Biopolymer Development from Agro-Food and Aquaculture By-Products with Antioxidant Hydrolysates of Cyprinus carpio, Produced via Enzymatic Preparations of Pineapple and Papaya
Abstract
1. Introduction
2. Results
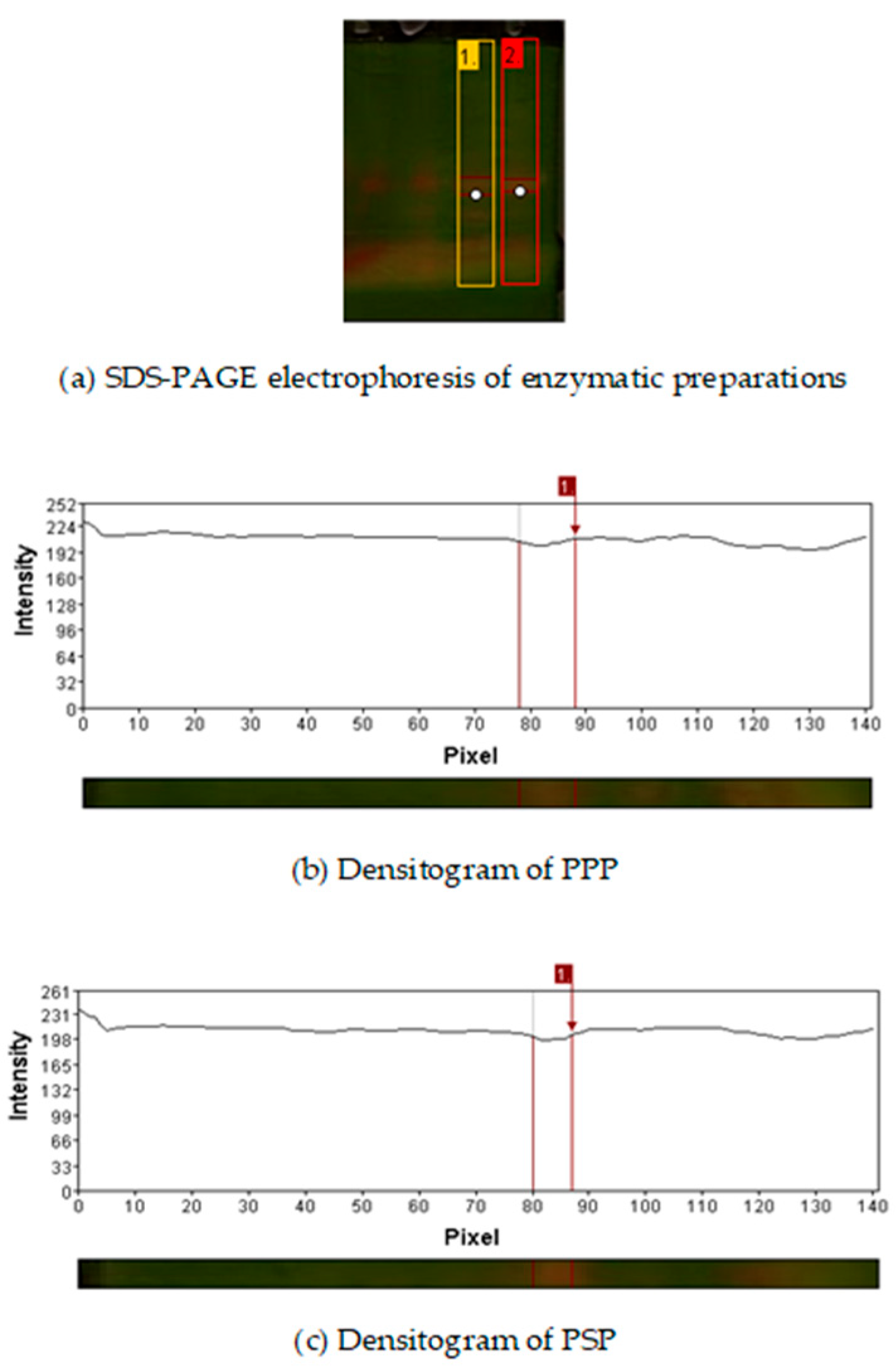
2.1. Electrophoretic Profile of Enzymatic Preparations
2.2. Proteolytic Activity of the Enzymatic Preparations
2.3. Hydrolysates
2.3.1. FTIR Analysis
2.3.2. In Silico Analysis
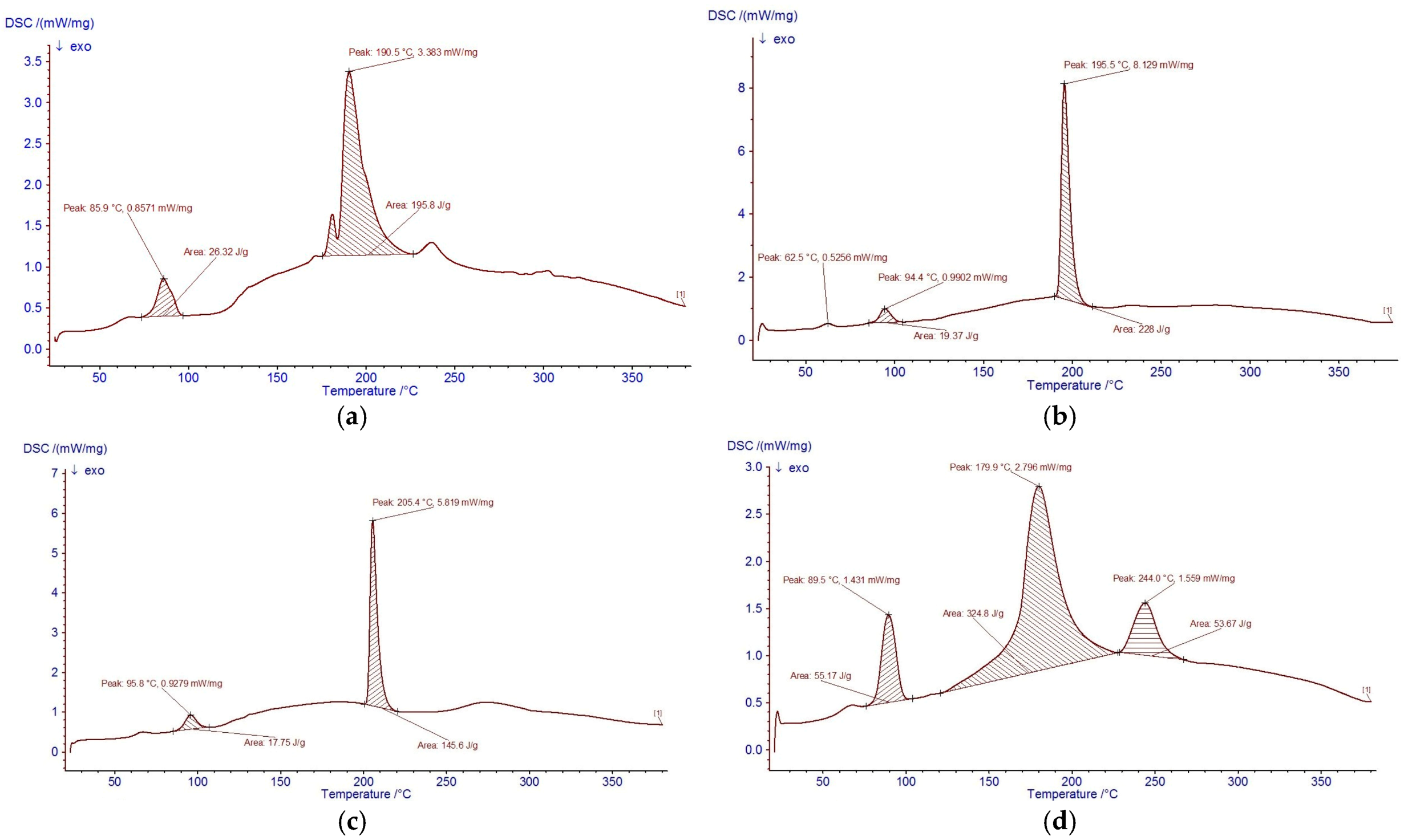
2.3.3. Thermal Stability (DSC) and Molecular Relationship with FTIR
2.3.4. Thermal Stability of Carp Hydrolysates and Molecular Relationship with FTIR
2.3.5. Soluble Protein Content of Carp Protein Hydrolysates
2.3.6. Antioxidant Activity (ABTS•+ and DPPH• Inhibition)
2.3.7. Reducing Power of Carp Protein Hydrolysates
2.4. Characterization of Raw Materials for Biopolymer Production
2.4.1. Orange Fiber
| Parameter | Orange Fiber | Casein | Grenetin | Specification Range |
|---|---|---|---|---|
| Moisture (%) | 3.61 ± 0.2 | 4.0 ± 0.1 | 5.9 ± 0.2 | 1.0–12.5 [58,59] |
| Ashes (%) | 3.54 ± 0.1 | 4.0 ± 0.5 | 1.6 ± 0.4 | <15.0 [58,59] |
| Lipids (%) | 0.60 ± 0.1 | 32.0 ± 3.5 | — | 26.0–42.0 [58,59] |
| pH | — | — | 4.6 ± 0.1 | ≥4.0 [60,61] |
| Transmittance (%) | — | — | 1.1 ± 0.1 | By agreement [60,61] |
| Viscosity (cps) | — | — | 29.0 ± 0.8 | 30 [60,61] |
| Gel strength (N) | — | — | 5.0 ± 0.4 | 1.5–5.8 [60,61] |
2.4.2. Casein
2.4.3. Grenetin
2.5. Biopolymer Based on Agrifood Byproducts
Biopolymer Characterization
2.6. Physical Properties
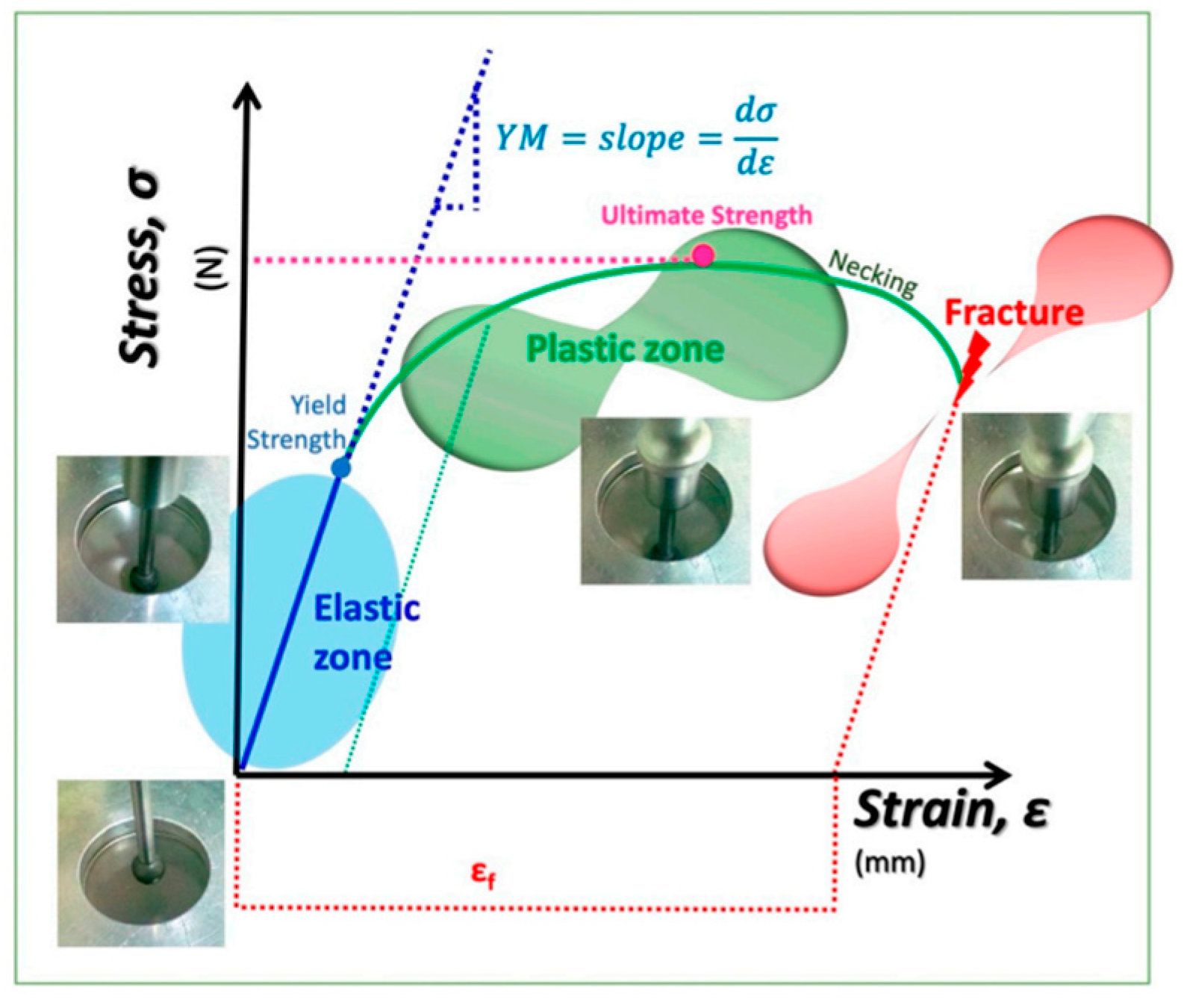
2.7. Mechanical Properties
Shelf Life Extension Assessment
2.8. Titratable Acidity and pH
2.9. Total Soluble Solids
2.10. Colour (L*, a*, b*)
2.11. Changes in Ascorbic Acid Content During Storage
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Enzymatic Preparations
4.2.1. Proteolytic Activity of Enzymatic Preparations
4.2.2. Electrophoresis of Enzymatic Preparations
4.3. Acquisition of Common Carp Subproduct Hydrolysates
4.3.1. Substrate
4.3.2. Enzymatic Hydrolysis
4.4. Identification of Hydrolysates by FTIR and In Silico Analysis
4.5. Thermal Analysis
4.6. Soluble Protein Content
4.7. Antioxidant Activity
4.7.1. ABTS•+ (2,2′-Azino-bis 3-Ethylenebenzothiazoline-6-sulfonic Acid)
4.7.2. DPPH• (2,2-Diphenyl-1-picrylhydrazyl)
4.7.3. Reducing Power (RP)
4.8. Obtaining Raw Materials Based on Agrifood By-Products for Biopolymers
4.8.1. Obtaining and Characterization of Agrifood By-Product Fiber (Orange Peel)
4.8.2. Obtaining and Characterization of Grenetin from Fish By-Products
4.8.3. Obtaining and Characterization of Whey Protein Isolate
4.9. Biopolymer Development
Physical and Mechanical Properties
4.10. Application of Biopolymer Coating to Raspberries
4.11. Physicochemical Characteristics of Coated Raspberries Stored Under Refrigeration
4.11.1. Colour Measurement
4.11.2. pH and Titratable Acidity
4.11.3. Total Soluble Solids (°Brix)
4.11.4. Ascorbic Acid Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid content |
| ABTS•+ | 2,2′-Azino-bis 3-ethylenebenzothiazoline-6-sulfonic acid |
| BOD | Biochemical oxygen demand |
| CP | Commercial pepsin |
| DSC | Differential Scanning Calorimetry |
| DPPH• | 1,1-diphenyl-2-picrylhydrazyl |
| % E | Percentage elongation |
| FRAP | Ferric Reducing Antioxidant Power |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GSH, | glutathione standard |
| IC50 | Mean inhibitory concentration |
| MY | Young’s modulus |
| P+CP | Common carp protein with the added commercial pepsin |
| P-E | Carp protein in absence of enzymes |
| PPP | Pineapple peel preparations |
| P+PPP | Common carp protein + pineapple peel enzymatic preparation |
| PSP | Papaya seed preparations |
| P+PSP | Common carp protein + papaya seed enzymatic preparation |
