Seasonal and Interspecific Variation in Frugivory by a Mixed Resident-Migrant Overwintering Songbird Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Design
2.3. Defining Fall and Winter
2.4. Bird Capture and Sample Collection
2.5. Fecal Analysis
2.6. Isotope Sample Preparation and Analysis
2.7. Comparing Community-Level Diet Divergence between Summer, Fall, and Winter
2.8. Potential Foods and Their Isotopic Values
2.9. Assessing Distinctiveness of Dietary Endpoints
2.10. Dietary Discrimination Values
2.11. Mixing Model Diet Estimation and Comparison of Fall and Winter Resource Use
3. Results
3.1. Characterizing Dietary Endpoints
3.2. Seasonal Changes in Diet Dispersion
3.3. Variability and Seasonal Patterns of Resource Use
3.4. Comparison of Fecal and Mixing Model Estimates of Songbird Fruit Consumption
4. Discussion
4.1. Fruit and Seed Contributions to Assimilated Songbird Diets
4.2. Comparing Estimates Derived from Stable Isotope Modeling to Those from Feces
4.3. Seasonal Changes in Diet: The Importance of Fruits and Seeds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renton, K. Lilac-crowned parrot diet and food resource availability: Resource tracking by a parrot seed predator. Condor 2001, 103, 62–69. [Google Scholar] [CrossRef]
- Paltridge, R. The diets of cats, foxes and dingoes in relation to prey availability in the Tanami Desert, Northern Territory. Wildl. Res. 2002, 29, 389–403. [Google Scholar] [CrossRef] [Green Version]
- Soininen, E.M.; Ravolainen, V.T.; Bråthen, K.A.; Yoccoz, N.G.; Gielly, L.; Ims, R.A. Arctic small rodents have diverse diets and flexible food selection. PLoS ONE 2013, 8, e68128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasov, W.H.; Martínez del Rio, C.; Caviedes-Vidal, E. Ecological physiology of diet and digestive systems. Annu. Rev. Physiol. 2011, 73, 69–93. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.A.; Martin, L.B.; Wingfield, J.C.; McWilliams, S.R.; Dunne, J.A. Physiological regulatory networks: Ecological roles and evolutionary constraints. Trends Ecol. Evol. 2012, 27, 428–435. [Google Scholar] [CrossRef]
- Barboza, P.S.; Parker, K.L.; Hume, I.D. (Eds.) Integrative Wildlife Nutrition; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-87884-1. [Google Scholar]
- Karasov, W.H.; Martínez del Rio, C. Physiological Ecology; Princeton University Press: Princeton, NJ, USA, 2007; ISBN 9780691213316. [Google Scholar]
- Raubenheimer, D.; Simpson, S.J.; Mayntz, D. Nutrition, ecology and nutritional ecology: Toward an integrated framework. Funct. Ecol. 2009, 23, 4–16. [Google Scholar] [CrossRef]
- Nwaogu, C.J.; Galema, A.; Cresswell, W.; Dietz, M.W.; Tieleman, B.I. A fruit diet rather than invertebrate diet maintains a robust innate immunity in an omnivorous tropical songbird. J. Anim. Ecol. 2020, 83, 867–883. [Google Scholar] [CrossRef]
- Bairlein, F. How to get fat: Nutritional mechanisms of seasonal fat accumulation in migratory songbirds. Naturwissenschaften 2004, 89, 1–10. [Google Scholar] [CrossRef]
- Stephens, R.B.; Hobbie, E.A.; Lee, T.D.; Rowe, R.J. Pulsed resource availability changes dietary niche breadth and partitioning between generalist rodent consumers. Ecol. Evol. 2019, 9, 10681–10693. [Google Scholar] [CrossRef] [Green Version]
- Bairlein, F. Fruit-Eating in Birds and Its Nutritional Consequences. Biochem. Physiol. 1996, 113, 215–224. [Google Scholar] [CrossRef]
