Differing Life-History Strategies of Two Mycoheterotrophic Orchid Species Associated with Leaf Litter- and Wood-Decaying Fungi
Abstract
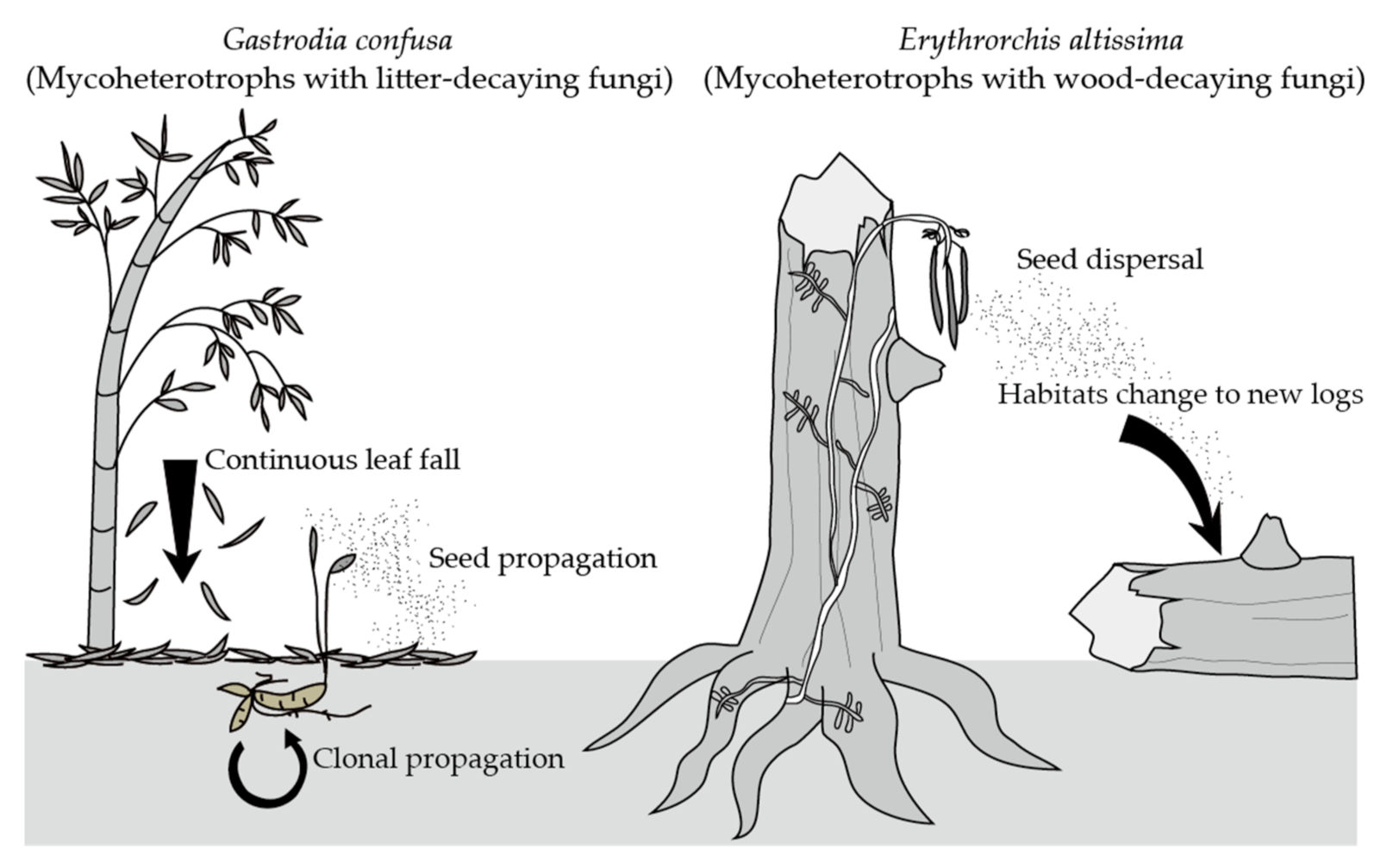
1. Introduction
2. Materials and Methods
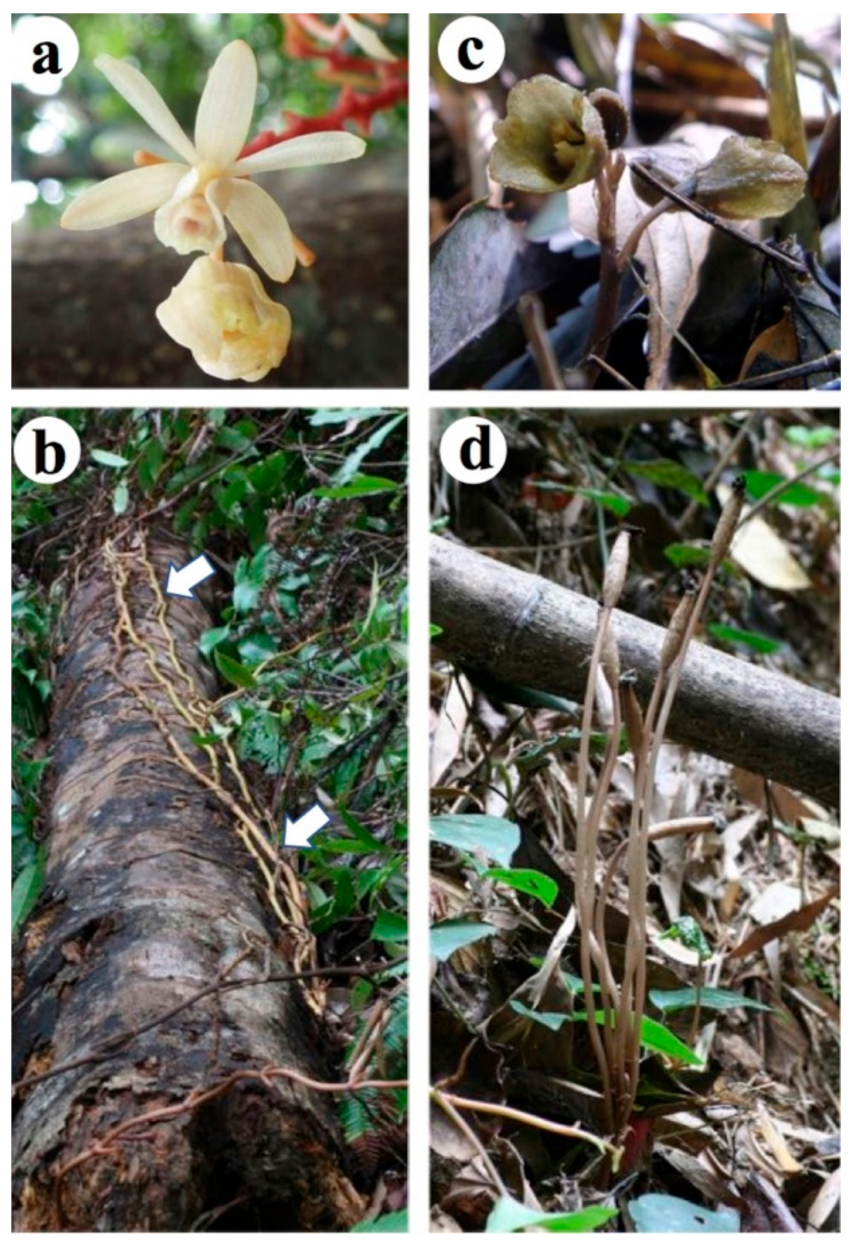
2.1. Erythrorchis altissima
2.2. Gastrodia confusa
3. Results
3.1. Erythrorchis altissima
3.2. Gastrodia confusa
4. Discussion
4.1. Erythrorchis altissima
4.2. Gastrodia confusa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leake, J. Tansley Review No. 69. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol. 1994, 127, 171–216. [Google Scholar] [CrossRef]
- Merckx, V.S.F.T. Mycoheterotrophy: The Biology of Plants Living on Fungi; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9781461452096. [Google Scholar]
- Ogura-Tsujita, Y.; Yukawa, T.; Kinoshita, A. Evolutionary histories and mycorrhizal associations of mycoheterotrophic plants dependent on saprotrophic fungi. J. Plant Res. 2021, 134, 19–41. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.K.; Whigham, D.F.; O’Neill, J.P.; Becker, J.J.; Sarah, W.; Rasmussen, H.N.; Bruns, T.D.; Taylor, D.L. Abundance and distribution of Corallorhiza odontorhiza reflect variations in climate and ectomycorrhizae. Ecol. Monogr. 2009, 79, 619–635. [Google Scholar] [CrossRef]
- Shefferson, R.P.; McCormick, M.K.; Whigham, D.F.; O’Neill, J.P. Life history strategy in herbaceous perennials: Inferring demographic patterns from the aboveground dynamics of a primarily subterranean, myco-heterotrophic orchid. Oikos 2011, 120, 1291–1300. [Google Scholar] [CrossRef]
- Verrier, J.T. A mycoheterotrophic orchid, Tomentelloid fungi, and drought in an Arizona Sky Island. Desert Plants 2017, 33, 3–9. [Google Scholar] [CrossRef]