| RP | Reducing power |
| SB | Blanck control |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| TCA | Trichloroacetic acid |
| TSS | Total soluble solids |
| Trolox | 6-hydroxy-2,3,7,8-tetramethylchroman-2-carboxylic acid |
| WRC | Water holding capacity |
References
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2018; FAO Flagship Publication: Rome, Italy, 2018; p. 220. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/26cd480f-6cf1-40bf-a565-200d11e01ef1/content (accessed on 7 February 2025).
- Food and Agriculture Organization of the United Nations (FAO). The State of Food and Agriculture 2019: Progress Towards Reducing Food Loss and Waste; FAO Flagship Publication: Rome, Italy, 2019; pp. 2–149. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/2120f787-5a49-41f5-a9fb-f4ceaac98b2c/content (accessed on 7 February 2025).
- Secretaría de Agricultura y Desarrollo Rural (SADER). Naranja, Más Presente Que Nunca. Available online: https://www.gob.mx/agricultura/es/articulos/naranja-mas-presente-que-nunca (accessed on 7 February 2025).
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Producción Agrícola. Available online: https://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119 (accessed on 7 February 2025).
- Wong, F.-C.; Ng, W.-J.; Ooi, A.-L.; Lem, F.-F.; Chai, T.-T. Carp-Derived Antioxidant Peptides and Hydrolysates: Biological Effects and Potential Applications in Health and Food. Antioxidants 2025, 14, 1095. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Tavano, O.; Murcia, A.B.; Torrestina-Sánchez, B.; Fernandez-Lafuente, R. Peptides with biological and technofunctional properties produced by bromelain hydrolysis of proteins from different sources: A review. Int. J. Biol. Macromol. 2023, 253, 127244. [Google Scholar] [CrossRef] [PubMed]
- Łubek-Nguyen, A.; Ziemichód, W.; Olech, M. Application of enzyme-assisted extraction for the recovery of natural bioactive compounds for nutraceutical and pharmaceutical applications. Appl. Sci. 2022, 12, 3232. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castaneda-Valbuena, D.; Morellon-Sterling, R.; Tavano, O.; Berenguer-Murcia, Á.; Vela-Gutiérrez, G.; Fernandez-Lafuente, R. Bioactive peptides from fisheries residues: A review of use of papain in proteolysis reactions. Int. J. Biol. Macromol. 2021, 184, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Selamassakul, O.; Laohakunjit, N.; Kerdchoechuen, O.; Yang, L.; Maier, C.S. Bioactive peptides from brown rice protein hydrolyzed by bromelain: Relationship between biofunctional activities and flavor characteristics. J Food Sci. 2020, 85, 707–717. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure–activity relationship of antioxidant peptides. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Kim, S.-K. (Ed.) Marine Proteins and Peptides: Biological Activities and Applications; MDPI AG: Basel, Switzerland, 2018; ISBN 978-3-03842-646-2. [Google Scholar] [CrossRef]
- Xu, B.; Dong, Q.; Yu, C.; Chen, H.; Zhao, Y.; Zhang, B.; Yu, P.; Chen, M. Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides. Antioxidants 2024, 13, 479. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef]
- Mahrokh, N.; Seyed, R.S.; Peiman, A. Review of fish protein hydrolysates: Production methods, antioxidant and antimicrobial activity and nanoencapsulation. Food Sci. Biotechnol. 2024, 33, 1789–1803. [Google Scholar] [CrossRef]
- Borawska, J.; Darewicz, M.; Vegarud, G.E.; Minkiewicz, P. Antioxidant properties of carp (Cyprinus carpio L.) protein Ex Vivo and In Vitro hydrolysates. Food Chem. 2016, 194, 770–779. [Google Scholar] [CrossRef]
- Sullivan, G.A.; Calkins, C.R. Application of exogenous enzymes to beef muscle of high and low-connective tissue. Meat Sci. 2010, 85, 730–734. [Google Scholar] [CrossRef]