- Parrish, J.D. Patterns of frugivory and energetic condition in nearctic landbirds during autumn migration. Condor 1997, 99, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.B.; McPherson, K.H.; Backer, J.M.; Pierce, B.J.; Podlesak, D.W.; McWilliams, S.R. Fruit quality and consumption by songbirds during autumn migration. Wilson J. Ornithol. 2007, 119, 419–428. [Google Scholar] [CrossRef]
- Strong, C.M.; Brown, D.R.; Stouffer, P.C. Frugivory by wintering Hermit Thrush in Louisiana. Southeast. Nat. 2005, 4, 627–638. [Google Scholar] [CrossRef]
- Mudrzynski, B.M.; Norment, C.J. Influence of habitat structure and fruit availability on use of a northeastern stopover site by fall songbirds. Wilson J. Ornithol. 2013, 125, 744–754. [Google Scholar] [CrossRef]
- Smith, A.D.; McWilliams, S.R. Fruit removal rate depends on neighborhood fruit density, frugivore abundance, and spatial context. Oecologia 2014, 174, 931–942. [Google Scholar] [CrossRef]
- Levey, D.J.; Martinez del Rio, C. It takes guts (and more) to eat fruit: Lessons from avian nutritional ecology. Auk 2001, 118, 819–831. [Google Scholar] [CrossRef]
- Podlesak, D.W.; McWilliams, S.R.; Hatch, K.A. Stable isotopes in breath, blood, feces and feathers can indicate intra-individual changes in the diet of migratory songbirds. Oecologia 2005, 142, 501–510. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Isagi, Y. Dietary breadth of frugivorous birds in relation to their feeding strategies in the lowland forests of central Honshu, Japan. Oikos 2012, 121, 1041–1052. [Google Scholar] [CrossRef]
- Plein, M.; Langsfeld, L.; Neuschulz, E.L.; Schulthei, C.; Ingmann, L.; Topfer, T.; Bohning-Gaese, K.; Schleuning, M. Constant properties of plant-frugivore networks despite fluctuations in fruit and bird communities in space and time. Ecology 2013, 94, 1296–1306. [Google Scholar] [CrossRef]
- Levey, D.J.; Karasov, W.H. Gut passage of insects by European Starlings and comparison with other species. Auk 1994, 111, 478–481. [Google Scholar] [CrossRef]
- Afik, D.; Karasov, W.H. The trade-offs between digestion rate and efficiency in warblers and their ecological implications. Ecology 1995, 76, 2247–2257. [Google Scholar] [CrossRef]
- Klasing, K.C. Comparative Avian Nutrition; CAB International: Wallingford, UK, 1998; ISBN 9780851992198. [Google Scholar]
- Hobson, K.A.; Bairlein, F. Isotopic fractionation and turnover in captive Garden Warblers (Sylvia borin): Implications for delineating dietary and migratory associations in wild passerines. Can. J. Zool. 2003, 81, 1630–1635. [Google Scholar] [CrossRef]
- Ayliffe, L.K.; Cerling, T.E.; Robinson, T.; West, A.G.; Sponheimer, M.; Passey, B.H.; Hammer, J.; Roeder, B.; Dearing, M.D.; Ehleringer, J.R. Turnover of carbon isotopes in tail hair and breath CO 2 of horses fed an isotopically varied diet. Oecologia 2004, 139, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W.; Semmens, B.X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 2008, 11, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L. Converting isotope values to diet composition: The use of mixing models. J. Mammal. 2012, 93, 342–352. [Google Scholar] [CrossRef]
- Gagnon, C.; Hobson, K.A. Using stable isotopes to track frugivory in migratory passerines. Can. J. Zool. 2009, 87, 981–992. [Google Scholar] [CrossRef]
- Gómez, C.; Larsen, T.; Popp, B.; Hobson, K.A.; Cadena, C.D. Assessing seasonal changes in animal diets with stable—Isotope analysis of amino acids: A migratory boreal songbird switches diet over its annual cycle. Oecologia 2018, 187, 1–13. [Google Scholar] [CrossRef]
