Current and Future Distribution Modeling of Socotra Cormorants Using MaxEnt
Abstract
1. Introduction
2. Materials and Methods
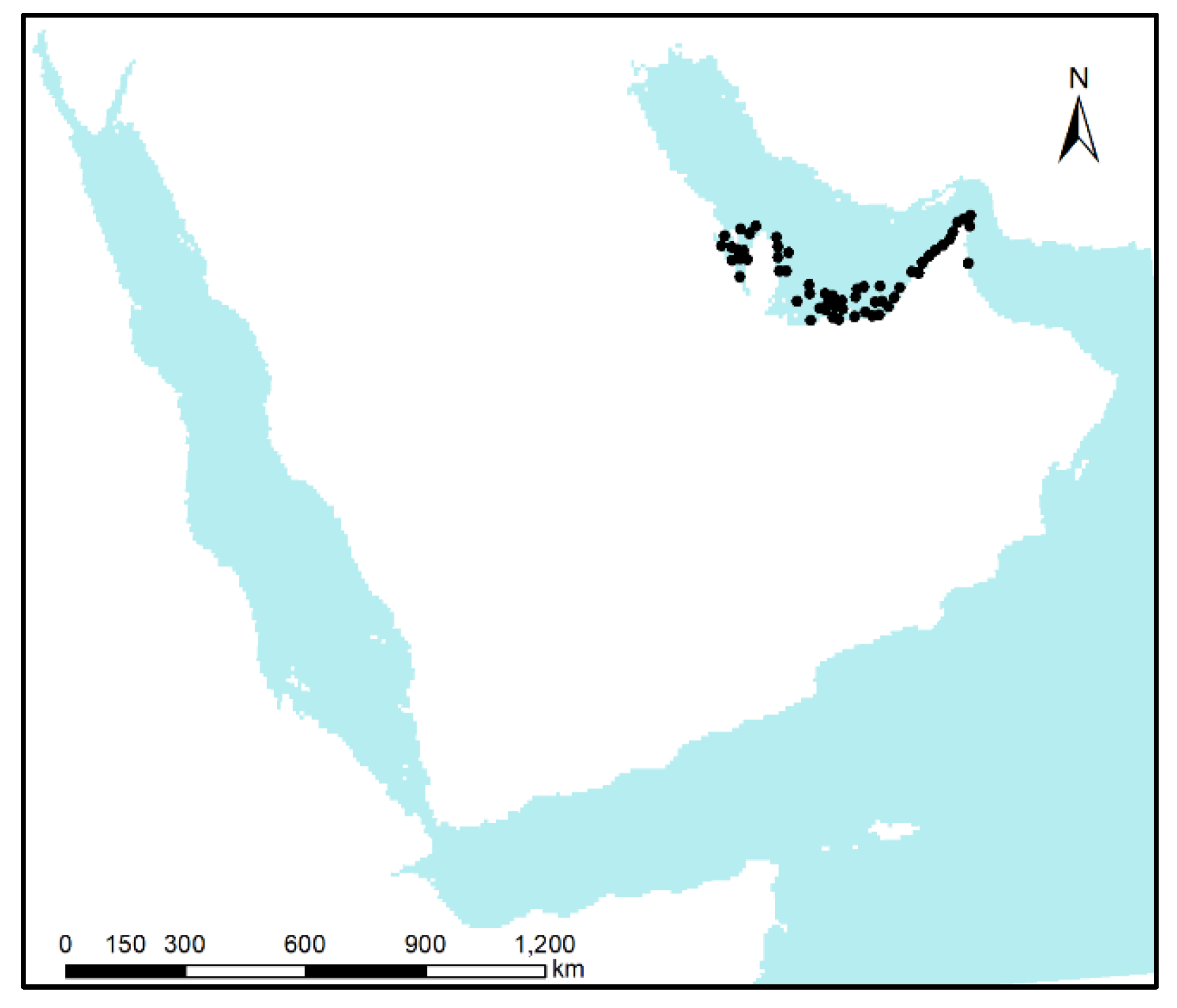
2.1. Collection and Preparation of Occurrence Data
2.2. Collection and Preparation of Predictor Variables
2.3. Modeling Procedures and Calibration
2.4. Model Evaluation
2.5. Model Exploration
3. Results
3.1. Autocorrelation Tests
3.2. Model Evaluation and Sensitivity Analysis
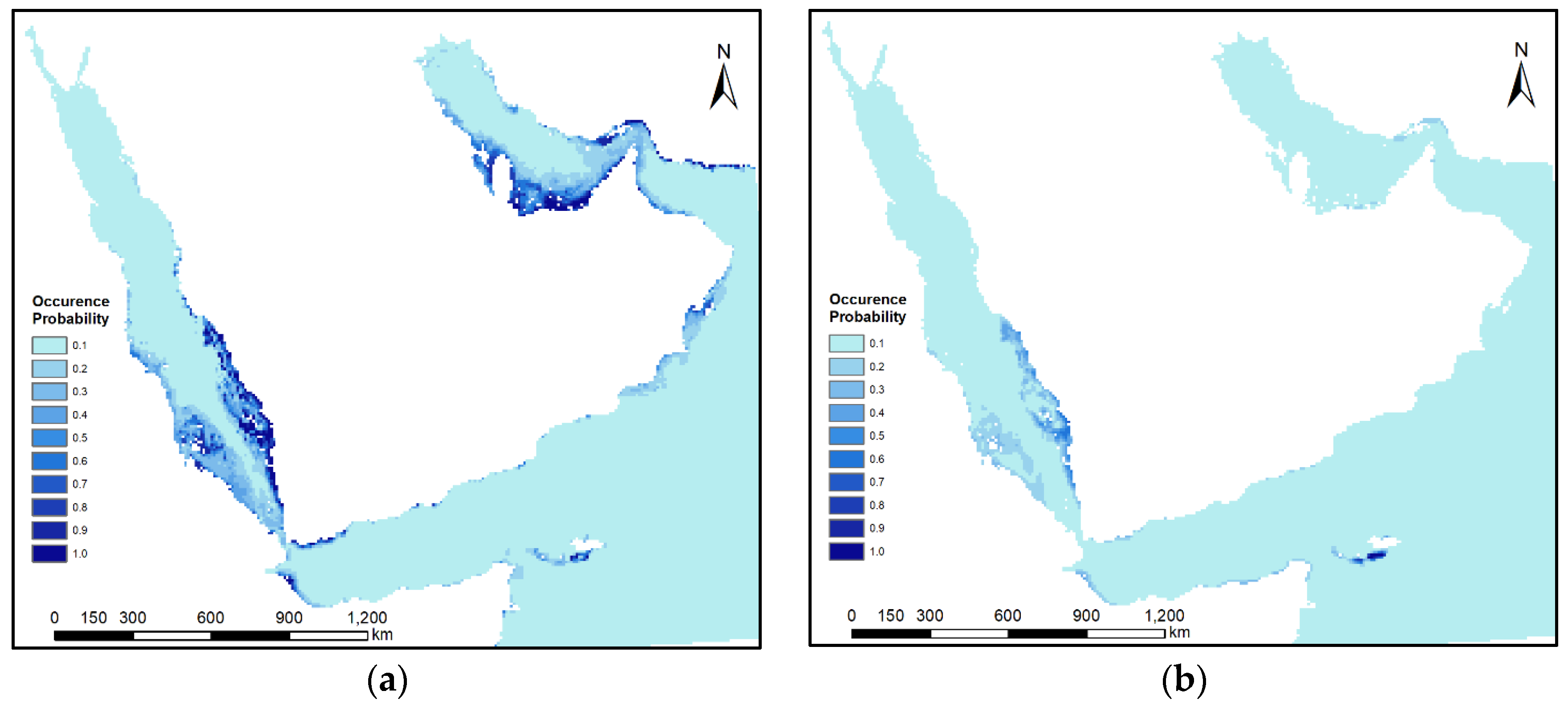
3.3. Predicted Potential Suitability
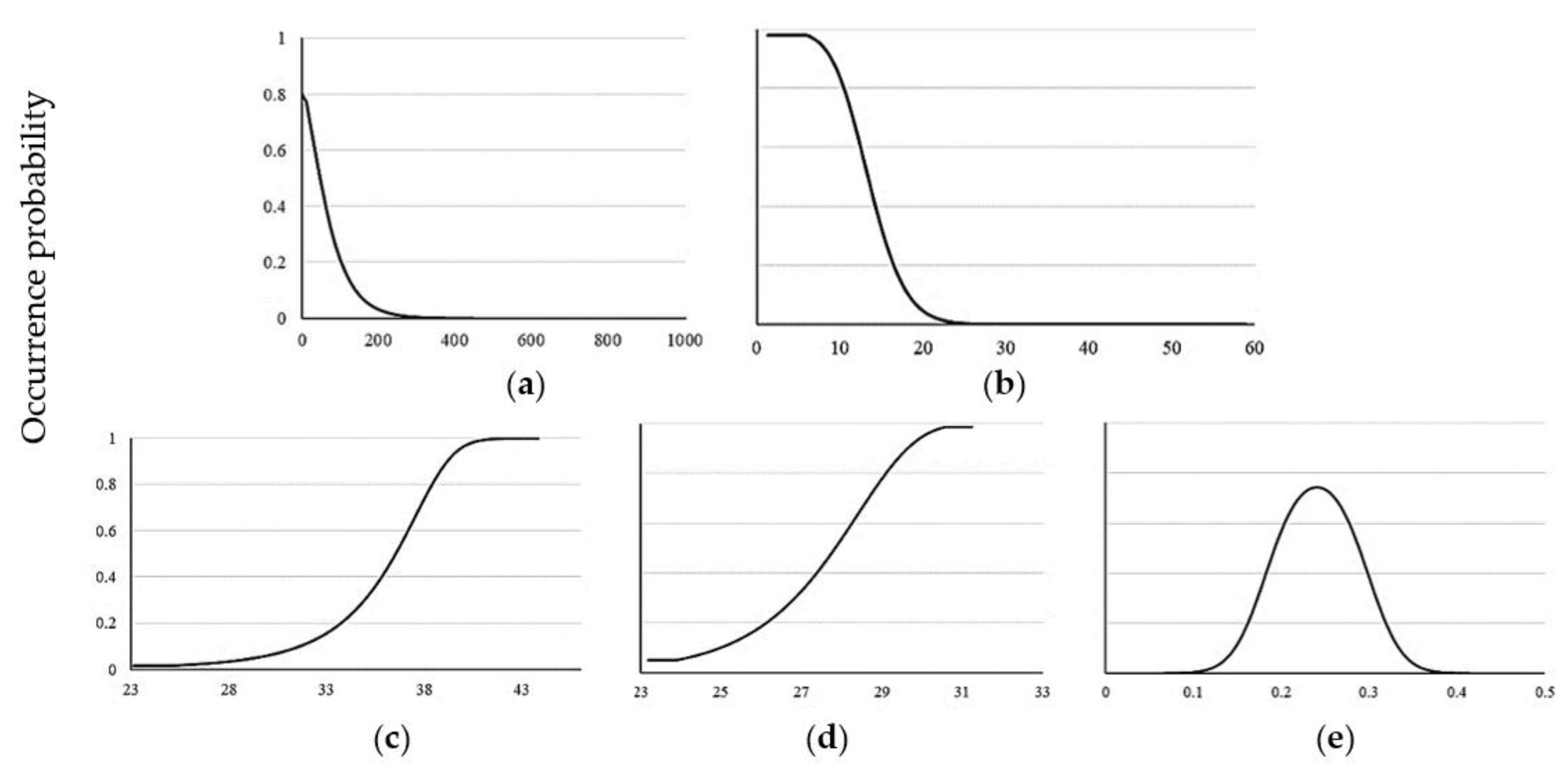
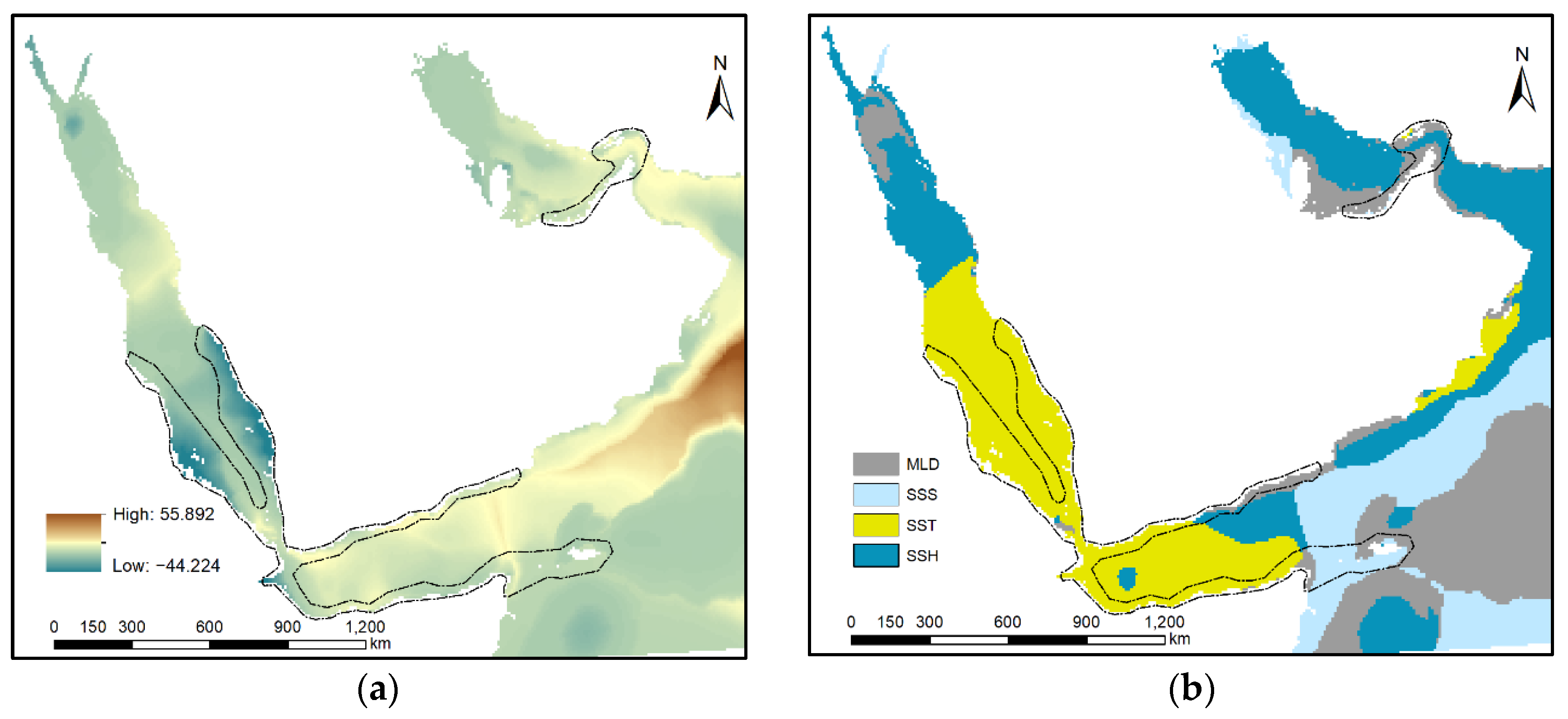
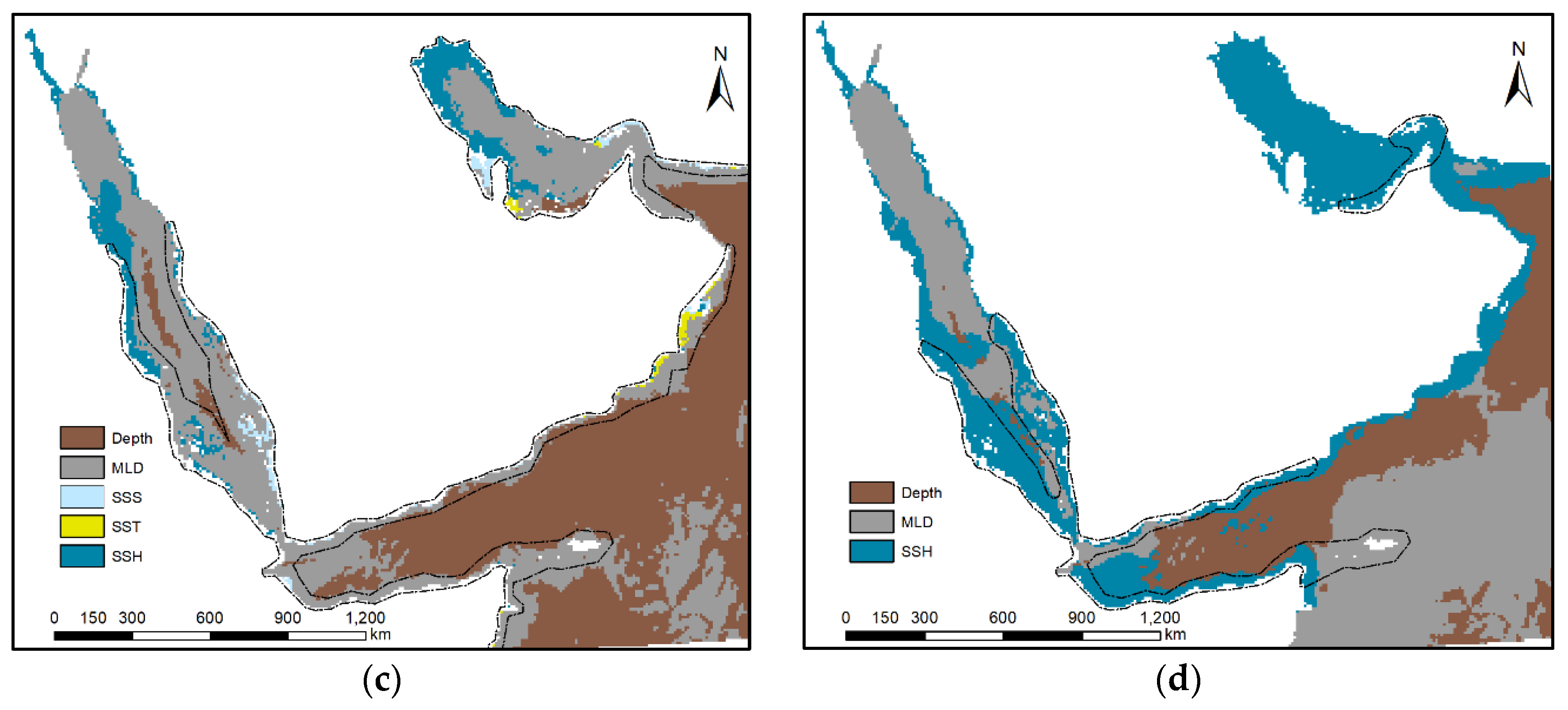
3.4. Model Exploration
4. Discussion
4.1. Predicted Suitability and Re/Colonization
4.2. Influence of Predictor Variables
4.3. Limitations and Unresolved Questions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Croxall, J.P.; Butchart, S.H.; Lascelles, B.E.N.; Stattersfield, A.J.; Sullivan, B.E.N.; Symes, A.; Taylor, P.H.I.L. Seabird