Cardiovascular Biomarkers: Tools for Precision Diagnosis and Prognosis
Abstract
1. Introduction
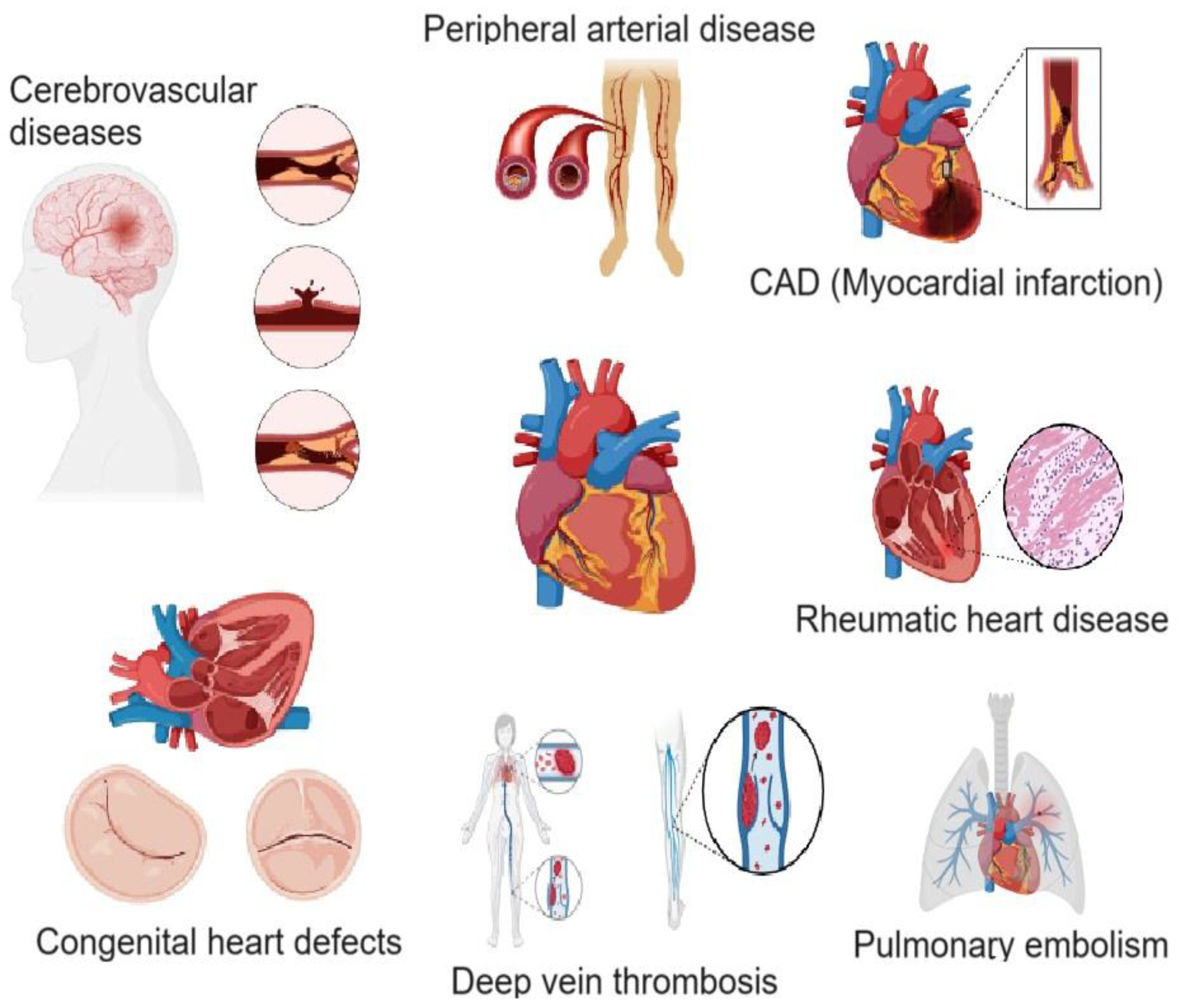
2. Cardiovascular Diseases
3. Diagnosis of CVDs and the Role of Biomarkers
4. Cardiac Biomarkers
4.1. Aspartate Aminotransferase (AST)
4.2. Creatine Kinase MB (CK-MB)
