Abstract
Mexico is one of the richest countries in amphibian species (420 spp.), with a high level of endemism (69%). The order Anura represents the most diverse and widespread of the three extant amphibian orders (257 spp.). The anurofauna of Mexico’s tropical dry forest ecosystem host a high proportion of the species and endemism registered in the country. In terms of conservation, both dry forests and amphibians are at risk due to climate change because it is expected that as the temperature becomes higher and precipitation decreases, this vegetation type may experience water stress. We applied the MaxEnt algorithm to estimate the potential current and future (year 2070) geographic distribution patterns of 95 endemic Mexican anuran species inhabiting the country’s tropical dry forests by considering two representative concentration pathway scenarios (RCP4.5/RCP8.5) and analyzed the potential distributional pattern changes. The results indicated that overall, species would experience enough of a significant warming effect to cause a reduction in the original distribution area, with 44% of species losing an average of 50% of their original range (9 spp. in threatened category); additionally, 22% of the species in the dry forest ecosystem will experience an average increase of almost 50% in their original area, two species will lose more than 80% of their range, and one will disappear.
1. Introduction
In the 21st century, the environmental changes driven by climate change pose major risks to global biodiversity, affecting ecosystem processes, disturbing natural habitats, and driving shifts in species distribution [1,2]. The climate is an extremely important factor for species, since it acts as a natural selection factor, and this may be because all species experience physiological limits to environmental factors. Both temperatures and humidity are related to the development and physiology of organisms, as well as their spatial distribution, population dynamics, and interactions between species. It is expected, then, that major changes in the Earth’s climate patterns—such as those we are currently experiencing—will influence the biology, ecology, and geography of species [3,4,5]. There are numerous studies that show that species are responding to the effects of climate change by moving their distribution limits, sometimes increasing and sometimes reducing their ranges, but these changes may lead to the local disappearance or even extinction of species [6,7].
In the last two decades, amphibians have received international attention because in many parts of the world many populations are threatened and declining at faster rates compared to pre-Anthropocene mass extinction rates [8,9]. It is estimated that about 41% of all amphibian species are in danger of extinction [10] and perhaps one-fifth of the species have either become extinct or are on the brink of extinction [11]. Evidence suggests that climate change is one of the factors explaining these declines and that it will significantly increase the risk of extinction in the short term [12,13,14]. Is considered that more than 50% of the amphibian species are sensitive to climate change, and a 64% reduction in the current geographical range of endemic amphibians is projected by the year 2080, with 50% of the species losing more than 60% of their distribution [13]. This can be explained by the fact that amphibians are ectotherms and thermal conformers, thus rendering them sensitive to environmental temperature changes. Furthermore, many species are habitat specialists with limited geographic ranges, and they have a limited capacity for dispersal or colonization of new or more favorable areas, in addition to the fact that they are stenoic species, meaning that they present a reduced tolerance to environmental factors [9,15].
There are approximately 8000 known species of living amphibians, and the class Amphibia is constituted by three majors orders: Anura (~7000 species), Caudata, (~700 species), and Gymnophiona (>200 species) [16]. Mexico is considered a mega-diverse country and occupies the fifth place in terms of amphibian richness, with approximately 420 species, 291 of which are endemic, and ranks third in amphibian endemic species, comprising around 65% of the amphibian species [17]. The order Anura is the best represented and most widespread of the three extant amphibian orders, with 257 species [17,18,19]. Most of the endemic amphibian species in Mexico have restricted geographic distributions, and many of them are micro-endemic, which makes this group particularly threatened by climate change [14].
Mexico also is one of the countries that is most vulnerable to climate change because of its geographic location and topography, which make it particularly fragile to major damage resulting from extreme climatic events [15]. For example, according to climate change predictions, for the period 2015–2039, it is expected that around 50% of Mexico’s territory will have a higher temperature and less precipitation, leading to conditions of increased water stress [20,21]. For most of the country, the annual temperature increases are expected to be 1–1.5 °C and up to 2 °C for the northern zone, and the precipitation is projected to decrease by 10–20%. One of the ecosystems that is most threatened by climate change is the tropical deciduous forest (TDF), since it is estimated that the dry season will become more severe. This ecosystem has relatively high endemic species richness, and the species composition differs among sites and is the most important ecosystem currently occupied by the endemic amphibian species [13]. Other factors threatening TDF areas are the change in land use, habitat fragmentation (which is mainly due to agricultural activities and overgrazing, as well as the increase in human settlements), and forest fires, among others [21,22]. Therefore, the populations of amphibian species with very specific habitats and a restricted range have started to decrease in size and are becoming isolated, which can put them at risk of extinction; therefore, endemic species could become strongly affected, making their study and conservation a priority. Due to the high number of endemic species as well as the high rates of deforestation, this ecosystem is considered one of the highest priorities of conservation in Mexico and in the world [23].
Therefore, the goals of this study are (1) to obtain the current distribution of endemic anurans from Mexico across the TDF and project to the year 2070 under two Representative Concentration Pathways (RCP 4.5 and RCP 8.5) to obtain the future potential distribution by applying spatial distribution modelsand (2) to identify changes in potential species richness between current and future distribution.