- Bergman, E.; Ackerman, J.D.; Thompson, J.; Zimmerman, J.K. Land-use history affects the distribution of the saprophytic orchid Wullschlaegelia calcarata in Puerto Rico’s tabonuco forest. Biotropica 2006, 38, 492–499. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, H.; Fan, X.L.; Gao, J.Y. Autonomous self-pollination and insect visitation in a saprophytic orchid, Epipogium roseum (D.Don) Lindl. Aust. J. Bot. 2012, 60, 154–159. [Google Scholar] [CrossRef]
- Suetsugu, K. Autogamous fruit set in a mycoheterotrophic orchid Cyrtosia septentrionalis. Plant Syst. Evol. 2013, 299, 481–486. [Google Scholar] [CrossRef]
- Suetsugu, K. Gynomonoecy in a mycoheterotrophic orchid Eulophia zollingeri with autonomous selfing hermaphroditic flowers and putatively outcrossing female flowers. PeerJ 2020, 8, e10272. [Google Scholar] [CrossRef]
- Stokland, J.N.; Siitonen, J.; Jonsson, B.G. Biodiversity in Dead Wood; Cambridge University Press: Cambridge, UK, 2012; ISBN 9781139025843. [Google Scholar]
- Osono, T. Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol. Res. 2007, 22, 955–974. [Google Scholar] [CrossRef]
- Ogura-Tsujita, Y.; Gebauer, G.; Xu, H.; Fukasawa, Y.; Umata, H.; Tetsuka, K.; Kubota, M.; Schweiger, J.M.I.; Yamashita, S.; Maekawa, N.; et al. The giant mycoheterotrophic orchid Erythrorchis altissima is associated mainly with a divergent set of wood-decaying fungi. Mol. Ecol. 2018, 27, 1324–1337. [Google Scholar] [CrossRef]
- Comber, J.B. Orchids of Java; Betham-Moxon Trust: Surrey, UK, 1990. [Google Scholar]
- Ogura-Tsujita, Y.; Gebauer, G.; Hashimoto, T.; Umata, H.; Yukawa, T. Evidence for novel and specialized mycorrhizal parasitism: The orchid Gastrodia confusa gains carbon from saprotrophic Mycena. Proc. R. Soc. B Biol. Sci. 2009, 276, 761–767. [Google Scholar] [CrossRef]
