The Legacy of the Past Logging: How Forest Structure Affects Different Facets of Understory Plant Diversity in Abandoned Coppice Forests
Abstract
1. Introduction
2. Materials and Methods
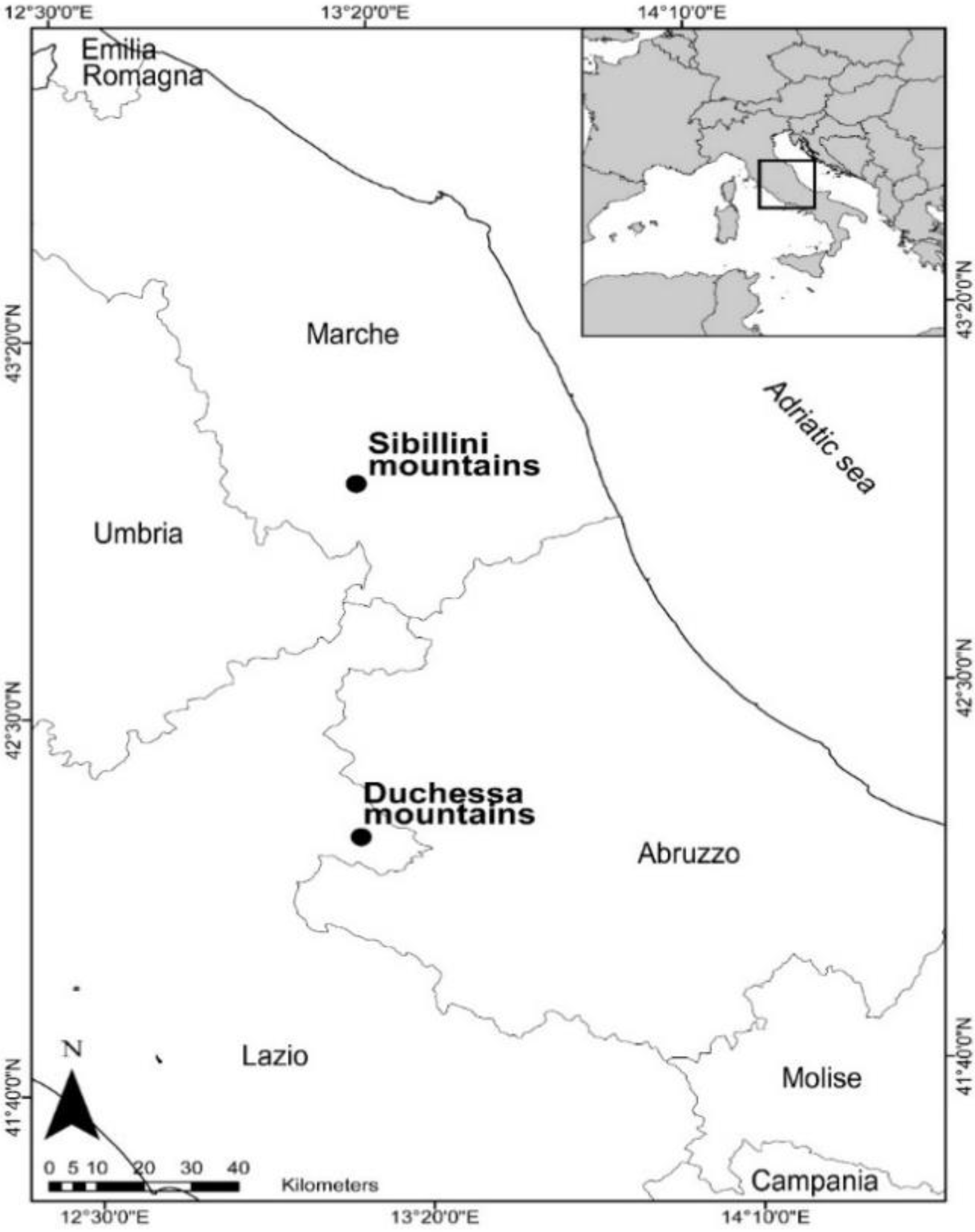
2.1. Study Area
2.2. Vegetation Data
2.3. Trait Data
2.4. Calculation of Indices
2.5. Data Analysis
3. Results
3.1. Taxonomic Diversity
3.2. Functional Diversity
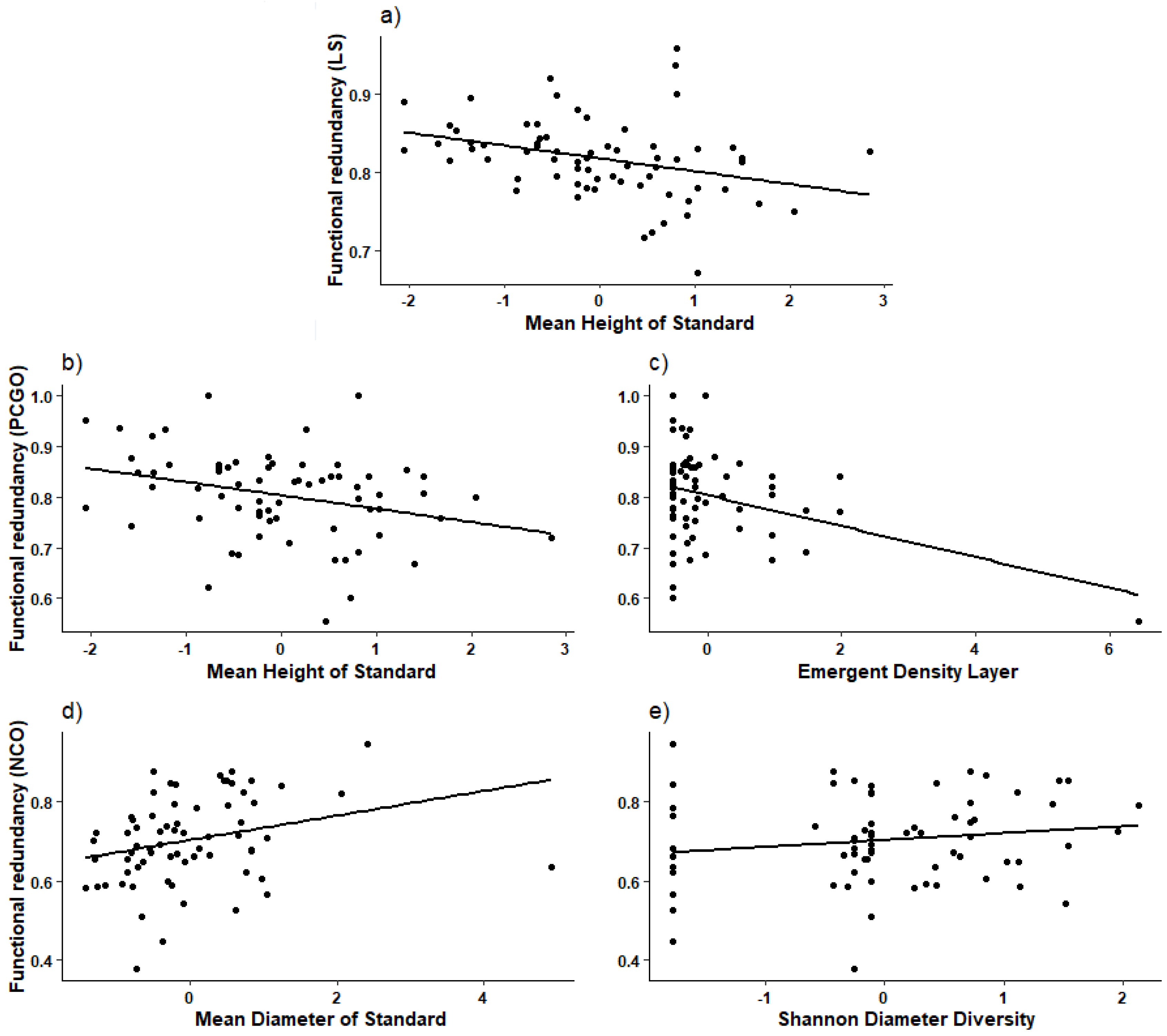
3.3. Functional Redundancy
4. Discussion
4.1. Taxonomic Diversity
4.2. Functional Diversity
4.3. Functional Redundancy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Leemans, R. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Hughes, L.; McIntyre, S.; Thomas, C.D. Assisted colonization and rapid climate change. Science 2008, 321, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Mersey, B. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51. [Google Scholar] [CrossRef] [PubMed]
- Shipley, B.; De Bello, F.; Cornelissen, J.H.C.; Reich, P.B. Reinforcing loose foundation stones in trait-based plant ecology. Oecologia 2016, 180, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Cabido, M. Vive la difference: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Carmona, C.P.; Azcárate, F.M.; de Bello, F.; Peco, B. Taxonomical and functional diversity turnover in Mediterranean grasslands: Interactions between grazing, habitat type and rainfall. J. Appl. Ecol. 2012, 49, 1084–1093. [Google Scholar] [CrossRef]
- de Bello, F.; Buchmann, N.; Casals, P.; Lepš, J.; Sebastià, M.T. Relating plant species and functional diversity to community δ 13 C in NE Spain pastures. Agric. Ecosyst. Environ. 2009, 131, 303–307. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Diaz, S.; Gamier, E.; Urcelay, C. New handbook for stand-ardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Stanisci, A.; Bricca, A.; Calabrese, V.; Cutini, M.; Pauli, H.; Steinbauer, K.; Carranza, M.L. Functional composition and diversity of leaf traits in subalpine versus alpine vegetation in the Apennines. AoB Plants 2020. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Chelli, S.; Marignani, M.; Barni, E.; Chiarucci, A. Plant–environment interactions through a functional traits perspective: A review of Italian studies. Plant Biosyst. 2019, 153, 853–869. [Google Scholar] [CrossRef]
- de Bello, F.; Lavorel, S.; Díaz, S.; da Silva, P.M. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Ricotta, C.; de Bello, F.; Moretti, M.; Caccianiga, M.; Cerabolini, B.E.; Pavoine, S. Measuring the functional redundancy of biological communities: A quantitative guide. Methods Ecol. Evol. 2016, 7, 1386–1395. [Google Scholar] [CrossRef]
