Morphological Convergence and Divergence in Galaxias Fishes in Lentic and Lotic Habitats
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Galaxias Sampling
2.3. Galaxias Genetics
2.4. Aquatic Invertebrates
2.5. Hydrological Environment Data
2.6. Galaxias Morphometrics
2.7. Hydrological Environment–Galaxias Morphology Relationships
3. Results
3.1. Hydrological Environment of Lentic and Lotic Habitats
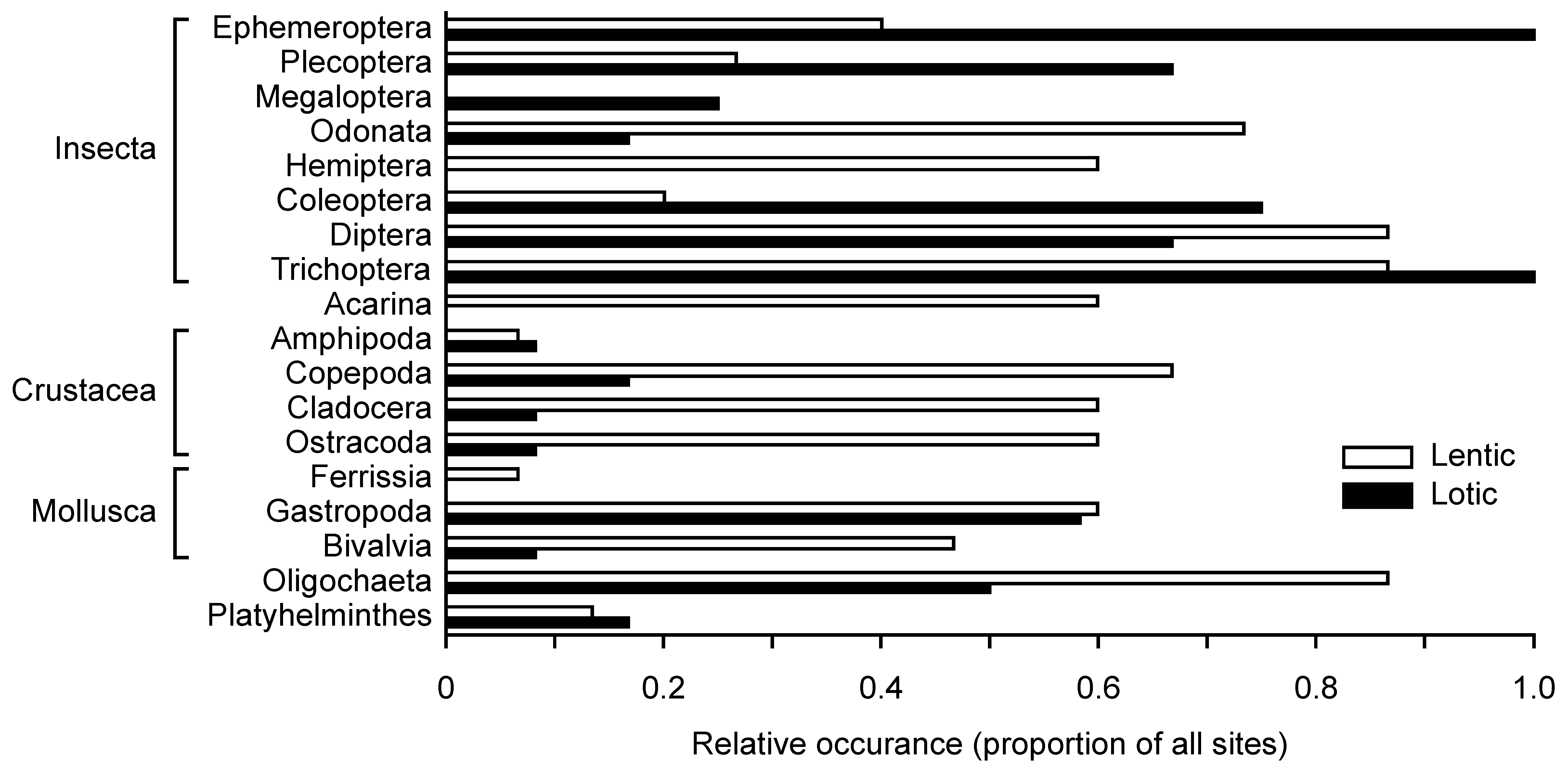
3.2. Aquatic Invertebrates
3.3. Galaxias Morphology in Lentic and Lotic Habitats
3.4. Galaxias Genetic Considerations
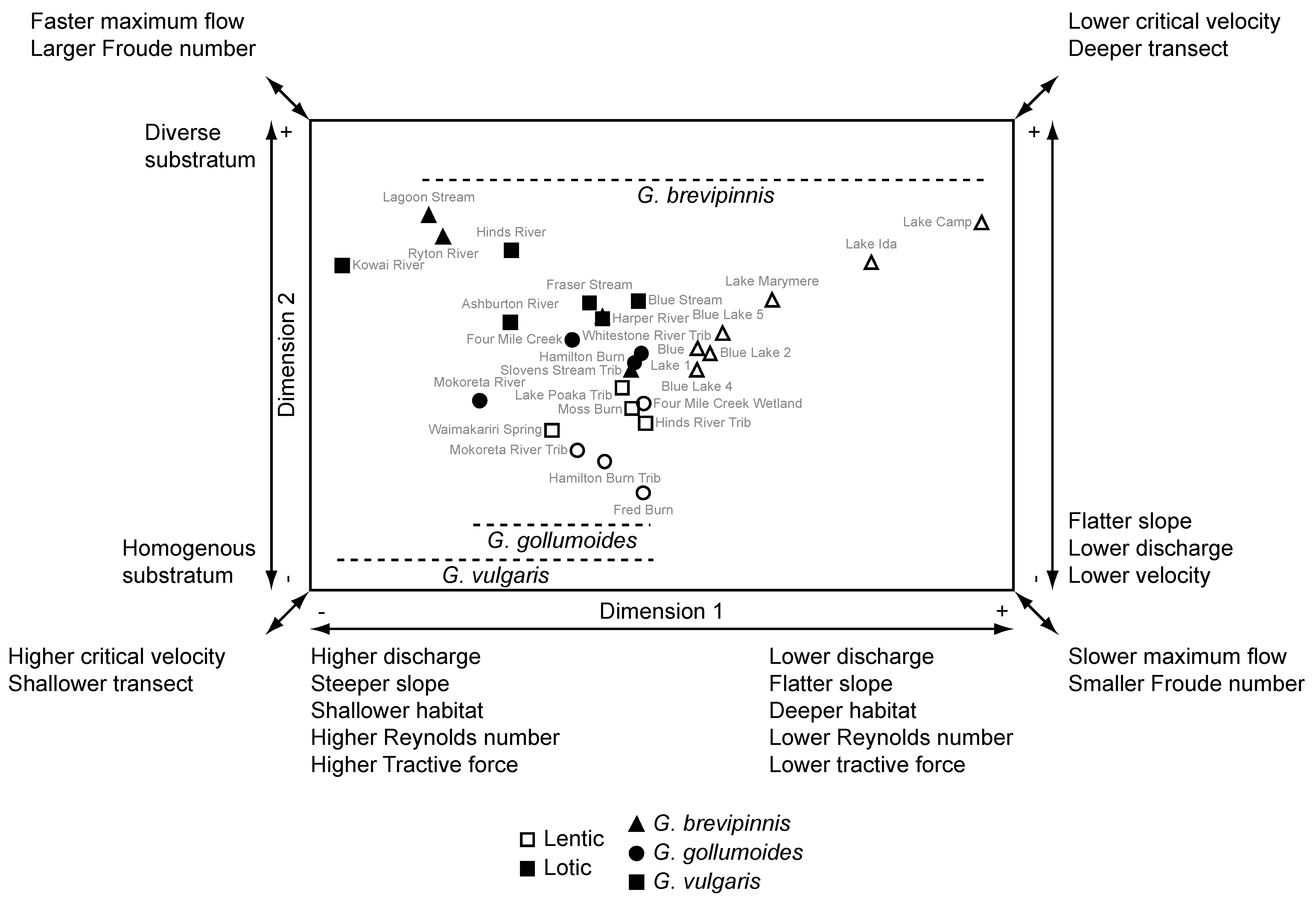
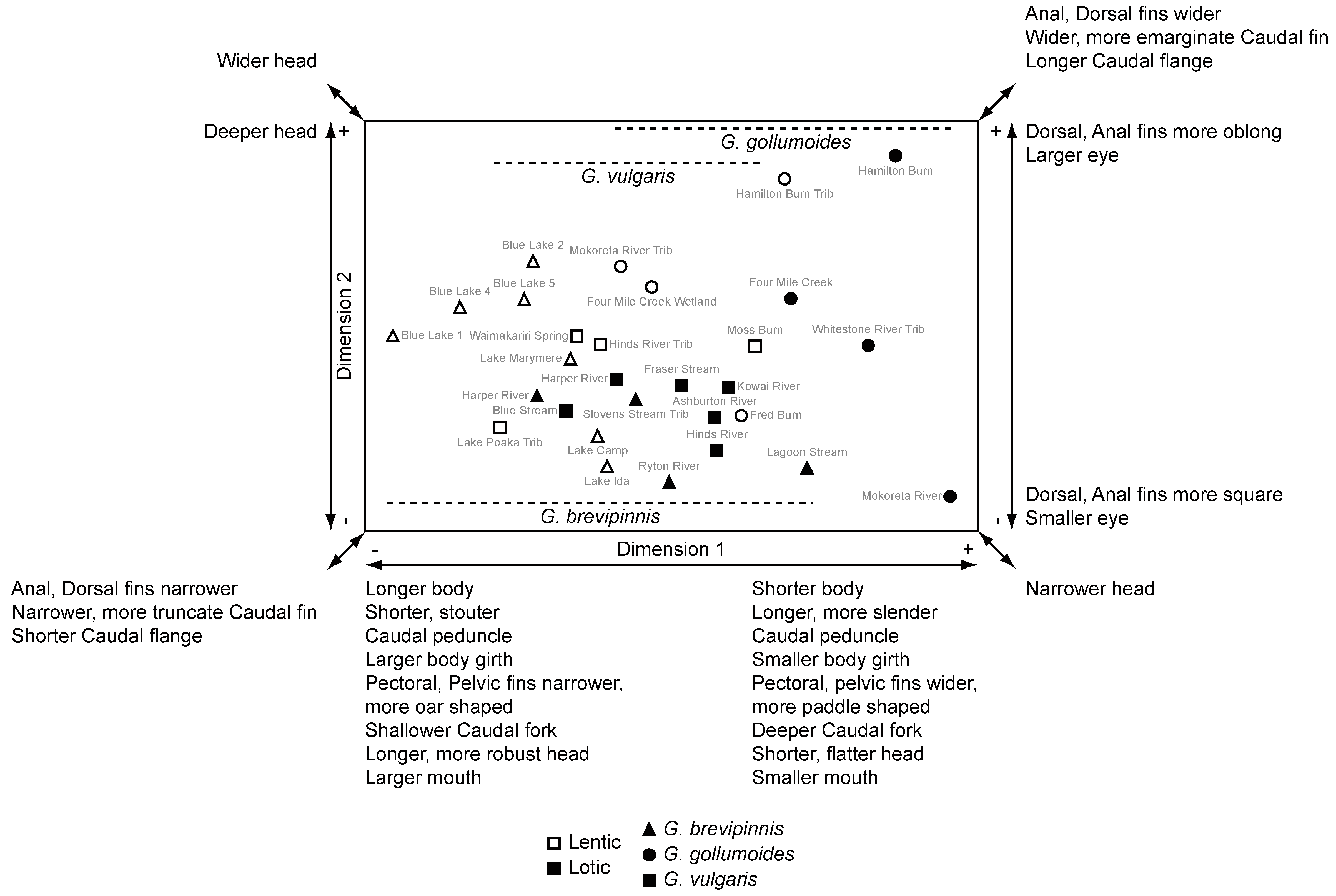
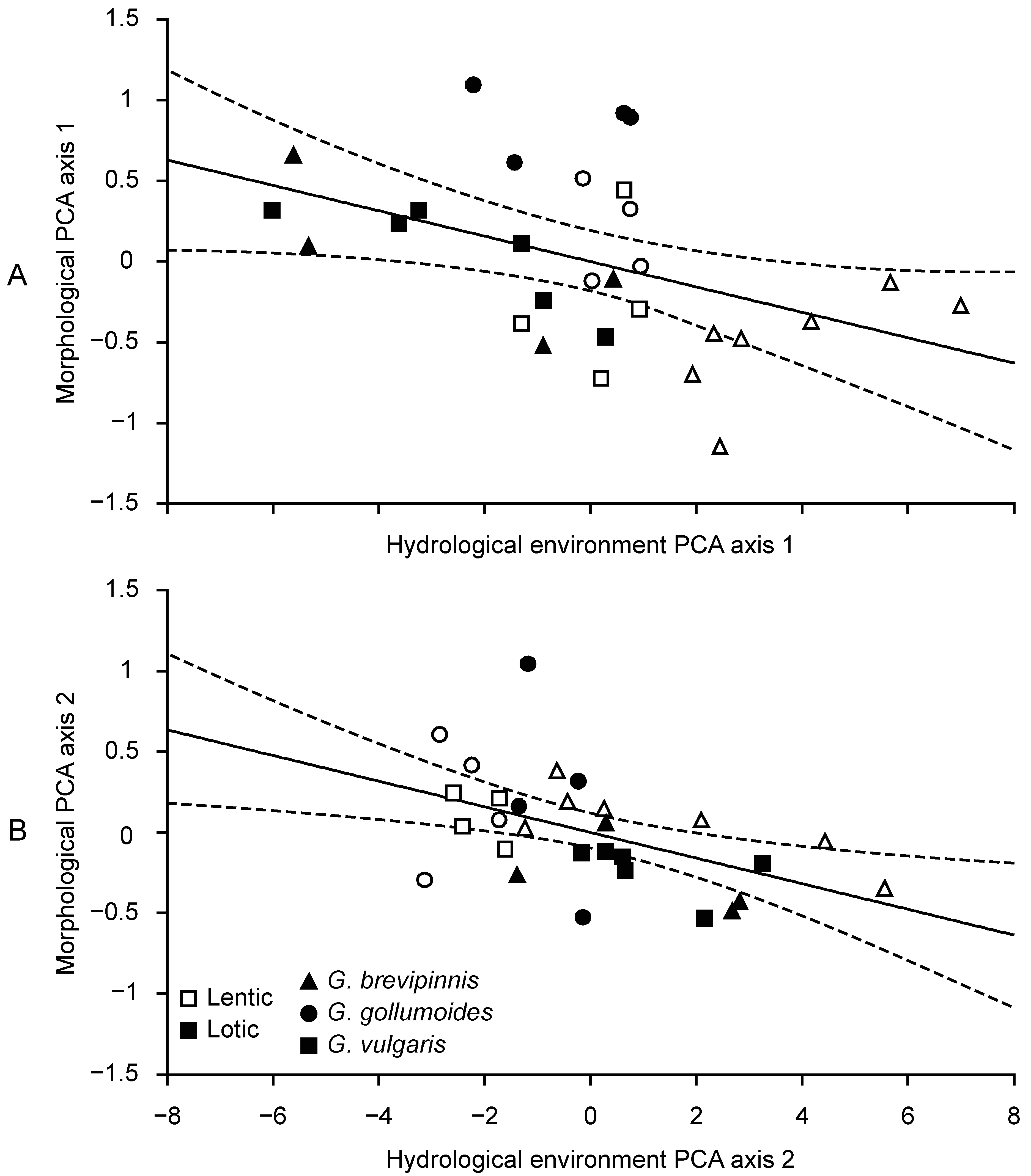
3.5. Relationships between the Hydrological Environment and Galaxias Body Form
4. Discussion
4.1. Convergence and Divergence of Form
4.2. Phenotypic Basis for Morphological Shifts
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lagler, K.F.; Bardach, J.E.; Miller, R.R. Ichthyology; John Wiley and Sons Inc.: New York, NY, USA, 1962; p. 545. [Google Scholar]
- Moyle, P.B.; Cech, J.C. Fishes. An Introduction to Ichthyology, Fifth ed.; Prentice-Hall Incorporated: Upper Saddle River, NJ, USA, 2004; p. 726. [Google Scholar]
- Wootton, R.J. Ecology of Teleost Fishes, Second ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; Volume 24, p. 386. [Google Scholar]
- Page, L.M.; Swofford, D.L. Morphological correlates of ecological specialization in darters. Environ. Biol. Fish. 1984, 11, 139–159. [Google Scholar] [CrossRef]
- McLaughlin, R.L.; Grant, J.W.A. Morphological and behavioural differences among recently-emerged brook charr, Salvelinus fontinalis, foraging in slow- vs. fast-running water. Environ. Biol. Fish. 1994, 39, 289–300. [Google Scholar] [CrossRef]
- Imre, I.; McLaughlin, R.L.; Noakes, D.L.G. Phenotypic plasticity in brook charr: Changes in caudal fin induced by water flow. J. Fish Biol. 2002, 61, 1171–1181. [Google Scholar] [CrossRef]
- Hubbs, C.L. Speciation of fishes. Am. Nat. 1940, 74, 198–211. [Google Scholar] [CrossRef]
- Douglas, M.E.; Matthews, W.J. Does morphology predict ecology? Hypothesis testing within a freshwater stream fish assemblage. Oikos 1992, 65, 213–224. [Google Scholar] [CrossRef]
- Langerhans, R.B.; Layman, C.A.; Langerhans, A.K.; DeWitt, T.J. Habitat-associated morphological divergence in two Neotropical fish species. Biol. J. Linn. Soc. 2003, 80, 689–698. [Google Scholar] [CrossRef]
- Keenleyside, M.H.A. Diversity and Adaptation in Fish Behaviour; Springer-Verlag: Berlin, German, 1979; Volume 11, p. 208. [Google Scholar]
- Matthews, W.J. Patterns in Freshwater Fish Ecology; Chapman & Hall: New York, NY, USA, 1998; p. 756. [Google Scholar]
- Alexander, R.M. Functional Design in Fishes; Hutchinson & Co. Ltd.: London, UK, 1967; p. 160. [Google Scholar]