4.3. Lactate Dehydrogenase (LDH)
4.4. Carbonic Anhydrase III (CA-III)
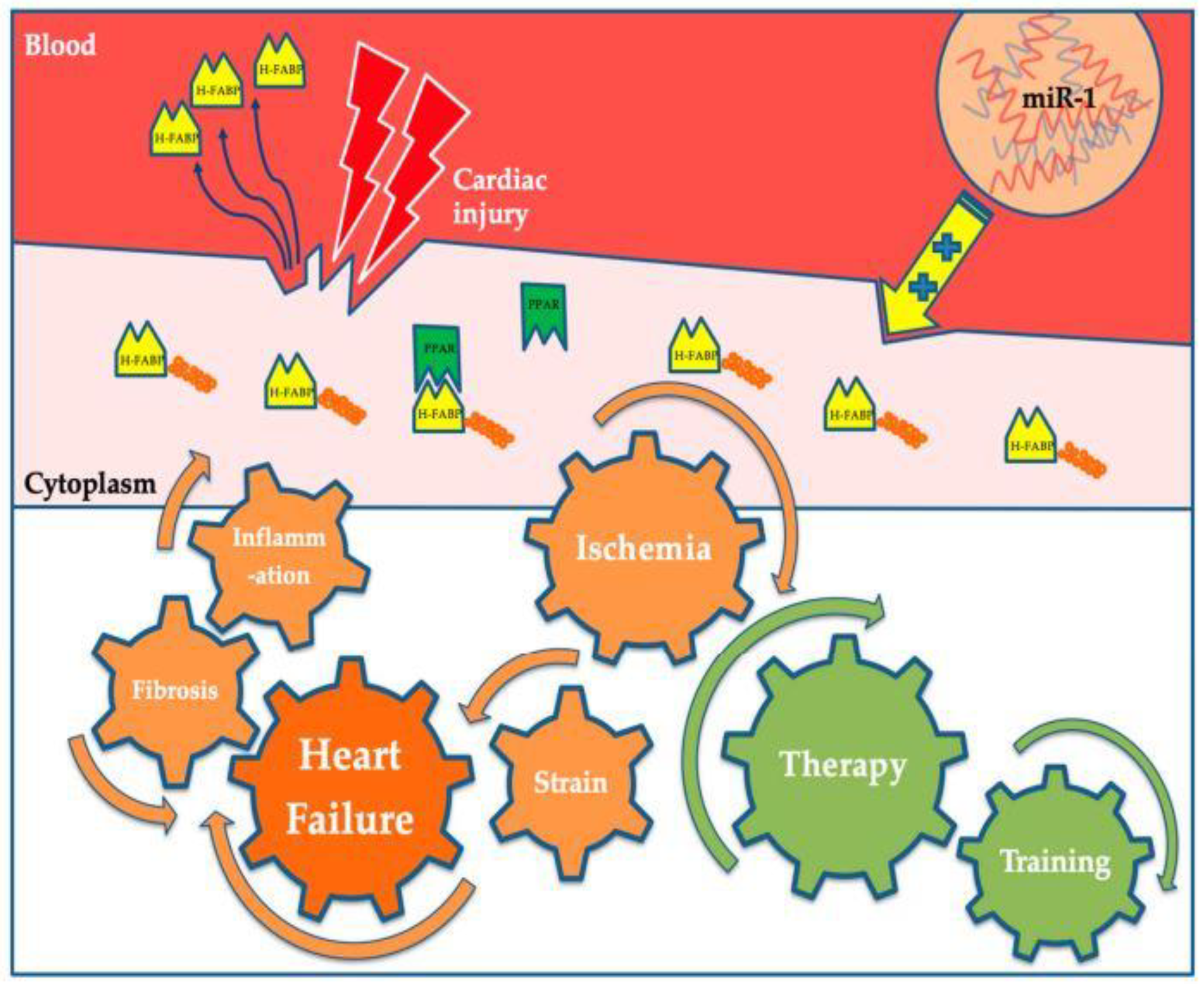
4.5. Heart-Type Fatty Acid-Binding Protein (H-FABP)
4.6. C-Reactive Protein (CRP)
4.7. Myeloperoxidase (MPO)
4.8. Cardiac Troponins
4.9. Hydroxybutyrate Dehydrogenase (HBDH)