- Ruhl, P.J.; Flaherty, E.A.; Dunning, J.B. Using stable isotopes of plasma, red blood cells, feces, and feathers to assess mature-forest bird diet during the post-fledging period. Can. J. Zool. 2020, 98, 39–46. [Google Scholar] [CrossRef]
- Bond, A.L.; Diamond, A.W. Recent bayesian stable-isotope mixing models are highly sensitive to variation in discrimination factors. Ecol. Appl. 2011, 21, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.L.; Inger, R.; Bearhop, S.; Jackson, A.L.; Moore, J.W.; Parnell, A.C.; Semmens, B.X.; Ward, E.J. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 2014, 835, 823–835. [Google Scholar] [CrossRef] [Green Version]
- Law, R.; Morton, R.D. Permanence and the assembly of ecological communities. Ecology 1996, 77, 762–775. [Google Scholar] [CrossRef]
- Valdovinos, F.S.; Ramos-Jiliberto, R.; Garay-Narváez, L.; Urbani, P.; Dunne, J.A. Consequences of adaptive behaviour for the structure and dynamics of food webs. Ecol. Lett. 2010, 13, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Semmens, B.X.; Ward, E.J.; Moore, J.W.; Darimont, C.T. Quantifying inter-and intra-population niche variability using hierarchical bayesian stable isotope mixing models. PLoS ONE 2009, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Derbridge, J.J.; Krausman, P.R.; Darimont, C.T. Using bayesian stable isotope mixing models to estimate wolf diet in a multi-prey ecosystem. J. Wildl. Manag. 2012, 76, 1277–1289. [Google Scholar] [CrossRef]
- Larroque, J.; Ruette, S.; Vandel, J.M.; Devillard, S. Level- and scale-dependent habitat selection for resting sites by 2 syntopic Martes species. J. Mammal. 2017, 98, 1709–1720. [Google Scholar] [CrossRef]
- McCarty, J.P.; Levey, D.J.; Greenberg, C.H.; Sargent, S. Spatial and temporal variation in fruit use by wildlife in a forested landscape. For. Ecol. Manag. 2002, 164, 277–291. [Google Scholar] [CrossRef]
- Kilgo, J.C.; Blake, J.I. (Eds.) Ecology and Management of a Forested Landscape: Fifty Years on the Savannah River Site; Island Press: Washington, DC, USA, 2005; ISBN 9781597260114. [Google Scholar]
- Rohwer, S.; Ricklefs, R.E.; Rohwer, V.G.; Copple, M.M. Allometry of the duration of flight feather molt in birds. PLoS Biol. 2009, 7, e1000132. [Google Scholar] [CrossRef] [Green Version]
- Vanderhoff, N.; Pyle, P.; Patten, M.A.; Sallabanks, R.; James, F.C. American Robin (Turdus migratorius). In Birds of the World; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Nolan, V., Jr.; Ketterson, E.D.; Cristol, D.A.; Rogers, C.M.; Clotfelter, E.D.; Titus, R.C.; Schoech, S.J.; Snajdr, E. Dark-eyed Junco (Junco hyemalis). In Birds of the World; Poole, A.F., Gill, F.B., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Greenlaw, J.S. Eastern Towhee (Pipilo erythrophthalmus). In Birds of the World; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Ritchison, G.; Grubb, T.C., Jr.; Pravosudov, V.V. Tufted Titmouse (Baeolophus bicolor). In Birds of the World; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Smith, R.J.; Hatch, M.I.; Cimprich, D.A.; Moore, F.R. Gray Catbird (Dumetella carolinensis). In Birds of the World; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Dellinger, R.; Wood, P.B.; Jones, P.W.; Donovan, T.M. Hermit Thrush (Catharus guttatus). In Birds of the World; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Halkin, S.L.; Shustack, D.P.; DeVries, M.S.; Jawor, J.M.; Linville, S.U. Northern Cardinal (Cardinalis cardinalis). In Birds of the World; Rodewald, P.G., Keeney, B.K., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2021. [Google Scholar]
