Piceatannol Inhibits the Immunostimulatory Functions of Dendritic Cells and Alleviates Experimental Arthritis
Abstract
1. Introduction
2. Results
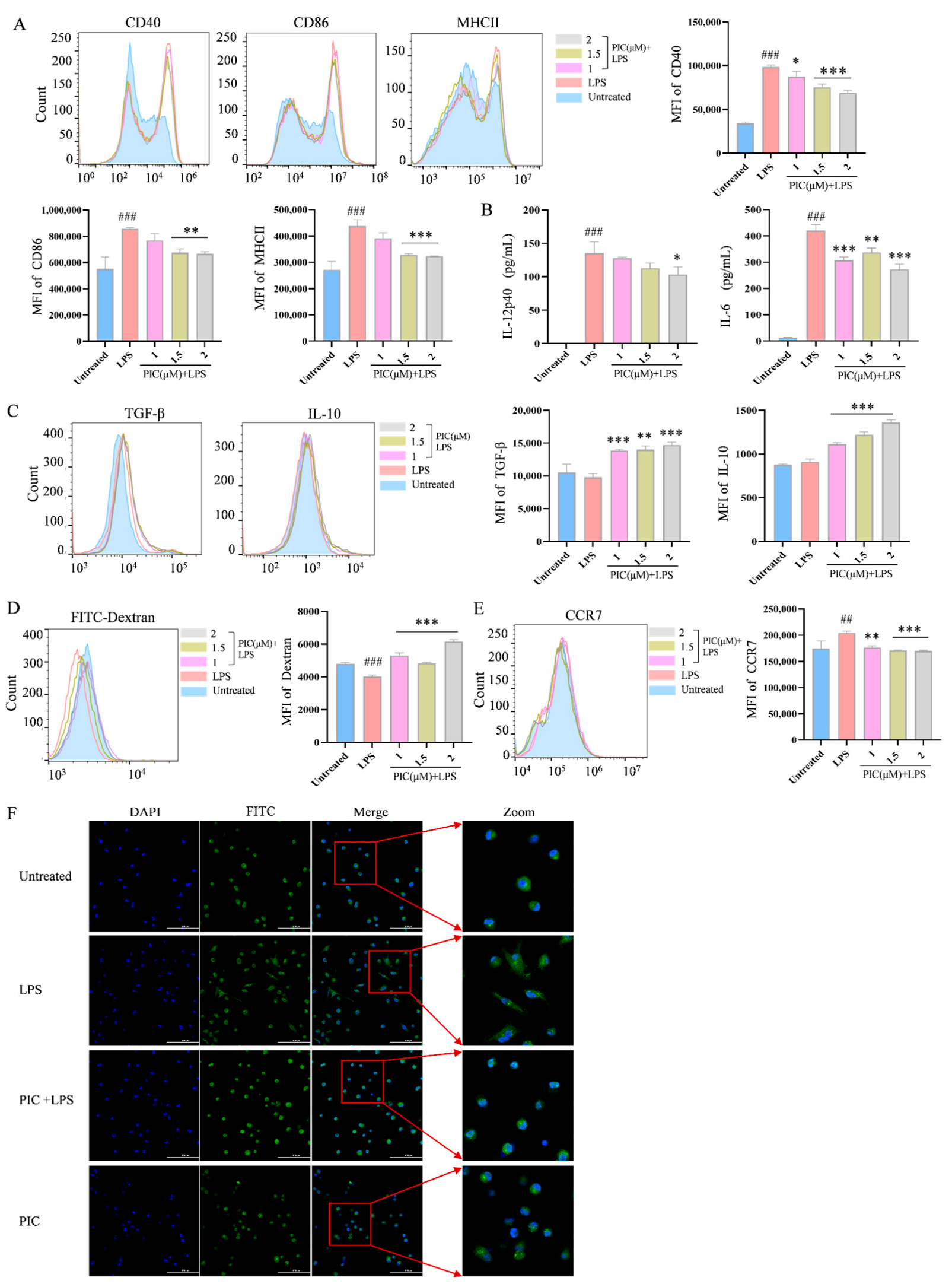
2.1. PIC Inhibits LPS-Induced DC Maturation and Function In Vitro
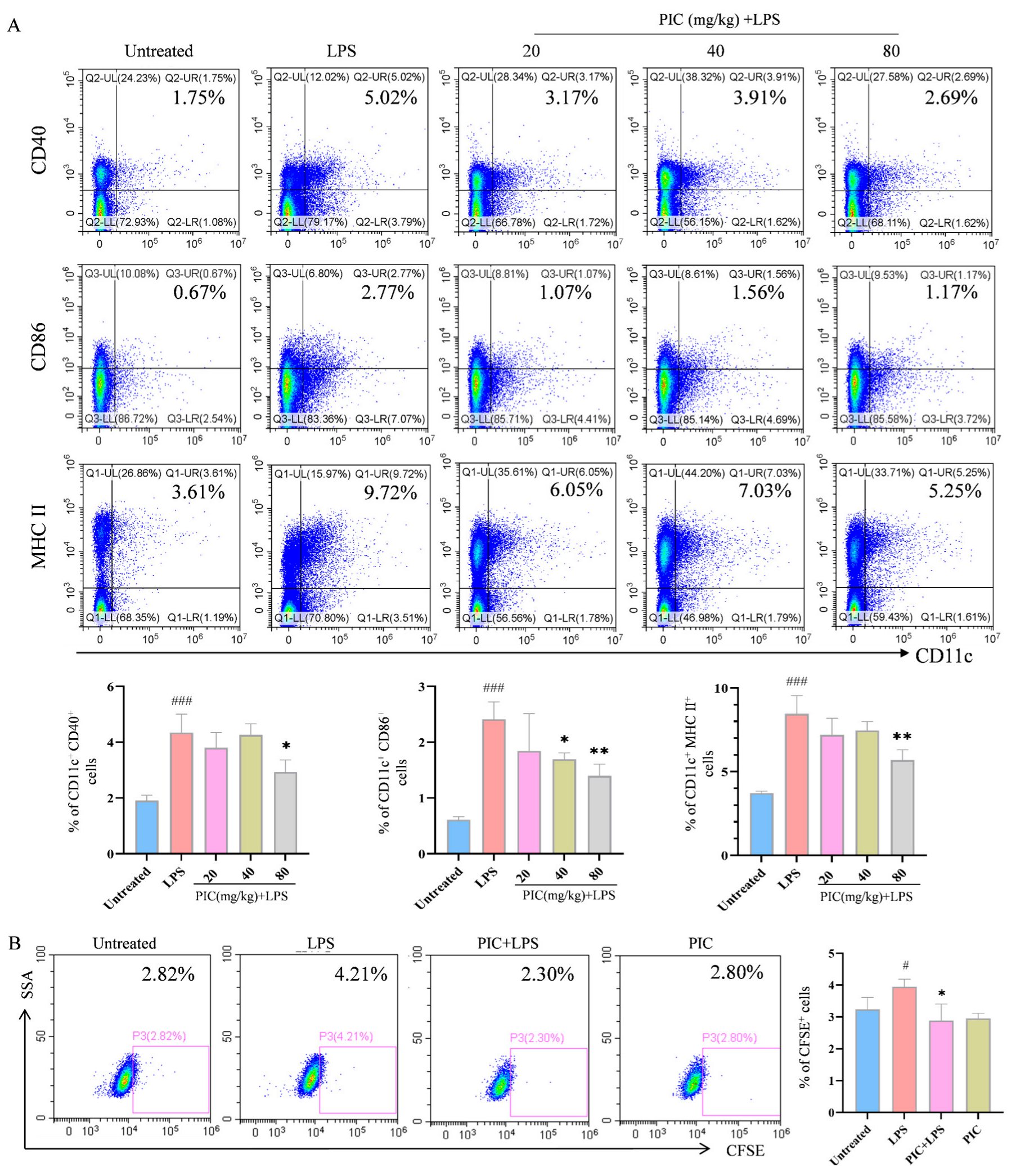
2.2. PIC Inhibited LPS-Induced DC Maturation and Function In Vivo
2.3. Evaluation of PIC’s Therapeutic Effect on the AIA Mouse Model
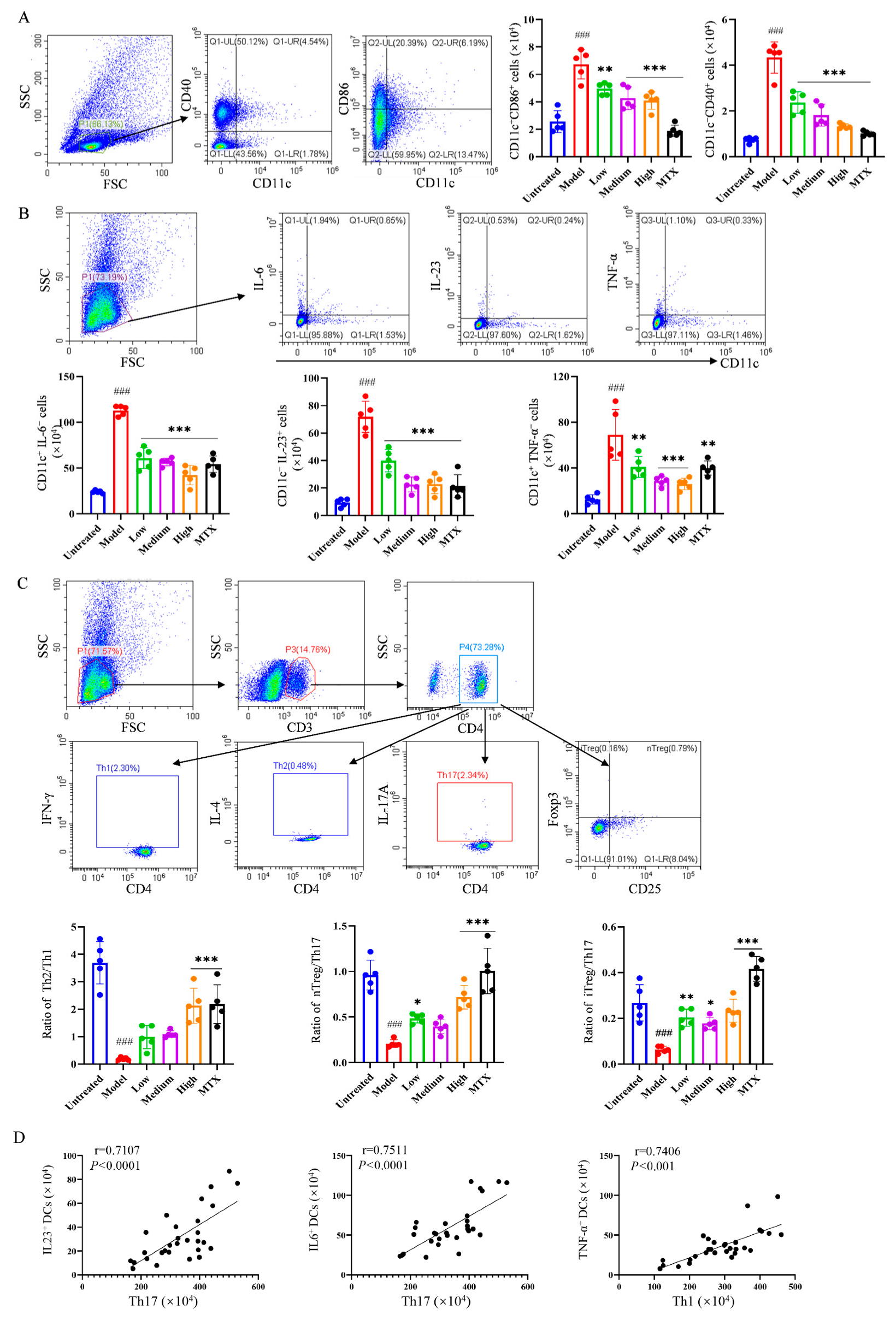
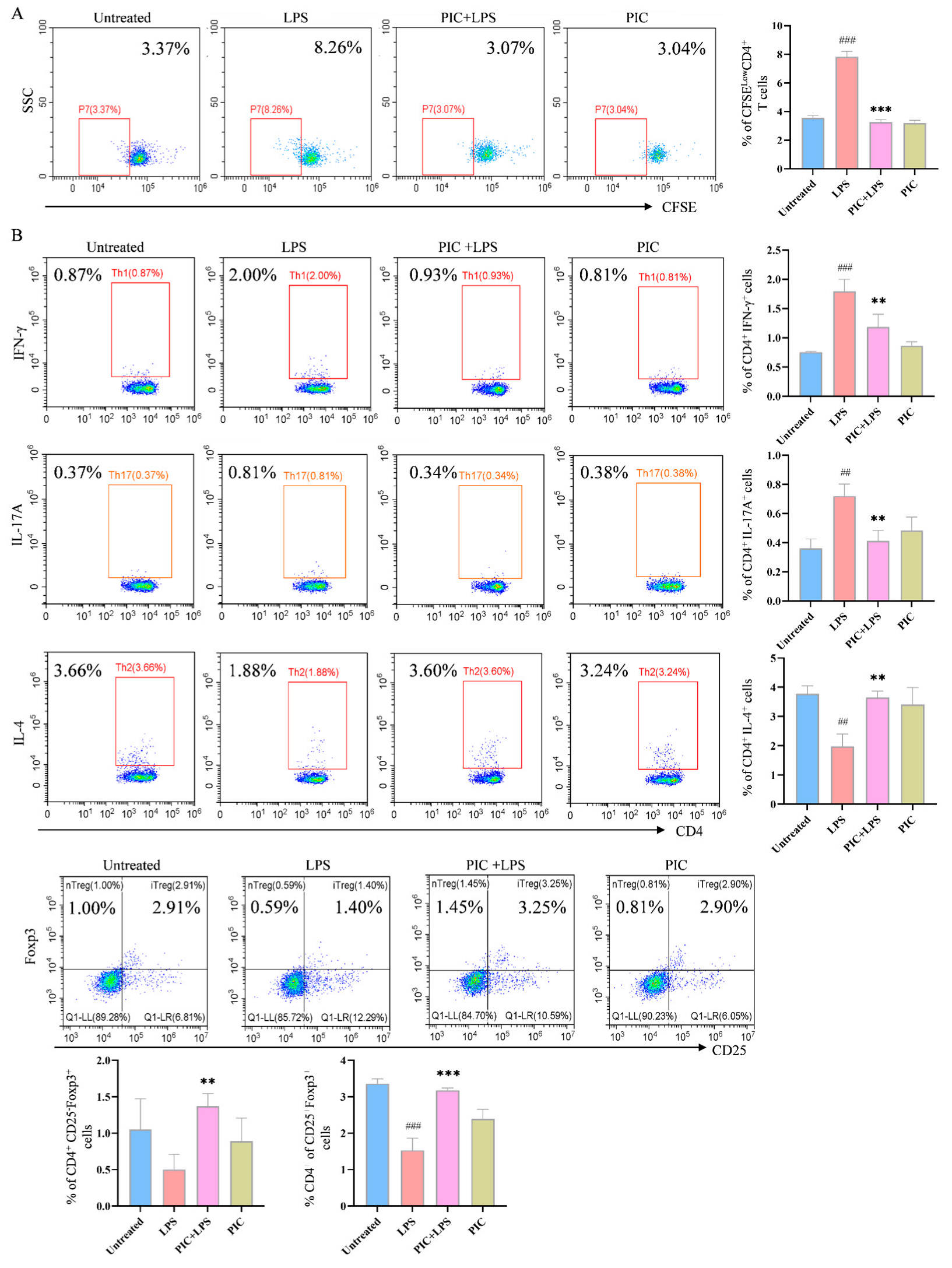
2.4. PIC-Treated DCs Inhibited Th17 and Upregulated Treg Differentiation from T Cells in Experimental Arthritis Mouse Model
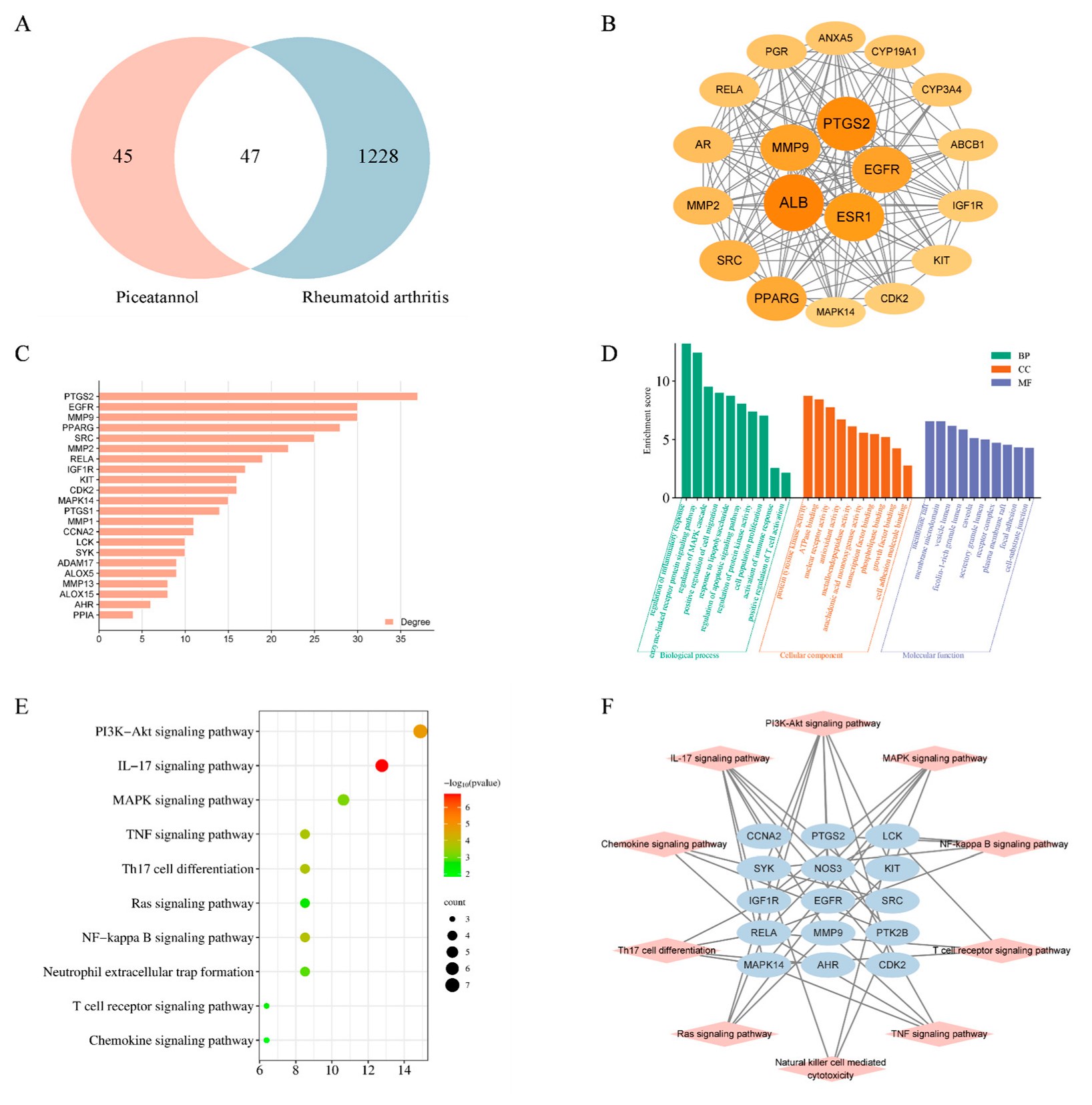
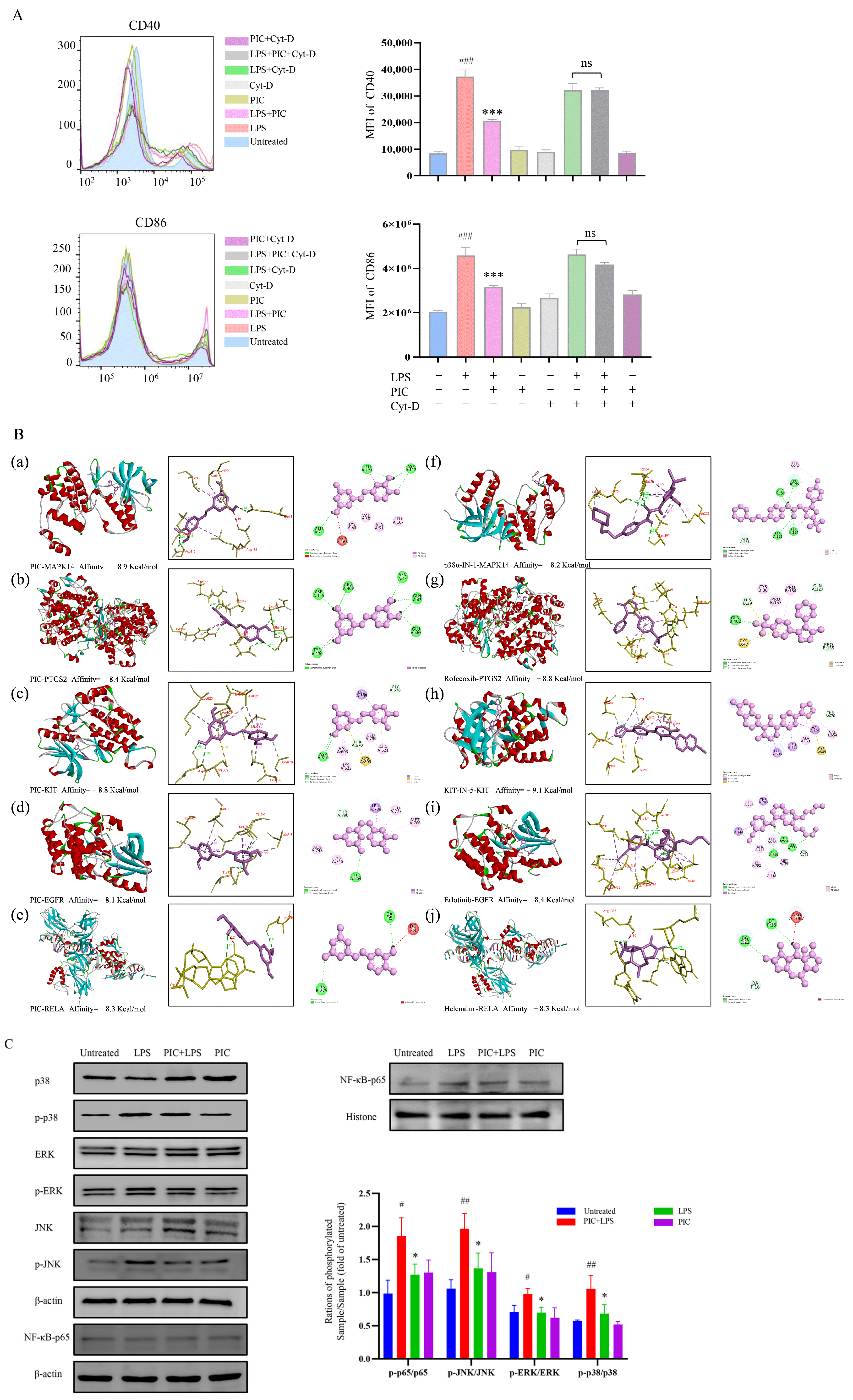
2.5. Network Pharmacology and Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Induction and Culture of Mouse Bone Marrow-Derived DCs
4.3. In Vitro Maturation of DCs
4.4. In Vivo Maturation of DCs
4.5. Validation of DC Migration Ability
4.6. PIC Inhibits the Proliferation of Specific CD4+T Cells
4.7. DCs Treated with PIC Inhibited T Cell Differentiation
4.8. Network Pharmacology and Molecular Docking
4.9. Western Blots
4.10. CFA-Induced Mouse Model of Arthritis
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PIC | Piceatannol |
| RA | Rheumatoid arthritis |
| DCs | Dendritic cells |
| LC-MS | Liquid chromatography–mass spectrometry |
| APC | Antigen-presenting cell |
| CCR-7 | CC chemokine receptor 7 |
| CIA | CFA-induced arthritis |
| DMARDs | Disease-modifying anti-rheumatic drugs |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| TCRs | T cell receptors |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear Factor kappa-B |
| LPS | Lipopolysaccharide |
| CFSE | 5,6-CarboxyfluoresceinDiacetate Succinimidyl Ester |