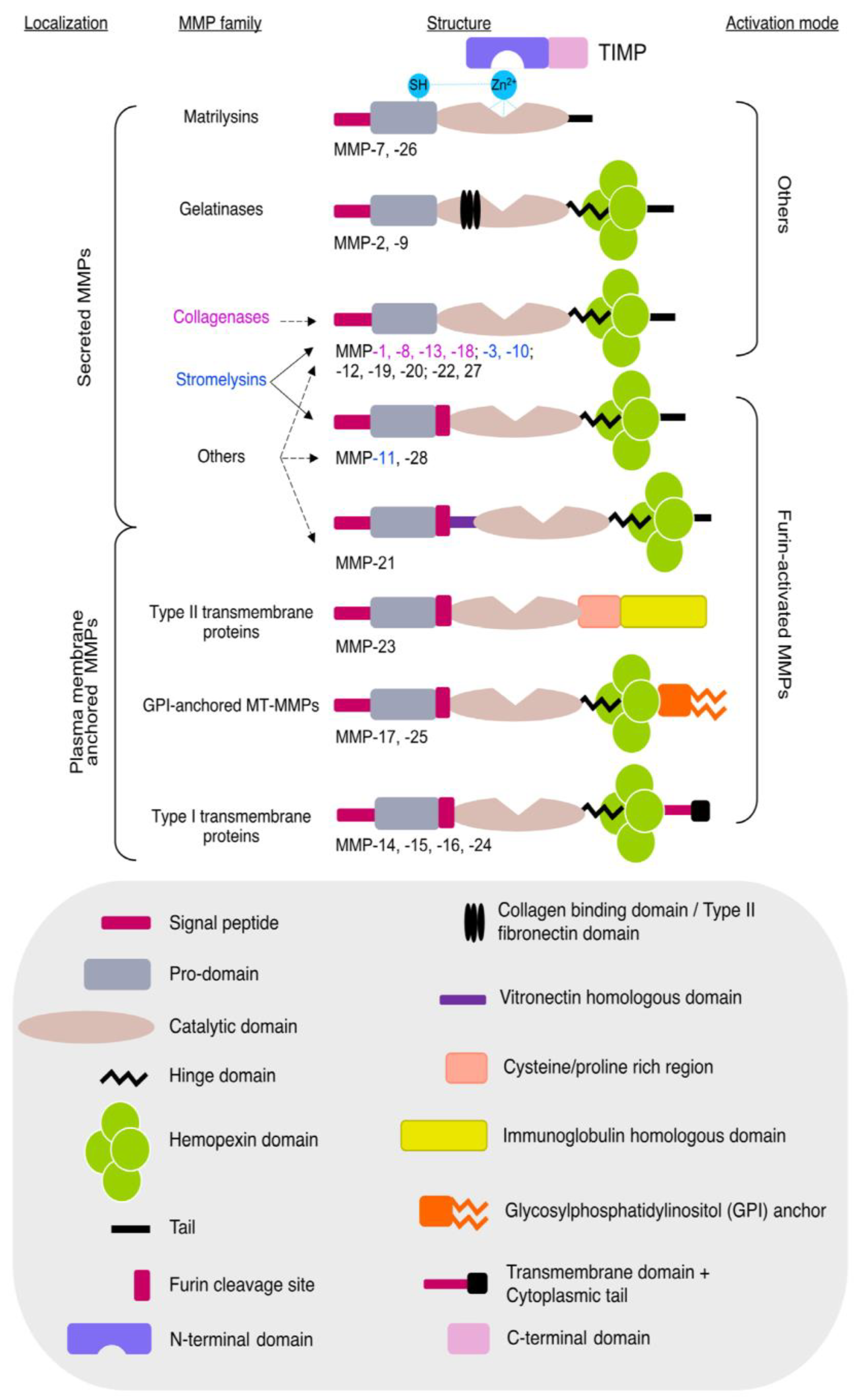
4.10. Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of Metalloproteinases (TIMPs)
4.11. Fibrinogen Levels
4.12. Myoglobin
4.13. Ischemia-Modified Albumin (IMA)
4.14. Glycogen Phosphorylase Isoenzyme BB (GPBB)
4.15. Oxylipins