2. Materials and Methods
2.1. Study Area
Tropical deciduous forests, also known as dry forests, low deciduous forests, or seasonally dry tropical forests, are characterized by communities that live in areas with a marked environmental seasonality and have a long dry season that lasts from four to eight months, while the rainy season lasts four to six months. The rain is concentrated within a few months, with the total annual precipitation being less than 1600 mm [22,24]. This seasonal water shortage determines the growth patterns and the phenological and physiological behavior of vegetation. The TDF community is dominated by tropical elements, with trees that vary in height from 8 to 12 m on average, and has canopy cover greater than 30% [22,25]. Deciduousness is an important adaptation undertaken by plants during periods of extended drought by alternating between deciduousness during the dry season and evergreen physiognomy during the rainy season. Most of the vegetation loses 50–100% of its foliage and many types of plants disperse their seeds during the dry season [26,27].
In Mexico, the TDF has a wide distribution, occupying approximately 11.7% (226, 898 km2) of the national surface, and is generally found from sea level to 1200 mamsl, although occasionally reaching up to 1900 mamsl. TDF is distributed from 29° N, in an almost continuous strip on the Pacific slope, from southern Sonora and southwestern Chihuahua to Chiapas, and continues to the border with Guatemala, in the south of México [24]. It is also found in the less dry areas of the Tehuacán-Cuicatlán Valley, in the Bajío region of the Mexican Plateau, in the Balsas Depression, in the southern portion of the Baja California peninsula (Figure 1) [13], and also on the slope of the Gulf of Mexico, from Tamaulipas to the Yucatán peninsula, in more isolated and discontinuous areas [25].
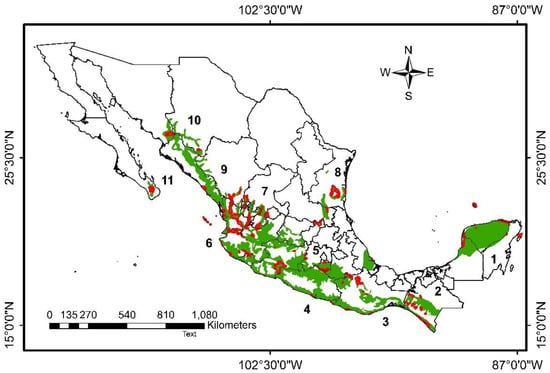
Figure 1.
Location of the tropical dry forest (TDF) in Mexico (green areas) and the Federal Protected Natural Areas in the TDF (red polygons). Numbers correspond to ecoregions included in the study region according to [13]: Yucatán forests (1), Chiapas forests (2), Centro American Pacific forests (3), Pacific South forests (4), Balsas River basin forests (5), Jalisco forest (6), Bajío forests (7), Tamaulipas-Veracruz forests (8), Sinaloa forests (9), Sonora forests (10), and Cape forests (11).
2.2. Species Records and Occurrence Data
We compiled a list of endemic anuran species from the Mexican tropical dry forest ecosystem based on the known species distribution. The occurrence data for each species were obtained from (a) published literature, (b) the Global Biodiversity Information Facility (http://www.gbif.org, accessed on 19 March 2020), (c) the Sistema Nacional de Información sobre Biodiversidad de México (www.snib.mx, accessed on 8 July 2021), and (d) unpublished data obtained from our field surveys. We select the occurrences records collected between 1970 and 2020, and each record was reviewed to identify uncertainty and mistakes in the geographic or taxonomic information. We eliminated records with (a) imprecise geographic information, (b) duplicate records, (c) records outside the natural range of the species, (d) records that could not be georeferenced, and (e) species with <5 records available for modeling purposes [28]. When the geographical coordinates were incomplete, we used the locality descriptions provided by collectors to georeference them using Global Gazetteer Version 2.3 (http://www.fallingrain.com, accessed on 15 July 2021) and Google Earth.
To avoid the over-adjustment and the sampling bias that occurs with data derived from field collection and to reduce their spatial autocorrelation, two filtering approaches were selected based on range size, as estimated by the minimum convex polygon (MCP) around all of the occurrence points of each species: (1) for widely distributed species, whose MCP values were higher than 30 km2, a thinning distance of 5 km was applied and (2) for micro endemic species, whose MCP values were lower than 30 km2, a thinning distance of 1 km was applied [29,30]. Once the databases were refined, we only included the species with a minimum of five records.
2.3. Environmental Data and Determination of the Accessible Area (M)
We obtained 19 environmental variables from the WorldClim database (http://www.worldclim.org/, accessed on 10 Novembrer 2013), which were interpolated from global climate datasets at a resolution of ~1 km2 (Table 1). These variables contain monthly information on precipitation and the average temperature, as well as their seasonality [31]. For the distribution model with climate change projections, the future climate variables were obtained for the general circulation model (GCM) GFDL-CM3 (Geophysical Fluid Dynamics Laboratory Climate Model), which has shown good performance in projecting the climatic conditions of North America [32]. We used two Representative Concentration Pathways (RCP), one moderate-forcing stabilization scenario (RCP4.5), and another a high-forcing scenario (RCP8.5) for the year 2070 (2045–2069). RCPs refer to the global radiation of energy expressed in W/m2 and they assume that this radiation varies as greenhouse gases (GHG) increase. All of these layers were clipped according to a “Potential vegetation” land cover map [33] and included a buffer zone of 30 km around this coverage (Figure 2). For each species, we delimited the area to calibrate the models, an approximation of the “M” or part of the world that is accessible to the species and those that have the potential to disperse [34]. For these, we clipped the cover by drawing a buffer of 30 km around the MCP of each species. This region includes environments that are probably accessible to the species given its dispersal limitations [35].

