Abstract
Stable-hydrogen (δ2H), nitrogen (δ15N), and carbon (δ13C) isotopes are used to decipher broad movement patterns and trophic relationships among diverse species, and an improved understanding of factors controlling natural variation in tissue-isotope measurements will enhance these applications. To evaluate the rearing environment and family-related effects on the isotopic composition of tissues, we cross-fostered nestling tree swallows (Tachycineta bicolor, Vieillot 1808) and American kestrels (Falco sparverius, Linnaeus 1758) by swapping recently hatched birds (<4 days old) among nest boxes and collecting blood and feathers prior to fledging. To assess developmental effects, we measured δ2H in blood and feathers of captive mallard (Anas platyrhynchos, Linnaeus 1758) ducklings challenged energetically during growth. Stable isotope composition was not strongly related to nest box type or natal nest (i.e., family of origin) effects in swallows and kestrels; tissue-isotope composition was related to rearing environment, indicative of differences in nest and parental quality or parental provisioning tactics. Blood and feather δ2H values in swallows were positively related to antecedent maximum ambient temperature, and unrelated to elevated energy expenditure in mallards. The average differences between δ2H in blood and feathers were similar for nestling swallows (27‰, 32‰; two sites) and mallards (26‰, 30‰; two age groups), and lower than in nestling kestrels (50‰). Strong species-specific patterns in blood-feather differences were not observed for δ15N and δ13C in swallows or kestrels; divergent δ2H results may be related to differences in nest ambient conditions, diet composition, or physiological processes affecting hydrogen assimilation during growth and feather synthesis. In swallows, tissue-isotope values reflected parental prey selection from spatially distinct food webs during nestling development with little effect(s) of family of origin, egg composition, or early growth.
Keywords:
American kestrel; cross-fostering; growth; mallard; nest site; stable isotope; tree swallow 1. Introduction
Stable nitrogen (δ15N) and carbon (δ13C) isotopes are routinely used to investigate trophic relationships in birds and other animals, while hydrogen (δ2H) isotopes have been used primarily to ascertain broad migration and movement patterns [1]. Applications of stable isotopes have been greatly strengthened by experimental and comparative studies of diverse factors affecting natural variation in stable isotope measurements of precipitation, surface waters, and food webs, as well as tissue-specific discrimination values [1,2,3,4]. Application of δ2H as an additional tracer in trophic studies is promising, and assessing the environmental and endogenous drivers of δ2H variation continues to be an important area of research (e.g., [5,6]). In birds, variation in feather δ2H values may be attributed to environmental factors such as year, habitat use, and foraging mode, in some instances producing isotopic differences among individuals, both within and among species, sampled in the same locations and years [7,8,9]. Far less is known about how early-life conditions influence tissue-δ2H variation in wild and captive birds during growth.
In growing birds, the δ2H value of tissues is sensitive to habitat microclimate and local evaporative conditions. In general, thermally stressed individuals show higher diet-tissue 2H discrimination than less stressed individuals [10,11]. For δ15N, diet quality effects often occur, with higher diet-tissue isotope discrimination factors associated with low dietary protein content than those with higher protein content [3]. Beyond direct dietary influences, sources of δ13C variation are less clear. To our knowledge, there are no comparisons of how rearing environment versus genetic or early-life effects control the isotopic composition of tissues of wild birds. This contrasts sharply with several other physiological processes where, for instance, numerous studies have examined the heritability of size, and stress and immune responses [12,13,14,15], and, in domestic chickens (Gallus gallus domesticus, Linnaeus 1758), where the genetics of digestive efficiency and diet assimilation have been thoroughly quantified [16].
Our aim was to investigate sources of stable isotope variation (δ2H, δ13C, δ15N) of whole blood and feathers in wild nestling tree swallows (Tachycineta bicolor (Vieillot 1880)) and American kestrels (Falco sparverius, Linnaeus 1758). We conducted partial cross-fostering experiments to determine the relative contribution of rearing environments versus family-specific effects (including genetic and early-life conditions) on the stable isotope composition of blood and feathers. It was unknown whether genetically determined growth rate, assimilation efficiency, or other physiological processes might contribute to tissue-isotope variation among individuals. Furthermore, by swapping individuals among nest boxes that differed in their microclimate, we tested whether nesting thermal conditions affected tissue δ2H, under the hypothesis that nestlings exposed to hotter (more evaporative) conditions during development would have higher δ2H values [5,11]. We evaluated the hypothesis that nestlings raised in the same nest box type (plywood or aspen) would be isotopically similar to each other compared to siblings raised in different box types since nest-mates experience the same nest microclimate and parental feeding conditions [17]. This cross-fostering design created an opportunity to gain insights into the effects of family origin in shaping tissue stable isotope composition. If air temperature affects tissue-isotopes, we predicted that variation in blood and feather isotopes would be closely related to nest box microclimate effects [18] and positively related to maximum ambient air temperature prior to sampling [10]. We also examined experimentally how variation in energy expenditure during growth affected blood and feather δ2H relationships in growing mallard (Anas platyrhynchos, Linnaeus 1758) ducklings exposed to energetic challenges in captivity [19].
We compared differences between measurements of blood and feather δ2H values in pre-fledging tree swallows, American kestrels, and mallards. Small-bodied insectivores like swallows may have different diet-to-tissue discrimination factors than larger-bodied carnivores (kestrel) or omnivores (mallard); differences could also arise due to metabolic rate or H routing associated with dietary protein [20]. However, we had limited information about the H isotope composition of swallow and kestrel prey items, so we focused on possible differences in blood-feather relationships rather than on mechanisms, reasoning that the processes occurring during feather formation could produce the distinctive feather δ2H patterns observed in raptors that often differ markedly from other species [21,22,23].
2. Materials and Methods
2.1. Study Sites
The tree swallow experiments were conducted at St. Denis National Wildlife Area, Saskatchewan (SK), Canada (hereafter SK; 52.20° N, 106.08° W; [24]) during 2008 and 2009, and near Prince George, British Columbia, Canada (BC; 53.79° N, 122.77° W; [25]) during 2008. Swallows at both sites nested in wooden boxes mounted on metal or wooden posts ca. 1.25 m above ground, with nest holes generally facing the southeast. The SK site was characterized as having moderately undulating topography with numerous small ponds and groves of trembling aspen (Populus tremuloides Michx.), and surrounded by fields of planted and native grasses, and cropland [17,24]. Nest boxes at the SK site were constructed of plywood or untreated whole trembling aspen boles, but both had identical entrance hole sizes and internal floor dimensions (details in [26]). Aspen nest boxes had less variation in air temperature than plywood boxes [18,26] with a more stable microclimate that buffered diel conditions more efficiently than plywood. The median distance between nest boxes used for cross-fostering was 309 m (range: 30–760 m, years combined).
The BC tree swallow study site was in the interior of the province, in a forested region interspersed with open fields of hayland, pasture, and crops along valleys and flatter terrain [27]. Nest boxes, constructed of plywood with the same dimensions as those at the SK site were placed along the edge of managed wetlands [25]. Wetlands were interconnected hydrologically via surface and ground water flow, and nest boxes used for cross-fostering were 60–170 m apart.