- Ha, M.; Bekhit, A.E.D.A.; Carne, A.; Hopkins, D.L. Characterization of commercial papain, bromelain, actinidin and zingibain protease preparations and their activities toward meat proteins. Food Chem. 2012, 134, 95–105. [Google Scholar] [CrossRef]
- Liu, Y.; Pallarés, N.; Ferrer, E.; Martínez-Culebras, P.V.; Roig, P.; Castagnini, J.M.; Barba, F.J. Enzymatic Hydrolysis Enhances Antioxidant Bioactive Sequences and Mineral Recovery from SC-CO2 Defatted Salmon Side Streams. Food Bioprocess Technol. 2025, 18, 7518–7530. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Ghosh, T.; Roy, S.; Rhim, J.-W. Chapter 3—Biopolymer-based antimicrobial nanocomposite materials for food packaging and preservation. In Food Packaging and Preservation; Jaiswal, A.K., Shankar, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 33–52. ISBN 9780323900447. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; Colín-Alvarez, M.d.L.; Gaona-Sánchez, V.A.; Chalapud, M.C.; García-Hernández, A.B.; León-Espinosa, E.B.; Valdespino-León, M.; Serrano-Villa, F.S.; Calderón-Domínguez, G. Characterization and Applications of the Pectin Extracted from the Peel of Passiflora tripartita var. mollissima. Membranes 2023, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, C.; Márquez, M.; Pérez, A.T.; Chávez, M.A.; Hernández, M. Kinetic characterization of a semipurified preparation from bromelain for antitumor use. Rev. Cub. Plantas Med. 2010, 15, 27–41. [Google Scholar]
- Mejía-Aguilar, R.B.; Vega-Ramos, C.X. Medición de La Actividad Proteolítica de La Enzima Papaína Natural Extraída Del Látex de Papayo (Carica papaya) E Inmovilizada En Gel de Agar. Bachelor’s Thesis, Universidad de El Salvador, San Salvador, El Salvador, 2010. Available online: https://agris.fao.org/search/en/providers/122525/records/6511b0e958c30050e8a3e6b7 (accessed on 14 December 2025).
- Navarro-Cruz, A.; Rojas-Zenteno, E.; Lazcano-Hernández, M.; Vera-López, O. Propiedades funcionales de semillas de papaya (Carica papaya L.). Rev. Cienc. Salud 2016, 3, 48–56. Available online: https://www.ecorfan.org/bolivia/researchjournals/Ciencias_de_la_Salud/vol3num7/Revista_Ciencias_de_la_Salud_V3_N7_7.pdf (accessed on 14 December 2025).
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Shiao, W.C.; Wu, T.; Kuo, C.H.; Tsai, Y.H.; Tsai, M.L.; Hong, Y.H.; Huang, C.Y. Physicochemical and Antioxidant Properties of Gelatin and Gelatin Hydrolysates Obtained from Extrusion-Pretreated Fish (Oreochromis sp.) Scales. Mar. Drugs 2021, 19, 275. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Ma, Y.Z.; Jiang, X.Y.; Wang, C.Y.; Li, Y.Q.; Liang, Y.; Ren, X.D.; Qin, L.C.; Zhao, X.Z. Proteolytic and structural mechanisms in tuna tenderization by papain, bromelain and ficin. Food Chem. X 2025, 30, 103010. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, Q.; Shen, Y.; Wu, Y.; Gao, L.; Xu, X.; Hao, G. Research progress on antioxidant peptides from fish by-products: Purification, identification, and structure-activity relationship. Metabolites 2024, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.R.; Cansian, R.L.; Mello, R.d.O.; Demiate, I.M.; Kempka, A.P.; Dornelles, R.C.P.; Rodriguez, J.M.L.; Campagnol, P.C.B. Production of collagens and protein hydrolysates with antimicrobial and antioxidant activity from sheep slaughter by-products. Antioxidants 2022, 11, 1173. [Google Scholar] [CrossRef] [PubMed]
- Bhimrao, M.A.; Bhalchandra, P.A.; Satishchandra, S.R.; Govind, D.S. Production of biologically active peptides by hydrolysis of whey protein isolates using hydrodynamic cavitation. Ultrason. Sonochem. 2021, 71, 105385. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Yasemi, M. Protein hydrolysates from fishery processing by-products: Production, characteristics, food applications, and challenges. Foods 2023, 12, 4470. [Google Scholar] [CrossRef]