Table 1.
Environmental variables considered modeling the potential distribution.

Figure 2.
Locality records used for model performance (red dots). The black areas represent tropical dry forest [33], and the calibration area is shown in light gray. Numbers correspond to ecoregions included in the study region according to [13]: Yucatán forests (1), Chiapas forests (2), Centro American Pacific forests (3), Pacific South forests (4), Balsas River basin forests (5), Jalisco forest (6), Bajío forests (7), Tamaulipas-Veracruz forests (8), Sinaloa forests (9), Sonora forests (10), and Cape forests (11).
2.4. Potential Distribution Modeling
One of the most used methods to project how the distributions of species may change under different scenarios of climate change to predict the future distribution of species in the face of climate change is “species distribution models” (SDMs) that use environmental variables and localities of occurrence of the species to characterize places where a species occurs that can then be extrapolated to project future occurrences at locations where the correlated environmental features are projected to be present [36].We used the maximum entropy algorithm (MaxEnt v3.4.3) [37] to develop the potential distribution models (SDM). MaxEnt is one of the most used algorithms and is considered one of the most robust to generate models of the ecological niche and to obtain the species distribution with adequate results, even for species with small sample sizes [38,39]. MaxEnt is a software based on the principle of maximum entropy which calculates the most probable distribution for species by considering two data inputs: occurrence data and digital environmental layers [40].
Prior to modeling the potential distribution and considering the possibility of multicollinearity among the variables, we performed a correlation analysis (Pearson’s coefficient) to reduce over-fitting and the collinearity of the environmental layers as well as redundant variables. We used those variables with values of |r| < 0.85 [41]. To obtain estimates of the percentages of contribution for each variable and to determine which variables were most important for the performance of each potential distribution model we used a Jackknife analysis [42].
To generate the SDM, the species were classified into two groups depending on the number of occurrences: (1) species with >20 different occurrences and (2) species with <20. For group 1, modeling was performed by dividing the data into 75% for training and 25% for validation [43], while for group 2, due to the low number of occurrences, 80% of the data were used for training data and 20% were used for test data. To configure MaxEnt the following parameters were used: maximum number of iterations = 500, convergence threshold = 0.00001, and 10 replicates [40]. The final models were validated through the cross-validation of the area under the curve (AUC) of the receiver operating characteristic curve (ROC); the values that the AUC can take range from 1, when there is a perfect fit of the model, to 0. An AUC value of 0.5 indicates a low predictive value, since the adjustment is equal to that of a model made with points taken at random [40]. To obtain the prediction maps, the 10th percentile value was used as the cut-off point, to maximize sensitivity and minimize specificity. Finally, the maps that were obtained were converted to binary maps (presence/absence), where a value equal to 1 refers to a possible presence (suitable habitat) while a value of 0 indicates absence (unsuitable habitat). Additionally, changes in potential species richness between the present and the climate change scenario for the future (RPC 4.5 and 8.5) were analyzed. For this, potential richness maps were constructed for each RCP by adding the binary models of each species. Therefore, the value of each pixel was equivalent to the number of species present in that region. Changes in potential richness were evaluated by calculating the difference between the percentage of individual areas and comparing the present and the future (with respect to RCP 4.5 and RCP 8.5).
3. Results
3.1. Species and Model Performance
A list of 95 species of anurans endemic to Mexico inhabiting the TDF ecosystem and that are distributed in 24 states of the Republic was obtained, with the states of Oaxaca (49 spp.), Guerrero (44 spp.), Jalisco (33 spp.), and Michoacán (30 spp.) having the highest number of species. However, the final base used to generate the SMD (species that had more than five different records) was made up of 6040 occurrence data points, including five families (Bufonidae, Craugastoridae, Eleutherodactylidae, Hylidae, and Ranidae), 17 genera, and 62 species. Of the 62 endemic anuran species considered in the analysis, 61 were in some category of risk according to the International Union for Conservation of Nature (IUCN): 37 species are in Least Concern (LC); 4 Near Threatened (NT); 10 Vulnerable (Vu); 8 Endangered (En); and 2 Critically Endangered (CR). The number of occurrences per species ranged from a minimum of 5 records, as in Charadrahyla pinorum and Plectrohyla celata, to a maximum of 831 records, such as Pachymedusa dacnicolor, with an average of 97 records (Table S1).