American kestrels were studied in 2008 in the mixedwood boreal forest near Besnard Lake, Saskatchewan (SK), Canada (55° N, 106° W; [28]), where kestrels nested in boxes constructed of plywood placed approximately 3.6 m above ground in trees or on decommissioned power poles [23]. Nest boxes used for cross-fostering were ~1600 m apart.
2.2. Monitoring of Breeding Birds in Nest Boxes
Swallow nest boxes were visited every 2 days from early May until an egg was found and then checked daily. When the number of eggs was unchanged in a box for 3 consecutive days, indicating the onset of incubation and a completed clutch, the nest was not visited again until 2 days before the expected hatch date and then checked daily until all eggs had hatched. The numbers of nestlings and unhatched eggs were recorded. Further details are reported by Shutler and Clark [24] and Griebel and Dawson [25].
Kestrel nest boxes were visited every 3–5 days, beginning in early May to determine clutch initiation dates, and then visited during incubation to ascertain clutch size. The date of hatching of the first nestling for each nest was determined by daily visits starting 1 to 2 days before the predicted hatch date (see Greenwood and Dawson [23] for further details).
2.3. Cross-Fostering in Swallows and Kestrels
When swallow nestlings were 2–4 days old (i.e., 2–4 days after the first egg hatched), three average-size nestlings were uniquely marked with colored non-toxic ink and then swapped between the two plywood boxes in BC, or between the plywood and aspen boxes in SK [18], as outlined in Figure S1 (Supplementary Materials). When the swallows were 15–16 days old, 2–3 flank feathers were taken from two natal and two cross-fostered nestlings per nest box and stored in labeled paper envelopes, and blood was collected (2008 only) from the brachial vein using heparinized capillary tubes. Whole blood was stored on ice until frozen (−20 °C) prior to stable isotope analysis.
In American kestrels, two nestlings uniquely marked with colored non-toxic ink were exchanged among the four nest boxes (Supplementary Materials, Figure S1). Nestlings at one pair of boxes were swapped when they were 1 day old, while the exchange at the other boxes took place when nestlings were 3 days old. Prior to fledging at 24 days of age, 2–3 flank feathers were taken from two natal and two cross-fostered nestlings per nest box and stored in labeled paper envelopes. Blood was also collected from the brachial vein of these nestlings in heparinized capillary tubes and frozen (–20 °C) until isotope analysis.
2.4. Dietary Samples—Swallows and Kestrels
In 2009, aerial insects were captured in passive nets at the SK site and stored in a 70:30 ethanol:water mixture until processed in the laboratory. These insect samples contained chironomids, damselflies, and other flying insects. Terrestrial insect species were obtained via standardized sweep netting in herbaceous cover. Details are provided in Bortolotti et al. [17].
In 2008, during routine visits to active kestrel nest boxes at Besnard Lake, SK, representative samples of common prey items were collected from nests. These prey items comprised southern red-backed voles (Myodes gapperi (Vigors 1830)), frogs (Lithobates spp., Fitzinger 1983), and dragonflies (Odonata, superfamily Aeshnoidea). These three types of prey constitute the majority of total biomass provisioned to offspring by parent kestrels (voles: 44.1%, frogs: 9.3%, dragonflies: 21.5%; [29], R.D. Dawson unpublished data). Prey samples were kept frozen until processing occurred in the laboratory, at which time they were thawed. Tissues of prey that are normally discarded by kestrels prior to consumption or being provisioned to offspring, or are cast in pellets (wings and legs of dragonflies, skin, and bones of voles and frogs) were removed from samples such that only soft tissues remained. Each sample was dried in a freeze drier, and then pulverized into a homogenized product using a mortar and pestle and stored in a microfuge tube until isotope analysis.
2.5. Captive Mallards
One-day-old female mallards were raised in captivity to measure the effects of work-load treatments on corticosterone deposition [19], and δ2H in blood and feathers. Half of the ducklings carried backpacks with added weight (10–15% of body mass), and half of these also scaled obstacles and towers to access ad libitum food and water; control ducklings accessed food and water placed at ground level at opposite ends of pens. This created four treatment groups: control-control, control-tower; backpack-control, backpack-tower (Supplementary materials, Figure S1). Otherwise, the rearing environment, including food and water sources, were identical for all of the ducklings. Daily energy expenditure was ~7–10% higher in ducklings carrying backpacks and climbing ramps to access food compared with controls [19]. Blood and greater secondary covert feathers were sampled randomly from selected birds from treatment groups when ducklings were 36 or 51 days old. Whole blood was placed in capillary tubes and frozen at –20 °C; feathers were stored in paper envelopes.
2.6. Stable Isotope Analyses
Prior to all isotope analyses, feathers were soaked and rinsed in a 2:1 chloroform:methanol solution for 24 hrs and then air-dried in a fume hood. Whole blood samples were freeze-dried. Blood lipids were removed from pooled whole blood taken from a subset of swallows (n = 5 nest boxes in SK, n = 9 boxes in BC) and kestrels (n = 4 boxes) using a similar solvent soak and rinse. Stable hydrogen isotope analyses of tissues from tree swallows and American kestrels followed the comparative-equilibration method described by Wassenaar and Hobson [30] through the use of calibrated protein (keratin) hydrogen isotope reference materials. Hydrogen isotopes were measured on H2 derived from high temperature (1350 °C) flash pyrolysis of 350 μg subsamples (±20 μg range in mass) of insects and feathers by means of continuous-flow isotope-ratio mass spectrometry. Measurements of keratin in three Environment and Climate Change Canada laboratory reference materials (cow hoof, chicken feather, and bowhead whale (Balaena mysticetus, Linnaeus 1758) baleen, corrected for linear instrumental drift, were both accurate and precise, with typical δ2H values (mean ± SD) of –187 ± 0.9‰ (n = 5), –147.7 ± 0.9‰ (n = 5), and –109 ± 0.8‰ (n = 5) per autorun, respectively. A control keratin reference yielded a 6-month running SD of ±3.3‰ (n = 76). The repeatability of non-homogenized tissue samples was ±2‰, on average, for n = 27 samples of insect tissue (no difference between tissue types). Samples were analyzed at the Environment and Climate Change Canada laboratory, Saskatoon, SK, Canada. Feather and blood samples were combusted using continuous-flow isotope-ratio mass spectrometry to determine stable nitrogen (15N/14N) and carbon (13C/12C) isotope ratios. Bowhead whale baleen and egg albumen were used as standards for feather nitrogen and carbon, and egg albumen was used as the standard for invertebrate nitrogen and carbon. The 95% confidence interval for both δ15N and δ13C was ±0.2‰. Samples were analyzed for carbon and nitrogen isotopes (2008 only) at the University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
Mallard whole blood and greater secondary covert feathers were prepared as above and analyzed for hydrogen isotopes at the Stable Isotope Facility at the University of California-Davis, Davis, California, using an Elementar PyroCube (Elementar Analysensysteme GmbH, Hanau, Germany) interfaced to an Isoprime VisION (Isoprime Ltd., Stockport, UK). Samples were combusted pyrolytically to H2 at 1450 °C. Feather samples (keratin) were allowed to equilibrate with keratin reference materials (IAEA-CH7, USGS-42, USGS-43, CBS, and KHS) for at least 96 hr before analysis [30,31]. Equilibration standards were not available for blood, so the non-exchangeable fraction was determined by equilibrating samples and standards with two waters with different 2H values. Measurement precision of at least four within-run keratin standards, interspersed within each run, was ±2.1‰. Keratin laboratory reference measurements were accurate, with a within-run precision of ±1.9‰.