- Pollard, D.A. The biology of a landlocked form of the normally catadromous Salmoniform fish Galaxias maculatus (Jenyns). II. Morphology and systematic relationships. Aust. J. Mar. Freshwater Res. 1971, 22, 91–123. [Google Scholar] [CrossRef]
- Berra, T.M. A home range study of Galaxias bongbong in Australia. Copeia 1973, 1973, 363–366. [Google Scholar] [CrossRef]
- Cadwallader, P.L. Home range and movements of the common river galaxias, Galaxias vulgaris Stokell (Pisces: Salmoniformes), in the Glentui River, New Zealand. Aust. J. Mar. Freshwater Res. 1976, 27, 23–33. [Google Scholar] [CrossRef]
- Swain, D.P.; Holtby, L.B. Differences in morphology and behavior between juvenile coho salmon (Oncorhynchus kisutch) rearing in a lake and in its tributary stream. Can. J. Fish. Aquat. Sci. 1989, 46, 1406–1414. [Google Scholar] [CrossRef]
- Robinson, B.W.; Wilson, D.S. Character release and displacement in fishes: A neglected literature. Am. Nat. 1994, 144, 596–627. [Google Scholar] [CrossRef]
- Jobling, M. Environmental Biology of Fishes; Chapman & Hall: London, UK, 1995; p. 455. [Google Scholar]
- McDowall, R.M. New Zealand freshwater fishes an historical and ecological biogeography. Fish Fish. 2010, 32, 1–449. [Google Scholar]
- Blaxter, J.H.S. Pattern and variety in development. In Fish physiology. Volume XI. The Physiology of Developing Fish. Part A. Eggs and Larvae; Hoar, W.S., Randall, D.J., Eds.; Academic Press: San Diego, CA, USA, 1988; Volume 11, pp. 1–58. [Google Scholar]
- Scheiner, S.M. Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 1993, 24, 35–68. [Google Scholar] [CrossRef]
- Pigliucci, M. Phenotypic Plasticity: Beyond Nature and Nurture; The Johns Hopkins University Press: Baltimore, MD, USA, 2001; p. 328. [Google Scholar]
- Robinson, B.W.; Parsons, K.J. Changing times, spaces, and faces: Tests and implications of adaptive morphological plasticity in the fishes of northern postglacial lakes. Can. J. Fish. Aquat. Sci. 2002, 59, 1819–1833. [Google Scholar] [CrossRef]
- DeWitt, T.J.; Scheiner, S.M. Phenotypic variation from single genotypes: A primer. In Phenotypic Plasticity. Functional and Conceptual Approaches; DeWitt, T.J.; Scheiner, S.M. Oxford University Press: New York, NY, USA, 2004; pp. 1–9. [Google Scholar]
- McDowall, R.M. The status of Nesogalaxias neocaledonicus (Weber and De Beaufort) (Pisces, Galaxiidae). Breviora 1968, 286, 1–8. [Google Scholar]
- McDowall, R.M. The galaxiid fishes of New Zealand. Bull. Mus. Compar. Zool. 1970, 139, 341–432. [Google Scholar]
- McDowall, R.M. The galaxiid fishes of South America. Zool. J. Linn. Soc. 1971, 50, 33–73. [Google Scholar] [CrossRef]
- McDowall, R.M. The status of the South African galaxiid (Pisces, Galaxiidae). Ann. Cape Prov. Mus. 1973, 9, 91–101. [Google Scholar]
- McDowall, R.M. New Zealand Freshwater Fishes: A Natural History and Guide; Heinemann Reed and MAF Publishing Group: Auckland, New Zealand, 1990; p. 553. [Google Scholar]