- Swanson, D.L.; Ingold, J.L.; Wallace, G.E. Ruby-crowned Kinglet (Regulus calendula). In Birds of the World; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Mack, D.E.; Yong, W. Swainson’s Thrush (Catharus ustulatus). In Birds of the World; Poole, A.F., Gill, F.B., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Falls, J.B.; Kopachena, J.G. White-throated Sparrow (Zonotrichia albicollis). In Birds of the World; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Hunt, P.D.; Flaspohler, D.J. Yellow-rumped Warbler (Setophaga coronata). In Birds of the World; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Hobson, K.A.; Wassenaar, L.I. Linking breeding and wintering grounds of neotropical migrant songbirds using stable hydrogen isotopic analysis of feathers. Oecologia 1997, 109, 142–148. [Google Scholar] [CrossRef]
- Graves, G.R.; Romanek, C.S.; Rodriguez Navarro, A. Stable isotope signature of philopatry and dispersal in a migratory songbird. Proc. Natl. Acad. Sci. USA 2002, 99, 8096–8100. [Google Scholar] [CrossRef] [Green Version]
- Hobson, K.A.; Clark, R.G. Turnover of 13C in cellular and plasma fractions of blood: Implications for nondestructive sampling in avian dietary studies. Auk 1993, 110, 638–641. [Google Scholar] [CrossRef]
- Pearson, S.F.; Levey, D.J.; Greenberg, C.H.; Martínez del Rio, C. Effects of elemental composition on the incorporation of dietary nitrogen and carbon isotopic signatures in an omnivorous songbird. Oecologia 2003, 135, 516–523. [Google Scholar] [CrossRef]
- Zurovchak, J.G.; Stiles, E.W.; Place, A.R. Effect of dietary lipid level on lipid passage rate and lipid absorption efficiency in American robins (Turdus migratorius). J. Exp. Zool. 1999, 283, 408–417. [Google Scholar] [CrossRef]
- Taylor, W. Change-Point Analyzer Shareware Program version 2.0; Taylor Enterprises: Libertyville, IL, USA, 2000. [Google Scholar]
- Brown, D.R.; Stouffer, P.C.; Strong, C.M. Movement and territoriality of wintering hermit thrushes in southeastern Louisiana. Wilson Bull. 2000, 112, 347–353. [Google Scholar] [CrossRef]
- Moorman, C.E.; Bowen, L.T.; Kilgo, J.C.; Sorenson, C.E.; Hanula, J.L.; Horn, S.; Ulyshen, M.D. Seasonal diets of insectivorous birds using canopy gaps in a bottomland forest. J. Field Ornithol. 2007, 78, 11–20. [Google Scholar] [CrossRef]
- Hobson, K.A.; Clark, R.G. Assessing avian diets using stable isotopes II: Factors influencing diet-tissue frationation. Condor 1992, 94, 189–197. [Google Scholar] [CrossRef]
- Podlesak, D.W.; McWilliams, S.R. Metabolic routing of dietary nutrients in birds: Effects of diet quality and macronutrient composition revealed using stable isotopes. Physiol. Biochem. Zool. 2006, 79, 534–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caut, S.; Angulo, E.; Courchamp, F. Caution on isotopic model use for analyses of consumer diet. Can. J. Zool. 2008, 86, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Carleton, S.A.; Martínez del Rio, C. The effect of cold-induced increased metabolic rate on the rate of 13C and 15N incorporation in house sparrows (Passer domesticus). Oecologia 2005, 144, 226–232. [Google Scholar] [CrossRef]
- Parnell, A.C.; Jackson, A.L. Package “Siar” Version 4.2 2013. pp. 467–504. Available online: http://CRAN.R-project.org/package=siar, (accessed on 1 May 2013).