2.7. Statistical Analyses
We partitioned the variation in tissue-isotope values using linear mixed-effects models (swallows, kestrels; restricted maximum likelihood method) and general linear models (mallards) in SAS version 9.4 [32]. In swallows and kestrels, the fixed effects included sampling date (swallows only; continuous variable), whether or not a nestling was cross-fostered (swallows and kestrels; categorical variable with two levels) and, at SK only, nest box type (i.e., plywood, aspen). Random effects were attributed to nest box of rearing or origin (i.e., family or nest-specific effects occurring prior to 2–4 days old when swaps occurred). Blood and feather δ2H values for swallows from SK were also related to antecedent ambient air temperatures using mixed-effects models accounting for random effects of rearing nest box identity; hourly air temperature data were obtained from an on-site weather station and converted to daily mean and maximum air temperatures for 1 day (Mean T1, MaxT1) and 0–5 days (MeanT5, MaxT5) before sampling [11]. In mallard ducklings, fixed effects were treatment group, age, and treatment × age interaction. In exploratory analyses, species- and site-specific differences between blood and feather isotope values were compared with general linear models. Pearson correlation coefficients (r) were calculated to measure associations between variables. In all analyses, we used significance at p ≤ 0.05 and trends at p ≤ 0.10.
3. Results
3.1. Cross-Fostering Experiments
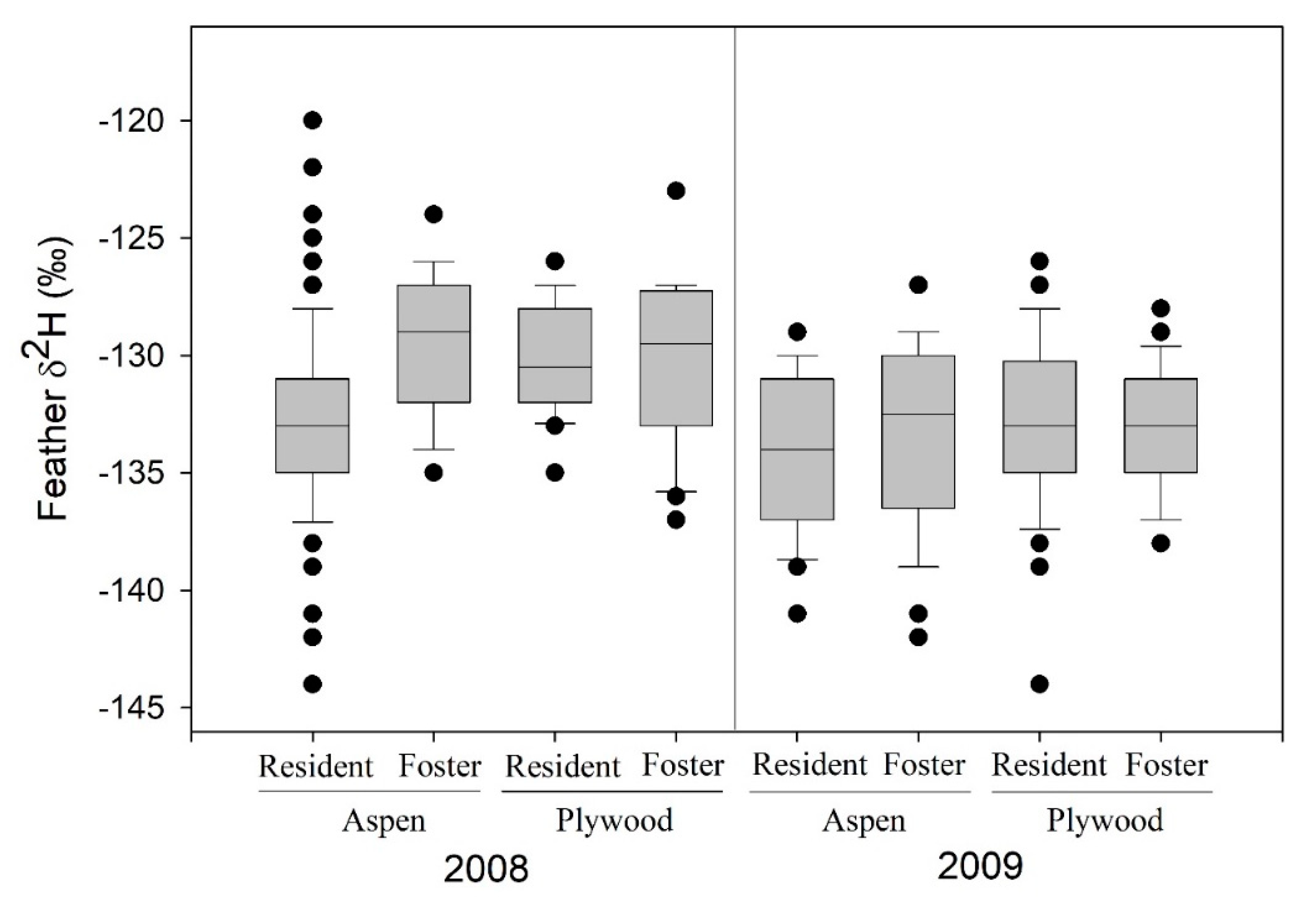
Among tree swallows at St. Denis, SK, 232 nestlings were swapped among nest boxes (28:30 aspen:plywood boxes) in the 2008 (n = 96) and 2009 (n = 136) breeding periods. However, prior to analyses, we removed 4 nestlings from a single 2009 nest in a plywood box that hatched 8 days later than other nests and had no matched nestlings from an aspen box. A mixed-effects model indicated that feather δ2H values were more positive in 2008 than 2009, but not related to sampling date, box type, or whether or not a nestling was swapped between box types (Figure 1, Table 1). A stronger random effect was associated with nest of rearing, with weaker effects of natal nest identity. In 2008, δ13C values of feathers were positively related to sampling date, and unrelated to box type or swap status (Table 1); random effects were attributed to rearing nest but not to natal nest. In 2008, variation in feather δ15N values was unrelated to fixed effects of sampling date, box type and swap status (Table 1); random effects of rearing nest were detected whereas natal nest effects were inestimable given low variation and sample size. The coefficient of variation (CV) in feather δ2H was 2.7% in 2008 (4.7% for δ15N and 2.1% for δ13C), and 2.8% in 2009.
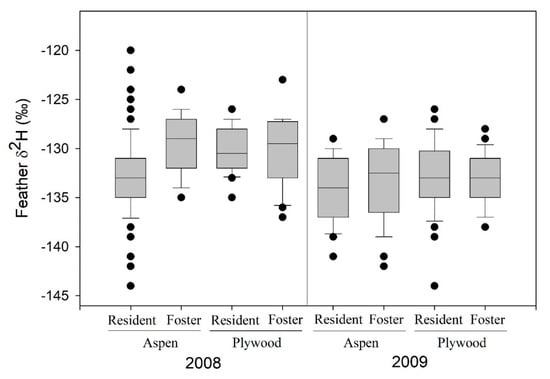
Figure 1.