- Laliberté, E.; Wells, J.A.; De Clerck, F.; McNamara, S. Land-use intensification reduces functional redundancy and response diversity in plant communities. Ecol. Lett. 2010, 13, 76–86. [Google Scholar] [CrossRef]
- Naeem, S. Species redundancy and ecosystem reliability. Conserv. Biol. 1998, 12, 39–45. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef]
- Fonseca, C.R.; Ganade, G. Species functional redundancy, random extinctions and the stability of ecosystems. J. Ecol. 2001, 89, 118–125. [Google Scholar] [CrossRef]
- Pillar, V.D.; Blanco, C.C.; Müller, S.C.; Duarte, L.D. Functional redundancy and stability in plant communities. J. Veg. Sci. 2013, 24, 963–974. [Google Scholar] [CrossRef]
- Oldeman, R.A.A. Forests: Elements of Silvology; Springer: Berlin, Germany, 1990. [Google Scholar]
- Decocq, G.; Aubert, M.; Dupont, F.; Bardat, J. Plant diversity in a managed temperate deciduous forest: Understorey response to two silvicultural systems. J. Appl. Ecol. 2004, 41, 1065–1079. [Google Scholar] [CrossRef]
- Bartha, S.; Merolli, A.; Campetella, G.; Canullo, R. Changes of vascular plant diversity along a chronosequence of beech coppice stands, central Apennines, Italy. Plant Biosyst. 2008, 142, 572–583. [Google Scholar] [CrossRef]
- Cervellini, M.; Fiorini, S.; Cavicchi, A.; Gimona, A. Relationships between understory specialist species and local management practices in coppiced forests–Evidence from the Italian Apennines. For. Ecol. Manag. 2017, 385, 35–45. [Google Scholar] [CrossRef]
- Warde, P. Fear of Wood Shortage and the Reality of the Woodland in Europe, c. 1450–1850. In History Workshop Journal; Oxford University Press: Oxford, UK, 2006; pp. 28–57. [Google Scholar]
- Nocentini, S. Structure and management of beech (Fagus sylvatica L.) forests in Italy. iFOREST 2009, 2, 105. [Google Scholar] [CrossRef]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Burton, J.I.; Scheller, R.M.; Amatangelo, K.L.; Mladenoff, D.J. Functional diversity of ground-layer plant communities in old-growth and managed northern hardwood forests. Appl. Veg. Sci. 2014, 17, 398–407. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: Consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. BioScience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Campetella, G.; Botta-Dukát, Z.; Wellstein, C.; Bartha, S. Patterns of plant trait–environment relationships along a forest succession chronosequence. Agric. Ecosyst. Environ. 2011, 145, 38–48. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Lebrija-Trejos, E.; Bongers, F. Successional changes in functional composition contrast for dry and wet tropical forest. Ecology 2013, 94, 1211–1216. [Google Scholar] [CrossRef]