- Alahmad, K.; Xia, W.; Jiang, Q.; Xu, Y. Effect of the Degree of hydrolysis on nutritional, functional, and morphological characteristics of protein hydrolysate produced from bighead barp (Hypophthalmichthys nobilis) using ficin enzyme. Foods 2022, 11, 1320. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Bukowski, M.; Mak, P. Identification of Antioxidant Peptides in Enzymatic Hydrolysates of Carp (Cyprinus carpio) Skin Gelatin. Molecules 2019, 24, 97. [Google Scholar] [CrossRef]
- Ming, X.; Xu, F.; Jiang, Y.; Zong, P.; Wang, B.; Li, J.; Tian, Y. Thermal degradation of food waste by TG-FTIR and Py-GC/MS: Pyrolysis behaviors, products, kinetic and thermodynamic analysis. J. Clean. Prod. 2020, 244, 118713. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166, Erratum in Thermochimica Act 2004, 409, 95–97. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Vázquez-Torres, H.; Licea-Claveríe, A. TGA/FTIR study on thermal degradation of polymethacrylates containing carboxylic groups. Polym. Degrad. Stab. 2006, 91, 3312–3321. [Google Scholar] [CrossRef]
- Jiang, D.D.; Yao, Q.; McKinney, M.A.; Wilkie, C.A. TGA/FTIR studies on the thermal degradation of some polymeric sulfonic and phosphonic acids and their sodium salts. Polym. Degrad. Stab. 1999, 63, 423–434. [Google Scholar] [CrossRef]
- Nguyen, M.V.; Liceaga, A.M. Impact of microwave-assisted enzymatic hydrolysis on functional and antioxidant properties of rainbow trout (Oncorhynchus mykiss) by-products. Fish. Sci. 2017, 83, 343–356, Correction in Fish. Sci. 2022, 88, 665. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Chen, Z.; Yu, J.; Wang, F.; Wang, J. Characterization of structural and functional properties of fish protein hydrolysates from surimi processing by-products. Food Chem. 2014, 151, 459–465. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing radical scavenging and chelation properties of in vitro digests of Alcalase-treated zein hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721, Erratum in J. Agric. Food Chem. 2008, 56, 3884. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.P.F.; Daroit, D.J.; Coelho, J.; Meira, S.M.; Lopes, F.C.; Segalin, J.; Risso, P.H.; Brandelli, A. Antioxidant, antihypertensive and antimicrobial properties of ovine milk caseinate hydrolyzed with a microbial protease. J. Sci. Food Agric. 2011, 91, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Tirtawijaya, G.; Christine, E.; Putra, S.E.D.; Askitosari, T.D. Valorization of Keting fish (Mystus nigriceps) viscera using papain for antioxidant protein hydrolysate production. IOP Conf. Ser Earth Environ. Sci. 2025, 1478, 012005. [Google Scholar] [CrossRef]
- Ali, A.; Bukhsh, K.K.; Raza, M.M.; Afraz, M.T.; Diana, T.; Waseem, M.; Abdi, G. Exploring the structure–activity relationships and molecular mechanisms of food-derived antioxidative peptides in mitigating oxidative stress: A comprehensive review. J. Funct. Foods 2025, 127, 106751. [Google Scholar] [CrossRef]
- Sampath, K.N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2012, 42, 1641–1649. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Jung, W.; Je, J.; Kim, S. Purification of radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Wijesekara, I.; Kim, S.-K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Rawdkuen, S. Application of Bromelain Extract for Muscle Foods Tenderization. Food Nutr. Sci. 2011, 2, 393–401. [Google Scholar] [CrossRef]
- Aryanti, R.; Perdana, F.; Syamsudin, R.A.M.R. Study of antioxidant activity testing methods of green tea (Camellia sinensis (L.) Kuntze). J. Surya Med. 2021, 7, 15–24. [Google Scholar] [CrossRef]