Feather δ2H values (‰) for resident and cross-fostered nestling tree swallows at St. Denis, Saskatchewan, 2008 (n = 96) and 2009 (n = 136), in relation to nest box type of rearing (aspen, plywood). Boxplot shows median (horizontal line), 25% and 75% values (lower and upper edges of shaded box), 5% and 95% values (horizontal whiskers), and extreme values (filled circles).

Table 1.
Results of mixed-effects models for δ2H, δ13C, and δ15N values in feathers of wild tree swallows and American kestrels.1 Feather δ2H was measured over two years only in swallows at the SK site. Cross-foster refers to whether or not a nestling was cross-fostered, and box type refers to aspen versus plywood boxes (SK only). Shown are model F values for fixed effects, z values for random terms, and number of nestlings (n), of which ~50% were cross-fostered.
At St. Denis, SK, swallow blood δ2H (2008 only, n = 96) was positively related (βTmax1 = 0.173 ± 0.083 [SE], p = 0.040) to maximum ambient air temperature one day before sampling, controlling for fixed effects of nest box type (p = 0.018) and random effects of rearing nest box (p = 0.055). Likewise, feather δ2H was positively related (βTmax5 = 0.807 ± 0.382, p = 0.036, n = 232, 2008 and 2009 combined) to antecedent maximum air temperatures, after controlling for fixed effects of year (p = 0.05) and random effects of nest box of rearing (p < 0.001); effects of nest box type or interaction between nest box type and MaxT5 were not detected (p > 0.30). In 2008, there was no correlation (r = 0.185, p = 0.39, n = 24 per-box mean values) between blood and feather δ2H values but differences between blood and feather δ2H increased following 5 days of warmer temperatures (βTmax5 = 1.125 ± 0.521, p = 0.034, n = 96), controlling for random effects of nest box of rearing (p = 0.009), with no detectable fixed effects (p > 0.29) of box type or interaction between box type and MaxT5.
At Prince George, BC (2008; n = 35 nestlings, 10 nest boxes), a mixed-effects model indicated that nestlings transferred to a new plywood box had lower feather δ2H values (Table 1) than nestlings raised in their natal box; sampling date effects were negligible. Nest box of rearing was more influential than natal nest box in explaining feather δ2H variation. Feather δ13C values were positively related to sampling date and more positive in swapped nestlings, with random effects of rearing nest but not natal nest (Table 1). Values of feather δ15N tended to increase with sampling date and were not related to swap status; natal nest random effects were inestimable while those of rearing box were evident (Table 1). The CV for feather δ2H was 3.6%, which was similar to δ13C (2.7%) and less than δ15N (9.0%).
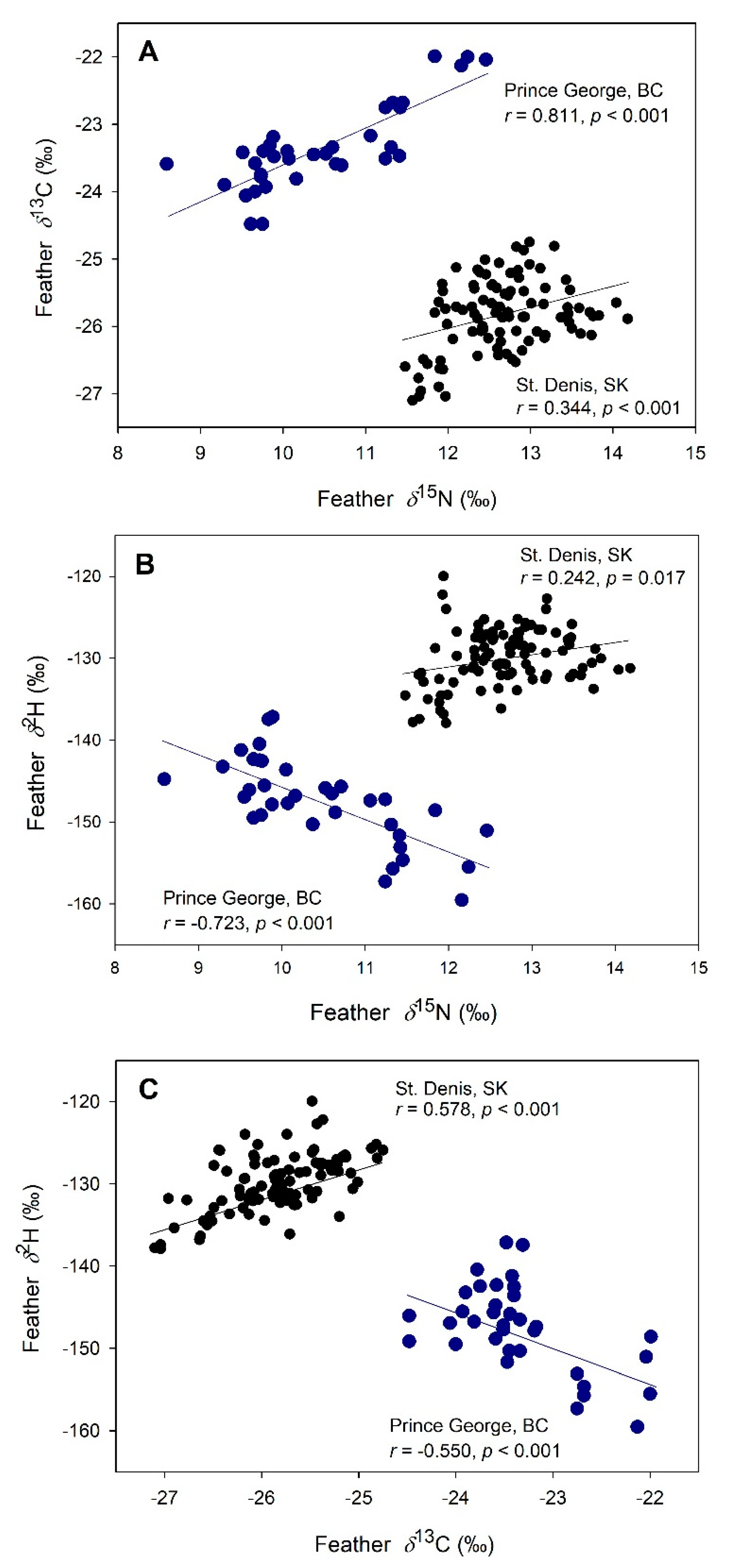
Among nestling tree swallows, baseline isotope values in feathers showed strong site-specific patterns (Figure 2). A positive correlation was detected between 15N and 13C at both sites. Positive associations were also observed between 2H and both 15N and 13C at St. Denis, SK, but these relationships were negative among nestlings from Prince George, BC. Similar correlation results were obtained for blood isotope values for nestlings at both sites (results not shown).
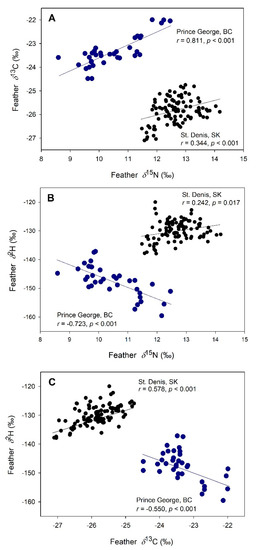
Figure 2.