- Ottaviani, G.; Götzenberger, L.; Bacaro, G.; Chiarucci, A.; de Bello, F.; Marcantonio, M. A multifaceted approach for beech forest conservation: Environmental drivers of understorey plant diversity. Flora 2019, 256, 85–91. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y.H. Is understory plant species diversity driven by resource quantity or resource heterogeneity? Ecology 2010, 91, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Scolastri, A.; Bricca, A.; Cancellieri, L.; Cutini, M. Understory functional response to different management strategies in Mediterranean beech forests (central Apennines, Italy). For. Ecol. Manag. 2017, 400, 665–676. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Garnier, E. The global spectrum of plant form and function. Nature 2016, 529, 167. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E. Below-ground frontiers in trait-based plant ecology. New Phytol. 2017, 213, 1597–1603. [Google Scholar] [CrossRef]
- Klimešová, J.; Martínková, J.; Ottaviani, G. Belowground plant functional ecology: Towards an integrated perspective. Funct. Ecol. 2018, 32, 2115–2126. [Google Scholar] [CrossRef]
- Chelli, S.; Ottaviani, G.; Simonetti, E.; Campetella, G. Climate is the main driver of clonal and bud bank traits in Italian forest understories. Perspect. Plant Ecol. Evol. Syst. 2019, 40, 125478. [Google Scholar] [CrossRef]
- Vojktó, A.-E.; Freitag, M.; Bricca, A.; Götzenberger, L. Clonal vs leaf-height-seed (LHS) traits: Which are filtered more strongly across habitats? Folia Geobat. 2017, 52, 269–281. [Google Scholar]
- Klimešová, J.; Herben, T. Clonal and bud bank traits: Patterns across temperate plant communities. J. Veg. Sci. 2015, 26, 243–253. [Google Scholar] [CrossRef]
- Klimešová, J.; Danihelka, J.; Chrtek, J.; de Bello, F.; Herben, T. CLO-PLA: A database of clonal and bud-bank traits of the Central European flora. Ecology 2017, 98, 1179. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Canullo, R.; Campetella, G.; Mucina, L.; Chelli, S.; Wellstein, C.; Bartha, S. Patterns of clonal growth modes along a chronosequence of post-coppice forest regeneration in beech forests of Central Italy. Folia Geobot. 2011, 46, 271–288. [Google Scholar] [CrossRef]
- Canullo, R.; Simonetti, E.; Cervellini, M.; Campetella, G. Unravelling mechanisms of short-term vegetation dynamics in complex coppice forest systems. Folia Geobot. 2017, 52, 71–81. [Google Scholar] [CrossRef]
- Catorci, A.; Vitanzi, A.; Tardella, F.M.; Hrsak, V. Regeneration of Ostrya carpinifolia Scop. Forest after coppicing: Modelling of changes in species diversity and composition. Pol. J. Ecol. 2011, 59, 483–494. [Google Scholar]