- McDowall, R.M. Crying wolf, crying foul, or crying shame: Alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes? Rev. Fish Biol. Fish. 2006, 16, 233–422. [Google Scholar] [CrossRef]
- McDowall, R.M.; Frankenberg, R.S. The Galaxiid fishes of Australia (Pisces: Galaxiidae). Rec. Austr. Mus. 1981, 33, 443–605. [Google Scholar] [CrossRef]
- Humphries, P. Morphological variation in diadromous and landlocked populations of the spotted galaxias, Galaxias truttaceus, in Tasmania, south-eastern Australia. Environ. Biol. Fish. 1990, 27, 97–105. [Google Scholar] [CrossRef]
- McDowall, R.M. The composition of the New Zealand whitebait catch, 1964. New Zeal. J. Sci. 1965, 8, 285–300. [Google Scholar]
- McDowall, R.M. New land-locked fish species of the genus Galaxias from North Auckland, New Zealand. Breviora 1967, 265, 1–11. [Google Scholar]
- McDowall, R.M. The species problem in freshwater fishes and the taxonomy of diadromous and lacustrine populations of Galaxias maculatus (Jenyns). J. Royal Soc. New Zeal. 1972, 2, 325–367. [Google Scholar] [CrossRef]
- Crow, S.K.; Waters, J.M.; Closs, G.P.; Wallis, G.P. Morphological and genetic analysis of Galaxias ‘southern’ and G. gollumoides: Interspecific differentiation and intraspecific structuring. J. Royal Soc. New Zeal. 2009, 39, 43–62. [Google Scholar] [CrossRef]
- McDowall, R.M. Phylogenetic relationships and ecomorphological divergence in sympatric and allopatric species of Paragalaxias (Teleostei: Galaxiidae) in high elevation Tasmanian lakes. Environ. Biol. Fish. 1998, 53, 235–257. [Google Scholar] [CrossRef]
- McDowall, R.M. An accessory lateral line in some New Zealand and Australian galaxiids (Teleostei: Galaxiidae). Ecol. Freshwater Fish. 1997, 6, 217–224. [Google Scholar] [CrossRef]
- McDowall, R.M.; Nakaya, K. Morphological divergence in the two species of Aplochiton Jenyns (Salmoniformes: Aplochitonidae): A generalist and a specialist. Copeia 1988, 1988, 233–236. [Google Scholar] [CrossRef]
- Lattuca, M.E.; Ortubay, S.G.; Battini, M.A.; Barriga, J.P.; Cussac, V.E. Presumptive environmental effects on body shape of Aplochiton zebra (Pisces, Galaxiidae) in northern Patagonian lakes. J. Appl. Ichthyol. 2007, 23, 25–33. [Google Scholar] [CrossRef]
- McDowall, R.M.; Pankhurst, N.W. Loss of negative eye-size allometry in a population of Aplochiton zebra (Teleostei: Galaxiidae) from the Falkland Islands. New Zeal. J. Zool. 2005, 32, 17–22. [Google Scholar] [CrossRef]
- Milano, D.; Cussac, V.E.; Macchi, P.J.; Ruzzante, D.E.; Alonso, M.F.; Vigliano, P.H.; Denegri, M.A. Predator associated morphology in Galaxias platei in Patagonian lakes. J. Fish Biol. 2002, 61, 138–156. [Google Scholar] [CrossRef]
- Jones, P.E.; Closs, G.P. Interspecific differences in early life-history traits in a species complex of stream-resident galaxiids. Ecol. Freshwater Fish. 2016, 25, 211–224. [Google Scholar] [CrossRef]