Feather isotope values (‰) for nestling tree swallows from St. Denis, SK (black circles) and Prince George, BC (blue circles), 2008. Pearson correlations (r) are given for relationships between δ13C and δ15N (A), δ2H and δ15N (B), and δ2H and δ13C (C); regression lines are shown for illustration.
No cross-fostering effect was detected for feather δ2H values of American kestrels swapped between four plywood boxes near Besnard Lake, SK (Table 1). Random effects of natal nest box and box of rearing were not found or inestimable. Similar results were obtained for feather δ13C and δ15N values. The CVs of feather isotope values were 3.1% for δ2H, 4.5% for δ15N, and 2.1% for δ13C.
3.2. δ2H in Tree Swallow and American Kestrel Dietary Samples
In 2009, the δ2H values of swallow foods at St. Denis, SK, were more negative than those in swallow feathers (mean = −133‰, standard deviation [SD] = 3‰, n = 132), with aquatic and terrestrial insects averaging −163‰ (SD = 42‰, n = 51) and −167‰ (SD = 41‰, n = 18), respectively (Table 2). With a possible exception of frogs (mean = −157‰, SD = 3‰, n = 4), representative kestrel prey species such as voles (mean = −178‰, SD = 5‰, n = 4) and dragonflies (mean = −207‰, SD = 27‰, n = 4) had more negative tissue-δ2H values than kestrel blood and, especially, feathers (Table 2).

Table 2.
Stable isotope values (‰) of whole blood (lipids not removed) and feathers for wild nestling tree swallows and American kestrels, 2008. Shown are the mean ± standard deviation (sample size).
3.3. Captive Mallard Ducklings
For blood and feather δ2H values of mallards, there was no interaction (p > 0.53) between treatment and age, and no differences were detected among work-related treatment groups (p > 0.37; p = 0.46 when comparing only feather δ2H of control birds with those carrying backpacks and scaling towers). Blood δ2H was more negative (F1,22 = 12.81, p < 0.01) in 36-day-old ducklings (mean ± SD = −189 ± 7‰, n = 16) relative to 51-day-old ducklings (−181 ± 5‰, n = 14), but no difference was found for feather δ2H values (F1,23 = 0.05, p = 0.82; −155 ± 2‰, age groups pooled). Thus, blood-feather δ2H differences were somewhat larger for younger (+34 ± 8‰) than older (+26 ± 4‰) ducklings (p < 0.01), and these were similar to those obtained for wild tree swallows (Table 2), assuming that mallard blood δ2H values (which lacked equilibration standards at the UC Davis laboratory) approximated true values. CVs for feather δ2H values were 1.2% and 1.8% for 36- and 51-day-old ducklings, respectively, which were within measurement error and comparable to other isotopes (respective values were 2.5% and 3.5% for δ15N, and 0.5% and 0.7% for δ13C; corresponding blood values not available).
3.4. Exploring Blood-Feather Isotope Differences among Species and Sites
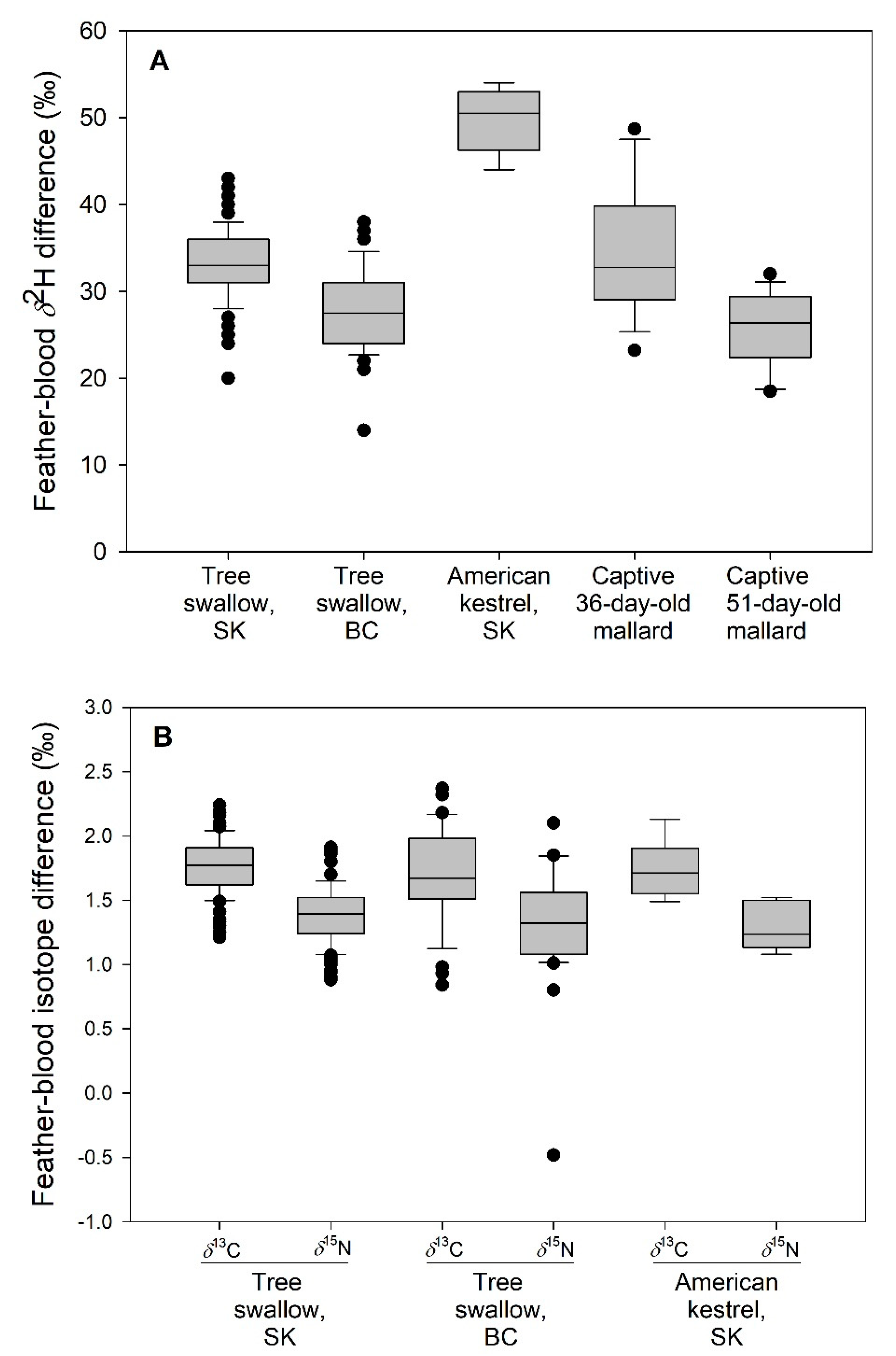
American kestrel blood-feather differences in δ2H values were nearly double (F2,133 = 82.15, p < 0.001) those recorded in wild swallows and captive mallards (Table 2, Figure 3). Differences between swallows from SK and BC were less than for kestrels but SK values were larger (t = −5.92, p < 0.01) than at BC. Species and site-specific blood-feather differences in δ13C or δ15N values were not found in comparisons of swallows or kestrels (F2,133 < 1.09, p > 0.34) and, overall, averaged 1.74‰ ± 0.04‰ (SE) for δ13C and 1.36‰ ± 0.04‰ (SE) for δ15N. C:N ratios for whole blood of kestrels (mean ± SD = 3.46 ± 0.02, n = 8) were intermediate and similar to those of tree swallows (3.48 ± 0.03 [n = 36] and 3.45 ± 0.03 [n = 43] for BC and SK, respectively).