| RBC Lysate | Red blood cell lysate |
| OVA antigen | Ovalbumin antigen |
| BCA kit | Bicinchoninic Acid Kit |
| CFA | Complete Freund’s adjuvant |
| MTX | Methotrexate |
| Treg | Regulatory T cells |
| EGCG | Epigallocatechin gallate |
| C3G | Cyanidin-3-O-glucoside |
| AIA | Adjuvant-induced arthritis |
| PPI | Protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Petrelli, F.; Mariani, F.M.; Alunno, A.; Puxeddu, I. Pathogenesis of rheumatoid arthritis: One year in review 2022. Clin. Exp. Rheumatol. 2022, 40, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef]
- Firestein, G.S. The disease formerly known as rheumatoid arthritis. Arthritis Res. Ther. 2014, 16, 114. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.H.; Morand, E.; Leech, M. Annexin A1: Potential for glucocorticoid sparing in RA. Nat. Rev. Rheumatol. 2013, 9, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Flórez, J.C.; Seija-Butnaru, D.; Valero, E.G.; Acosta, C.D.P.A.; Amaya, S. Pain Management Strategies in Rheumatoid Arthritis: A Narrative Review. J. Pain. Palliat. Care Pharmacother. 2021, 35, 291–299. [Google Scholar] [CrossRef]
- Friedman, B.; Cronstein, B. Methotrexate mechanism in treatment of rheumatoid arthritis. Jt. Bone Spine 2019, 86, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Grabiec, A.M.; Korchynskyi, O.; Tak, P.P.; Reedquist, K.A. Histone deacetylase inhibitors suppress rheumatoid arthritis fibroblast-like synoviocyte and macrophage IL-6 production by accelerating mRNA decay. Ann. Rheum. Dis. 2012, 71, 424–431. [Google Scholar] [CrossRef]
- Wang, T.; Rui, J.; Shan, W.; Xue, F.; Feng, D.; Dong, L.; Mao, J.; Shu, Y.; Mao, C.; Wang, X. Imbalance of Th17, Treg, and helper innate lymphoid cell in the peripheral blood of patients with rheumatoid arthritis. Clin. Rheumatol. 2022, 41, 3837–3849. [Google Scholar] [CrossRef]