conservation status, threats and priority actions: A global assessment. Bird Conserv. Int. 2012, 22, 1–34. [Google Scholar] [CrossRef]
- Dias, M.P.; Martin, R.; Pearmain, E.J.; Burfield, I.J.; Small, C.; Phillips, R.A.; Yates, O.; Lascelles, B.; Borboroglu, P.G.; Croxall, J.P. Threats to seabirds: A global assessment. Biol. Conserv. 2019, 237, 525–537. [Google Scholar] [CrossRef]
- Schreiber, E.A.; Burger, J. Seabirds in the Marine Environment. In Biology of Marine Birds; Schreiber, E.A., Burger, J., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–12. [Google Scholar]
- Cursach, J.A.; Arriagada, A.; Rau, J.R.; Ojeda, J.; Bizama, G.; Becerra, A. Predicting the potential distribution of the endemic seabird Pelecanus thagus in the Humboldt Current Large Marine Ecosystem under different climate change scenarios. Peer J. 2019, 7, e7642. [Google Scholar] [CrossRef] [PubMed]
- Quillfeldt, P.; Masello, J.F. Impacts of climate variation and potential effects of climate change on South American seabirds–a review. Mar. Biol. Res. 2013, 9, 337–357. [Google Scholar] [CrossRef]
- Krüger, L.; Ramos, J.A.; Xavier, J.C.; Gremillet, D.; González-Solís, J.; Petry, M.V.; Phillips, R.A.; Wanless, R.M.; Paiva, V.H. Projected distributions of Southern Ocean albatrosses, petrels and fisheries as a consequence of climatic change. Ecography 2018, 41, 195–208. [Google Scholar] [CrossRef]
- Glencross, J.S.; Lavers, J.L.; Woehler, E.J. Breeding success of short-tailed shearwaters following extreme environmental conditions. Mar. Ecol. Prog. Ser. 2021, 672, 193–203. [Google Scholar] [CrossRef]
- BirdLife International. Phalacrocorax nigrogularis (amended version of 2018 assessment). The IUCN Red List of Threatened Species 2019, e.T22696802A155525071. Available online: https://www.iucnredlist.org/species/22696802/155525071 (accessed on 20 March 2022).