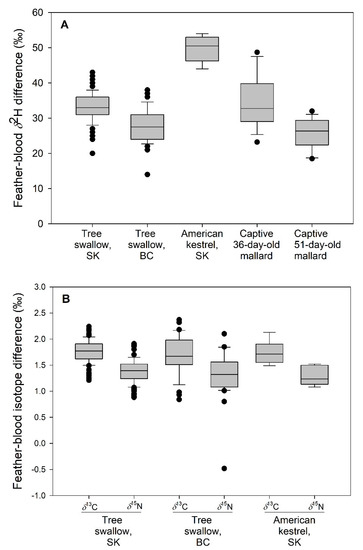
Figure 3.
Blood-feather differences (‰) for δ2H (A) and δ13C and δ15N (B) in wild tree swallows from British Columbia (BC) and Saskatchewan (SK), nesting in plywood and aspen boxes; wild American kestrels nesting in plywood boxes in boreal forest, SK, and female mallard ducklings raised in captivity (δ2H difference only). Differences may be slightly smaller for δ2H in swallows but not kestrels due to inclusion of blood lipids (see Table 2), 2008; n = 16 and 14, respectively, for 36- and 51-day-old mallard duckling age groups. Boxplot shows median (horizontal line), 25% and 75% values (lower and upper edges of shaded box), 5% and 95% values (horizontal whiskers), and extreme values (filled circles).
4. Discussion
Whether isotope assimilation is genetically determined is an open question, and contrasts with extensive investigations of several other components of avian digestive, stress, and immune systems. Our experimental findings with wild tree swallows indicate that isotopic variation is related to the rearing environment more than to genetic early-life (i.e., family) effects. Furthermore, our estimates of the relative importance of rearing environment versus family-of-origin effects may be conservative because we were only able to cross-foster swallow nestlings when birds were 2–4 days old. Thus, experiments to more fully evaluate these effects should rely on methods that allow swaps to occur with confidence when nestlings are younger and ideally when hatching. Our results for American kestrels were inconclusive, due mainly to the low sample size in the cross-fostering experiment (Table 1). It would be informative to evaluate contributions of rearing environment and natal effects in other species, but we suspect that results would be similar to swallows given the overwhelming contributions of local water and food sources during nestling growth. That rearing environment effects superseded those of natal origin in mixed-effects models of feather δ13C and δ15N values are consistent with expectations that parental food choices during nestling development control the isotopic composition of growing birds. Moreover, Vander Zanden et al. [5] advanced the idea of using δ2H in trophic studies, and our results emphasize their view, given comparable results obtained with δ13C and δ15N, isotopes that are traditionally used to characterize trophic relationships.
Given the close proximity of experimental boxes at St. Denis, SK, and known foraging patterns of female swallows (when feeding nestlings, 90% of female excursions are <500 m from nests [33], we assumed that all nestlings would receive isotopically similar diets. Indeed, there was no correlation regarding distance between matched nest boxes and the difference between these nests in mean per-nest feather δ2H for nestlings in either year (2008: r = 0.353, p = 0.31, n = 10; 2009: r = 0.142, p = 0.60, n = 16) or overall (r = 0.174, p = 0.39). Furthermore, tissue-isotopes in swallow nestlings at Prince George, BC, were highly variable even though all nest boxes were <200 m apart. Thus, we conclude that the consistently strong effect of rearing nest is related to the distinct foraging and provisioning tactics of parents, as inferred previously for swallows breeding at the SK site (Bortolotti et al. [17] for δ2H; Michelson et al. [34] for δ13C and δ15N). The St. Denis site was, as expected, more enriched in 2H compared to the Prince George site due to its lower latitude. The generally higher δ13C and δ15N values at St. Denis likely reflected the much more intensive industrial agriculture of the region. The negative relationships observed between tissue δ2H and both δ13C and δ15N values for the Prince George sites are intriguing. We are unsure of the causes of this pattern but clearly, diets higher in δ13C and δ15N values involved lower δ2H and may relate to inputs from groundwater and local sewage treatment plants (R.D. Dawson, pers. obs.).
As predicted, warmer ambient air temperatures experienced by nestlings prior to sampling had positive effects on blood δ2H values, confirming Betini et al.’s [11] reported positive effects of maximum temperature 1 day before sampling on δ2H of blood in tree swallow nestlings. However, we also demonstrated that antecedent air temperature extended to feather δ2H, with positive maximum temperature effects during rapid feather growth. Despite known microclimate differences between aspen and plywood boxes [18,26], we did not detect any differences in feather δ2H between tree swallow nestlings raised in these box types. Relative to ambient air temperature extremes, perhaps any differences in the thermal environment between aspen and plywood nest box types were too subtle to produce isotopic effects associated with, for example, differential body water loss via metabolism or evapotranspiration. Nestling swallows raised in aspen boxes at our SK site tended to have lower feather corticosterone when compared with birds raised in plywood boxes [15,18], yet determining how hormones and isotopes respond to energetic challenges and body water turnover warrants further attention. Similarly, treatment effects on body mass and corticosterone deposition observed in captive mallard ducklings [19] coupled with a lack of such effects on feather-δ2H reported herein presumably reflect a relatively low impact of an estimated 7–10% increase in energy expenditure on H assimilation in feathers. It also seems plausible that captive birds with unrestricted access to drinking water may be able to buffer the effects of higher respiration and water turnover. The low relative variability (i.e., CV) of δ2H in feathers of captive mallard ducklings – birds obtained from a common source and raised on identical diets - could indicate that intrinsic physiological processes associated with abundant drinking or dietary water produce low variation in H assimilation in growing feathers.
Exploratory Blood-Feather Relationships
The average differences recorded between δ2H in blood and feather were similar for swallows from the two sites (+27‰ and +32‰) and for captive-reared mallard ducklings (+26‰ and +34‰), and these values were lower than those observed in kestrels (+50‰). These species-specific patterns in blood-feather differences were not observed for δ15N and δ13C in swallows or kestrels. We recognize that the derived difference between blood and feathers is arbitrary, and change could be due to variation in isotopic discrimination to both tissues [35]. Both feathers and whole blood in developing nestlings represented similar periods of dietary integration but, in addition to differences expected from isotopic discrimination during formation, the opportunity for H exchange with ambient body water is greater for blood than for keratins which are metabolically inert following formation. For ecological interpretation of δ2H measurements, our results emphasize the need to understand such tissue-specific isotopic effects and underscore the need to consider tissue-specific isotopic models [35].