- Joseph, A.; Brasington, R.; Kahl, L.; Ranganathan, P.; Cheng, T.P.; Atkinson, J. Immunologic rheumatic disorders. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S204–S215. [Google Scholar] [CrossRef]
- Angelotti, F.; Parma, A.; Cafaro, G.; Capecchi, R.; Alunno, A.; Puxeddu, I. One year in review 2017: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2017, 35, 368–378. [Google Scholar] [PubMed]
- Moret, F.M.; Hack, C.E.; van der Wurff-Jacobs, K.M.; de Jager, W.; Radstake, T.R.; Lafeber, F.P.; van Roon, J.A. Intra-articular CD1c-expressing myeloid dendritic cells from rheumatoid arthritis patients express a unique set of T cell-attracting chemokines and spontaneously induce Th1, Th17 and Th2 cell activity. Arthritis Res. Ther. 2013, 15, R155. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.X.; Wu, Y.J.; Zhang, J.; Wei, W. T-cells interact with B cells, dendritic cells, and fibroblast-like synoviocytes as hub-like key cells in rheumatoid arthritis. Int. Immunopharmacol. 2019, 70, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A.; Dutertre, C.A., 3rd; Ginhoux, F.; Murphy, K.M. Genetic models of human and mouse dendritic cell development and function. Nat. Rev. Immunol. 2021, 21, 101–115. [Google Scholar] [CrossRef]
- Puhr, S.; Lee, J.; Zvezdova, E.; Zhou, Y.J.; Liu, K. Dendritic cell development-History, advances, and open questions. Semin. Immunol. 2015, 27, 388–396. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef]