- Allibone, R.M.; Wallis, G.P. Genetic variation and diadromy in some native New Zealand galaxiids (Teleostei: Galaxiidae). Biol. J. Linn. Soc. 1993, 50, 13–33. [Google Scholar] [CrossRef]
- McDowall, R.M.; Hewitt, J. Attempts to Distinguish Morphotypes of the Canterbury–Otago Non-Migratory Galaxias Species Complex; Department of Conservation: Wellington, New Zealand, 2004; Volume 165, pp. 1–19. [Google Scholar]
- McDowall, R.M. The Taxonomic Status, Distribution and Identification of the Galaxias Vulgaris Species Complex in the Eastern/Southern South Island and Stewart Island; NIWA Client Report: CHCDOC2006-081; National Institute of Water & Atmospheric Research Ltd.: Christchurch, New Zealand, 2006; p. 41. [Google Scholar]
- McDowall, R.M.; Wallis, G.P. Description and redescription of Galaxias species (Teleostei: Galaxiidae) from Otago and Southland. J. Royal Soc. New Zeal. 1996, 26, 401–427. [Google Scholar] [CrossRef]
- Waters, J.M.; Wallis, G.P. Mitochondrial DNA phylogenetics of the Galaxias vulgaris complex from South Island, New Zealand: Rapid radiation of a species flock. J. Fish Biol. 2001, 58, 1166–1180. [Google Scholar] [CrossRef]
- Department of Conservation. New Zealand Non-Migratory Galaxiid Fishes Recovery Plan 2003-13; Threatened Species Recovery Plan 53; Department of Conservation: Wellington, New Zealand, 2004; p. 45. [Google Scholar]
- McDowall, R.M. The Reed Field Guide to New Zealand Freshwater Fishes; Reed Books: Auckland, New Zealand, 2000; p. 224. [Google Scholar]
- Burridge, C.P.; Craw, D.; Waters, J.M. River capture, range expansion, and cladogenesis: The genetic signature of freshwater vicariance. Evolution 2006, 60, 1038–1049. [Google Scholar] [CrossRef]
- Waters, J.M.; Wallis, G.P. Cladogenesis and loss of the marine life-history phase in freshwater galaxiid fishes (Osmeriformes: Galaxiidae). Evolution 2001, 55, 587–597. [Google Scholar] [CrossRef]
- Burridge, C.P.; Craw, D.; Waters, J.M. An empirical test of freshwater vicariance via river capture. Mol. Ecol. 2007, 16, 1883–1895. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods); Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2003. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.1). An Integrated Software Package for Population Genetics Data Analysis; Computational and Molecular Population Genetics Lab (CMPG), University of Berne: Bern, Switzerland, 2006; p. 145. [Google Scholar]
- Stark, J.D.; Boothroyd, I.K.G.; Harding, J.S.; Maxted, J.R.; Scarsbrook, M.R. Protocols For Sampling Macro Invertebrates in Wadeable Streams. New Zealand Macro Invertebrate Working Group Report No. 1. Prepared for the Ministry for the Environment; Sustainable Management Fund Project No. 5103. Wellington, New Zealand, 2001. Available online: https://www.researchgate.net/publication/288969348_Protocols_for_Sampling_Macroinvertebrates_in_Wadeable_Streams (accessed on 31 January 2001).