- Crawford, R.J.M.; Sabarros, P.S.; Fairweather, T.; Underhill, L.G.; Wolfaardt, A.C. Implications for seabirds of a long term change in the distribution of sardine: A South African experience. Afr. J. Mar. Sci. 2008, 30, 177–184. [Google Scholar] [CrossRef]
- Muzaffar, S.B. Socotra Cormorants in the Arabian Gulf: A review of breeding biology, feeding ecology, movements and conservation. Aquat. Ecosyst. Health Manag. 2020, 23, 220–228. [Google Scholar] [CrossRef]
- Paleczny, M.; Hammill, E.; Karpouzi, V.; Pauly, D. Population trend of the world’s monitored seabirds, 1950–2010. PLoS ONE 2015, 10, e0129342. [Google Scholar] [CrossRef] [PubMed]
- Oppel, S.; Meirinho, A.; Ramírez, I.; Gardner, B.; O’Connell, A.F.; Miller, P.I.; Louzao, M. Comparison of five modelling techniques to predict the spatial distribution and abundance of seabirds. Biol. Conserv. 2012, 156, 94–104. [Google Scholar] [CrossRef]
- Robinson, L.M.; Elith, J.; Hobday, A.J.; Pearson, R.G.; Kendall, B.E.; Possingham, H.P.; Richardson, A.J. Pushing the limits in marine species distribution modelling: Lessons from the land present challenges and opportunities. Glob. Ecol. Biogeogr. 2011, 20, 789–802. [Google Scholar] [CrossRef]
- Jennings, M.C. Atlas of the breeding birds of Arabia. Br. Birds 2010, 103, 549–553. [Google Scholar]
- Nelson, J.B. Pelicans, Cormorants, and Their Relatives: Pelecanidae, Sulidae, Phalacrocoracidae, Anhingidae, Fregatidae, Phaethontidae; OUP Oxford: New York, NY, USA, 2005; pp. 429–431. [Google Scholar]
- BirdLife International. Species factsheet: Phalacrocorax nigrogularis. Available online: http://datazone.birdlife.org/species/factsheet/socotra-cormorant-phalacrocorax-nigrogularis/text (accessed on 20 March 2022).