- Yu, M.B.; Langridge, W.H.R. The function of myeloid dendritic cells in rheumatoid arthritis. Rheumatol. Int. 2017, 37, 1043–1051. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.C.; Li, S.; Zhan, J.; Ho, C.T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Zhang, G.; Wu, H.; Chang, X. Potential therapeutic effects of cyanidin-3-O-glucoside on rheumatoid arthritis by relieving inhibition of CD38+ NK cells on Treg cell differentiation. Arthritis Res. Ther. 2019, 21, 220. [Google Scholar] [CrossRef]
- Rodríguez Mesa, X.M.; Moreno Vergara, A.F.; Contreras Bolaños, L.A.; Guevara Moriones, N.; Mejía Piñeros, A.L.; Santander González, S.P. Therapeutic Prospects of Cannabinoids in the Immunomodulation of Prevalent Autoimmune Diseases. Cannabis Cannabinoid Res. 2021, 6, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, T.; Fritzer-Szekeres, M.; Saiko, P.; Jäger, W. Resveratrol and resveratrol analogues--structure-activity relationship. Pharm. Res. 2010, 27, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kang, X.; Lu, H.; Xue, E.; Chen, R.; Pan, J.; Ma, J. Piceatannol suppresses inflammation and promotes apoptosis in rheumatoid arthritis-fibroblast-like synoviocytes by inhibiting the NF-κB and MAPK signaling pathways. Mol. Med. Rep. 2022, 25, 180. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Y.; Zhang, X.; Chen, Y.; Zhu, H.; Chen, K.; Liu, S.; Li, Z.; Cao, X. Glycosyltransferase Extl1 promotes CCR7-mediated dendritic cell migration to restrain infection and autoimmunity. Cell Rep. 2023, 42, 111991. [Google Scholar] [CrossRef]
- Lopez, S.; Gomez, E.; Torres, M.J.; Pozo, D.; Fernandez, T.D.; Ariza, A.; Sanz, M.L.; Blanca, M.; Mayorga, C. Betalactam antibiotics affect human dendritic cells maturation through MAPK/NF-kB systems. Role Allerg. React. Drugs Toxicol. Appl. Pharmacol. 2015, 288, 289–299. [Google Scholar] [CrossRef]