The validation tests for the 62 species indicated that the models have a good predictive capacity. The analysis of the ROC curves of all the species provided high values of the AUC, greater than 0.9, except for five species, whose values ranged between 0.74 and 0.893. Based on this, we assume that the models were robust enough to predict the presence of species in the study area. Regarding the results of the climatic variables, in general, it was observed that under the present conditions, the variables Bio 18 (precipitation of the warmest quarter), Bio 15 (seasonality of precipitation), and Bio 04 (temperature seasonality) were the variables that presented the greatest contributions (Table S1).
3.2. Current and Future Suitable Habitat
Under current climate conditions, the habitat suitability for the species indicates that the sizes of the distribution areas range from 3205 km2 for Eleutherodactylus pallidus to 501,962 km2 for E. verruculatus (average area for all species = 75,294.6 km2). The richness patterns for the present indicate a greater number of species in the part of the forest along the Pacific Coast, where up to 20 species are distributed, particularly in the provinces of the Sierra Madre del Sur and the Pacific, in the Balsas River basin (18 spp.), in the forests of the Central American Pacific (18 spp.), in the Jalisco forest (19 spp.), and in the forests of the South Pacific (20 spp.). On the other hand, the lowest richness was recorded in the Yucatán forest (2 spp.) and in the Tamaulipas-Veracruz forest (4 spp.), where four species were distributed (Figure 3A). For the year 2070, according to RCP 4.5, a decrease is generally predicted in all of the TDFs; however, the greatest differences in richness are predicted to occur in the Jalisco forest (9 spp.) and in the Pacific South forests. (13 spp.). These areas will potentially experience the greatest species loss and range reductions, although a hotspot will remain in the Central American Pacific forests with a predicted species distribution of 19 species (Figure 3B). Regarding potential distribution areas, the smallest size distribution area obtained was 819 km2 for E. nivicolimae, while the species with the largest area of 714,214 km2 was E. verruculatus (mean = 65,343 km2). These results indicate that for the year 2070 and under RCP 4.5, the predicted area size change between species ranged from an increase of 249% to a decrease of 89.7% (mean = 86.9%; Table S1, Figure 4). In particular, 17 species (27.4%) were predicted to experience an increase in range size, with a mean increase of 69.5%. The 45 species that show habitat contraction (the remaining 72.6%) show an average reduction in their range of 44.2%. Of these, 28 species (45%) could potentially show a decrease of more than 50% of their current range. The RCP 8.5 results show a pattern similar to RCP 4.5 but more pronounced (Figure 4), as well as a general decrease in richness in the TDF, although the hotspot in the Central American Pacific forests will potentially increase its richness with 21 species (Figure 3C). E. nivicolimae still has the smallest potential distribution area of 71 km2, while E. verruculatus has the largest distribution area of 777,239 km2 (average = 62,756 km2). In addition, 18 species will potentially increase their areas between 1.4% and 391.4% (mean = 111.4%). Of the 44 shelf species, 28 species are expected to lose more than 50% of their current range (mean = 76.9%) and 16 species are expected to lose between 6.6% and 30.6% of their current range. In total, we found that of the 62 species studied, 6 will be especially affected by the contraction of their distribution area, losing more than 70% of their distribution area in both RCPs: Exerodonta xera, Eleutherodactylus dilatus, E. nivicolimae, Lithobates spectabilis, Craugastor rugulosus, and C. yucatanensis. The most affected species could be C. yucatanensis and E. dilatus, since by RCP 8.5 it is predicted that they will lose between 98% and 99% of their distribution, respectively.
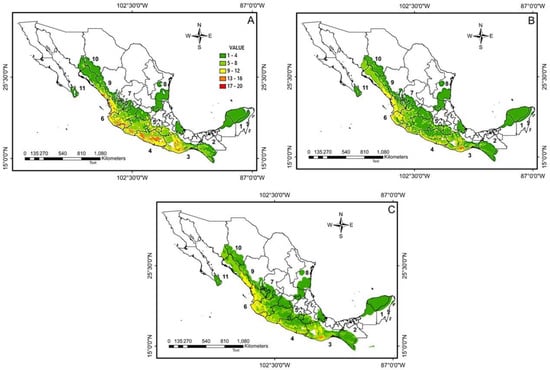
Figure 3.
Species richness predicted for 62 anurans endemic to the Mexican tropical dry forest is shown for: (A) present, (B) RCP 4.5 in 2070, and (C) RCP 8.5 in 2070.

Figure 4.
Differences in rate of range change (%) between the two Representative Concentration Pathways (RCPs), dots represent those species that will increase their area more than the average.
4. Discussion
We are currently in the sixth mass extinction period caused by factors such as climate change, land use change, the overexploitation of natural resources, pollution, and introduced species [11]. Although climate change is one of the greatest topics of interest today, its study is complicated due to the global scale on which it is presented, as well as the impossibility of determining a single future climate change scenario. The species that are supposed to be more susceptible to climate change are those that present more specific physiological requirements, dependence on a specialized habitat, reduced tolerance to environmental factors, and a limited vagility or ability to colonize new favorable areas [5]. In 2010 the IUCN determined that 52% of amphibian species are susceptible to climate change. This high susceptibility makes amphibians ideal species to analyze and measure the effects of disturbances in the environment. For this reason, they are called bioindicator species [44]. Within amphibians, the Anura family represents an important case in the study of the impacts of climate change, since this taxon is one of the most abundant and diverse groups within amphibians and is found in aquatic, terrestrial, arboreal, fossorial, and marine habitats on practically all continents [18,45]. The degree of endemism in the herpetofauna in TDFs is one of the highest in the country, representing more than half of all endemic species in Mexico, surpassed only by tropical forests [13,17].