- Muzaffar, S.B.; Gubiani, R.; Benjamin, S.; AlShihi, R.; Al-Romithi, A.; Al Kaabi, F.H. Food consumption patterns of the Vulnerable Socotra cormorant Phalacrocorax nigrogularis indicate minimal overlap with fisheries in the eastern Arabian Gulf. Oryx 2015, 51, 115–123. [Google Scholar] [CrossRef]
- Cook, T.R.; Gubiani, R.; Ryan, P.G.; Muzaffar, S.B. Group foraging in Socotra cormorants: A biologging approach to the study of a complex behavior. Ecol. Evolut. 2017, 7, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Al Shehhi, H.; Muzaffar, S.B. Impact of nesting Socotra Cormorants on terrestrial invertebrate communities. Insects 2021, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, S. Why the Socotra Cormorant Phalacrocorax nigrogularis should be formally protected. Notes Contrib. 1995, 5, 10–12. [Google Scholar]
- Ksiksi, T.S.; Muzaffar, S.B.; Gubiani, R.; Alshihi, R.M. The impact of nesting Socotra cormorants on soil chemistry and vegetation in a large colony in the United Arab Emirates. Diversity 2015, 7, 60–73. [Google Scholar] [CrossRef]
- Khan, S.B.; Javed, S.; Ahmed, S.; Al Hammadi, E.A.; Al Hammadi, A.A.; Al Dhaheri, S. Does a recent surge in Socotra Cormorant Phalacrocorax nigrogularis nesting population and establishment of new breeding colonies ensure long term conservation? Pragmatic assessment of recent augmentation in Abu Dhabi Emirate, UAE. Bird Conserv. Int. 2018, 29, 361–369. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Alizada, N.; Malik, S.; Muzaffar, S.B. Bioaccumulation of heavy metals in tissues of Indian anchovy (Stolephorus indicus) from the UAE coast, Arabian Gulf. Mar. Pollut. Bull. 2020, 154, 111033. [Google Scholar] [CrossRef]
- Malik, S.; Alizada, N.; Muzaffar, S.B. Bioaccumulation of trace elements in tissues of Indian oil sardine (Sardinella longiceps) from the northern United Arab Emirates. Mar. Pollut. Bull. 2020, 161, 111771. [Google Scholar] [CrossRef]
- Diaz Lopez, B.; Methion, S.; Das, H.; Bugla, I.; Al Hameli, M.; Al Ameri, H.; Al Hashmi, A.; Grandcourt, E. Vulnerability of a top marine predator in one of the world’s most impacted marine environments (Arabian Gulf). Mar. Biol. 2021, 168, 1–11. [Google Scholar] [CrossRef]
- Riegl, B.M.; Purkis, S.J. Coral reefs of the Gulf: Adaptation to climatic extremes in the world’s hottest sea. In Coral Reefs of the Gulf; Springer: Dordrecht, The Netherlands, 2012; Volume 3, pp. 1–4. [Google Scholar] [CrossRef]
- Del Hoyo, J.; Elliott, A.; Sargatal, J. Handbook of the Birds of the World; Lynx edicions: Barcelona, Spain, 1992. [Google Scholar]
- Muzaffar, S.B.; Clarke, C.; Whelan, R.; Gubiani, R.; Cook, T.R. Short distance directional migration in the threatened Socotra cormorant: Link to primary productivity and implications for conservation. Mar. Ecol. Prog. Ser. 2017, 575, 181–194. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Veloz, S.D. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Gilmour, M.E.; Castillo-Guerrero, J.A.; Fleishman, A.B.; Hernández-Vázquez, S.; Young, H.S.; Shaffer, S.A. Plasticity of foraging behaviors in response to diverse environmental conditions. Ecosphere 2018, 9, e02301. [Google Scholar] [CrossRef]
- Tremblay, Y.; Bertrand, S.; Henry, R.W.; Kappes, M.A.; Costa, D.P.; Shaffer, S.A. Analytical approaches to investigating seabird–environment interactions: A review. Mar. Ecol. Prog. Ser. 2009, 391, 153–163. [Google Scholar] [CrossRef]
- Wakefield, E.D.; Phillips, R.A.; Matthiopoulos, J. Quantifying habitat use and preferences of pelagic seabirds using individual movement data: A review. Mar. Ecol. Prog. Ser. 2009, 391, 165–182. [Google Scholar] [CrossRef]
- E.U. Copernicus Marine Service Information. Global Ocean Physics Reanalysis. Available online: https://resources.marine.copernicus.eu/product-detail/GLOBAL_MULTIYEAR_PHY_001_030/INFORMATION (accessed on 10 June 2021).
- E.U. Copernicus Marine Service Information. Global Ocean 1/12° Physics Analysis and Forecast updated Daily. Available online: https://resources.marine.copernicus.eu/product-detail/GLOBAL_ANALYSIS_FORECAST_PHY_001_024/INFORMATION (accessed on 10 June 2021).
