Effects of Vetiveria zizanioides on the Restoration and Succession of Coal Gangue Mountain Plant Communities in Different Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Plot Setting and Quadrat Investigation
2.3. Data Analysis
2.3.1. α Diversity
2.3.2. β Diversity
3. Results
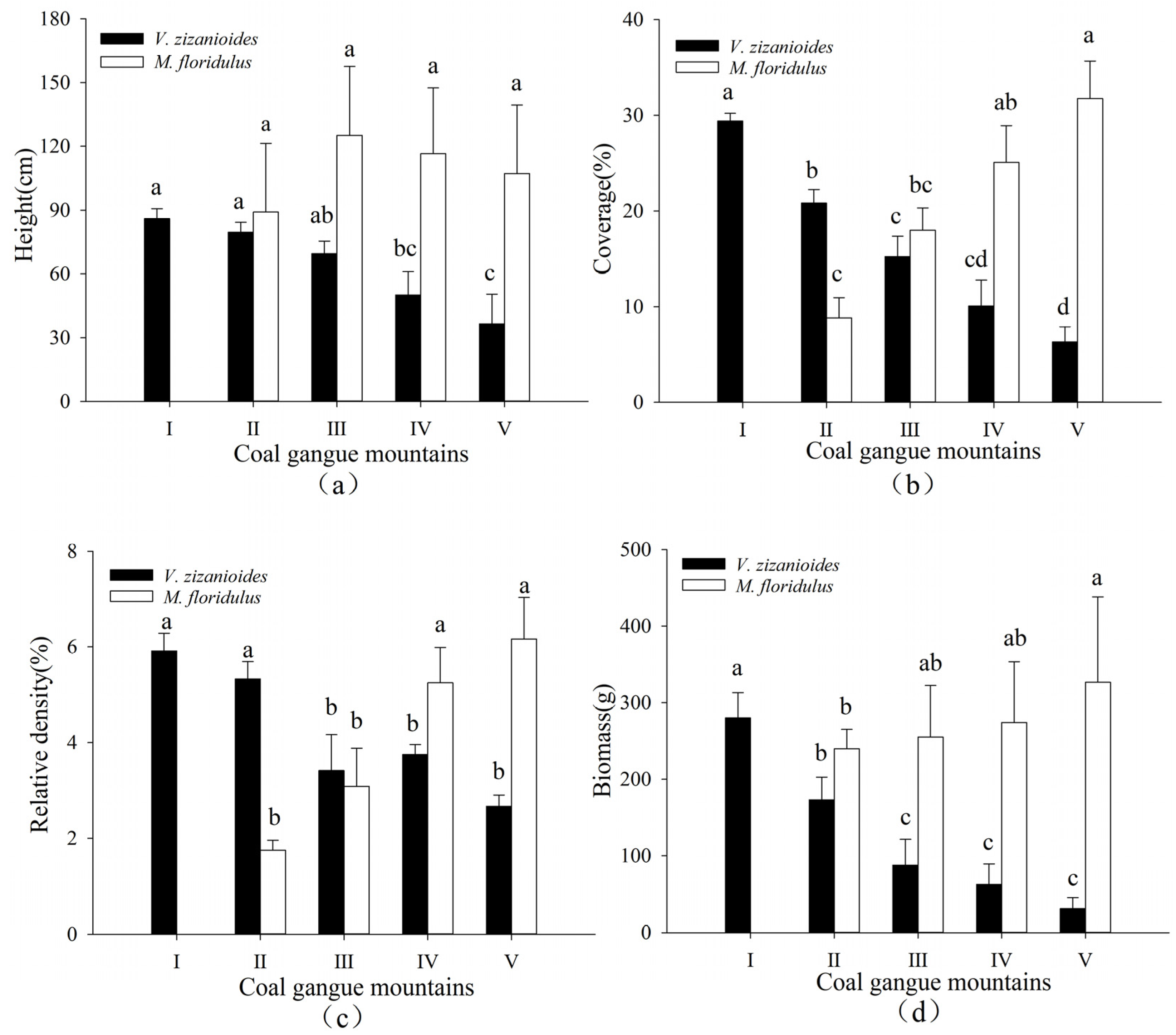
3.1. Changes in Species Composition and the Importance Value in Different Coal Gangue Mountains
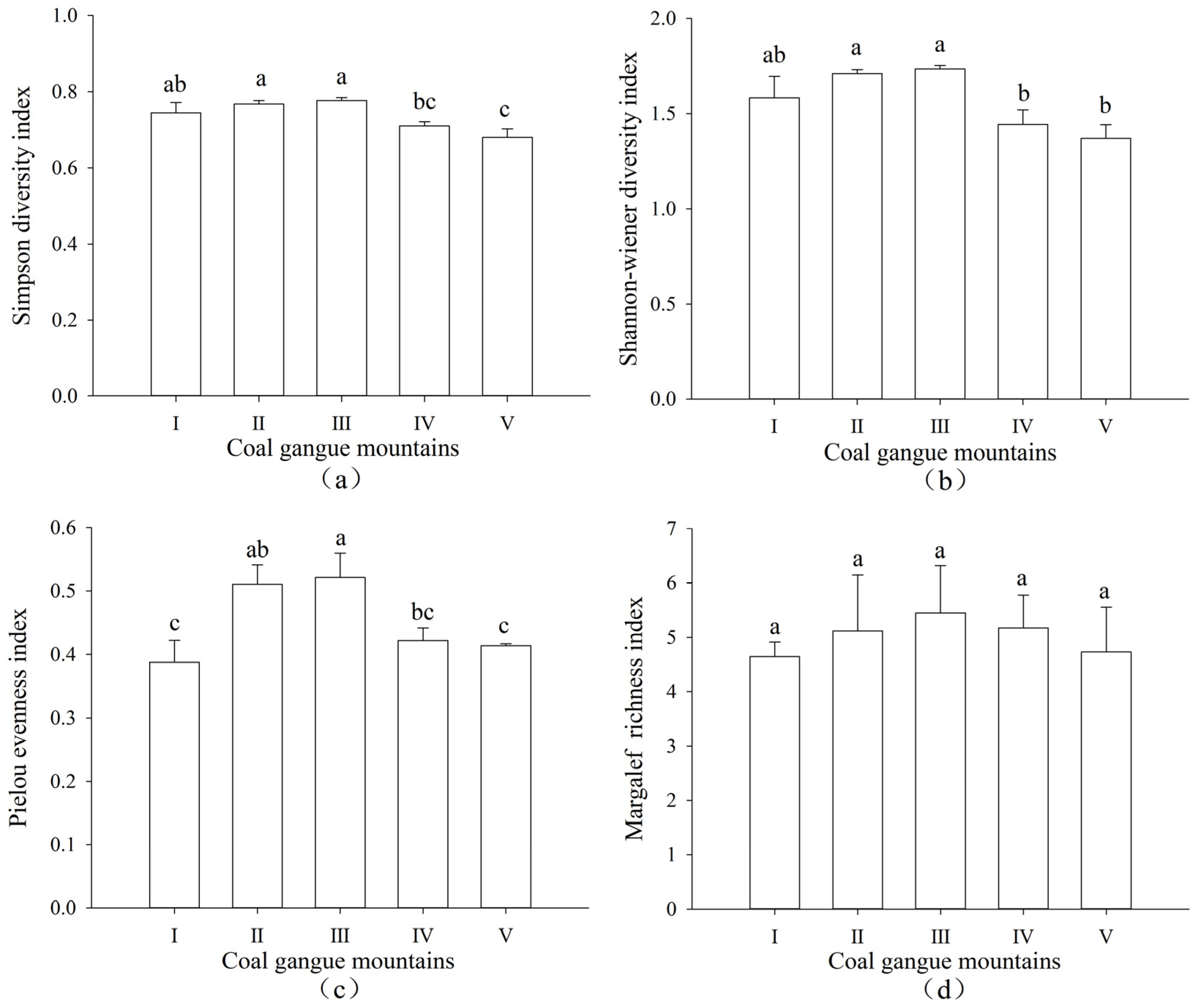
3.2. Changes of Plant Community Diversity in Different Coal Gangue Mountains
3.3. Dynamic Changes of Community Succession in Different Coal Gangue Mountains
4. Discussion
4.1. Influence of V. zizanioides on Community Characteristics in Different Coal Gangue Mountains
4.2. Influence of V. zizanioides on Community Succession in the Coal Gangue Mountain
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Zhao, B.; Bai, Z.; Shang-Guan, T.; Guo, D. Correlation of soil nutrients and plant community in ecological reclamation of opencast coal mine in a semiarid area. Chin. J. Ecol. 2014, 33, 2369–2375. [Google Scholar] [CrossRef]
- Saini, V.; Gupta, R.P.; Arora, M.K. Environmental impact studies in coalfields in India: A case study from Jharia coal-field. Renew. Sust. Energ. Rev. 2016, 53, 1222–1239. [Google Scholar] [CrossRef]
- Hao, Z.; Li, S. Ecological relationship of herbaceous plant community under different vegetation in coal gangue mountain reclaimed land in Yangquan mining area. Chin. J. Appl. Environ. Biol. 2018, 24, 1158–1164. [Google Scholar] [CrossRef]
- Xu, L.; Feng, F.; Liu, Y.; Yang, Y.; Zheng, W. Relationship between plant species diversity and soil chemical properties in coal gangue dump: Early stage of ecological restoration in Lingwu mining area. Coal Sci. Technol. 2020, 48, 97–104. [Google Scholar] [CrossRef]
- Li, H.; Shen, W.; Si, W.; Yan, Q. Investigation of driving factors of land degradation in mine areas in China: Concept, types and approaches. J. Ecol. Rural Environ. 2015, 31, 445–451. [Google Scholar] [CrossRef]
- Ding, W.; Huang, Z.; Tan, H.; Zhang, J.; Pu, Z.; Zheng, G.; Liu, X. Current situation and countermeasures of comprehensive utilization of coal cangue in Liupanshui city, Guizhou province. Acta Mineral. Sin. 2011, 31, 4. [Google Scholar] [CrossRef]