The present study provides information about the impact of climate change on the distribution of the endemic anurans of Mexico that inhabit one of the most vulnerable ecosystems due to climate change and deforestation: the TDF systems. TDFs have also been poorly studied and are one of the least preserved ecosystems of tropical ecosystems [46]. The results suggest potentially significant climatic effects for the year 2070 for both RCPs (4.5 and 8.5); for example, the highest potential richness values for the present are in areas between 1100–1600 mamsl. However, the predicted changes for the future in both RCPs show that the richest sites will be found at altitudes between 1600–2100 mamsl (Figure 2). These results are consistent with the documented effects of climate change on the distribution of various species, which suggest that species will move to higher altitudes [5,47] and that species that inhabit tropical areas can invade areas of coniferous forests [48]. However, if there are no higher altitudes within the geography where they can colonize, as is the case in the Yucatán Peninsula, the species will reduce their distribution area, as in the case of C. yucatanensis, which could disappear. For both RCPs, the same general richness pattern is presented for the endemic anuran species of TDFs: the species richness will be diminished, and the areas with the greatest potential richness will be considerably smaller and restricted to the areas of the Central American Pacific forests in the state of Oaxaca, which coincides with the pattern found in previous studies [13] for amphibians endemic to western Mexico. Our study suggests an average reduction in the current geographic distribution of around 44% for RCP 4.5 and 60% for RCP 8.5. These data are similar to those reported in another study, which report a reduction of 64% in the current geographical range of endemic amphibians in the TDF of the Pacific Coast by 2080 as a consequence of climate change [13]. This is explained by the increase in temperature due to the climatic conditions in the temperate zones towards warmer conditions, which will allow various species to colonize areas near the Sierra Madre Occidental and the western part of the Trans-Mexican Volcanic Belt. This is explained by the fact that the increase in temperature occurring in temperate zones will eventually shift them to warmer areas that are favorable for several species. Although our results indicate that 45% of the species in the TDF ecosystem will lose more than 50% of their current distribution area, it is also expected that there are some species (18%) that could increase their area by an average of 111%, but species such Lithobates omiltemanus, Exerodonta xera, Plectrohyla chryse, Charadrahyla altipotens, E. melanomma, P. crassa, P. ciclada, and Quilticohyla erythromma are predicted to be more vulnerable by potentially losing more than 50% of their current distribution, and are in the critical risk category. These highly vulnerable species should be the priority in future conservation activities and would require special protection plans and research of life history traits, population status, and habitat conditions.
In general, the government mitigation strategy for the effects of climate change focuses on establishing protected natural areas. However, the PNAs that currently exist in Mexico are still insufficient to guarantee the protection of endemic anuran species, since the analysis carried out shows a low representativeness of the potential distribution area within the ANPs, and the PNAs do not cover the sites of greatest predicted potential richness of the endemic anurans. Another problem is that the many of the PNAs are very small and already have some degree of anthropogenic management, in addition to being in the form of discontinuous patches or ecological islands (Figure 1). This lack of connectivity between the conserved low deciduous forest patches may affect the viability of the species or that they may move to sites with suitable environmental conditions in the future. It is clear, therefore, that given the vulnerability of the amphibian species, as well as the habitat, monitoring and search of the populations are required, as well as the establishment of new PNAs that conserve the TDF, to prevent reserves from remaining as isolated patches. In addition, it must be taken into account that the climatic condition of the current PNAs may possibly no longer be suitable in the future for amphibians due to climate change, requiring PNA planning to conserve habitats. For example, it is necessary to increase the number of these protection zones, especially in the states of Campeche, Estado de México, Guanajuato, Guerrero, Sinaloa, and Yucatán and propose PNAs in the zones with the greatest potential richness predicted in the future, such as in the Central American Pacific forests in the state of Oaxaca.
These results could be used as a basis to carry out studies on the ecology and physiology of the most vulnerable species, or to compare the biology of species that are benefited in terms of increasing the area with adequate environmental conditions against species that will have a negative response. It is important to take into account that changes in the environmental conditions of the TDF caused by climate change with high temperatures and seasonality in the rain will have repercussions on the biology of the amphibians. In general amphibians tend to be more susceptible to increases in temperature than to its decreases, since species located in the tropics could be especially threatened by increases in temperatures, because tropical species live closer to their upper limits of thermal tolerance than mid-latitude species and thus are more likely to decline in fitness [49]. Some studies have recorded the effects of global warming in changes in their reproduction, as because amphibians arrive at breeding sites before the spring rains, their chances of desiccation may increase. In addition, an increase in temperatures and less precipitation in seasonal ecosystems can decrease the bodies of water where amphibians lay eggs, with which the progeny would be lost [50], which could happen in the future in the bodies of water in the TDF of Mexico. For example, on the south coast of Jalisco extreme climatic variations are already perceived; in recent years they have recorded rainfall up to 40% lower than average [51]. Additionally, amphibians often exhibit water-conserving behaviors when conditions are dry, such as increasing their use of their roost and burying and decreasing their activity and exposed surface area [52]. These behaviors, in most cases, prevent the search for food and reproductive activities. The reduction in populations due to the mentioned factors and their low vagility could prevent them from moving to areas with adequate environmental conditions.