- Chapman, A.; Lewis, M. An Introduction to the Freshwater Crustacea of New Zealand; Collins: Auckland, New Zealand, 1976; p. 261. [Google Scholar]
- Winterbourn, M.J.; Gregson, K.L.D.; Dolphin, C.H. Guide to the aquatic insects of New Zealand. Third edition. Bull. ESNZ 2000, 13, 1–102. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; p. 190. [Google Scholar]
- StatSoft Inc. STATISTICA for Windows, Version 6.0; Statsoft Inc.: Tulsa, OK, USA, 2001.
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- McDowall, R.M. Affinities, generic classification and biogeography of the Australian and New Zealand mudfishes (Salmoniformes: Galaxiidae). Rec. Aust. Mus. 1997, 49, 121–137. [Google Scholar] [CrossRef]
- McDowall, R.M.; Chadderton, W.L. Galaxias gollumoides (Teleostei: Galaxiidae), a new fish species from Stewart Island, with notes on other non-migratory freshwater fishes present on the island. J. Royal Soc. New Zeal. 1999, 29, 77–88. [Google Scholar] [CrossRef]
- Raadik, T.A. Fifteen from one: A revision of the Galaxias olidus Günther, 1866 complex (Teleostei, Galaxiidae) in south-eastern Australia recognises three previously described taxa and describes 12 new species. Zootaxa 2014, 3898, 1–198. [Google Scholar] [CrossRef]
- Langerhans, R.B. Predictability of phenotypic differentiation across flow regimes in fishes. Integr. Comp. Biol. 2008, 48, 750–768. [Google Scholar] [CrossRef]
- O’Brien, L.K.; Dunn, N.R. Mudfish (Neochanna Galaxiidae) Literature Review; Department of Conservation: Wellington, New Zealand, 2007; p. 8. [Google Scholar]
- Waters, J.M.; McDowall, R.M. Phylogenetics of the Australasian mudfishes: Evolution of an eel-like body plan. Mol. Phylogenet. Evol. 2005, 37, 417–425. [Google Scholar] [CrossRef]
- Keast, A.; Webb, D. Mouth and body form relative to feeding ecology in the fish fauna of a small lake, Lake Opinicon, Ontario. J. Fish. Res. Board Can. 1966, 23, 1845–1874. [Google Scholar] [CrossRef]
- McDowall, R.M.; Pole, M. A large galaxiid fossil (Teleostei) from the Miocene of Central Otago, New Zealand. J. Royal Soc. New Zeal. 1997, 27, 193–198. [Google Scholar] [CrossRef]
- McDowall, R.M. Variation in vertebral number in galaxiid fishes, how fishes swim and a possible reason for pleomerism. Rev. Fish Biol. Fish. 2003, 13, 247–263. [Google Scholar] [CrossRef]
- Brinsmead, J.; Fox, M.G. Morphological variation between lake- and stream-dwelling rock bass and pumpkinseed populations. J. Fish Biol. 2002, 61, 1619–1638. [Google Scholar] [CrossRef]
- Sagnes, P.; Champagne, J.-Y.; Morel, R. Shifts in drag and swimming potential during grayling ontogenesis: Relations with habitat use. J. Fish Biol. 2000, 57, 52–68. [Google Scholar] [CrossRef]
- McKenzie, M.K. Embryonic and larval structures of Galaxias attenuatus (Jenyns). Master’s Thesis, Victoria University College, Wellington, New Zealand, 1933. [Google Scholar]
- McDowall, R.M. Variation in vertebral number in galaxiid fishes (Teleostei: Galaxiidae): A legacy of life history, latitude and length. Environ. Biol. Fish. 2003, 66, 361–381. [Google Scholar] [CrossRef]
- Webb, P.W. Form and function in fish swimming. Sci. Am. 1984, 251, 72–82. [Google Scholar] [CrossRef]
- McDowall, R.M. The Chatham Islands endemic galaxiid: A Neochanna mudfish (Teleostei: Galaxiidae). J. Royal Soc. New Zeal. 2004, 34, 315–331. [Google Scholar] [CrossRef]
- McDowall, R.M.; Burridge, C.P. Osteology and relationships of the southern freshwater lower euteleostean fishes. Zoosyst. Evol. 2011, 87, 7–185. [Google Scholar] [CrossRef]
- McDowall, R.M.; Waters, J.M. A new species of Galaxias (Teleostei: Galaxiidae) from the Mackenzie Basin, New Zealand. J. Royal Soc. New Zeal. 2003, 33, 675–691. [Google Scholar] [CrossRef]
- McDowall, R.M.; Waters, J.M. Phylogenetic relationships in a small group of diminutive galaxiid fishes and the evolution of sexual dimorphism. J. Royal Soc. New Zeal. 2004, 34, 23–57. [Google Scholar] [CrossRef]
- McDowall, R.M. Relationships of galaxioid fishes with a further discussion of salmoniform classification. Copeia 1969, 1969, 796–824. [Google Scholar] [CrossRef]
- Crow, S.K.; McDowall, R.M. Ontogenetic changes in morphology of flathead galaxiid fishes (Osmeriformes: Galaxiidae) in South Island, New Zealand. New Zeal. J. Mar. Freshwater Res. 2011, 45, 689–702. [Google Scholar] [CrossRef]
- McIntosh, A.R.; McHugh, P.A.; Dunn, N.R.; Goodman, J.M.; Howard, S.W.; Jellyman, P.G.; O’Brien, L.K.; Nyström, P.; Woodford, D.J. The impact of trout on galaxiid fishes in New Zealand. New Zeal. J. Ecol. 2010, 34, 195–206. [Google Scholar]