- Greenberg, C.H.; Levey, D.J.; Kwit, C.; Mccarty, J.P.; Pearson, S.F.; Sargent, S.; Kilgo, J. Long-term patterns of fruit production in five forest types of the South Carolina upper coastal plain. J. Wildl. Manag. 2012, 76, 1036–1046. [Google Scholar] [CrossRef]
- Kwit, C.; Levey, D.J.; Greenberg, C.H.; Pearson, S.F.; McCarty, J.P.; Sargent, S. Cold temperature increases winter fruit removal rate of a bird-dispersed shrub. Oecologia 2004, 139, 30–34. [Google Scholar] [CrossRef]
- Kwit, C.; Levey, D.J.; Greenberg, C.H.; Pearson, S.F.; McCarty, J.P.; Sargent, S.; Mumme, R.L. Fruit abundance and local distribution of wintering Hermit Thrushes (Catharus guttatus) and Yellow-Rumped Warblers (Dendroica coronata) in South Carolina. Auk 2004, 121, 46–57. [Google Scholar] [CrossRef]
- Borgmann, K.L.; Pearson, S.F.; Levey, D.J.; Greenberg, C.H. Wintering Yellow-Rumped Warblers (Dendroica Coronata) track manipulated abundance of Myrica Cerifera fruits. Auk 2004, 121, 74–87. [Google Scholar] [CrossRef]
- Bechtoldt, C.L.; Stouffer, P.C. Home-range size, response to fire, and habitat preferences of wintering Henslow’s Sparrows. Wilson Bull. 2005, 117, 211–225. [Google Scholar] [CrossRef]
- McKinnon, E.A.; Kurt Kyser, T.; Stutchbury, B.J.M. Does the proportion of arthropods versus fruit in the diet influence overwintering condition of an omnivorous songbird? J. Field Ornithol. 2017, 88, 65–79. [Google Scholar] [CrossRef]
- Ting, S.; Hartley, S.; Burns, K.C. Global patterns in fruiting seasons. Glob. Ecol. Biogeogr. 2008, 17, 648–657. [Google Scholar] [CrossRef]
- Newell Wohner, P.J.; Cooper, R.J.; Greenberg, R.S.; Schweitzer, S.H. Weather affects diet composition of rusty blackbirds wintering in suburban landscapes. J. Wildl. Manag. 2016, 80, 91–100. [Google Scholar] [CrossRef]