- Song, M.; Dong, M.; Jiang, G. Importance of clonal plants and plant species diversity in the Northeast China Transect. Ecol. Res. 2002, 17, 705–716. [Google Scholar] [CrossRef]
- Graae, B.J.; Sunde, P.B. The impact of forest continuity and management on forest floor vegetation evaluated by species traits. Ecography 2000, 23, 720–731. [Google Scholar] [CrossRef]
- Aubin, I.; Gachet, S.; Messier, C.; Bouchard, A. How resilient are northern hardwood forests to human disturbance? An evaluation using a plant functional group approach. Ecoscience 2007, 14, 259–271. [Google Scholar] [CrossRef]
- Aubin, I.; Ouellette, M.H.; Legendre, P.; Messier, C.; Bouchard, A. Comparison of two plant functional approaches to evaluate natural restoration along an old-field–deciduous forest chronosequence. J. Veg. Sci. 2009, 20, 185–198. [Google Scholar] [CrossRef]
- Malavasi, M.; Carranza, M.L.; Moravec, D.; Cutini, M. Reforestation dynamics after land abandonment: A trajectory analysis in Mediterranean mountain landscapes. Reg. Environ. Chang. 2018, 18, 2459–2469. [Google Scholar] [CrossRef]
- Bracchetti, L.; Carotenuto, L.; Catorci, A. Land-cover changes in a remote area of central Apennines (Italy) and management directions. Landsc. Urban Plan. 2012, 104, 157–170. [Google Scholar] [CrossRef]
- Scolastri, A.; Cancellieri, L.; Iocchi, M.; Cutini, M. Patterns of floristic variation on a montane beech forest in the central Apennines (central Italy). Plant Sociol. 2014, 51, 69–82. [Google Scholar]
- Campetella, G.; Canullo, R.; Gimona, A.; Bartha, S. Scale-dependent effects of coppicing on the species pool of late successional beech forests in the central Apennines, Italy. Appl. Veg. Sci. 2016, 19, 474–485. [Google Scholar] [CrossRef]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Chytrý, M. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Scolatri, A.; Cancellieri, L.; Iocchi, M.; Cutini, M. Old coppice versus high forest: The impact of beech forest management on plant species diversity in central Apennines (Italy). J. Plant Ecol. 2017, 10, 271–280. [Google Scholar]
- Ciancio, O.; Corona, P.; Lamonaca, A.; Portoghesi, L.; Travaglini, D. Conversion of clearcut beech coppices into high forests with continuous cover: A case study in central Italy. For. Ecol. Manag. 2006, 224, 235–240. [Google Scholar] [CrossRef]
- Swenson, N.G.; Anglada-Cordero, P.; Barone, J.A. Deterministic tropical tree community turnover: Evidence from patterns of functional beta diversity along an elevational gradient. Proc. R. Soc. B 2011, 278, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Bricca, A.; Conti, L.; Tardella, M.F.; Cutini, M. Community assembly processes along a sub-Mediterranean elevation gradient: Analyzing the interdependence of trait community weighted mean and functional diversity. Plant Ecol. 2019, 220, 1139–1151. [Google Scholar] [CrossRef]