- Norton, S.F. A functional approach to ecomorphological patterns of feeding in cottid fishes. Environ. Biol. Fish. 1995, 44, 61–78. [Google Scholar] [CrossRef]
- Sagnes, P.; Gaudin, P.; Statzner, B. Shifts in morphometrics and their relation to hydrodynamic potential and habitat use during grayling ontogenesis. J. Fish Biol. 1997, 50, 846–858. [Google Scholar] [CrossRef]
- Dunn, N.R.; O’Brien, L.K.; Closs, G.P. Phenotypically induced intraspecific variation in the morphological development of wetland and stream Galaxias gollumoides McDowall and Chadderton. Diversity 2020, in press. [Google Scholar]
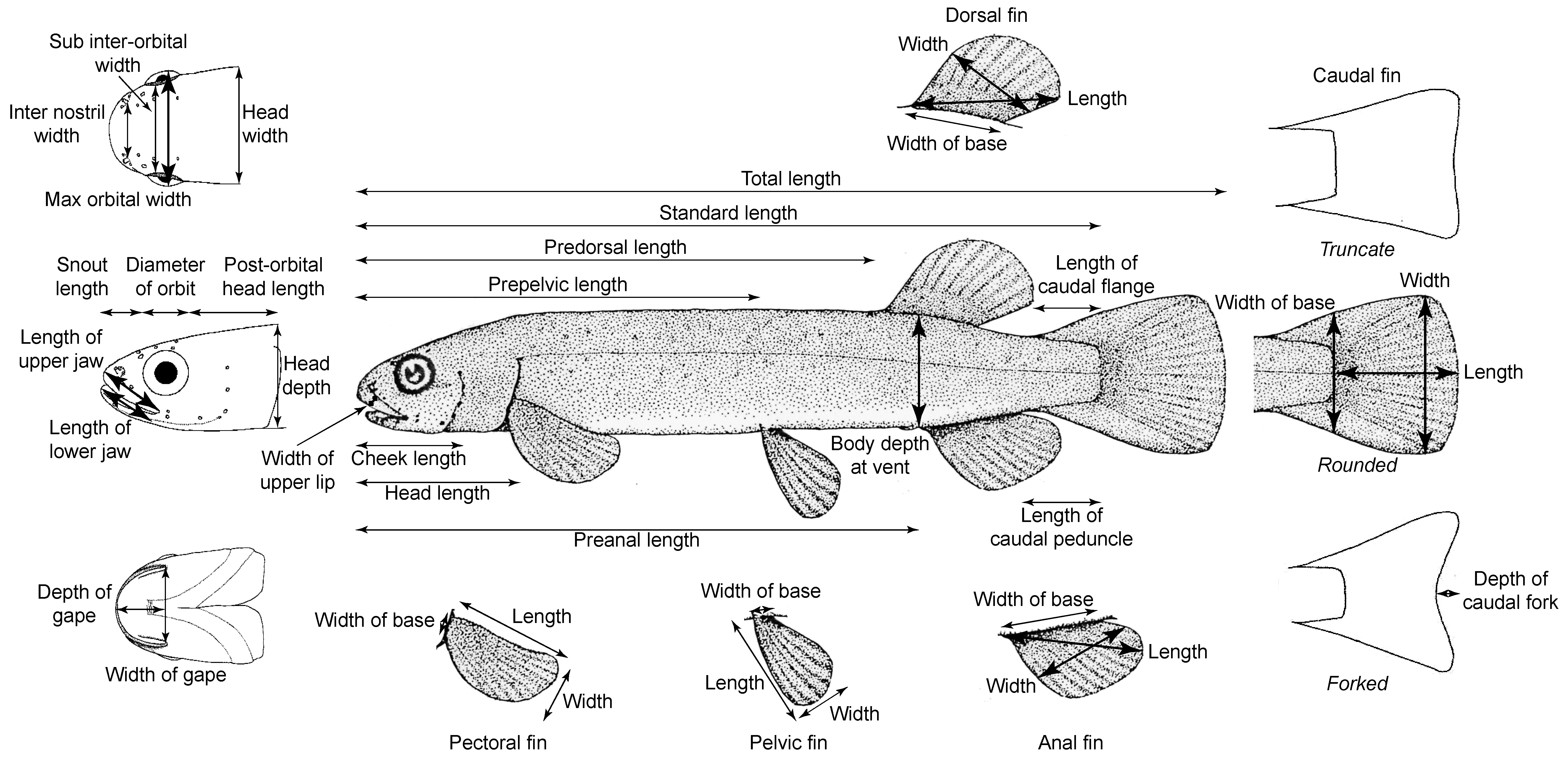

| Catchment and Habitat | Habitat Type | Species | Latitude | Longitude | Altitude (m) | All Other Fish Species Present in Study Habitat |
|---|---|---|---|---|---|---|
| Waimakariri River | ||||||
| Lake Marymere | Lentic | G. brevipinnis | 43°06’56.91’’ | 171°51’27.98’’ | 616 | Gobiomorphus breviceps |
| Slovens Stream Tributary | Lotic | G. brevipinnis | 43°05’40.18’’ | 171°50’55.65’’ | 579 | Anguilla dieffenbachii, G. breviceps, Salmo trutta |
| Waimakariri Spring 1 | Lentic | G. vulgaris | 43°00’55.00’’ | 171°48’39.44’’ | 503 | A. dieffenbachii, S. trutta |
| Kowai River | Lotic | G. vulgaris | 43°19’53.69’’ | 171°51’55.45’’ | 438 | G. breviceps |
| Rakaia River | ||||||
| Lake Ida | Lentic | G. brevipinnis | 43°14’08.93’’ | 171°32’33.11’’ | 679 | G. breviceps, Oncorhynchus mykiss, S. trutta |
| Ryton River | Lotic | G. brevipinnis | 43°16’41.83’’ | 171°32’29.81’’ | 537 | O. mykiss, S. trutta |
| Moss Burn | Lentic | G. vulgaris | 43°13’38.19’’ | 171°29’22.38’’ | 561 | G. breviceps |
| Harper River | Lotic | G. brevipinnis2,G. vulgaris | 43°13’04.44’’ | 171°28’18.15’’ | 539 | Galaxias paucispondylus, G. breviceps, O. mykiss |
| Ashburton River | ||||||
| Lake Camp | Lentic | G. brevipinnis2 | 43°36’49.65’’ | 171°03’26.86’’ | 680 | Gobiomorphus cotidianus, G. breviceps |
| Ashburton River | Lotic | G. vulgaris2 | 43°35’08.37’’ | 171°09’53.74’’ | 629 | G. paucispondylus, G. breviceps, S. trutta |
| Hinds River | ||||||
| Hinds River Tributary | Lentic | G. vulgaris | 43°42’12.35’’ | 171°20’12.85’’ | 413 | G. breviceps |
| Hinds River | Lotic | G. vulgaris | 43°43’16.16’’ | 171°21’25.50’’ | 382 | A. dieffenbachii, G. breviceps |
| Waitaki River | ||||||
| Blue Lake 1 1 | Lentic | G. brevipinnis | 43°41’35.61’’ | 170°09’51.20’’ | 760 | – |
| Blue Lake 2 1 | Lentic | G. brevipinnis2 | 43°41’36.77’’ | 170°09’53.47’’ | 754 | – |
| Blue Lake 4 1 | Lentic | G. brevipinnis2 | 43°41’41.36’’ | 170°09’55.71’’ | 758 | – |
| Blue Lake 5 1 | Lentic | G. brevipinnis2 | 43°41’43.95’’ | 170°09’56.89’’ | 756 | – |
| Lagoon Stream 1 | Lotic | G. brevipinnis | 43°47’26.29’’ | 170°06’45.50’’ | 617 | S. trutta |
| Lake Poaka Tributary | Lentic | G. vulgaris | 44°12’13.48’’ | 170°05’24.82’’ | 495 | Galaxias macronasus, G. breviceps, O. mykiss |
| Fraser Stream | Lotic | G. vulgaris | 44°12’51.47’’ | 170°02’03.81’’ | 527 | G. paucispondylus, G. breviceps, S. trutta |
| Blue Stream | Lotic | G. vulgaris2 | 43°41’53.99’’ | 170°09’43.55’’ | 717 | – |
| Big Creek | Lotic | G. brevipinnis2 | 46°09’31.68’’ | 170°09’06.33’’ | 2 | Gobiomorphus huttoni |
| Mokoreta River | ||||||
| Mokoreta River Tributary | Lentic | G. gollumoides | 46°19’36.85’’ | 169°15’21.86’’ | 211 | – |
| Mokoreta River | Lotic | G. gollumoides | 46°20’12.74’’ | 169°17’47.75’’ | 321 | A. dieffenbachii |
| Mataura River | ||||||
| Four Mile Creek Wetland 1 | Lentic | G. gollumoides | 45°22’55.12’’ | 168°40’56.16’’ | 347 | G. breviceps |
| Four Mile Creek 1 | Lotic | G. gollumoides | 45°21’38.64’’ | 168°42’33.19’’ | 373 | G. breviceps |
| Aparima River | ||||||
| Hamilton Burn Tributary | Lentic | G. gollumoides | 45°38’15.59’’ | 168°05’38.04’’ | 382 | – |
| Hamilton Burn | Lotic | G. gollumoides | 45°36’45.49’’ | 168°04’57.89’’ | 433 | – |
| Waiau River | ||||||
| Fred Burn | Lentic | G. gollumoides | 45°20’13.72’’ | 167°55’35.26’’ | 414 | – |
| Whitestone River Tributary | Lotic | G. gollumoides | 45°21’30.10’’ | 167°54’05.73’’ | 434 | A. dieffenbachii, G. breviceps |
| Freshwater River | ||||||
| Island Hill Wetland 1 | Lentic | G. gollumoides2 | 46°54’26.55’’ | 167°50’37.17’’ | 32 | G. breviceps |
| Hydrological Environment Variable | Dimension 1 | Dimension 2 | ||
|---|---|---|---|---|
| Spearman ρ | p-Value | Spearman ρ | p-Value | |
| Flow | ||||
| Discharge (m3·s−1) | −0.961 | <0.0001 | 0.257 | 0.179 |
| Maximum velocity (m·s−1) | −0.905 | <0.0001 | 0.372 | 0.047 |
| Hydraulic radius (m) | −0.619 | <0.0001 | −0.525 | 0.003 |
| Flow depth (m) | −0.600 | 0.001 | −0.532 | 0.003 |
| Channel form | ||||