- Venson, G.R.; Marenzi, R.C.; Almeida, T.; Deschamps-Schmidt, A.; Testolin, R.C.; Rorig, L.R.; Radetski, C.M. Restoration of areas degraded by alluvial sand mining: Use of soil microbiological activity and plant biomass growth to assess evolution of restored riparian vegetation. Environ. Monit. Assess. 2017, 189, 120. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Bai, Z.; Lv, C. Effects of vegetation on runoff and soil erosion on reclaimed land in an opencast coal-mine dump in a loess area. Catena 2015, 128, 44–53. [Google Scholar] [CrossRef]
- Vipin, K.; Avantika, C.; Zeba, U. Impact of coal mining on soil properties and their efficient eco-restoration. Int. J. Energy Technol. Policy 2017, 13, 158–165. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mastob, R.E.; Yadav, A.; George, J.; Ram, L.C.; Shukla, S.P. Soil quality index for evaluation of reclaimed coal mine spoil. Sci. Total Environ. 2016, 542, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, T.; Yuan, X. Problems and countermeasures of environmental risk management in tailing pond. Environ. Prot. 2021, 49, 52–53. [Google Scholar] [CrossRef]
- Are, K.S.; Adelana, A.O.; Adeyolanu, O.D.; Oyeogbe, I.A.; Adelabu, L. Comparative effects of vetiver grass (Chrysopogon zizanioides) strips, vetiver mulch and veticompost on soil quality and erodibility of a sloping land. Agric. Trop. Subtrop. 2012, 45, 189–198. [Google Scholar] [CrossRef]
- Xu, D.; Zhan, J.; Chen, Z.; Gao, Y.; Xie, X.; Sun, Q.; Dou, C. Effects of Vetiveria zizanioides L. growth on chemical and biological properties of copper mine tailing wastelands. Acta Ecol. Sin. 2012, 32, 5683–5691. [Google Scholar] [CrossRef]
- Nayak, A.K.; Panda, S.S.; Basu, A.; Dhal, N.K. Enhancement of toxic Cr (VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Vetiveria zizanioides L. Int. J. Phytoremediat. 2018, 20, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Melato, F.A.; Mokgalaka, N.S.; Mccrindle, R.I. Adaptation and detoxification mechanisms of vetiver grass (Chrysopogon zizanioides) growing on gold mine tailings. Int. J. Phytoremediat. 2016, 18, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Aksorn, E.; Chitsomboon, B. Bioaccumulation of heavy metal uptake by two different vetiver grass (Vetiveria zizanioides and Vetiveria nemoralis) species. Acad. J. 2013, 24, 3166–3171. [Google Scholar] [CrossRef]