- de Bello, F.; Lavergne, S.; Meynard, C.N.; Lepš, J.; Thuiller, W. The partitioning of diversity: Showing Theseus a way out of the labyrinth. J. Veg. Sci. 2010, 21, 992–1000. [Google Scholar] [CrossRef]
- Bacaro, G.; Ricotta, C.; Marignani, M.; Torri, D.; Chiarucci, A. Using Shannon’s recursivity to summarize forest structural diversity. For. Trees Livelihoods 2014, 23, 211–216. [Google Scholar] [CrossRef]
- Daly, A.J.; Baetens, J.M.; De Baets, B. Ecological diversity: Measuring the unmeasurable. Mathematics 2018, 6, 119. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science & Business Media: Berlin, Germany, 2009; pp. 121–122. [Google Scholar]
- Botta-Dukát, Z.; Czúcz, B. Testing the ability of functional diversity indices to detect trait convergence and divergence using individual-based simulation. Methods Ecol. Evol. 2016, 7, 114–126. [Google Scholar] [CrossRef]
- de Bello, F. The quest for trait convergence and divergence in community assembly: Are null-models the magic wand? Glob. Ecol. Biogeogr. 2012, 21, 312–317. [Google Scholar] [CrossRef]
- Landuyt, D.; Perring, M.P.; Seidl, R.; Taubert, F.; Verbeeck, H.; Verheyen, K. Modelling understorey dynamics in temperate forests under global change–Challenges and perspectives. Perspect. Plant Ecol. Evol. Syst. 2018, 31, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Lieffers, V.J.; Messier, C.; Stadt, K.J.; Gendron, F.; Comeau, P.G. Predicting and managing light in the understory of boreal forests. Can. J. For. Res. 1999, 29, 796–811. [Google Scholar] [CrossRef]
- Way, D.A.; Pearcy, R.W. Sunflecks in trees and forests: From photosynthetic physiology to global change biology. Tree Physiol. 2012, 32, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Albert, C.H.; Dubuis, A.; Randin, C.; Guisan, A. Variation in habitat suitability does not always relate to variation in species’ plant functional traits. Biol. Lett. 2009, 6, 120–123. [Google Scholar] [CrossRef]
- Kermavnar, J.; Kutnar, L. Patterns of Understory Community Assembly and Plant Trait-Environment Relationships in Temperate SE European Forests. Diversity 2020, 12, 91. [Google Scholar] [CrossRef]
- MacArthur, R.; Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Conti, L.; de Bello, F.; Lepš, J.; Acosta, A.T.R.; Carboni, M. Environmental gradients and micro-heterogeneity shape fine-scale plant community assembly on coastal dunes. J. Veg. Sci. 2017, 28, 762–773. [Google Scholar] [CrossRef]
