2 | 3 |
| Construction (C) | 18 | 14 | 18 | 15 |
Table 3.
Informant Consensus Factor (ICF) results.
Table 3.
Informant Consensus Factor (ICF) results.
| ICF |
|---|
| Cabinetmaking (CM) | 0.4 |
| Firewood or fuel (F) | 0.1 |
| Tools (T) | 0.2 |
| Medicinal or ritual (R) | 0.06 |
| Shade or shelter (S) | 0 |
| Food (Fo) | 0.1 |
| Construction (C) | 0.3 |
Table 4.
Cultural Importance Index (CII) results.
Table 4.
Cultural Importance Index (CII) results.
| CII |
|---|
| Cedrela odorata | 2.3 |
| Gliricidia sepium (Jacq.) Steud. | 1.3 |
| Havardia pallens (Benth.) Britton and Rose | 1.3 |
| Ocotea tampicensis (Meisn.) Hemsl. | 1.3 |
| Guazuma ulmifolia Lam. | 1.3 |
Table 5.
Shannon–Wiener Biodiversity Index (SWI) results.
Table 5.
Shannon–Wiener Biodiversity Index (SWI) results.
| SWI |
|---|
| Low deciduous forest | 3.4 |
| Tropical semi-evergreen forest | 3.4 |
| Oak forest | 1.1 |
| Sub-montane scrubland | 1.4 |
| Agroforestry system | 2.6 |
| Others | 0.7 |
Table 6.
Shannon–Wiener Biodiversity Index (SWI) statistics by biome.
Table 6.
Shannon–Wiener Biodiversity Index (SWI) statistics by biome.
| | Mean | Var | St. Dev. | Interval |
|---|
| Low deciduous forest | 0.4079 | 0.2447 | 0.4947 | (0.3148, 0.5010) |
| Tropical semi-evergreen forest | 0.3947 | 0.2421 | 0.4920 | (0.3022, 0.4873) |
| Oak forest | 0.0394 | 0.0384 | 0.1960 | (0.0026, 0.0763) |
| Sub-montane scrubland | 0.0526 | 0.0505 | 0.2248 | (0.0103, 0.0949) |
| Agroforestry system | 0.1710 | 0.1436 | 0.3791 | (0.0997, 0.3791) |
| Others | 0.0263 | 0.0259 | 0.1611 | (−0.0040, 0.0566) |
Table 7.
Statistical summary.
Table 7.
Statistical summary.
| Biome | Count | Sum | Mean | Var |
|---|
| Low deciduous forest | 76 | 31 | 0.407894737 | 0.24473684 |
| Tropical semi-evergreen forest | 76 | 30 | 0.394736842 | 0.24210526 |
| Oak forest | 76 | 3 | 0.039473684 | 0.03842105 |
| Sub-montane scrubland | 76 | 4 | 0.052631579 | 0.05052632 |
| Agroforestry system | 76 | 13 | 0.171052632 | 0.14368421 |
| Others | 76 | 2 | 0.026315789 | 0.02596491 |
Table 8.
ANOVA statistical test results.
Table 8.
ANOVA statistical test results.
| F | Probability | Critical Value for F |
|---|
| 19.2928 | 2.05738 × 10−17 | 2.2340 |
Table 9.
Tukey value results.
Table 9.
Tukey value results.
| | Low Deciduous Forest | Tropical Semi-evergreen Forest | Oak Forest | Sub-Montane Scrubland | Agroforestry | Others |
|---|
| Low deciduous forest | | 0.01316 | 0.36842 | 0.35526 | 0.23684 | 0.38158 |
| Tropical semi-evergreen forest | | | 0.35526 | 0.34211 | 0.22368 | 0.36842 |
| Oak forest | | | | −0.01316 | −0.13158 | 0.01316 |
| Sub-montane scrubland | | | | | −0.11842 | 0.02632 |
| Agroforestry | | | | | | 0.14474 |
| Others | | | | | | |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).