- Liu, S.; Ma, H.; Zhang, H.; Deng, C.; Xin, P. Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin. Immunol. 2021, 230, 108793. [Google Scholar] [CrossRef]
- Jain, S.; Mallick, S.; Ramteke, P.; Gogia, A. Neoplasms of follicular helper T-cells: An insight into the pathobiology. Am. J. Blood Res. 2022, 12, 64–81. [Google Scholar]
- Klarenbeek, P.L.; de Hair, M.J.; Doorenspleet, M.E.; van Schaik, B.D.; Esveldt, R.E.; van de Sande, M.G.; Cantaert, T.; Gerlag, D.M.; Baeten, D.; van Kampen, A.H.; et al. Inflamed target tissue provides a specific niche for highly expanded T-cell clones in early human autoimmune disease. Ann. Rheum. Dis. 2012, 71, 1088–1093. [Google Scholar] [CrossRef]
- Basu, A.; Ramamoorthi, G.; Albert, G.; Gallen, C.; Beyer, A.; Snyder, C.; Koski, G.; Disis, M.L.; Czerniecki, B.J.; Kodumudi, K. Differentiation and Regulation of TH Cells: A Balancing Act for Cancer Immunotherapy. Front. Immunol. 2021, 12, 669474. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. Lasker Basic Medical Research Award. Dendritic cells: Versatile controllers of the immune system. Nat. Med. 2007, 13, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Quah, B.J.; O’Neill, H.C. Maturation of function in dendritic cells for tolerance and immunity. J. Cell. Mol. Med. 2005, 9, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Villadangos, J.A.; Cardoso, M.; Steptoe, R.J.; van Berkel, D.; Pooley, J.; Carbone, F.R.; Shortman, K. MHC class II expression is regulated in dendritic cells independently of invariant chain degradation. Immunity 2001, 14, 739–749. [Google Scholar] [CrossRef]
- Brandum, E.P.; Jørgensen, A.S.; Rosenkilde, M.M.; Hjortø, G.M. Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. Int. J. Mol. Sci. 2021, 22, 8340. [Google Scholar] [CrossRef]