- Wang, L.; Tay, J.H.; Tay, S.; Hung, Y.T. Phytoremediation of heavy metal contaminated soils and water using vetiver grass. Handb. Environ. Eng. 2010, 11, 233–275. [Google Scholar] [CrossRef]
- Cain, M.D.; Shelton, M.G. Secondary forest succession following reproduction cutting on the Upper Coastal Plain of southeastern Arkansas, USA. For. Ecol. Manag. 2001, 146, 223–238. [Google Scholar] [CrossRef]
- Gao, D.; Cai, T.; Wang, X. Regulation on abrasive material of vegetation succession and countermeasure of vegetation restoration of opencast gold ore in Gingili River Watershed. Res. Soil Water Conserv. 2005, 6, 37–39. [Google Scholar] [CrossRef]
- Guan, J.; Cao, Y.; Wu, T.; Dong, L. An investigation of revegetation in Shouyun Iron Mine Wasteland in Beijing. Chin. Landsc. Archit. 2017, 33, 13–18. [Google Scholar] [CrossRef]
- Hodačová, D.; Prach, K. Spoil heaps from brown coal mining: Technical reclamation versus spontaneous revegetation. Restor. Ecol. 2003, 11, 385–391. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.; Hao, J.; Zhao, L.; Cheng, W. Effect of different planting years of Vetiveria zizanioides L. on the distribution of heavy metals in coal spoil-heap soil. J. China Coal Soc. 2016, 41, 3101–3107. [Google Scholar] [CrossRef]
- Sheng, M.; Hao, J.; Long, Y.; Xu, Z.; Mao, Y.; Cheng, W. Dynamic changes in nutritional distribution and allometric relationships in different organs of Vetiveria zizanioides in coal gangue. Pratac. Sci. 2019, 36, 1999–2007. [Google Scholar] [CrossRef]
- Zhang, J. Quantitative Ecology, 2nd ed.; Science Press: Beijing, China, 2011; p. 372. [Google Scholar]
- Zhang, Z.; Gao, B.; Lin, C.; Wang, Q.; Liu, J.; Yang, N.; Su, D.; Ping, X. Effects of grazing intensity on biomass allocation patterns of six plant species in a typical grassland. Acta Agrestia Sin. 2021, 29, 149–155. [Google Scholar] [CrossRef]
- Lou, J. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef]