- Van Hemert, C.; Handel, C.M.; O’Brien, D.M. Stable isotopes identify dietary changes associated with beak deformities in Black-capped Chickadees (Poecile atricapillus). Auk 2012, 129, 460–466. [Google Scholar] [CrossRef]
- Castro, G.; Stoyan, N.; Myers, J.P. Assimilation efficiency in birds: A function of taxon or food type? Comp. Biochem. Physiol. Part A Physiol. 1989, 92, 271–278. [Google Scholar] [CrossRef]
- Levey, D.J.; Karasov, W.H. Digestive responses of temperate birds switched to fruit or insect diets. Auk 1989, 106, 675–686. [Google Scholar]
- Robbins, C.T.; Felicetti, L.A.; Sponheimer, M. The effect of dietary protein quality on nitrogen isotope discrimination in mammals and birds. Oecologia 2005, 144, 534–540. [Google Scholar] [CrossRef]
- Willson, J.D.; Winne, C.T.; Pilgrim, M.A.; Romanek, C.S.; Gibbons, J.W. Seasonal variation in terrestrial resource subsidies influences trophic niche width and overlap in two aquatic snake species: A stable isotope approach. Oikos 2010, 119, 1161–1171. [Google Scholar] [CrossRef]
- Inger, R.; Harrison, X.A.; Ruxton, G.D.; Newton, J.; Colhoun, K.; Gudmundsson, G.A.; McElwaine, G.; Pickford, M.; Hodgson, D.; Bearhop, S. Carry-over effects reveal reproductive costs in a long-distance migrant. J. Anim. Ecol. 2010, 79, 974–982. [Google Scholar] [CrossRef]
- Russi, L.; Cocks, P.S.; Roberts, E.H. Seed bank dynamics in a Mediterranean grassland. J. Appl. Ecol. 1992, 29, 763–771. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Kelly, E.F.; Knapp, A.K. Geographic variation in growth and phenology of two dominant central US grasses: Consequences for climate change. J. Plant Ecol. 2014, 7, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Crosby, S.C.; Ivens-Duran, M.; Bertness, M.D.; Davey, E.; Deegan, L.A.; Leslie, H.M. Flowering and biomass allocation in U.S. Atlantic coast Spartina alterniflora. Am. J. Bot. 2015, 102, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Pimm, S.L.; Rosenzweig, M.L.; Mitchell, W. Competition and food selection: Field tests of a theory. Ecology 1985, 66, 798–807. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef] [Green Version]
- Gurd, D.B. Mechanistic analysis of interspecific competition using foraging trade-offs: Implications for duck assemblages. Ecology 2008, 89, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Raveling, D.G. The annual cycle of body composition of canada geese with special reference to control of reproduction. Auk 1979, 96, 234–252. [Google Scholar]
- Marra, P.P.; Hobson, K.A.; Holmes, R.T. Linking winter and wummer events in a migratory bird by using stable-carbon isotopes. Science 1998, 282, 1884–1886. [Google Scholar] [CrossRef] [Green Version]
- Parrish, J.D. Behavioral, energetic, and conservation implications of foraging plasticity during migration. Stud. Avian Biol. 2000, 20, 53–70. [Google Scholar]
- Inger, R.; Ruxton, G.D.; Newton, J.; Colhoun, K.; Robinson, J.A.; Jackson, A.L.; Bearhop, S. Temporal and intrapopulation variation in prey choice of wintering geese determined by stable isotope analysis. J. Anim. Ecol. 2006, 75, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.P.; de Melo, C. Individual-resource networks reveal distinct fruit preferences of selective individuals from a generalist population of the Helmeted Manakin. Ibis 2020, 162, 713–722. [Google Scholar] [CrossRef]
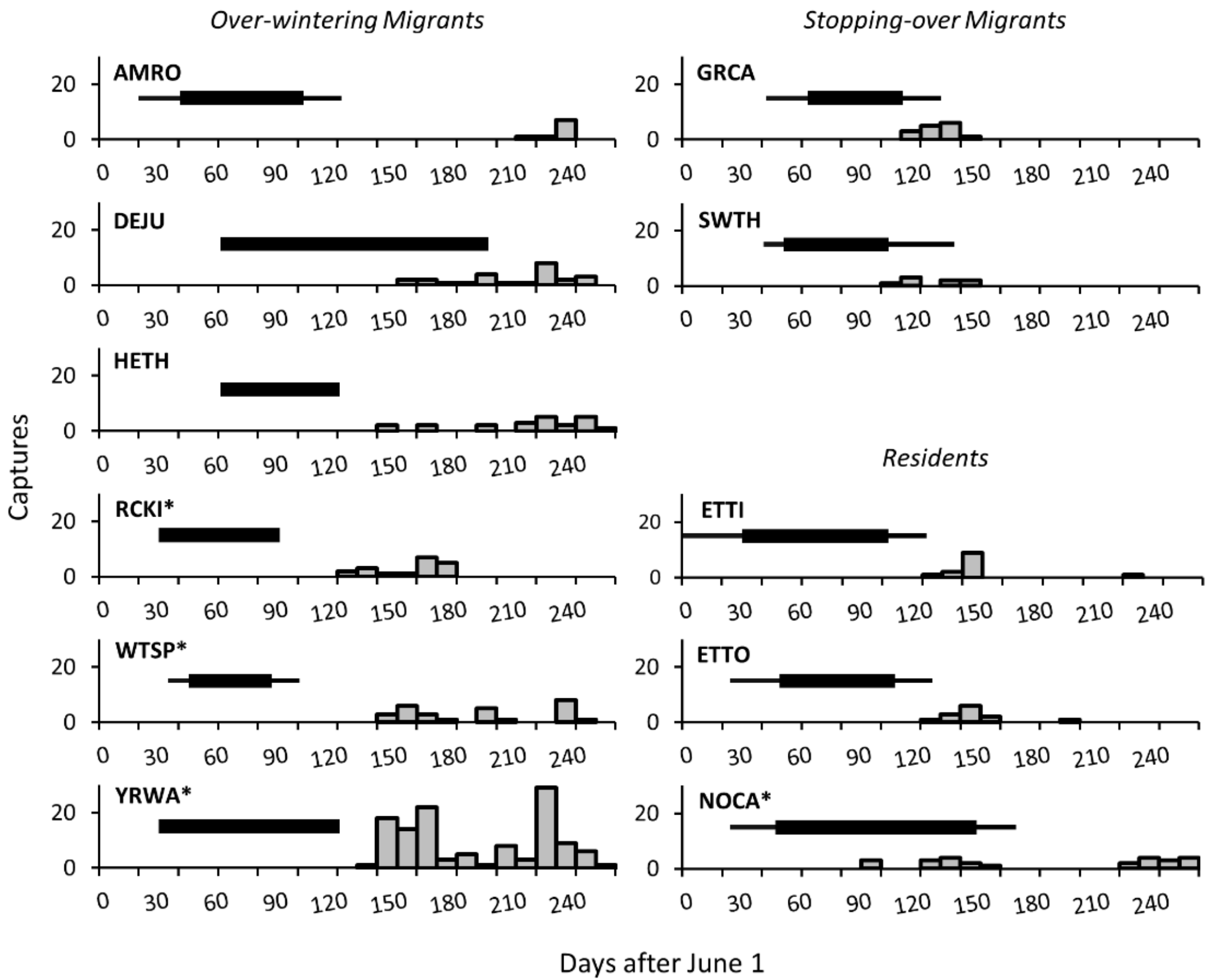