- Jang, M.H.; Sougawa, N.; Tanaka, T.; Hirata, T.; Hiroi, T.; Tohya, K.; Guo, Z.; Umemoto, E.; Ebisuno, Y.; Yang, B.G.; et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J. Immunol. 2006, 176, 803–810. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Qin, L.; Wang, T.; Bai, O. Insights into the mechanisms of Th17 differentiation and the Yin-Yang of Th17 cells in human diseases. Mol. Immunol. 2021, 134, 109–117. [Google Scholar] [CrossRef]
- Mbongue, J.C.; Nieves, H.A.; Torrez, T.W.; Langridge, W.H. The Role of Dendritic Cell Maturation in the Induction of Insulin-Dependent Diabetes Mellitus. Front. Immunol. 2017, 8, 327. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, G.; Liu, Q.; Wang, S.; Cui, D. Function and Role of Regulatory T Cells in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 626193. [Google Scholar] [CrossRef]
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 2020, 153, 104635. [Google Scholar] [CrossRef]
- Radkar, V.; Hardej, D.; Lau-Cam, C.; Billack, B. Evaluation of resveratrol and piceatannol cytotoxicity in macrophages, T cells, and skin cells. Arh. Hig. Rada Toksikol. 2007, 58, 293–304. [Google Scholar] [CrossRef][Green Version]
- Yao, L.; Cai, H.; Fang, Q.; Liu, D.; Zhan, M.; Chen, L.; Du, J. Piceatannol alleviates liver ischaemia/reperfusion injury by inhibiting TLR4/NF-κB/NLRP3 in hepatic macrophages. Eur. J. Pharmacol. 2023, 960, 176149. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Han, Y.; Jo, H.; Lee, K.W.; Song, Y.S. Piceatannol Is Superior to Resveratrol at Suppressing Adipogenesis in Human Visceral Adipose-Derived Stem Cells. Plants 2021, 10, 366. [Google Scholar] [CrossRef]
- Jancinova, V.; Perecko, T.; Nosal, R.; Svitekova, K.; Drabikova, K. The natural stilbenoid piceatannol decreases activity and accelerates apoptosis of human neutrophils: Involvement of protein kinase C. Oxid. Med. Cell Longev. 2013, 2013, 136539. [Google Scholar] [CrossRef]