| Habitat slope (m·m−1) | −0.736 | <0.0001 | 0.171 | 0.374 |
| Habitat depth (m) | 0.396 | 0.033 | −0.059 | 0.762 |
| Flow pattern | ||||
| Froude number | −0.902 | <0.0001 | 0.402 | 0.030 |
| Critical velocity | −0.599 | 0.001 | −0.517 | 0.004 |
| Reynolds number | −0.971 | <0.0001 | 0.254 | 0.183 |
| Substratum | ||||
| Substratum d25 (mm) | −0.059 | 0.761 | 0.773 | <0.0001 |
| Substratum d50 (mm) | 0.287 | 0.131 | 0.611 | <0.0001 |
| Substratum d84 (mm) | 0.234 | 0.222 | 0.270 | 0.157 |
| Substratum dmean (mm) | 0.066 | 0.734 | 0.286 | 0.133 |
| Physical disturbance | ||||
| Tractive force (N·m−2) | −0.896 | <0.0001 | −0.026 | 0.892 |
| Variable | Dimension 1 | Dimension 2 | G. brevipinnis | G. gollumoides | G. vulgaris | Convergence | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spearman ρ | p-Value | Spearman ρ | p-Value | Lentic | Lotic | Lentic | Lotic | Lentic | Lotic | ||
| Standard length | Longer | Shorter | Longer | Shorter | Longer | Shorter | Convergence | ||||
| Body lengths and depths | |||||||||||
| Prepelvic length | −0.374 | 0.045 | 0.174 | 0.366 | Longer | Shorter | Longer | Shorter | Longer | Shorter | Convergence |
| Predorsal length | −0.376 | 0.044 | −0.093 | 0.631 | Shorter | Longer | Longer | Shorter | Longer | Shorter | |
| Preanal length | −0.691 | <0.001 | −0.075 | 0.699 | Longer | Shorter | Longer | Shorter | Longer | Shorter | Convergence |
| Pectoral-pelvic length | 0.030 | 0.877 | 0.053 | 0.786 | Greater | Lesser | Greater | Lesser | Greater | Lesser | Convergence |
| Pelvic-anal length | −0.429 | 0.020 | −0.101 | 0.602 | Lesser | Greater | Lesser | Greater | Greater | Lesser | |
| Predorsal/Preanal length | 0.955 | <0.001 | −0.108 | 0.578 | Lesser | Greater | Lesser | Greater | Lesser | Greater | Convergence |
| Length of caudal peduncle | 0.515 | 0.004 | 0.038 | 0.843 | Shorter | Longer | Shorter | Longer | Shorter | Longer | Convergence |
| Depth of caudal peduncle | −0.133 | 0.493 | 0.083 | 0.668 | Deeper | Shallower | Deeper | Shallower | Deeper | Shallower | Convergence |
| Depth/Length of caudal peduncle | 0.524 | 0.004 | −0.029 | 0.883 | Stouter | Slenderer | Stouter | Slenderer | Stouter | Slenderer | Convergence |
| Body depth at vent | 0.004 | 0.982 | 0.240 | 0.209 | Shallower | Deeper | Deeper | Shallower | Deeper | Shallower | |
| Body width at vent | −0.186 | 0.335 | −0.221 | 0.250 | Narrower | Wider | Wider | Narrower | Wider | Narrower | |
| Position of the lateral line | 0.935 | <0.001 | −0.119 | 0.538 | More dorsal | More ventral | More dorsal | More ventral | More dorsal | More ventral | Convergence |
| Pectoral fin | |||||||||||
| Length of pectoral fin | −0.047 | 0.810 | −0.115 | 0.552 | Shorter | Longer | Shorter | Longer | Shorter | Longer | Convergence |
| Width of pectoral fin | 0.419 | 0.024 | −0.184 | 0.340 | Narrower | Wider | Narrower | Wider | Narrower | Wider | Convergence |
| Width of pectoral fin base | −0.255 | 0.182 | −0.246 | 0.198 | Narrower | Wider | Narrower | Wider | Narrower | Wider | Convergence |
| Shape of pectoral fin | 0.879 | <0.001 | −0.030 | 0.879 | More oar like | More paddle like | More oar like | More paddle like | More oar like | More paddle like | Convergence |
| Pelvic fin | |||||||||||
| Length of pelvic fin | −0.225 | 0.240 | 0.010 | 0.960 | Longer | Shorter | Shorter | Longer | Shorter | Longer | |
| Width of pelvic fin | 0.625 | <0.001 | −0.023 | 0.907 | Narrower | Wider | Narrower | Wider | Narrower | Wider | Convergence |
| Width of pelvic fin base | −0.049 | 0.800 | −0.183 | 0.341 | Narrower | Wider | Narrower | Wider | Wider | Narrower | |
| Shape of pelvic fin | 0.935 | <0.001 | −0.079 | 0.684 | More oar like | More paddle like | More oar like | More paddle like | More oar like | More paddle like | Convergence |
| Anal fin | |||||||||||
| Length of anal fin | 0.183 | 0.343 | 0.283 | 0.137 | Shorter | Longer | Shorter | Longer | Shorter | Longer | Convergence |
| Width of anal fin | 0.556 | 0.002 | 0.460 | 0.012 | Narrower | Wider | Narrower | Wider | Wider | Narrower | |
| Width of anal fin base | 0.499 | 0.006 | −0.058 | 0.766 | Shorter | Longer | Shorter | Longer | Shorter | Longer | Convergence |
| Shape of anal fin | 0.321 | 0.089 | −0.728 | <0.001 | More square | More oblong | More square | More oblong | More square | More oblong | Convergence |
| Caudal fin | |||||||||||
| Length of caudal fin | 0.540 | 0.003 | 0.211 | 0.271 | Longer | Shorter | Longer | Shorter | Shorter | Longer | |
| Width of caudal fin | 0.023 | 0.905 | 0.664 | <0.001 | Wider | Narrower | Narrower | Wider | Narrower | Wider | |
| Width of caudal fin base | 0.052 | 0.790 | 0.555 | 0.002 | Wider | Narrower | Wider | Narrower | Wider | Narrower | Convergence |