- de Bello, F.; Vandewalle, M.; Reitalu, T.; Sykes, M.T. Evidence for scale-and disturbance-dependent trait assembly patterns in dry semi-natural grasslands. J. Ecol. 2013, 101, 1237–1244. [Google Scholar] [CrossRef]
- Klimešová, J.; Martínková, J.; Pausas, J.G.; Altman, J. Handbook of standardized protocols for collecting plant modularity traits. Perspect. Plant Ecol. Evol. Syst. 2019, 40, 125485. [Google Scholar] [CrossRef]
- Chiarucci, A.; Bonini, I. Quantitative floristics as a tool for the assessment of plant diversity in Tuscan forests. For. Ecol. Manag. 2005, 212, 160–170. [Google Scholar] [CrossRef]
| Trait | Trait Code | Trait Definition |
|---|---|---|
| Lateral spread | LS | Distance between parental and offspring shoots (cm/year) |
| Persistence of clonal growth organs | PCGO | Lifespan of the physical connection between mother and daughter shoots (year) |
| Clonal offspring | CO | Number of offspring shoots produced per parent shoot per year (n/year) |
| Diversity’ Facet | Trait | Index | DBH | ShDBH | H | ShH | Em.d. | Sub.d. | Dom.d. | Tot.d. | R2 | AIC | Model Type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TD | S | −1.97 * | −1.34 n.s. | 19% | 440 | non-mixed | |||||||
| FD | LS | Q | 0.013 * | 7% | −230 | non-mixed | |||||||
| PCGO | Q | 0.02 * | 7% | −163 | non-mixed | ||||||||
| NCO | Q | −0.033 ** | −0.02 n.s. | 10% | −124 | non-mixed | |||||||
| FR | LS | R | −0.014 * | −0.1 n.s. | 13% | −218 | non-mixed | ||||||
| PCGO | R | −0.02 * | −0.03 ** | 16% | −146 | non-mixed | |||||||
| NCO | R | 0.04 ** | 0.03 * | 11% | −109 | non-mixed |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bricca, A.; Chelli, S.; Canullo, R.; Cutini, M. The Legacy of the Past Logging: How Forest Structure Affects Different Facets of Understory Plant Diversity in Abandoned Coppice Forests. Diversity 2020, 12, 109. https://doi.org/10.3390/d12030109
Bricca A, Chelli S, Canullo R, Cutini M. The Legacy of the Past Logging: How Forest Structure Affects Different Facets of Understory Plant Diversity in Abandoned Coppice Forests. Diversity. 2020; 12(3):109. https://doi.org/10.3390/d12030109
Chicago/Turabian StyleBricca, Alessandro, Stefano Chelli, Roberto Canullo, and Maurizio Cutini. 2020. "The Legacy of the Past Logging: How Forest Structure Affects Different Facets of Understory Plant Diversity in Abandoned Coppice Forests" Diversity 12, no. 3: 109. https://doi.org/10.3390/d12030109
APA StyleBricca, A., Chelli, S., Canullo, R., & Cutini, M. (2020). The Legacy of the Past Logging: How Forest Structure Affects Different Facets of Understory Plant Diversity in Abandoned Coppice Forests. Diversity, 12(3), 109. https://doi.org/10.3390/d12030109