Divergent δ2H results could be related to differences in nests, diet, reliance on environmental water, growth rate, or physiological processes affecting H assimilation and routing during growth and feather synthesis. Although the sample size was limited, species-specific differences in δ2H between blood and feather remained disparate when blood lipids were removed, with swallows at +19‰ (BC) and +24‰ (SK) and the kestrels at +49‰ (only two families were available for this comparison). This was similar to the original results with the difference being double in kestrels versus swallows. Furthermore, because C:N ratios for whole blood of kestrels and swallows were similar, we can reasonably rule out differential blood lipids as a mechanism for the difference between swallows and kestrels.
Could differences between kestrels and swallows be related to feather growth rates? In 2008, when feathers were collected from kestrels and swallows, we were able to compare the mean growth rate per nest of the 9th primary feather for tree swallows from BC with the mean growth rate per nest of the 10th primary for American kestrels (pooled sexes, although females grow feathers slightly faster than males; R.D. Dawson, unpublished data); swallows grew feathers at a rate of 5.44 mm/day (±0.09 SE, n = 18 nests) whereas kestrels were 3.79 mm/day (±0.04 SE, n = 43 nests), a significant difference (p < 0.001). Thus, the differential growth rate could be one of the mechanisms that help explain distinct blood-feather δ2H patterns, and possibly contribute to unique feather-precipitation δ2H relationships reported in several raptor species [23]. On the other hand, such differences reported between raptors and other species have been primarily associated with adults and not neonates [36].
Based on sensitivity modelling of a hypothetical small mammal, Magozzi et al. [20] concluded that (hair) keratin H was responsive to isotopic variation in the water content of diet relative to drinking water, and to a lesser extent metabolic rate. We suspect that environmental drinking water intake in wild kestrel and swallow nestlings was limited during growth in nest boxes. Magozzi et al. [20] further suggested that the difference between body water and keratin H (i.e., feather) was generally smaller among animals feeding at higher trophic levels. Given that avian blood is ~85% body water and assuming that kestrels feed at similar or higher trophic levels as do swallows (results above), our blood-feather results for swallows and kestrels appear to be inconsistent with this conclusion. Clearly, avian physiology models similar to that developed by Magozzi et al. [20] need to be developed specifically for birds [35].
5. Conclusions
It is well recognized that locally wide variation in feather δ2H values can complicate the assignment of birds to natal or moult origins if sources of variance are not propagated in isotope assignment models. As such, multiscale sources of feather δ2H variability have received considerable attention. These investigations have produced substantial improvements in our understanding and application of stable isotopes in the trophic and movement ecology of diverse taxa. In this respect, our work has revealed strong effects of rearing environment and temperature effects on (blood and) feather δ2H that, in turn, represent local variation in rearing temperature, food web characteristics, and foraging tactics of parent birds. Finally, we also substantiated clear relationships between an established isotopic indicator of trophic position (δ15N, as well as δ13C) and δ2H in tree swallows, but these patterns varied with site in opposite directions (also see [37]). This finding further reinforces the recommendation that local food web and hydrological features must be well-characterized to reliably use δ2H as a trophic tracer, both within (this study, [5]) and among [6] species.
Supplementary Materials
The following is available online at https://www.mdpi.com/article/10.3390/d13100495/s1, Figure S1: General layout of experiments with wild tree swallow nestlings, wild American kestrel nestlings and captive mallard ducklings (sample sizes). At St. Denis, SK, swallows were cross-fostered between aspen and plywood nest boxes in 2008 and 2009 (see Fairhurst et al. [18]). At Prince George, BC, swallows were cross-fostered between neighbouring plywood boxes, as were kestrels at Besnard Lake, SK, in 2008. Female mallard ducklings were raised in captivity and assigned to treatments that involved increasing workloads during growth, and were measured at two ages (see Johns et al. [19]). In some cases, sample sizes for individual isotopes varied because samples or data could not be analysed; see text for further details.
Author Contributions
Conceptualization, R.G.C., R.D.D. and K.A.H.; methodology, R.G.C., R.D.D. and K.A.H.; formal analysis, R.G.C. and R.D.D.; investigation, R.G.C., R.D.D., J.L.G. and D.W.J.; resources, R.G.C., R.D.D., L.I.W. and K.A.H.; data curation, R.G.C.; writing—original draft preparation, R.G.C., R.D.D. and K.A.H.; writing—review and editing, all authors; visualization, R.G.C.; funding acquisition, R.G.C., R.D.D., L.I.W. and K.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
Major funding was provided by Environment and Climate Change Canada, Natural Sciences and Engineering Research Council of Canada Discovery Grants to RGC and RDD, and other agencies listed in Greenwood and Dawson [23], and Johns et al. [19].
Institutional Review Board Statement
Work on wild and captive birds was conducted under scientific permits from Canadian Wildlife Service, and in accordance with approved animal care protocols administrated via the University of Saskatchewan.
Informed Consent Statement
Not applicable.
Data Availability Statement
Contact R.G.C. or R.D.D. for information about or requests for data sets.
Acknowledgments
We sincerely thank Vanessa Harriman and Lauren Bortolotti for expertise in swallow field studies, and colleagues acknowledged in Johns et al. [19]. We appreciate the constructive comments of two anonymous reviewers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hobson, K.A. Using stable isotopes to trace long-distance dispersal in birds and other taxa. Divers. Distrib. 2005, 11, 157–164. [Google Scholar] [CrossRef]
- Inger, R.; Bearhop, S. Applications of stable isotopes analyses to avian ecology. Ibis 2008, 150, 447–461. [Google Scholar] [CrossRef]
- Wolf, N.; Carleton, S.A.; Martínez del Rio, C.M. Ten years of experimental animal isotopic ecology. Funct. Ecol. 2009, 23, 17–26. [Google Scholar] [CrossRef]
- Boecklen, W.J.; Yarnes, C.T.; Cook, B.A.; James, A.C. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 411–440. [Google Scholar] [CrossRef] [Green Version]
- Vander Zanden, H.B.; Soto, D.X.; Bowen, G.J.; Hobson, K.A. Expanding the isotopic toolbox: Application of hydrogen and oxygen stable isotope ratios to food web studies. Front. Ecol. Evol. 2016, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Van Wijk, R.E.; Barshep, Y.; Hobson, K.A. On the use of stable hydrogen isotope measurements (δ2H) to discern trophic level in avian terrestrial food webs. Diversity 2021, 13, 202. [Google Scholar] [CrossRef]