- Ktari, N.; Fakhfakh, N.; Balti, R.; Ben Khélifa, K.; Nasri, M.; Bougatef, A. Effect of degree of hydrolysis and protease type on the antioxidant activity of protein hydrolysates from cuttlefish (Sepia officinalis) by-products. J. Aquat. Food Prod. Technol. 2012, 22, 436–448. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.H.; Tavano, O.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Use of alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- Bueno-Gavilá, R. Utilización de Proteasas de Cynara scolymus L. Para La Obtención de Péptidos Bioactivos a Partir de Ovoalbúmina, Caseína Y Leche. Ph.D. Thesis, Universidad Católica de Murcia, Murcia, Spain, 2017. Available online: https://repositorio.ucam.edu/bitstream/handle/10952/3101/Tesis%20Estefan%c3%ada%20Bueno%20Gavil%c3%a1.pdf (accessed on 14 December 2025).
- Gutiérrez, E.L.; Medina, G.B.; Roman, M.O.; Florez, O.A.; Martínez, O.L. Obtención y cuantificación de fibra dietaria a partir de residuos de algunas frutas comunes en Colombia. Vitae 2002, 9, 5–14. Available online: https://www.redalyc.org/pdf/1698/169818118001.pdf (accessed on 14 December 2025).
- Rincón, A.M.; Vásquez, A.; Padilla, M. Composición química y compuestos bioactivos de las harinas de cáscaras de naranja (Citrus sinensis), mandarina (Citrus reticulata) y toronja (Citrus paradisi) cultivadas en Venezuela. Arch. Latinoam. Nutr. 2005, 55, 305–310. Available online: https://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0004-06222005000300013 (accessed on 14 December 2025).
- Logesh, K.; Manjunatha, R.; Tuteja, G.; Siva, P.R.; Behera, R.; Kumar, K.P. Characterization and applications of Croton bonplandianus fiber for sustainable biomaterials. Results Eng. 2025, 20, 104765. [Google Scholar] [CrossRef]
- Lv, C.; Liu, J. Alkaline degradation of plant fiber reinforcements in geopolymer: A review. Molecules 2023, 28, 1868. [Google Scholar] [CrossRef]
- Valdez-Valdez, B.A. Efecto de un Bioempaque Eco-Friendly Con Características Antimicrobianas y Antioxidantes a Base de Fibra de Cítricos y Extracto de Orégano, Sobre Las Características Fisicoquímicas y Microbiológicas de Cyprinus carpio. Bachelor’s Thesis, Facultad de Química, Universidad Autónoma del Estado de México, Toluca, México, 2018. Available online: http://hdl.handle.net/20.500.11799/68343 (accessed on 14 December 2025).
- Secretaría de Economía; Secretaría de Agricultura y Desarrollo Rural (SAGARPA). NOM-222-SCFI/SAGARPA-2018: Leche en Polvo O Leche Deshidratada—Materia Prima—Especificaciones, Información Comercial Y Métodos de Prueba; Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 2019. Available online: https://www.dof.gob.mx/normasOficiales/7516/seeco3a13_C/seeco3a13_C.html (accessed on 14 December 2025).
- Secretaría de Economía. NMX-F-043-1970: Alimentos—Determinación de Acidez en Productos Alimenticios; Dirección General de Normas: Ciudad de Mexico, Mexico, 1970. Available online: https://www.dof.gob.mx/normasOficiales/4355/seeco/seeco.htm#:~:text=Objetivo%20y%20Campo%20de%20aplicaci%C3%B3n,y%20comercializaci%C3%B3n%20de%20la%20misma (accessed on 14 December 2025).
- Avena-Bustillos, R.J.; Krochta, J.M.; Mikal, E.S.; Rojas-Villegas, R.J.; Sauceda-Pérez, J.A. Optimization of edible coating formulations on zucchini to reduce water loss. J. Food Eng. 1994, 21, 197–214. [Google Scholar] [CrossRef]
- NOM-243-SSA1-2010; Secretaría de Salud: NORMA Oficial Mexicana. Productos y Servicios. Leche, Fórmula Láctea, Producto Lácteo Combinado y Derivados Lácteos. Disposiciones y Especificaciones Sanitarias. Métodos de Prueba. Secretaría de Salud: Ciudad de Mexico, Mexico, 2010. Available online: https://dof.gob.mx/normasOficiales/4156/salud2a/salud2a.htm (accessed on 14 December 2025).