4.16. Lipoprotein-Associated Phospholipase A2 (Lp-PLA2)
4.17. B-Type Natriuretic Peptide (BNP) and N-Terminal Pro-B-Type Natriuretic Peptide (NT-proBNP)
4.18. Mid-Regional Pro-Atrial Natriuretic Peptide (MR-proANP) and Mid-Regional Pro-Adrenomedullin (MR-proADM)
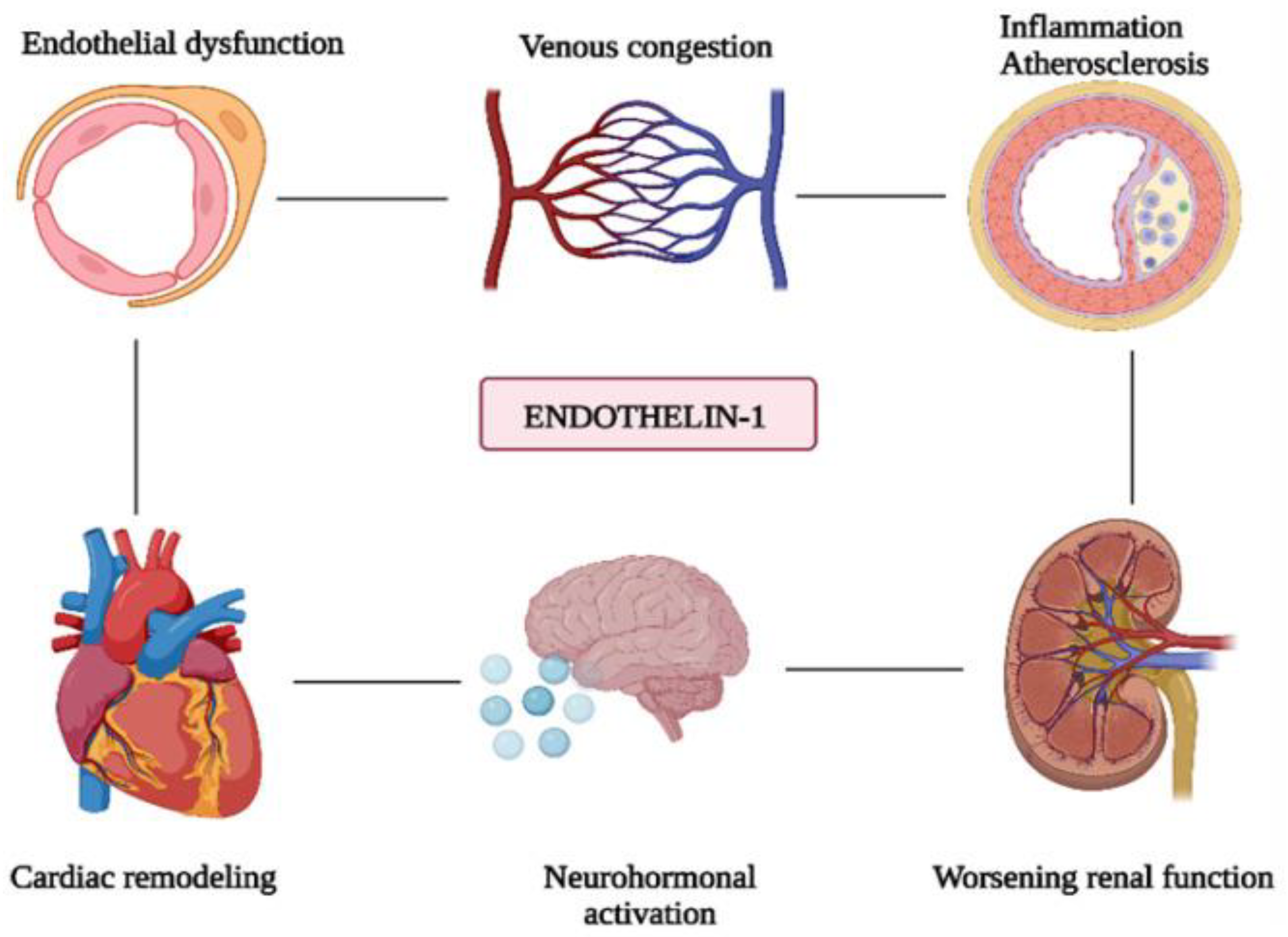