- Hobson, K.A.; Van Wilgenburg, S.L.; Wassenaar, L.I.; Larson, K. Linking hydrogen (δ2H) isotopes in feathers and precipitation: Sources of variance and consequences for assignment to isoscapes. PLoS ONE 2012, 7, e35137. [Google Scholar] [CrossRef]
- Vander Zanden, H.B.; Wunder, M.B.; Hobson, K.A.; Van Wilgenburg, S.L.; Wassenaar, L.I.; Welker, J.M.; Bowen, G.J. Contrasting assignment of migratory organisms to geographic origins using long-term versus year-specific precipitation isotope maps. Methods Ecol. Evol. 2014, 5, 891–900. [Google Scholar] [CrossRef] [Green Version]
- Nordell, C.J.; Haché, S.; Bayne, E.M.; Sólymos, P.; Foster, K.R.; Godwin, C.M.; Krikun, R.; Pyle, P.; Hobson, K.A. Within-site variation in feather stable hydrogen isotope (δ2Hf) values of boreal songbirds: Implications for assignment to molt origin. PLoS ONE 2016, 11, e0163957. [Google Scholar] [CrossRef]
- McKechnie, A.E.; Wolf, B.O.; Martínez del Rio, C. Deuterium stable isotope ratios as tracers of water resource use: An experimental test with rock doves. Oecologia 2004, 140, 191–200. [Google Scholar] [CrossRef]
- Betini, G.S.; Hobson, K.A.; Wassenaar, L.I.; Norris, D.R. Stable hydrogen isotope values (δD) in songbird nestlings: Effects of diet, temperature and body size. Can. J. Zool. 2009, 87, 767–772. [Google Scholar] [CrossRef]
- Criste, P.; Møller, A.P.; Saino, N.; De Lope, F. Genetic and environmental components of phenotypic variation in immune response and body size of a colonial bird, Delichon urbica (the house martin). Heredity 2000, 85, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Tella, J.L.; Bortolotti, G.R.; Forero, M.G.; Dawson, R.D. Environmental and genetic variation in T-cell mediated immune response of fledgling American kestrels. Oecologia 2000, 123, 453–459. [Google Scholar] [CrossRef]
- Evans, M.R.; Roberts, M.L.; Buchanan, K.L.; Goldsmith, A.R. Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. J. Evol. Biol. 2006, 19, 343–352. [Google Scholar] [CrossRef]
- Griebel, I.A.; Fairhurst, G.D.; Marchant, T.A.; Clark, R.G. Effects of parental and nest site characteristics on nestling quality in the tree swallow (Tachycineta bicolor). Can. J. Zool. 2018, 97, 63–71. [Google Scholar] [CrossRef]
- Pym, R.A.E. Nutritional Genetics. In Poultry Breeding and Genetics; Crawford, R.D., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 847–876. [Google Scholar]
- Bortolotti, L.E.; Clark, R.G.; Wassenaar, L.I. Hydrogen isotope variability in prairie wetland systems: Implications for studies of migratory connectivity. Ecol. Appl. 2013, 23, 110–121. [Google Scholar] [CrossRef]
- Fairhurst, G.D.; Treen, G.; Clark, R.G.; Bortolotti, G.R. Nestling corticosterone response to microclimate in an altricial bird. Can. J. Zool. 2012, 90, 1422–1430. [Google Scholar] [CrossRef]
- Johns, D.W.; Marchant, T.A.; Fairhurst, G.D.; Speakman, J.; Clark, R.G. Biomarker of burden: Feather corticosterone reflects energetic expenditure and allostatic overload in captive waterfowl. Funct. Ecol. 2017, 32, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Magozzi, C.; Vander Zanden, H.B.; Wunder, M.B.; Bowen, G.J. Mechanistic model predicts tissue–environment relationships and trophic shifts in animal hydrogen and oxygen isotope ratios. Oecologia 2019, 191, 777–789. [Google Scholar] [CrossRef]
- Meehan, T.D.; Rosenfield, R.N.; Atudorei, V.N.; Bielfeldt, J.; Rosenfield, L.J.; Stewart, A.C.; Stoudt, W.E.; Bozek, M.A. Variation in stable-hydrogen isotope ratios between adult and nestling Cooper’s hawks. Condor 2003, 105, 567–572. [Google Scholar] [CrossRef]
- Smith, A.D.; Dufty, A.M. Variation in the stable-hydrogen isotope composition of northern goshawk feathers: Relevance to the study of migratory origins. Condor 2005, 107, 547–558. [Google Scholar] [CrossRef]
- Greenwood, J.L.; Dawson, R.D. Correlates of deuterium enrichment in the feathers of adult American kestrels of known origin. Condor 2011, 113, 555–564. [Google Scholar] [CrossRef]
- Shutler, D.; Clark, R.G. Causes and consequences of tree swallow (Tachycineta bicolor) dispersal in Saskatchewan. Auk 2003, 120, 619–631. [Google Scholar] [CrossRef]
- Griebel, I.A.; Dawson, R.D. Experimental reduction of nestling hemoglobin concentration in combination with ectoparasite load manipulation affects nestling morphology and begging behavior, but not adult behavior. J. Ornithol. 2020, 161, 35–45. [Google Scholar] [CrossRef]
- Griebel, I.A.; Dawson, R.D.; Clark, R.G. Cavity type influences abundance of nest-dwelling avian blow flies: An experiment with tree swallows. Ecol. Entomol. 2020, 45, 434–443. [Google Scholar] [CrossRef]
- Dawson, R.D. Timing of breeding and environmental factors as determinants of reproductive performance of tree swallows. Can. J. Zool. 2008, 86, 843–850. [Google Scholar] [CrossRef]
- Dawson, R.D.; Bortolotti, G.R. Fire in the boreal forest: Proximate effects on reproduction and long-term consequences for territory occupancy of American kestrels. Ecoscience 2006, 13, 75–81. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Tella, J.L.; Forero, M.G.; Dawson, R.D.; Negro, J.J. Genetics, local environment and health as factors influencing plasma carotenoids in wild American kestrels (Falco sparverius). Proc. R. Soc. Lond. B 2000, 267, 1433–1438. [Google Scholar] [CrossRef] [Green Version]
- Wassenaar, L.I.; Hobson, K.A. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isot. Environ. Health Stud. 2003, 39, 211–217. [Google Scholar] [CrossRef]
- Soto, D.X.; Koehler, G.; Wassenaar, L.I.; Hobson, K.A. Re-evaluation of the hydrogen stable isotopic composition of keratin calibration standards for wildlife and forensic science applications. Rapid Commun. Mass Specrom. 2017, 31, 1193–1203. [Google Scholar] [CrossRef]
- SAS Institute. Statistical Analysis System, version 9.4; SAS Institute: Cary, NC, USA, 2016. [Google Scholar]
- Elgin, A.S.; Clark, R.G.; Morrissey, C.A. Tree swallow selection of wetlands in agricultural landscapes predicted by central place foraging theory. Condor Ornithol. Appl. 2020, 122, duaa039. [Google Scholar] [CrossRef]
- Michelson, C.I.; Clark, R.G.; Morrissey, C.A. Diets of adult and nestling tree swallows in contrasting agricultural environments: Evidence from stable isotope analyses. Condor Ornithol. Appl. 2018, 120, 751–764. [Google Scholar]
- Wolf, N.; Newsome, S.D.; Fogel, M.L.; Martínez del Rio, C.M. An experimental exploration of the incorporation of hydrogen isotopes from dietary sources into avian tissues. J. Exp. Biol. 2012, 215, 1915–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wunder, M.; Hobson, K.A.; Kelly, J.; Marra, P.; Wassenaar, L.I.; Stricker, C.; Doucette, R. Does a lack of design and repeatability compromise scientific criticism? A response to Smith et al. Auk 2009, 126, 922–926. [Google Scholar] [CrossRef]
- Grénier, C.S.V.; Guglielmo, C.G.; Mitchell, G.W.; Falconer, M.; Hobson, K.A. Nutritional consequences of breeding away from riparian habitats in bank swallows: New evidence from multiple endogenous markers. Conserv. Physiol. 2021, 9, coaa140. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).