- Ritesh, B.; Priya, G.; Khanindra, P.; Anita, M. Vetiver grass: An environment clean-up tool for heavy metal contaminated iron ore mine-soil. Ecol. Eng. 2016, 90, 25–34. [Google Scholar] [CrossRef]
- Yang, B.; Lan, C.; Shu, W. Growth and heavy metal accumulation of Vetiveria zizanioides grown on lead zinc mine tailings. Acta Ecol. Sin. 2005, 25, 45–50. [Google Scholar] [CrossRef]
- Huang, G.; Zheng, X.; Dong, X.; Peng, J.; Yang, J. Study on the reinforcement effect of herb and shrub root system on coal-bearing soil. J. B. Jiaotong Univ. 2020, 44, 49–56. [Google Scholar] [CrossRef]
- Miao, C.; Bai, Y.; Zhang, Y.; She, W.; Liu, L.; Qiao, Y.; Qin, S. Interspecific interactions alter plant functional strategies in a revegetated shrub-dominated community in the Mu Us Desert, China. Ann. Bot. 2022, 20, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Z.; Li, S.; Karsten, L. Interspecific association of dominant species in naturally colonized plant communities on coal gob piles of the Yangquan mining area in Shanxi, China. Chin. J. Appl. Environ. Biol. 2015, 21, 1143–1149. [Google Scholar] [CrossRef]
- Wang, X.; Cai, T.; Gu, J. Effects of soil properties on vegetation restoration in coal-gangue pile in Jixi area, China. Acta Ecol. Sin. 2007, 27, 8. [Google Scholar] [CrossRef]
- Abe, T.; Tanaka, N.; Shimizu, Y. Plant species diversity, community structure and invasion status in insular primary forests on the Sekimon uplifted limestone (Ogasawara islands). J. Plant Res. 2018, 131, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Cano, J.A.; Verdú, M.; Goberna, M. Trait-based selection of nurse plants to restore ecosystem functions in mine tailings. J. App. Ecolo. 2018, 55, 1195–1206. [Google Scholar] [CrossRef]
- Bashirzadeh, M.; Shefferson, R.P.; Farzam, M. Plant–plant interactions determine natural restoration of plant biodiversity over time, in a degraded mined land. Ecol. Evol. 2022, 12, e8878. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, J.; Zhou, W.; Bai, Z.; Cao, Y. Identification of land reclamation stages based on succession characteristics of rehabilitated vegetation in the Pingshuo opencast coal mine. J. Environ. Manag. 2022, 305, 114352. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Yue, M.; Liu, X.; Guo, Y.; Wang, M.; Xu, J.; Zhang, C.; Chen, Y.; Zhang, L.; Zhang, R. Patterns of taxonomic, phylogenetic diversity during a long-term succession of forest on the Loess Plateau, China: Insights into assembly process. Sci. Rep. 2016, 6, 27087. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Gong, H.; Zhang, G.; Dong, Z. Analysis of changes of the species diversity in the process of vegetation restoration in Antaibao Mining Field, China. Acta Ecol. Sin. 2005, 25, 763–770. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, H.; Zhang, T.; Zhao, X. Dynamics of species diversity of community in restoration processes in Horqin Sandy Land. Acta Phytoecol. Sin. 2004, 28, 86–92. [Google Scholar] [CrossRef]
- Anderson, K.J. Temporal patterns in rates of community change during succession. Am. Nat. 2007, 169, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Munford, K.E.; Asemaninejad, A.; Basiliko, N.; Mykytczuk, N.; Glasauer, S.; McGarry, S.; Watmough, S.A. Native plants facilitate vegetation succession on amended and unamended mine tailings. Int. J. Phytoremediat. 2022, 24, 963–974. [Google Scholar] [CrossRef]
- Burton, C.M.; Burton, P.J.; Hebda, R.; Tuner, N.J. Determining the optimal sowing density for a mixture of native plants used to revegetate degraded ecosystems. Restor. Ecol. 2010, 14, 379–390. [Google Scholar] [CrossRef]
- Coradini, K.; Krejčová, J.; Frouz, J. Potential of vegetation and woodland cover recovery during primary and secondary succession, a global quantitative review. Land Degrad. Dev. 2022, 33, 512–526. [Google Scholar] [CrossRef]
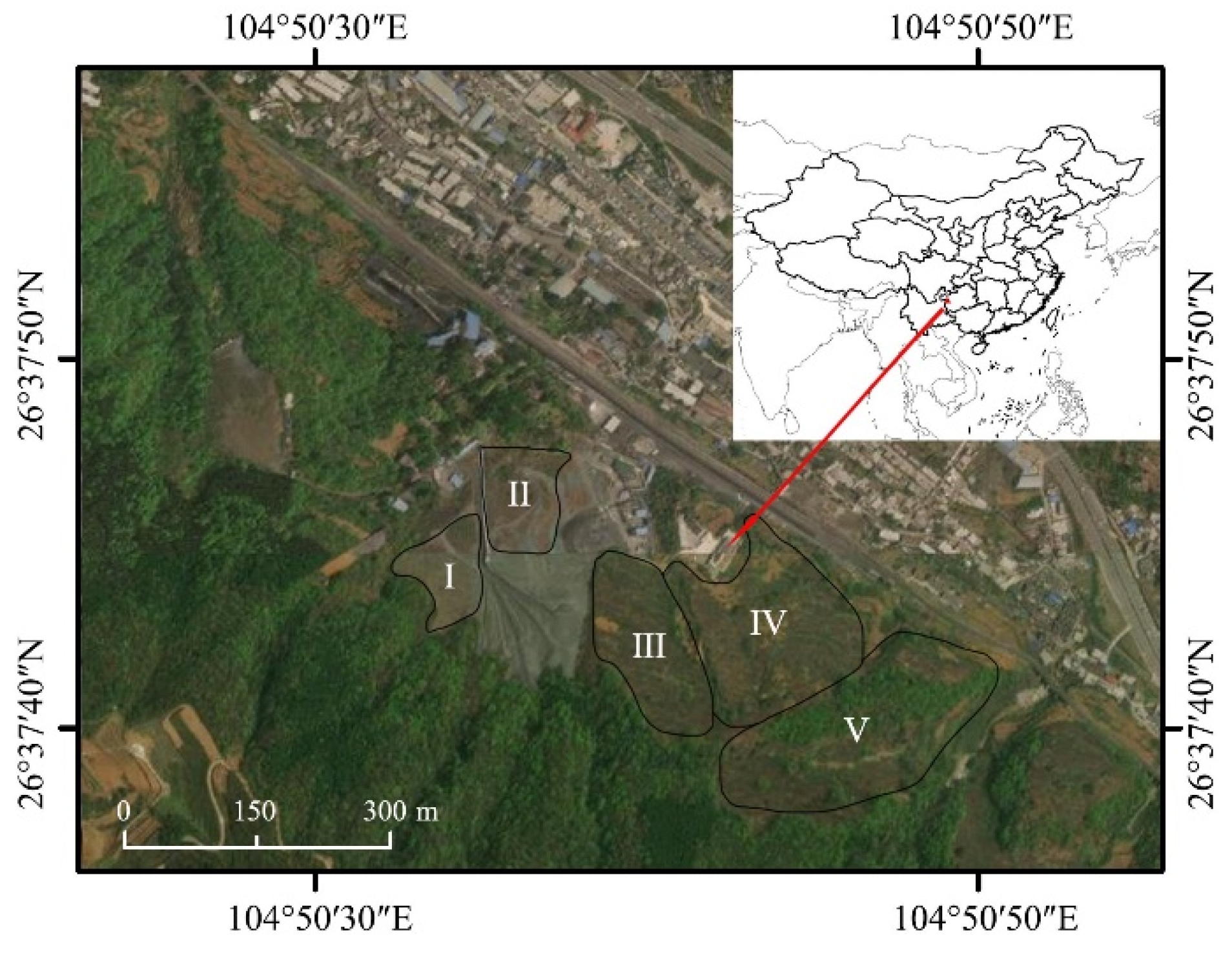

| Coal Gangue Mountains | Planting Time (Year) | Years after Planting (Year) | Altitude (m) | Planting Density (cm × cm) | Dominant Species |
|---|---|---|---|---|---|
| I | 2012 | 3 | 1860 | 50 × 20 | Vetiveria zizanioides |
| II | 2009 | 6 | 1879 | 50 × 20 | Vetiveria zizanioides |
| III | 2007 | 8 | 1880 | 50 × 20 | Vetiveria zizanioides |
| IV | 2005 | 10 | 1846 | 50 × 20 | Miscanthus floridulus |
| V | 2002 | 13 | 1793 | 50 × 20 | Miscanthus floridulus |
| Family Name | Speices Name | Plots | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Poaceae | Vetiveria zizanioides | 1 | 1 | 1 | 1 | 1 |
| Miscanthus floridulus | - | 1 | 1 | 1 | 1 | |