- Les, F.; Deleruyelle, S.; Cassagnes, L.-E.; Boutin, J.A.; Balogh, B.; Arbones-Mainar, J.M.; Biron, S.; Marceau, P.; Richard, D.; Nepveu, F.; et al. Piceatannol and resveratrol share inhibitory effects on hydrogen peroxide release, monoamine oxidase and lipogenic activities in adipose tissue, but differ in their antilipolytic properties. Chem. Biol. Interact. 2016, 258, 115–125. [Google Scholar] [CrossRef]
- Chan, B.S.; Bosco, A.A.; Buckley, N.A. Navigating methotrexate toxicity: Examining the therapeutic roles of folinic acid and glucarpidase. Br. J. Clin. Pharmacol. 2025, 91, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M.; Sauter, R.J.; Nording, H.; Olbrich, M.; Emschermann, F.; Langer, H.F. Protocol to isolate and analyze mouse bone marrow derived dendritic cells (BMDC). STAR Protoc. 2022, 3, 101664. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, X.; Han, P.; Aimaier, A.; Zhang, Y.; Li, J. Syringaldehyde ameliorates mouse arthritis by inhibiting dendritic cell maturation and proinflammatory cytokine secretion. Int. Immunopharmacol. 2023, 121, 110490. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, X.; Chen, J.; Zhao, J.; Liu, Y.; Zhang, J.; Wang, J. Utilizing the nanosecond pulse technique to improve antigen intracellular delivery and presentation to treat tongue squamous cell carcinoma. Med. Oral. Patol. Oral. Cir. Bucal. 2018, 23, e344–e350. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Jung, N.C.; Lee, J.H.; Choi, S.Y.; Choi, H.J.; Park, S.Y.; Jang, J.S.; Byun, S.H.; Hwang, S.U.; Noh, K.E.; et al. Pdlim4 is essential for CCR7-JNK-mediated dendritic cell migration and F-actin-related dendrite formation. FASEB J. 2019, 33, 11035–11044. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Wang, H.; Wu, Y.; Xia, S.; Zhang, M. CD8(+) T activation attenuates CD4(+) T proliferation through dendritic cells modification. Cell. Immunol. 2015, 296, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.P.N.; Nguyen, T.T.; Tran, G.B. Anti-Arthritis Effect of Ethanol Extract of Sacha Inchi (Plukenetia volubilis L.) Leaves Against Complete Freund’s Adjuvant-Induced Arthritis Model in Mice. Trop. Life Sci. Res. 2023, 34, 237–257. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Han, P.; Zhu, Y.; Dong, J.; Guan, Z.; Xu, Y.; Li, J.; Liu, X. Piceatannol Inhibits the Immunostimulatory Functions of Dendritic Cells and Alleviates Experimental Arthritis. Int. J. Mol. Sci. 2025, 26, 3626. https://doi.org/10.3390/ijms26083626
Han L, Han P, Zhu Y, Dong J, Guan Z, Xu Y, Li J, Liu X. Piceatannol Inhibits the Immunostimulatory Functions of Dendritic Cells and Alleviates Experimental Arthritis. International Journal of Molecular Sciences. 2025; 26(8):3626. https://doi.org/10.3390/ijms26083626
Chicago/Turabian StyleHan, Luyang, Peng Han, Yanbo Zhu, Jiawei Dong, Zhenyang Guan, Yuekang Xu, Jinyao Li, and Xiaoying Liu. 2025. "Piceatannol Inhibits the Immunostimulatory Functions of Dendritic Cells and Alleviates Experimental Arthritis" International Journal of Molecular Sciences 26, no. 8: 3626. https://doi.org/10.3390/ijms26083626
APA StyleHan, L., Han, P., Zhu, Y., Dong, J., Guan, Z., Xu, Y., Li, J., & Liu, X. (2025). Piceatannol Inhibits the Immunostimulatory Functions of Dendritic Cells and Alleviates Experimental Arthritis. International Journal of Molecular Sciences, 26(8), 3626. https://doi.org/10.3390/ijms26083626