- Zhao, X.; Ren, C.; Wu, T.; Zhu, H.; Qi, Y.; Jiang, H.; Han, R.; Yang, Y. Effects of homogenization and heat treatments on whey protein composition of bovine, buffalo, goat, camel and yak milk. LWT 2025, 215, 117178. [Google Scholar] [CrossRef]
- Lin, Y.; Geng, H.; Guo, Z.; Wang, Z.; Ban, Q.; Wang, N. Focusing on the formation mechanism and foaming properties of casein-hyaluronic acid complexes: Manufacturing highly moldable 3D aerated foods. Food Hydrocoll. 2025, 167, 111434. [Google Scholar] [CrossRef]
- Daniloski, D.; McCarthy, N.A.; O’Callaghan, T.F.; Vasiljevic, T. Authentication of β-casein milk phenotypes using FTIR spectroscopy. Int. Dairy J. 2022, 129, 105350. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación. NORMEX—Sociedad Mexicana de Normalización y Certificación, S.C.: NMX-F-043-NORMEX-2011 Alimentos—Grenetina comestible—Aspectos de calidad y seguridad alimentaria; Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 2011.
- Yin, Q.; Shi, H.; Zhao, Y.; Yu, G.; Wu, H.; Xia, G.; Yang, T. Physicochemical and functional properties of gelatin obtained from Auxis thazard, Katsuwonus pelamis, Thunnus tonggol and Thunnus albacares skin. Food Chem. X 2025, 27, 102360. [Google Scholar] [CrossRef]
- Bhargavi, P.K.; Banerjee, R.; Raziuddin, M.; Maheswarappa, N.B.; Verma, A.K.; Govindaiah, P.M.; Lalthanmawii, J. Sustainable gelatin extraction from poultry skin-head-feet blend: An ultrasound-assisted approach. Poult. Sci. 2025, 104, 104975. [Google Scholar] [CrossRef]
- Li, H.; Yang, M.; Chen, Y.; Liu, Y.; Wang, X.; Lei, W.; Yang, H.; Gao, Z. Biodegradable multifunctional hydroxypropyl-β-cyclodextrin@EGCG/lignin/gelatin composite films based on incorporating lignin and loaded EGCG for fruit preservation. Food Hydrocoll. 2025, 164, 111206. [Google Scholar] [CrossRef]
- Wang, L.; Kan, J.; Tang, L.; Abidin, S.Z. The effects of glycerol addition on the physicochemical, structural and mechanical properties of salt-gelatinized rice starch-based film. LWT 2025, 218, 117427. [Google Scholar] [CrossRef]
- González-Arana, L.A. Development of an Edible Biofilm Based on Agro-Industrial Byproducts with the Inclusion of Antioxidant and Antimicrobial Compounds. Bachelor’s Thesis, Facultad de Química, Universidad Autónoma del Estado de Mexico, Toluca, Mexico, 2018. [Google Scholar]
- Du, J.; Zhu, Q.; Guo, J.; Gu, J.; Wu, Y.; Ren, L.; Yang, S.; Jiang, J. Preparation and characterization of edible films from gelatin and hydroxypropyl methyl cellulose/sodium carboxymethyl cellulose. Heliyon 2025, 11, e01622. [Google Scholar] [CrossRef]
- Cazón, P.; Velázquez, G.; Vázquez, M. Regenerated cellulose films combined with glycerol and polyvinyl alcohol: Effect of moisture content on the physical properties. Food Hydrocoll. 2020, 103, 105657. [Google Scholar] [CrossRef]
- Herrera-Vázquez, S.E.; Dublán-García, O.; Arizmendi-Cotero, D.; Gómez-Oliván, L.M.; Islas-Flores, H.; Hernández-Navarro, M.D.; Ramírez-Durán, N. Optimization of the Physical, Optical and Mechanical Properties of Composite Edible Films of Gelatin, Whey Protein and Chitosan. Molecules 2022, 27, 869. [Google Scholar] [CrossRef]
- Ozdemir, M.; Floros, J.D. Optimization of edible whey protein films containing preservatives for mechanical and optical properties. J. Food Eng. 2008, 84, 116–123. [Google Scholar] [CrossRef]
- Jorge, A.M.; Gaspar, M.C.; Henriques, M.H.; Braga, M.E. Edible films produced from agrifood by-products and wastes. Innov. Food Sci. Emerg. Technol. 2023, 88, 103442. [Google Scholar] [CrossRef]
- Niño, K.A.; Huerta, M.E.A.; Verde, M.G.R.; Rodríguez, L.A.M. Películas biodegradables a partir de residuos de cítricos: Propuesta de empaques activos. Rev. Lat. Biotecnol. Ambient. Algal 2010, 1, 124–134. Available online: https://www.solabiaa.org/ojs3/index.php/RELBAA/article/view/19 (accessed on 14 December 2025).
- García-Argueta, I.; Dublán-García, O.; Quintero-Salazar, B.; Dominguez-Lopez, A.; Gómez-Oliván, L.M.; Salem, A.F.Z. Effect of lactic acid bacteria on the textural properties of an edible film based on whey, inulin and gelatin. Afr. J. Biotechnol. 2013, 12, 2659–2669. Available online: https://www.ajol.info/index.php/ajb/article/view/130124 (accessed on 14 December 2025).