| Cynodon dactylon | 1 | - | - | - | - | |
| Cymbopogon caesius | - | 1 | - | - | - | |
| Chrysopogon aciculatus | - | 1 | 1 | - | 1 | |
| Digitaria sanguinalis | - | - | 1 | - | - | |
| Zoysia japonica | 1 | - | 1 | 1 | 1 | |
| Fabaceae | Vicia sepium | - | 1 | - | - | - |
| Asteraceae | Erigeron annuus | 1 | - | 1 | - | - |
| Erigeron acris | - | - | - | 1 | 1 | |
| Artemisia argyi | 1 | 1 | - | - | - | |
| Artemisia sieversiana | - | - | 1 | 1 | 1 | |
| Sonchus arvensis | 1 | 1 | - | - | - | |
| Taraxacum mongolicum | - | 1 | - | - | - | |
| Senecio scandens | - | - | 1 | 1 | - | |
| Artemisia carvifolia | 1 | 1 | 1 | - | - | |
| Aster indicus | 1 | - | - | - | - | |
| Oxalidaceae | Oxalis corniculata | - | 1 | 1 | 1 | 1 |
| Polygonaceae | Fallopia multiflora | - | 1 | - | - | - |
| Amaranthaceae | Alternanthera philoxeroides | 1 | - | - | - | - |
| Clusiaceae | Hypericum monogynum | - | - | 1 | 1 | 1 |
| Oleaceae | Ligustrum lucidum | - | - | - | 1 | 1 |
| Loganiaceae | Buddleja fallowiana | 1 | - | 1 | 1 | - |
| Lamiaceae | Prunella vulgaris | - | 1 | 1 | - | - |
| Clinopodium chinense | - | 1 | 1 | - | - | |
| Convolvulaceae | Convolvulus arvensis | - | - | - | 1 | - |
| Geraniaceae | Geranium wilfordii | - | 1 | 1 | - | - |
| Apiaceae | Cnidium monnieri | - | - | 1 | 1 | - |
| Rosaceae | Duchesnea indica | - | 1 | - | 1 | - |
| Rubus idaeus | - | - | 1 | 1 | 1 | |
| Equisetaceae | Equisetum arvense | 1 | - | 1 | - | - |
| Ranunculaceae | Anemone hupehensis | - | - | - | 1 | - |
| Anemone vitifolia | 1 | 1 | 1 | - | - | |
| Betulaceae | Betula luminifera | - | - | - | - | 1 |
| Corylus heterophylla | - | - | - | - | 1 | |
| Total: 17 | 35 | 12 | 16 | 19 | 15 | 12 |
| Family Name | Speices Name | Quadrats | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Poaceae | Vetiveria zizanioides | 0.40 | 0.37 | 0.34 | 0.24 | 0.24 |
| Zoysia japonica | 0.15 | 0.04 | 0.01 | 0.06 | 0.04 | |
| Miscanthus floridulus | - | 0.21 | 0.23 | 0.44 | 0.47 | |
| Lamiaceae | Clinopodium chinense | 0.18 | 0.05 | 0.03 | 0.07 | 0.06 |
| Prunella vulgaris | - | 0.03 | 0.03 | - | - | |
| Ranunculaceae | Anemone vitifolia | 0.06 | 0.03 | 0.03 | - | - |
| Asteraceae | Artemisia carvifolia | 0.13 | 0.13 | 0.13 | - | - |