In general, our results should be taken with caution, since future predictions do not prove that changes in the distribution are taking place. Species distribution and ecological niche models provide static results that reveal where sites with suitable environmental conditions for species will exist in a climatically changing future but do not explicitly consider all the processes leading to the predicted changes [49]. The resulting maps are a geographic expression of the environmental niche and do not consider historical restrictions, geographic barriers, biotic interactions, or changes in land use [34]. Likewise, these predictions are subject to high levels of uncertainty [50]. Recent studies have shown that the projections derived from these models are sensitive to the assumptions on which they are based; the type, quantity, and spatial resolution of environmental variables; the future climate change scenarios selected; and the class of algorithm selected [53,54]. However, in general, these methods have demonstrated good predictive power [55], and our work also considered two climate change scenarios using a moderate scenario (RCP 4.5) and a pessimistic scenario (RCP 8.5) to try to cover different climate change projections.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14080650/s1. Table S1: List of the 62 endemic anuran species distributed within Mexican Tropical Dry Forest considered in this study.
Author Contributions
C.B.-B. conceived, designed, planned, performed analyses, and led the writing; O.T.-P. conceived, designed, planned, and performed analyses; A.L.-M. contributed records, revised taxonomy, and reviewed drafts of the paper and contributed to the data analyses; R.Z.-H., A.M.-B., B.V.-M. and S.O.-B. reviewed drafts of the paper and contributed to the data analyses; M.M.-C. contributed records and revised taxonomy. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the the Universidad Autónoma Metropolitana by the project “Environmental diversity, biological diversity and climate change: implications for conservation” (No. UAM 2015-2018), as well as the Postgraduate Master’s Degree in Biology program of the Universidad Autónoma Metropolitana and the National Council for Science and Technology (CONACyT, Mexico, No. 592795) for the academic and financial support for Oscar Tapia Pérez’s Master’s degree in Biology.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Scholes, R.J.; Agard, J.; Archer, E.; Arneth, A.; Bai, X.; Barnes, D.; Burrows, M.; Chan, L.; Cheung, W.L.; et al. IPBES-IPCC Co-Sponsored Workshop Report on Biodiversity and Climate Change. IPBES-IPCC Co-Sponsored Workshop Report on Biodiversity and Climate Change, 1st ed.; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2021; pp. 15–17. [Google Scholar] [CrossRef]
- Hansen, A.; Neilson, R.P.; Dale, V.H.; Flather, C.H.; Iverson, L.R.; Currie, D.J.; Shafer, S.; Cook, R.; Bartlein, P.J. Global changes in forest: Responses of species, communities and biomes. BioScience 2001, 51, 765–779. [Google Scholar] [CrossRef]
- Parmesan, C. Climate and species’ range. Nature 1996, 382, 765–766. [Google Scholar] [CrossRef]
- Parmesan, C.; Nils, R.; Stefanescus, C.; Hill, J.K.; Thomas, D.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Polewards shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Biggs, R.; Simons, H.; Bakkenes, M.; Scholesa, R.J.; Eickhout, B.; Van Vuuren, D.; Alkemade, R. Scenarios of biodiversity loss in southern Africa in the 21st century. Glob. Environ. Chang. 2008, 18, 296–309. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and Status and trends of amphibian declines and extinction worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 15, 11466–11473. Available online: www.pnas.org/cgi/doi10.1073/pnas.0801921105 (accessed on 20 February 2022). [CrossRef] [PubMed]
- Abarca-Alvarado, J.G. Endangered amphibians: Threats and effective conservation strategies. Biocenosis 2021, 32, 133–145. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Raven, P.H. Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 13596–13602. Available online: https://www.pnas.org/doi/full/10.1073/pnas.1922686117 (accessed on 10 February 2022). [CrossRef] [PubMed]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- García, A.; Ortega-Huerta, M.A.; Martínez-Meyer, E. Potential distributional changes and conservation priorities of endemic amphibians in western Mexico as a result of climate change. Environ. Conserv. 2013, 47, 1–12. [Google Scholar] [CrossRef]
- Ochoa-Ochoa, L.; Rodríguez, P.; Mora, F.; Flores-Villela, O.; Whittaker, R. Climate change and amphibian diversity patterns in Mexico. Biol. Conserv. 2012, 150, 94–102. [Google Scholar] [CrossRef]
- World Wildlife Fundation (WWF). Impactos y Vulnerabilidad al Cambio Climático en México [en línea]. 2010. Available online: http://d2ouvy59p0dg6k.cloudfront.net/downloads/03_impactos_nacionales_e_internacionales_del_cambio_climatico.pdf (accessed on 5 March 2022).