- Kinoshita, A.; Ogura-Tsujita, Y.; Umata, H.; Sato, H.; Hashimoto, T.; Yukawa, T. How do fungal partners affect the evolution and habitat preferences of mycoheterotrophic plants? A case study in Gastrodia. Am. J. Bot. 2016, 103, 207–220. [Google Scholar] [CrossRef]
- Yukawa, T. Orchidaceae. In Wild Flowers of Japan; Ohashi, H., Kadota, Y., Murata, J., Yonekura, K., Kihara, H., Eds.; Hoibonsha: Tokyo, Japan, 2015; pp. 178–231. [Google Scholar]
- Xu, J.; Guo, S. Retrospect on the research of the cultivation of Gastrodia elata Bl, a rare traditional Chinese medicine. Chin. Med. J. 2000, 113, 686–692. [Google Scholar]
- Fukasawa, Y.; Osono, T.; Takeda, H. Dynamics of physicochemical properties and occurrence of fungal fruit bodies during decomposition of coarse woody debris of Fagus crenata. J. For. Res. 2009, 14, 20–29. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Fiendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Available online: http://cran.r-project.org/package=vegan (accessed on 2 December 2020).
- Umata, H. The habitat and habit of a chlorophyll-free orchid, Erythrorchis altissima (Bl.) Bl. Acta Phytotax. Geobot. 1994, 45, 131–138. [Google Scholar]
- Dearnaley, J. The fungal endophytes of Erythrorchis cassythoides—Is this orchid saprophytic or parasitic? Australas. Mycol. 2006, 25, 51–57. [Google Scholar]
- Shimaoka, C.; Fukunaga, H.; Inagaki, S.; Sawa, S. Artificial cultivation system for Gastrodia spp. and identification of associated mycorrhizal fungi. Int. J. Biol. 2017, 9, 27–34. [Google Scholar] [CrossRef][Green Version]
- Martos, F.; Dulormne, M.; Pailler, T.; Bonfante, P.; Faccio, A.; Fournel, J.; Dubois, M.P.; Selosse, M.A. Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytol. 2009, 184, 668–681. [Google Scholar] [CrossRef]
- Hatté, C.; Zazzo, A.; Selosse, M.A. The radiocarbon age of mycoheterotrophic plants. New Phytol. 2020, 227, 1284–1288. [Google Scholar] [CrossRef]