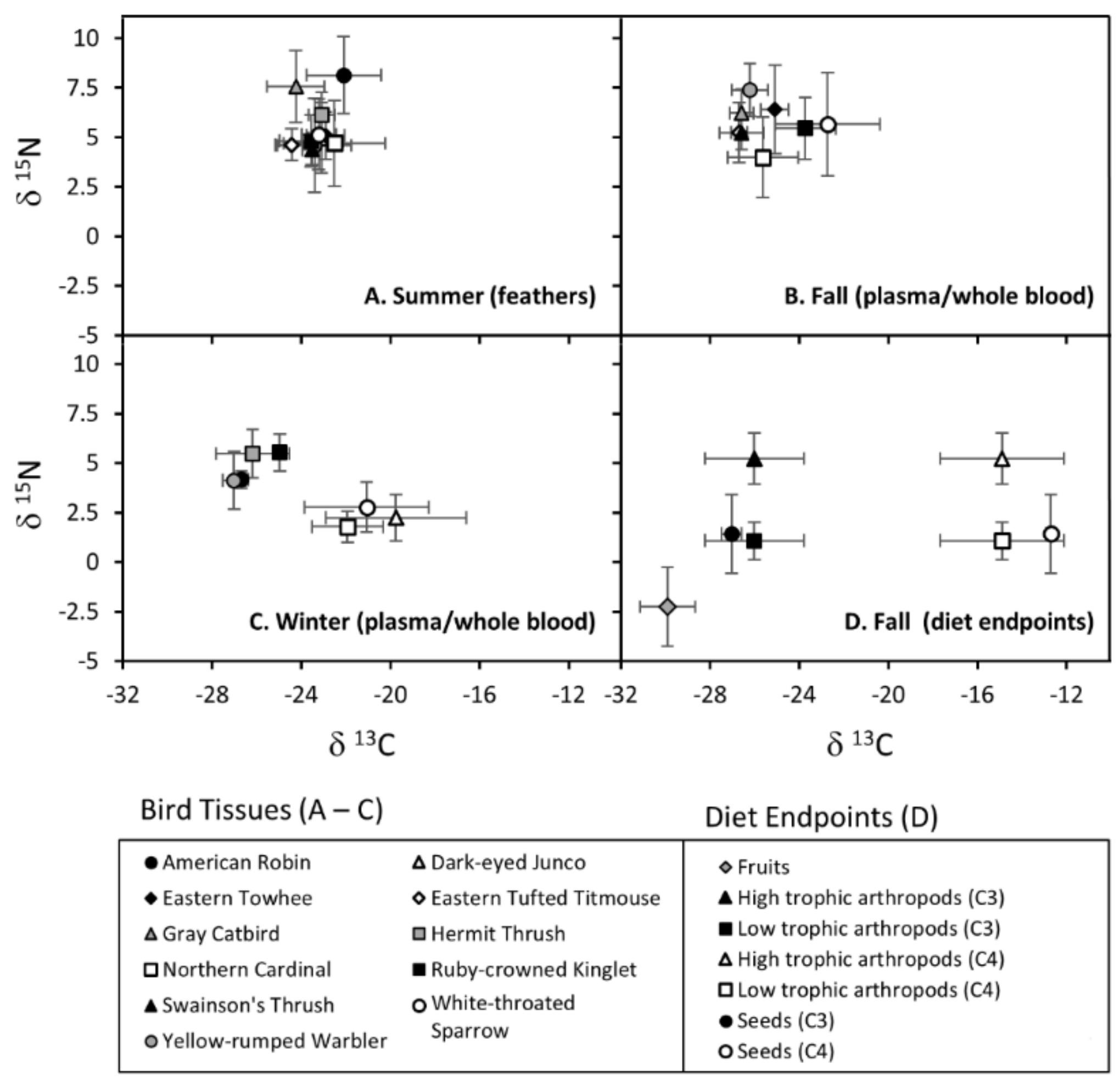


| Species | Comparison | Fruit | Arth | Arth (H-C3) | Arth (L-C3) | Arth (H-C4) | Arth (L-C4) | Seeds | Seeds (C3) | Seeds (C4) |
|---|---|---|---|---|---|---|---|---|---|---|
| Northern Cardinal | Winter > Fall? | 0.25 | 0.60 | 0.40 | 0.35 | 0.55 | 0.85 | 0.61 | 0.34 | 0.86 |
| Ruby-Crowned Kinglet | Winter > Fall? | 0.84 | 0.16 | 0.49 | 0.21 | - | - | - | - | - |
| Yellow-Rumped Warbler | Winter 1 > Fall? | 0.98 | 0.02 | 0.00 | 1.00 | - | - | - | - | - |
| Winter 2 > Fall? | 0.77 | 0.23 | 0.00 | 0.98 | - | - | - | - | - | |
| White-Throated Sparrow | Winter > Fall? | - | 0.43 | 0.28 | 0.50 | 0.43 | 0.66 | 0.57 | 0.46 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, W.A.; Pearson, S.F.; Smith, A.D.; McWilliams, S.R.; Levey, D.J. Seasonal and Interspecific Variation in Frugivory by a Mixed Resident-Migrant Overwintering Songbird Community. Diversity 2021, 13, 314. https://doi.org/10.3390/d13070314
Carter WA, Pearson SF, Smith AD, McWilliams SR, Levey DJ. Seasonal and Interspecific Variation in Frugivory by a Mixed Resident-Migrant Overwintering Songbird Community. Diversity. 2021; 13(7):314. https://doi.org/10.3390/d13070314
Chicago/Turabian StyleCarter, Wales A., Scott F. Pearson, Adam D. Smith, Scott R. McWilliams, and Douglas J. Levey. 2021. "Seasonal and Interspecific Variation in Frugivory by a Mixed Resident-Migrant Overwintering Songbird Community" Diversity 13, no. 7: 314. https://doi.org/10.3390/d13070314
APA StyleCarter, W. A., Pearson, S. F., Smith, A. D., McWilliams, S. R., & Levey, D. J. (2021). Seasonal and Interspecific Variation in Frugivory by a Mixed Resident-Migrant Overwintering Songbird Community. Diversity, 13(7), 314. https://doi.org/10.3390/d13070314