- Kumar, L.; Ramakanth, D.; Konala, A.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2021, 20, 26–52. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Adiba, A.; Ranjbar, A.; Dorostkar, M. Edible coatings to prolong the shelf life and improve the quality of subtropical fresh/fresh cut fruits: A review. Horticulturae 2025, 11, 577. [Google Scholar] [CrossRef]
- Romani, V.P.; Martins, V.G.; Goddard, J.M. Radical scavenging polyethylene films as antioxidant active packaging materials. Food Control 2020, 109, 106946. [Google Scholar] [CrossRef]
- Romero, J.; Albertos, I.; Díez-Méndez, A.; Poveda, J. Control of postharvest diseases in berries through edible coatings and bacterial probiotics. Sci. Hortic. 2022, 304, 111326. [Google Scholar] [CrossRef]
- Stavang, J.A.; Freitag, S.; Foito, A.; Verrall, S.; Heide, O.M.; Stewart, D.; Sønsteby, A. Raspberry fruit quality changes during ripening and storage as assessed by colour, sensory evaluation and chemical analyses. Sci. Hortic. 2015, 195, 216–225. [Google Scholar] [CrossRef]
- Gao, R.; Xie, P.; Liu, Y.; Li, Z.; Zhao, W.; Ren, J.; Liu, X. Production, bioactive properties and potential applications of fish protein hydrolysates and bioactive peptides. Food Res. Int. 2021, 150, 110–219. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Raghavan, S. Bioactive Peptides from Aquatic Food Proteins. In Antioxidants and Functional Components in Aquatic Foods; Kristinsson, H.G., Raghavan, S., Eds.; John Wiley & Sons: Chichester, UK, 2014; pp. 165–196. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.D.F.F.; Coimbra, J.S.R.; Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Bioactive peptides: Synthesis, properties, and applications in the aackaging and preservation of food. Compr. Rev. Food Sci. Food Saf. 2012, 11, 187–204. [Google Scholar] [CrossRef]
- Jeya, J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto, J.G.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Torres, F.G.; Arroyo, J.; Gómez, C. Challenges in upscaling biopolymer-based films: Processing and performance considerations. Food Packag. Shelf Life 2022, 33, 100920. [Google Scholar] [CrossRef]
- Gallardo, L.; Sánchez, A.; Montalvo, C.; Alonso, A. Extracción de bromelina a partir de residuos de piña. Cienc. Tecnol. Aliment. 2008, 18, 1–4. Available online: https://aquadocs.org/bitstreams/9d244c1f-1283-404b-9c68-d873ce141a80/viewer?itemid=9a67a753-aea5-4bd4-9ec1-358387a486ae (accessed on 14 December 2025).
- Galindo-Estrella, T.; Hernández, R.G.; Mateos-Díaz., J.; Sandoval-Fabian, G.; Chel-Guerrero, L.; Rodríguez-Buenfil, I.; Gallegos-Tintoré, S. Proteolytic activity in enzymatic extracts from Carica papaya L. cv. Maradol harvest by-products. Process Biochem. 2009, 44, 77–82. [Google Scholar] [CrossRef]
- Kunitz, M. Crystalline Soybean Trypsin Inhibitor: II. General Properties. J. Gen. Physiol. 1947, 30, 291–310. [Google Scholar] [CrossRef]
- López-Medina, F.A.; Dublán-García, A.G.; Morachis-Valdez, L.M.; Gómez-Oliván, H.; Islas-Flores, M.D.; Hernández-Navarro, M.D. Functional and physicochemical properties of protein from giant squid (Dosidicus gigas) extracted using foam-aided pH-shift processing. J. Food Sci. 2023, 88, 1409–1419. [Google Scholar] [CrossRef]
- Jae, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- López-García, G.; Dublán-García, O.; Arizmendi-Cotero, D.; Gómez-Oliván, L.M. Antioxidant and antimicrobial peptides derived from food proteins. Molecules 2022, 27, 1343. [Google Scholar] [CrossRef]
- Cerna, E.; Ochoa, Y.; Mendoza, R.; Badii, M.H.; Gallegos, G.; Landeros, J. Assessment of protein quantification methods in Tetranychus urticae, as a potential tool for resistance detection to pesticides. Int. J. Exp. Bot. 2010, 79, 149–152. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, J.N.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Mabel, P.C.N. Extracción de Fibra Comestible a Partir de La Pulpa de Arazá (Eugenia stipitata). Ph.D. Thesis, Universidad Agraria del Ecuador, Guayaquil, Ecuador, 2022. Available online: https://cia.uagraria.edu.ec/Archivos/CHICA%20PEREZ%20NERY%20MABEL.pdf (accessed on 14 December 2025).
- AOAC. Métodos Oficiales de Análisis, 17th ed; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- ASTM D5229/D5229M-92(2004); Standard Test Method for Moisture Content of Composites by the Gravimetric Method, 2004. ASTM International: Conshohocken, PA, USA, 2024. Available online: https://file.yizimg.com/175706/2014021816594640.pdf (accessed on 14 December 2025).
- Gómez-Estaca, J.; Montero, P.; Giménez, B.; Gómez-Guillén, M.C. Effect of functional edible films and high pressure processing on microbial and oxidative spoilage in cold-smoked sardine (Sardina pilchardus). Food Chem. 2007, 105, 511–520. [Google Scholar] [CrossRef]
- López-Medina, F.A.; Dublán-García, O.; Morachis-Valdez, A.G.; Saucedo-Vence, K.; López-García, G.; Díaz-Bandera, D.; Gómez-Espinoza, R.M. Biopolymeric Hydrolysates from Dosidicus gigas: Functional Applications and Shelf-Life Extension in Squid Sausages. Polymers 2025, 17, 839. [Google Scholar] [CrossRef]
- AOAC International. Method 981.13: pH of Solid and Semisolid Foods by Potentiometric Method. In Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- AOAC. Method 942.15: Acidity (Titratable) of Fruit Products. In Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Secretaría de Economía (SE). Norma Mexicana NMX-F-112-NORMEX-2010. Alimentos—Determinación de Sólidos Solubles—Método refractométrico; Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 2010.