- Roberts, M. MOHC HadGEM3-GC31-HH model output prepared for CMIP6 HighResMIP highres-future. Version 20200514. Earth System Grid Federation. 2019. Available online: https://www.wdc-climate.de/ui/cmip6?input=CMIP6.HighResMIP.MOHC.HadGEM3-GC31-HH.highres-future (accessed on 5 March 2021).
- Meinshausen, M.; Nicholls, Z.R.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N.; et al. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- Lima-Ribeiro, M.S.; Varela, S.; González-Hernández, J.; de Oliveira, G.; Diniz-Filho, J.A.F.; Terribile, L.C. EcoClimate: A database of climate data from multiple models for past, present, and future for macroecologists and biogeographers. Biodivers. Inform. 2015, 10, 1–21. [Google Scholar] [CrossRef]
- Varela, S.; Lima-Ribeiro, M.S.; Terribile, L.C. A short guide to the climatic variables of the last glacial maximum for biogeographers. PLoS ONE 2015, 10, e0129037. [Google Scholar] [CrossRef] [PubMed]
- Duque-Lazo, J.; Van Gils, H.A.M.J.; Groen, T.A.; Navarro-Cerrillo, R.M. Transferability of species distribution models: The case of Phytophthora cinnamomi in Southwest Spain and Southwest Australia. Ecol. Model. 2016, 320, 62–70. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent software for modeling species niches and distributions Version 3.4.3. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent (accessed on 26 February 2022).
- Bradie, J.; Leung, B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2017, 44, 1344–1361. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander Jr, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Peer J. 2017, 5, e4095. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Merckx, B.; Steyaert, M.; Vanreusel, A.; Vincx, M.; Vanaverbeke, J. Null models reveal preferential sampling, spatial autocorrelation and overfitting in habitat suitability modelling. Ecol. Model. 2011, 222, 588–597. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evolut. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Rodder, D.; Lawing, A.M.; Flecks, M.; Ahmadzadeh, F.; Dambach, J.; Engler, J.O.; Habel, J.C.; Hartmann, T.; Hornes, D.; Ihlow, F.; et al. Evaluating the significance of paleo phylogeographic species distribution models in reconstructing Quaternary range-shifts of Nearctic chelonians. PLoS ONE 2013, 8, e72855. [Google Scholar] [CrossRef] [PubMed]
- Rodda, G.H.; Jarnevich, C.S.; Reed, R.N. Challenges in identifying sites climatically matched to the native ranges of animal invaders. PLoS ONE 2011, 6, e14670. [Google Scholar] [CrossRef]
- Jarnevich, C.S.; Stohlgren, T.J.; Kumar, S.; Morisette, J.T.; Holcombe, T.R. Caveats for correlative species distribution modeling. Ecol. Inform. 2015, 29, 6–15. [Google Scholar] [CrossRef]
- Semere, D.; Hagos, T.; Seleba, G.; Gebrezgabhier, Y.; Haile, Z.; Chiozzi, G.; De Marchi, G. The status of breeding seabirds and waterbirds on the Eritrean Red Sea islands. Bull. Afr. Bird Club 2008, 15, 228–237. [Google Scholar] [CrossRef]