- Yagame, T.; Yamato, M.; Mii, M.; Suzuki, A.; Iwase, K. Developmental processes of achlorophyllous orchid, Epipogium roseum: From seed germination to flowering under symbiotic cultivation with mycorrhizal fungus. J. Plant Res. 2007, 120, 229–236. [Google Scholar] [CrossRef] [PubMed]
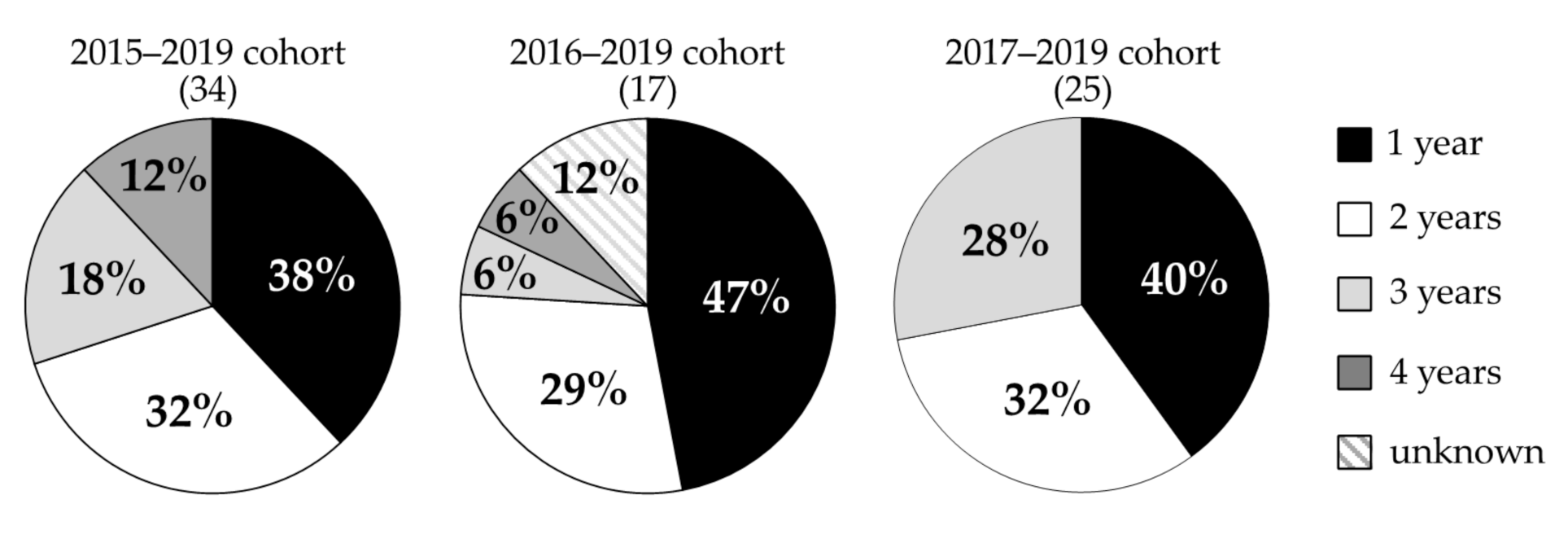


| Monitoring Year | No. of Individuals Monitored | No. of Individuals That Flowered | No. of Individuals That Died within a Year after Flowering |
|---|---|---|---|
| 2015–2019 | 34 | 28 | 21 |
| 2016–2019 | 17 | 8 | 6 |
| 2017–2019 | 25 | 11 | 6 |
| Total | 76 | 47 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogura-Tsujita, Y.; Tetsuka, K.; Tagane, S.; Kubota, M.; Anan, S.; Yamashita, Y.; Tone, K.; Yukawa, T. Differing Life-History Strategies of Two Mycoheterotrophic Orchid Species Associated with Leaf Litter- and Wood-Decaying Fungi. Diversity 2021, 13, 161. https://doi.org/10.3390/d13040161
Ogura-Tsujita Y, Tetsuka K, Tagane S, Kubota M, Anan S, Yamashita Y, Tone K, Yukawa T. Differing Life-History Strategies of Two Mycoheterotrophic Orchid Species Associated with Leaf Litter- and Wood-Decaying Fungi. Diversity. 2021; 13(4):161. https://doi.org/10.3390/d13040161
Chicago/Turabian StyleOgura-Tsujita, Yuki, Kenshi Tetsuka, Shuichiro Tagane, Miho Kubota, Shuichiro Anan, Yumi Yamashita, Koichi Tone, and Tomohisa Yukawa. 2021. "Differing Life-History Strategies of Two Mycoheterotrophic Orchid Species Associated with Leaf Litter- and Wood-Decaying Fungi" Diversity 13, no. 4: 161. https://doi.org/10.3390/d13040161
APA StyleOgura-Tsujita, Y., Tetsuka, K., Tagane, S., Kubota, M., Anan, S., Yamashita, Y., Tone, K., & Yukawa, T. (2021). Differing Life-History Strategies of Two Mycoheterotrophic Orchid Species Associated with Leaf Litter- and Wood-Decaying Fungi. Diversity, 13(4), 161. https://doi.org/10.3390/d13040161