| Sample | Specific Activity (U/mg Protein) |
|---|---|
| PPP | 969.4 ± 58.5 b |
| PSP | 420.2 ± 7.4 a |
| CP | 430.6 ± 15.9 a |
| Sample | IC50 ABTS•+ (mg Hydrolysate/mL) | IC50 DPPH• (mg Hydrolysate/mL) | Soluble Protein (mg/mL) |
|---|---|---|---|
| P-E | 1.1 ± 0.1 c | 71.3 ± 1.0 b | 3.6 ± 0.2 a |
| P+PPP | 1.3 ± 0.1 b | 58.1 ± 0.5 c | 3.1 ± 0.1 b |
| P+PSP | 0.8 ± 0.1 d | 31.6 ± 0.3 d | 2.4 ± 0.1 c |
| P+CP | 1.7 ± 0.1 a | 76.7 ± 0.9 a | 3.1 ± 0.1 b |
| Parameter | Results |
|---|---|
| Thickness (µm) | 0.17 ± 0.05 |
| Moisture (%) | 33.80 ± 1.24 |
| WHC (%) | 161.57 ± 3.30 |
| S (%) | 49.78 ± 4.75 |
| L* | 91.22 ± 0.31 |
| a* | −2.10 ± 0.49 |
| b* | 12.16 ± 2.20 |
| C | 12.51 ± 2.14 |
| °H | 100.02 ± 0.16 |
| ΔE | 12.50 ± 2.04 |
| Viscosity (mPa·s) | 143.96 ± 4.78 |
| Parameter | Results |
|---|---|
| Resistance (N) | 5.03 ± 1.87 |
| Elongation (mm) | 19.22 ± 0.73 |
| % of elongation | 51.31 ± 5.71 |
| YM (N/mm) | 0.32 ± 0.12 |
| Database | Web Address | Content |
|---|---|---|
| AHTPDB * | https://biochemia.uwm.edu.pl/biopep/start_biopep.php /(accessed on 14 December 2025) | Antihypertensive peptides |
| AntiTbPdb | http://webs.iiitd.edu.in/raghava/antitbpdb (accessed on 14 December 2025) | Antitubercular and mycobacterial peptides |
| APD | http://aps.unmc.edu/AP/ (accessed on 14 December 2025) | Antimicrobial and anticancer peptides |
| AVPdb | http://crdd.osdd.net/servers/avpdb/ (accessed on 14 December 2025) | Antiviral peptides |
| BaAMPs | http://www.baamps.it/ (accessed on 14 December 2025) | Antimicrobial peptides tested against microbial films |
| BactPepDB | http://bactpepdb.rpbs.univ-paris-diderot.fr/cgi-bin/home.pl (accessed on 14 December 2025) | Bacterial peptides |
| BIOPEP-UWMTM * | http://www.uwm.edu.pl/biochemia (accessed on 14 December 2025) | Bioactive peptides/sensory peptides and amino acids |
| Brainpeps | http://brainpeps.ugent.be/ (accessed on 14 December 2025) | Blood-brain barrier passing peptides |
| CAMPR3 | https://camp.bicnirrh.res.in/ (accessed on 14 December 2025) | Antimicrobial peptides |
| CancerPPD | http://crdd.osdd.net/raghava/cancerppd/index.php (accessed on 14 December 2025) | Anticancer peptides and proteins |
| CPPSite 2.0 | http://crdd.osdd.net/raghava/cppsite/ (accessed on 14 December 2025) | Cell-penetrating peptides |
| DBAASP | https://dbaasp.org/ (accessed on 14 December 2025) | Antimicrobial peptides |
| EROP-Moscow | https://academic.oup.com/nar/article/34/suppl_1/D261/1132217 / (accessed on 14 December 2025) | Bioactive peptides |
| Hemolytik | http://crdd.osdd.net/raghava/hemolytik/ (accessed on 14 December 2025) | Hemolytic and non-hemolytic peptides |
| MBPDB * | http://mbpdb.nws.oregonstate.edu/ (accessed on 14 December 2025) | Milk protein-derived bioactive peptides |
| NeuroPep | http://isyslab.info/NeuroPep/ (accessed on 14 December 2025) | Neuropeptides |
| PepBank | https://pubmed.ncbi.nlm.nih.gov/17678535/ (accessed on 14 December 2025) | Bioactive peptides |
| Quorumpeps | http://quorumpeps.ugent.be/ (accessed on 14 December 2025) | Quorum sensing signaling peptides |
| SATPdb | http://crdd.osdd.net/raghava/satpdb/links.php (accessed on 14 December 2025) | A metabase of therapeutic peptides |
| StraPep | http://isyslab.info/StraPep/ (accessed on 14 December 2025) | Structures of bioactive peptides |
| THPdb | http://crdd.osdd.net/raghava/thpdb/index.html (accessed on 14 December 2025) | FDA-approved therapeutic peptides |
| TumorHoPe | http://crdd.osdd.net/raghava/tumorhope/ (accessed on 14 December 2025) | Tumor homing peptides |
| YADAMP | http://yadamp.unisa.it/about.aspx (accessed on 14 December 2025) | Antimicrobial peptides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
López-García, G.; Dublán-García, O.; López-Medina, F.A.; Morachis-Valdez, A.G.; Saucedo-Vence, K.; Arizmendi-Cotero, D.; Díaz-Bandera, D.; Heredia-García, G.; Santillán-Álvarez, A.; Cira-Chávez, L.A.; et al. Biopolymer Development from Agro-Food and Aquaculture By-Products with Antioxidant Hydrolysates of Cyprinus carpio, Produced via Enzymatic Preparations of Pineapple and Papaya. Int. J. Mol. Sci. 2026, 27, 148. https://doi.org/10.3390/ijms27010148
López-García G, Dublán-García O, López-Medina FA, Morachis-Valdez AG, Saucedo-Vence K, Arizmendi-Cotero D, Díaz-Bandera D, Heredia-García G, Santillán-Álvarez A, Cira-Chávez LA, et al. Biopolymer Development from Agro-Food and Aquaculture By-Products with Antioxidant Hydrolysates of Cyprinus carpio, Produced via Enzymatic Preparations of Pineapple and Papaya. International Journal of Molecular Sciences. 2026; 27(1):148. https://doi.org/10.3390/ijms27010148
Chicago/Turabian StyleLópez-García, Guadalupe, Octavio Dublán-García, Francisco Antonio López-Medina, Ana Gabriela Morachis-Valdez, Karinne Saucedo-Vence, Daniel Arizmendi-Cotero, Daniel Díaz-Bandera, Gerardo Heredia-García, Angel Santillán-Álvarez, Luis Alberto Cira-Chávez, and et al. 2026. "Biopolymer Development from Agro-Food and Aquaculture By-Products with Antioxidant Hydrolysates of Cyprinus carpio, Produced via Enzymatic Preparations of Pineapple and Papaya" International Journal of Molecular Sciences 27, no. 1: 148. https://doi.org/10.3390/ijms27010148
APA StyleLópez-García, G., Dublán-García, O., López-Medina, F. A., Morachis-Valdez, A. G., Saucedo-Vence, K., Arizmendi-Cotero, D., Díaz-Bandera, D., Heredia-García, G., Santillán-Álvarez, A., Cira-Chávez, L. A., & Quintero-Salazar, B. (2026). Biopolymer Development from Agro-Food and Aquaculture By-Products with Antioxidant Hydrolysates of Cyprinus carpio, Produced via Enzymatic Preparations of Pineapple and Papaya. International Journal of Molecular Sciences, 27(1), 148. https://doi.org/10.3390/ijms27010148