- Wake, D.B.; Koo, M.S. Primer: Amphibians. Curr. Biol. 2018, 28, R1237–R1241. [Google Scholar] [CrossRef] [PubMed]
- Balderas-Valdivia, C.J.; González-Hernández, A. Inventario de la herpetofauna de México 2021. Herpetol. Mex. 2021, 2, 10–71. [Google Scholar]
- Parra-Olea, G.; Flores-Villela, O.; Mendoza-Almeralla, C. Biodiversidad de anfibios en México. Rev. Mex. Biodivers. Supl. 2014, 85, S460–S466. [Google Scholar] [CrossRef]
- Stuart, S.N.; Hoffmann, M.; Chanson, J.S.; Cox, N.A.; Berridge, R.J.; Ramani, P.; Young, B.E. Threatened Amphibians of the World, 1st ed.; Lynx Edicions: Barcelona, Spain, 2008; pp. 2–3. [Google Scholar]
- Balvanera, P.; Mass, M. Los Servicios Ecosistémicos Que Proveen las Selvas Secas. In Diversidad, Amenazas y Áreas Prioritarias Para la Conservación de las Selvas Secas del Pacífico de México, 1st ed.; Ceballos, G., Martínez, L., García, A., Espinoza, E., Bezaury Creel, J., Dirzo, R., Eds.; Fondo De Cultura Económica (FCE): Mexico City, México; Comisión Nacional para el Fomento y Uso de la Biodiversidad (CONABIO): Mexico City, México; Comisión Nacional de Áreas Naturales Protegidas (CONANP): Mexico City, México, 2010; pp. 251–270. ISBN 970-9000-38-1. [Google Scholar]
- Jaramillo, V.; García-Oliva, F.; Martínez-Yrízar, A. La selva seca y las perturbaciones antrópicas en un contexto funcional. In Diversidad, Amenazas y Áreas Prioritarias Para la Conservación de Las Sevas Secas del Pacífico de México, 1st ed.; Ceballos, G., Martínez, L., García, A., Espinoza, E., Bezaury Creel, J., Dirzo, R., Eds.; Fondo De Cultura Económica (FCE): Mexico City, México; Comisión Nacional para el Fomento y Uso de la Biodiversidad (CONABIO): Mexico City, México; Comisión Nacional de Áreas Naturales Protegidas (CONANP): Mexico City, México, 2010; pp. 235–250. ISBN 970-9000-38-1. [Google Scholar]
- Banda, R.K.; Delgado-Salinas, A.; Dexter, K.G.; Linares-Palomino, R.; Oliveira-Filho, A.; Prado, D.; Pullan, M.; Quintana, C.; Riina, R.; Rodriguez, M.G.M.; et al. Plant diversity patterns in neotropical dry forests and their conservation implications. Science 2016, 6306, 1383–1387. [Google Scholar] [CrossRef]
- Ceballos, G. Vertebrate diversity, ecology, and conservation in Neotropical dry forests. In Seasonally dry Tropical Forests, 1st ed.; Bullock, S.H., Mooney, H.A., Medina, E., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 195–220. [Google Scholar]
- Trejo, I. Las selvas secas del Pacífico mexicano. In Diversidad, Amenazas y Áreas Prioritarias Para la Conservación de las Selvas Secas del Pacífico de México, 1st ed.; Ceballos, G., Martínez, L., García, A., Espinoza, E., Bezaury Creel, J., Dirzo, R., Eds.; Fondo De Cultura Económica (FCE): Mexico City, México; Comisión Nacional para el Fomento y Uso de la Biodiversidad (CONABIO): Mexico City, México; Comisión Nacional de Áreas Naturales Protegidas (CONANP): Mexico City, México, 2010; pp. 41–52. ISBN 970-9000-38-1. [Google Scholar]
- Trejo-Vázquez, I. El clima de la selva baja caducifolia en México. Investig. Geográficas 1999, 39, 40–52. [Google Scholar] [CrossRef]
- Ceballos, G.; Valenzuela, D. Diversidad, ecología y conservación de los vertebrados de Latinoamérica. In Diversidad, Amenazas y Áreas Prioritarias Para la Conservación de las Selvas Secas del Pacífico de, 1st ed.; Ceballos, G., Martínez, L., García, A., Espinoza, E., Bezaury Creel, J., Dirzo, R., Eds.; Fondo De Cultura Económica (FCE): Mexico City, México; Comisión Nacional para el Fomento y Uso de la Biodiversidad (CONABIO): Mexico City, México; Comisión Nacional de Áreas Naturales Protegidas (CONANP): Mexico City, México, 2010; pp. 94–118. ISBN 970-9000-38-1. [Google Scholar]
- Huechacona-Ruiz, A.H.; Dupuy, J.M.; Schwartz, N.B.; Powers, J.S.; Reyes-García, C.; Tun-Dzul, F.; Hernández-Stefanoni, J.L. Mapping Tree Species Deciduousness of Tropical Dry Forests Combining Reflectance, Spectral Unmixing, and Texture Data from High-Resolution Imagery. Forest 2020, 11, 1234. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Benavides, E.; Breceda, A.; Anadón, J.D. Winners and losers in the predicted impact of climate change on cacti species in Baja California. Plant Ecol. 2021, 222, 29–44. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.A. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2015, 275, 73–77. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high-resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Griffies, S.M.; Winton, M.; Donner, L.J.; Horowitz, L.W.; Downes, S.M.; Farneti, R.; Gnanadesikan, A.; Hurlin, W.J.; Lee, H.; Liang, Z.; et al. The GFDL CM3 coupled climate model: Characteristics of the ocean and sea ice simulations. J. Clim. 2011, 24, 3520–3544. [Google Scholar] [CrossRef]
- Rzedowski, J. Vegetación Potencial. IV.8.2. Atlas Nacional de México. Vol II. Escala 1:4000000. Instituto de Geografía, UNAM. México. 1990. Available online: http://www.conabio.gob.mx/informacion/gis/ (accessed on 27 July 2021).