| Shape of caudal fin | 0.503 | 0.005 | 0.407 | 0.028 | More emarginate | More truncate | More truncate | More emarginate | More truncate | More emarginate | |
| Length of caudal flange | 0.162 | 0.402 | 0.379 | 0.042 | Longer | Shorter | Shorter | Longer | Longer | Shorter | |
| Depth of caudal fork | 0.442 | 0.016 | −0.236 | 0.217 | Shallower | Deeper | Deeper | Shallower | Shallower | Deeper | |
| Dorsal fin | |||||||||||
| Length of dorsal fin | −0.055 | 0.778 | 0.268 | 0.159 | Longer | Shorter | Longer | Shorter | Shorter | Longer | |
| Width of dorsal fin | 0.498 | 0.006 | 0.416 | 0.025 | Narrower | Wider | Narrower | Wider | Wider | Narrower | |
| Width of dorsal fin base | 0.028 | 0.885 | −0.015 | 0.937 | Wider | Narrower | Narrower | Wider | Narrower | Wider | |
| Shape of dorsal fin | 0.189 | 0.326 | −0.681 | <0.001 | More square | More oblong | More oblong | More square | More square | More oblong | |
| Head lengths and depths | |||||||||||
| Head length | −0.527 | 0.003 | 0.183 | 0.343 | Longer | Shorter | Longer | Shorter | Longer | Shorter | Convergence |
| Snout length | −0.548 | 0.002 | 0.041 | 0.831 | Longer | Shorter | Longer | Shorter | Longer | Shorter | Convergence |
| Snout length/Orbit diameter | 0.746 | <0.001 | −0.413 | 0.026 | Lesser | Greater | Lesser | Greater | Lesser | Greater | Convergence |
| Post-orbital head length | −0.563 | 0.001 | 0.304 | 0.109 | Longer | Shorter | Shorter | Longer | Longer | Shorter | |
| Cheek Length | −0.497 | 0.006 | 0.158 | 0.414 | Longer | Shorter | Shorter | Longer | Longer | Shorter | |
| Head width | −0.564 | 0.001 | 0.103 | 0.593 | Wider | Narrower | Wider | Narrower | Wider | Narrower | Convergence |
| Inter nostril width | −0.332 | 0.079 | −0.103 | 0.595 | Wider | Narrower | Wider | Narrower | Wider | Narrower | Convergence |
| Head depth | −0.159 | 0.410 | 0.656 | <0.001 | Deeper | Shallower | Deeper | Shallower | Deeper | Shallower | Convergence |
| Head width/Head depth | 0.860 | <0.001 | −0.281 | 0.140 | Robuster | Flatter | Robuster | Flatter | Robuster | Flatter | Convergence |
| Diameter of orbit | 0.237 | 0.216 | 0.453 | 0.014 | Larger | Smaller | Smaller | Larger | Smaller | Larger | |
| Sub interorbital width | −0.011 | 0.953 | 0.399 | 0.032 | Wider | Narrower | Narrower | Wider | Wider | Narrower | |
| Max orbital width | −0.224 | 0.244 | 0.534 | 0.003 | Wider | Narrower | Narrower | Wider | Wider | Narrower | |
| Position of the eye | 0.857 | <0.001 | 0.049 | 0.800 | Wider | Narrower | Wider | Narrower | Wider | Narrower | Convergence |
| Length of upper jaw | −0.678 | <0.001 | 0.001 | 0.994 | Longer | Shorter | Longer | Shorter | Shorter | Longer | |
| Length of lower jaw | −0.633 | <0.001 | 0.072 | 0.711 | Longer | Shorter | Longer | Shorter | Longer | Shorter | Convergence |
| Width of gape | −0.521 | 0.004 | 0.233 | 0.225 | Wider | Narrower | Wider | Narrower | Wider | Narrower | Convergence |
| Depth of gape | −0.604 | 0.001 | 0.078 | 0.686 | Deeper | Shallower | Deeper | Shallower | Deeper | Shallower | Convergence |
| Depth of gape/Width of gape | 0.839 | <0.001 | −0.222 | 0.247 | Smaller | Larger | Smaller | Larger | Smaller | Larger | Convergence |
| Width of upper lip | −0.388 | 0.038 | 0.084 | 0.664 | Wider | Narrower | Wider | Narrower | Narrower | Wider | |
| Variance Component | Observed Partition | p-Value | Φ Statistic | |
|---|---|---|---|---|
| Variance | % of total | |||
| Between catchments | 11.53 | 65.79 | <0.0001 | ΦCT = 0.658 |
| Between habitats within catchments | −2.06 | −11.73 | 0.929 | ΦSC = −0.343 |
| Within habitats | 8.05 | 45.94 | <0.0001 | ΦST = 0.541 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunn, N.R.; O’Brien, L.K.; Burridge, C.P.; Closs, G.P. Morphological Convergence and Divergence in Galaxias Fishes in Lentic and Lotic Habitats. Diversity 2020, 12, 183. https://doi.org/10.3390/d12050183
Dunn NR, O’Brien LK, Burridge CP, Closs GP. Morphological Convergence and Divergence in Galaxias Fishes in Lentic and Lotic Habitats. Diversity. 2020; 12(5):183. https://doi.org/10.3390/d12050183
Chicago/Turabian StyleDunn, Nicholas R., Leanne K. O’Brien, Christopher P. Burridge, and Gerard P. Closs. 2020. "Morphological Convergence and Divergence in Galaxias Fishes in Lentic and Lotic Habitats" Diversity 12, no. 5: 183. https://doi.org/10.3390/d12050183
APA StyleDunn, N. R., O’Brien, L. K., Burridge, C. P., & Closs, G. P. (2020). Morphological Convergence and Divergence in Galaxias Fishes in Lentic and Lotic Habitats. Diversity, 12(5), 183. https://doi.org/10.3390/d12050183