- Rasul, N.; Stewart, I.C.; Nawab, Z.A. Introduction to the Red Sea: Its origin, structure, and environment. In The Red Sea; Springer: Berlin, Germany, 2015; pp. 1–28. [Google Scholar] [CrossRef]
- Jenouvrier, S.; Holland, M.; Stroeve, J.; Serreze, M.; Barbraud, C.; Weimerskirch, H.; Caswell, H. Projected continent-wide declines of the emperor penguin under climate change. Nat. Clim. Change 2014, 4, 715–718. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Nat. Acad. Sci. U.S.A. 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Azizpour, J.; Chegini, V.; Khosravi, M.; Einali, A. Study of the physical oceanographic properties of the Persian Gulf, strait of Hormoz and Gulf of Oman based on PG-GOOS CTD measurements. J. Pers. Gulf 2014, 5, 37–48. [Google Scholar]
- Syah, A.F.; Siregar, E.S.Y.; Siregar, V.P.; Agus, S.B. Application of remotely sensed data and maximum entropy model in detecting potential fishing zones of Yellowfin tuna (Thunnus albacares) in the eastern Indian Ocean off Sumatera. J. Phys. Conf. Ser. 2020, 1569, 042097. [Google Scholar] [CrossRef]
- Tuanmu, M.N.; Vina, A.; Roloff, G.J.; Liu, W.; Ouyang, Z.; Zhang, H.; Liu, J. Temporal transferability of wildlife habitat models: Implications for habitat monitoring. J. Biogeogr. 2011, 38, 1510–1523. [Google Scholar] [CrossRef]
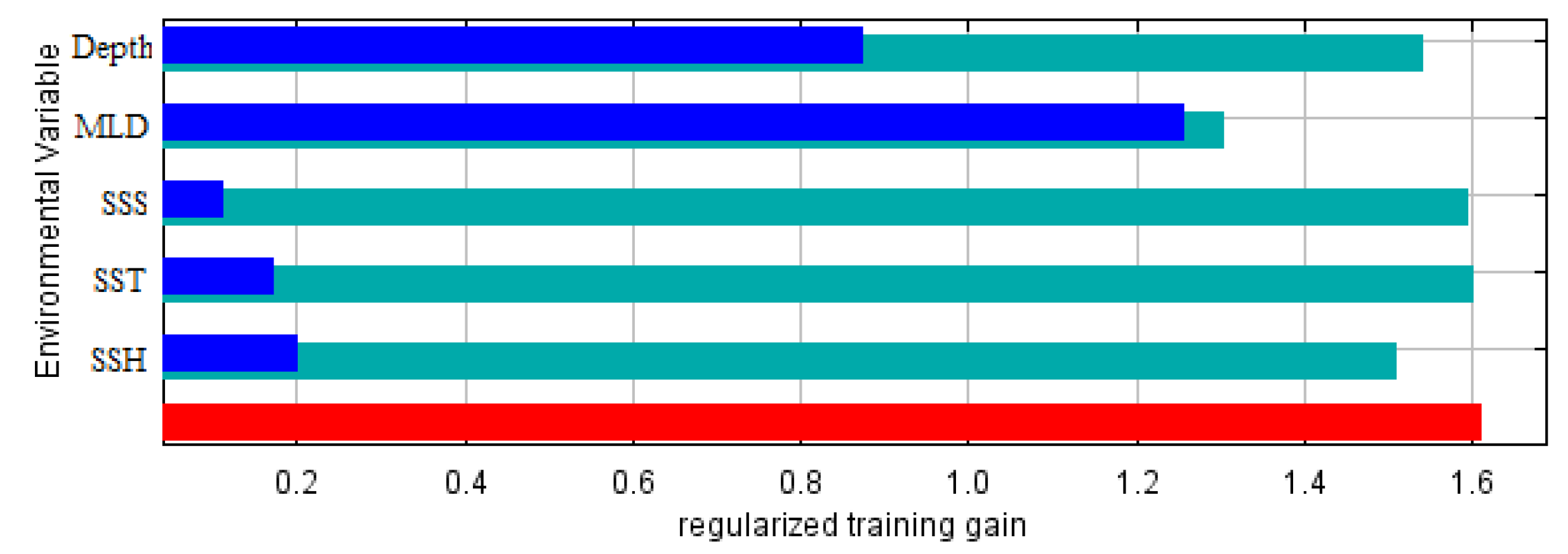
| Variable | Contribution to the Model (%) |
|---|---|
| MLD | 42.3 |
| Depth | 41.1 |
| SST | 9.6 |
| SSH | 6.4 |
| SSS | 0.6 |
| Evaluation Test | Result |
| AUC-test | 0.965 |
| AUC-train | 0.966 |
| TSS | 0.874 |
| Kappa max | 0.448 |
| Regions | Suitability Area (km2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unsuitable (<0.2) | Least Suitable (0.2–0.4) | Moderately Suitable (0.4–0.6) | Highly Suitable (>0.6) | |||||
| Current | Future | Current | Future | Current | Future | Current | Future | |
| All | 1,980,500 | 2,172,700 | 113,300 | 24,900 | 42,000 | 6000 | 64,100 | 1700 |
| Arabian Gulf | 156,200 | 225,600 | 27,300 | 0 | 16,400 | 0 | 24,000 | 0 |
| Gulf of Oman, Arabian Sea, Gulf of Aden | 1,488,100 | 1,524,600 | 25,500 | 4100 | 7800 | 800 | 8800 | 1600 |
| Red Sea | 336,200 | 422,500 | 60,500 | 20,800 | 17,800 | 5200 | 31,300 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaradat, A.; Ksiksi, T.; Muzaffar, S.B. Current and Future Distribution Modeling of Socotra Cormorants Using MaxEnt. Diversity 2022, 14, 840. https://doi.org/10.3390/d14100840
Jaradat A, Ksiksi T, Muzaffar SB. Current and Future Distribution Modeling of Socotra Cormorants Using MaxEnt. Diversity. 2022; 14(10):840. https://doi.org/10.3390/d14100840
Chicago/Turabian StyleJaradat, Areej, Taoufik Ksiksi, and Sabir Bin Muzaffar. 2022. "Current and Future Distribution Modeling of Socotra Cormorants Using MaxEnt" Diversity 14, no. 10: 840. https://doi.org/10.3390/d14100840
APA StyleJaradat, A., Ksiksi, T., & Muzaffar, S. B. (2022). Current and Future Distribution Modeling of Socotra Cormorants Using MaxEnt. Diversity, 14(10), 840. https://doi.org/10.3390/d14100840