| Aster indicus | - | 0.02 | 0.02 | - | - | |
| Artemisia argyi | - | 0.05 | 0.02 | 0.02 | 0.04 | |
| Erigeron annuus | - | - | 0.02 | 0.02 | 0.02 | |
| Erigeron acer | - | - | - | 0.04 | 0.04 | |
| Bidens pilosa | - | 0.03 | - | 0.03 | 0.02 | |
| Amaranthaceae | Alternanthera philoxeroides | 0.08 | - | - | - | - |
| Oxalidaceae | Oxalis corniculata | - | 0.03 | 0.06 | 0.07 | 0.08 |
| Apiaceae | Cnidium monnieri | - | - | 0.08 | - | - |
| Total: 7 | 15 | 6 | 11 | 12 | 9 | 9 |
| Measures | Coal Gangue Mountains | II | III | IV | V |
|---|---|---|---|---|---|
| βc | I | 8.50 | 8.50 | 11.00 | 11.50 |
| II | 7.00 | 10.50 | 11.00 | ||
| III | 7.00 | 7.50 | |||
| IV | 4.00 | ||||
| βws | I | 0.49 | 0.49 | 0.64 | 0.67 |
| II | 0.33 | 0.55 | 0.57 | ||
| III | 0.35 | 0.41 | |||
| IV | 0.26 | ||||
| Cj | I | 0.35 | 0.35 | 0.18 | 0.20 |
| II | 0.50 | 0.29 | 0.28 | ||
| III | 0.48 | 0.42 | |||
| IV | 0.59 | ||||
| Cs | I | 0.51 | 0.51 | 0.30 | 0.33 |
| II | 0.67 | 0.45 | 0.43 | ||
| III | 0.65 | 0.59 | |||
| IV | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, H.; Tian, S.; Jin, B.; Wang, Z.; Wang, J.; Zhang, Y.; Wang, Y.; Zhao, X. Effects of Vetiveria zizanioides on the Restoration and Succession of Coal Gangue Mountain Plant Communities in Different Years. Diversity 2022, 14, 843. https://doi.org/10.3390/d14100843
Shuai H, Tian S, Jin B, Wang Z, Wang J, Zhang Y, Wang Y, Zhao X. Effects of Vetiveria zizanioides on the Restoration and Succession of Coal Gangue Mountain Plant Communities in Different Years. Diversity. 2022; 14(10):843. https://doi.org/10.3390/d14100843
Chicago/Turabian StyleShuai, Honggang, Sihui Tian, Baocheng Jin, Zhaoyi Wang, Jigao Wang, Yaoyao Zhang, Yuefeng Wang, and Xuechun Zhao. 2022. "Effects of Vetiveria zizanioides on the Restoration and Succession of Coal Gangue Mountain Plant Communities in Different Years" Diversity 14, no. 10: 843. https://doi.org/10.3390/d14100843
APA StyleShuai, H., Tian, S., Jin, B., Wang, Z., Wang, J., Zhang, Y., Wang, Y., & Zhao, X. (2022). Effects of Vetiveria zizanioides on the Restoration and Succession of Coal Gangue Mountain Plant Communities in Different Years. Diversity, 14(10), 843. https://doi.org/10.3390/d14100843