- Soberón, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Guevara, L.; Gerstner, B.E.; Kass, J.M.; Anderson, R.P. Toward ecologically realistic predictions of species distributions: A cross-time example from tropical montane cloud forests. Glob. Chang. Biol. 2018, 24, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Wiensa, J.A.; Stralberga, D.; Jongsomjita, D.; Howella, C.A.; Snyderb, M.A. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc. Natl. Acad. Sci. USA 2009, 106, 19729–19736. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Zimmermann, N.E. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Pérez-García, B.; Liria, J. Modelos de nicho ecológico fundamental para especies del género Thraulodes (Ephemeroptera: Leptophlebiidae: Atalophlebiinae). Rev. Mex. Biodivers. 2013, 84, 600–611. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Meza-Joya, F.L.; Rojas-Morales, J.A.; Ramos, E. Predicting distributions of rare species: The case of the false coral snake Rhinobothryum bovallii (Serpentes: Colubridae). Phyllomedusa 2020, 19, 141–164. [Google Scholar] [CrossRef]
- Berriozabal-Islas, C.; Mota Rodrigues, J.F.; Ramírez-Bautista, A.; Becerra-López, J.L.; Nieto-Montes de Oca, A. Effect of climate change in lizards of the genus Xenosaurus (Xenosauridae) based on projected changes in climatic suitability and climatic niche conservatism. Ecol. Evol. 2018, 8, 6860–6871. [Google Scholar] [CrossRef] [PubMed]
- Correia, L.O.; Siqueira Júnior, S.; Carneiro, P.L.S.; Bezerra, M.A. Evaluation of the use of Leptodactylus ocellatus (Anura: Leptodactylidae) frog tissues as bioindicator of metal contamination in Contas River, Northeastern Brazil. An. Acad. Bras. Ciênc 2014, 86, 1549–1561. [Google Scholar] [CrossRef]
- García, A. Reptiles y anfibios. In Diversidad, Amenazas y Áreas Prioritarias Para la Conservación de las Selvas Secas del Pacífico de México, 1st ed.; Ceballos, G., Martínez, L., García, A., Espinoza, E., Bezaury Creel, J., Dirzo, R., Eds.; Fondo De Cultura Económica (FCE): Mexico City, México; Comisión Nacional para el Fomento y Uso de la Biodiversidad (CONABIO): Mexico City, México; Comisión Nacional de Áreas Naturales Protegidas (CONANP): Mexico City, México, 2010; pp. 165–178. ISBN 970-9000-38-1. [Google Scholar]
- Prieto-Torres, D.A.; Navarro-Sigüenza, A.G.; Santiago-Alarcón, D.; Rojas-Soto, O.R. Response of the endangered tropical dry forests to climate change and the role of Mexican Protected Areas for their conservation. Glob. Chang. Biol. 2016, 22, 364–379. [Google Scholar] [CrossRef]
- Pounds, J.A.; Fogden, M.P.L.; Campbell, J.H. Biological response to climate change on a tropical mountain. Nature 1999, 398, 611–615. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Climate Change and Biodiversity. IPCC Technical Paper V. IPCC-WMO-UNEP. 2002. Available online: https://www.tnrf.org/files/E-INFO_IPCC_2002_Climate_Change_and_Biodiversity_0.pdf (accessed on 28 July 2021).
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Li, Y.; Cohen, J.M.; Rohr, J.R. Review and synthesis of the effects of climate change on amphibians. Integr. Zool. 2013, 8, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Madison, D.M. Dryness increase predation risk in efts: Support for an amphibian decline hypothesis. Oecologia 2003, 135, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Theurillat, J.P. Monitoring Networks for Testing Model-Based Scenarios of Climate Change Impact on Mountain Plant Distribution. In Global Change and Mountain Regions. Advances in Global Change Research; Huber, U.M., Bugmann, H.K.M., Reasoner, M.A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 467–476. [Google Scholar]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species-climate impact models under climate change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Graham, C.H. The ability of climate envelope models to predict the effect of climate change on species distributions. Glob. Chang. Biol. 2006, 12, 2272–2281. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).