4.19. Endothelin-1 (ET-1)
4.20. Tumor Necrosis Factor-Alpha (TNFα)
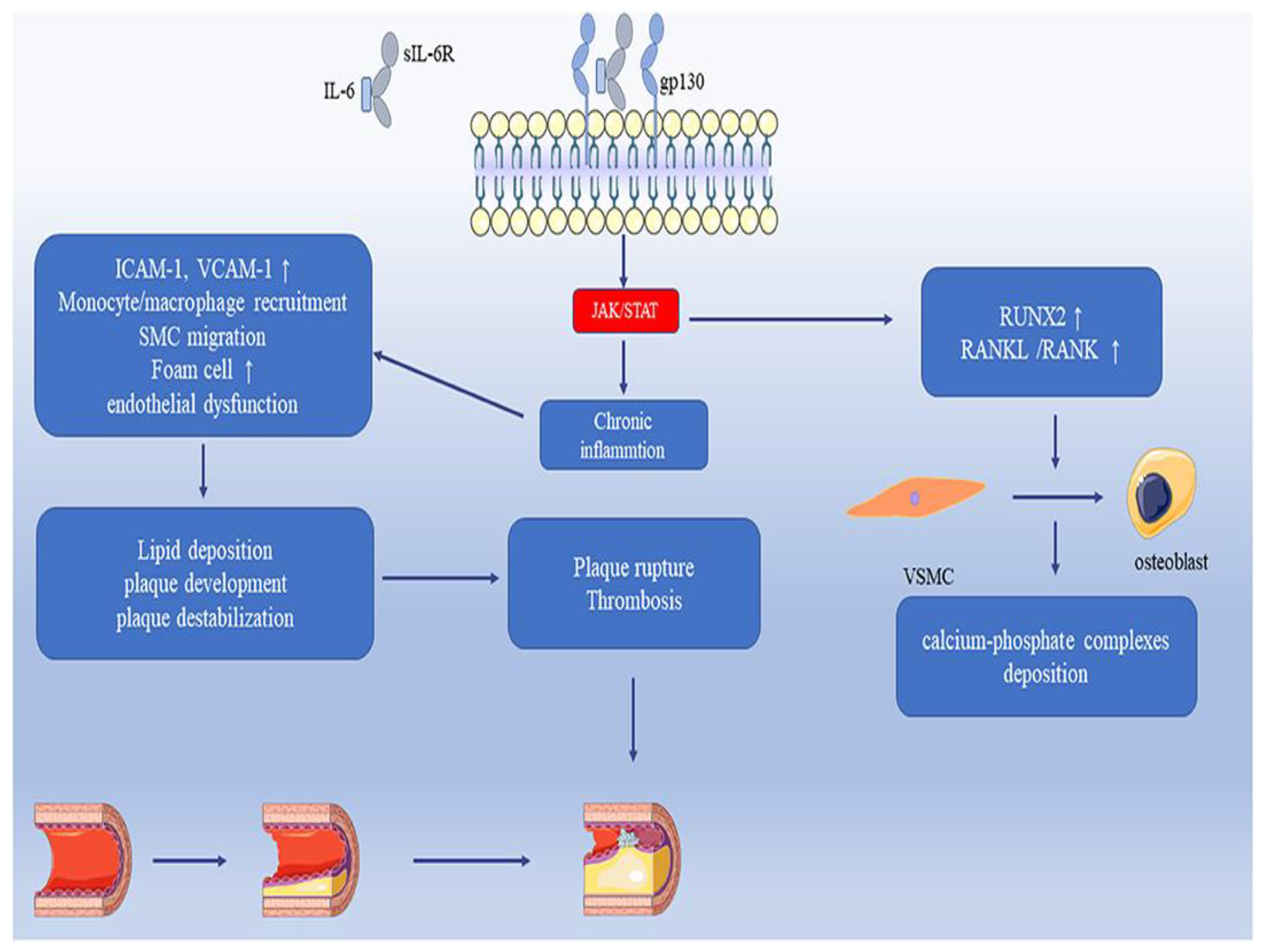
4.21. Interleukin-6 (IL-6)
4.22. Growth Differentiation Factor-15 (GDF-15)
4.23. Suppression of Tumorigenicity-2 (ST2)
4.24. Pentraxin 3 (PTX3)
4.25. Pregnancy-Associated Plasma Protein-A (PAPP-A)
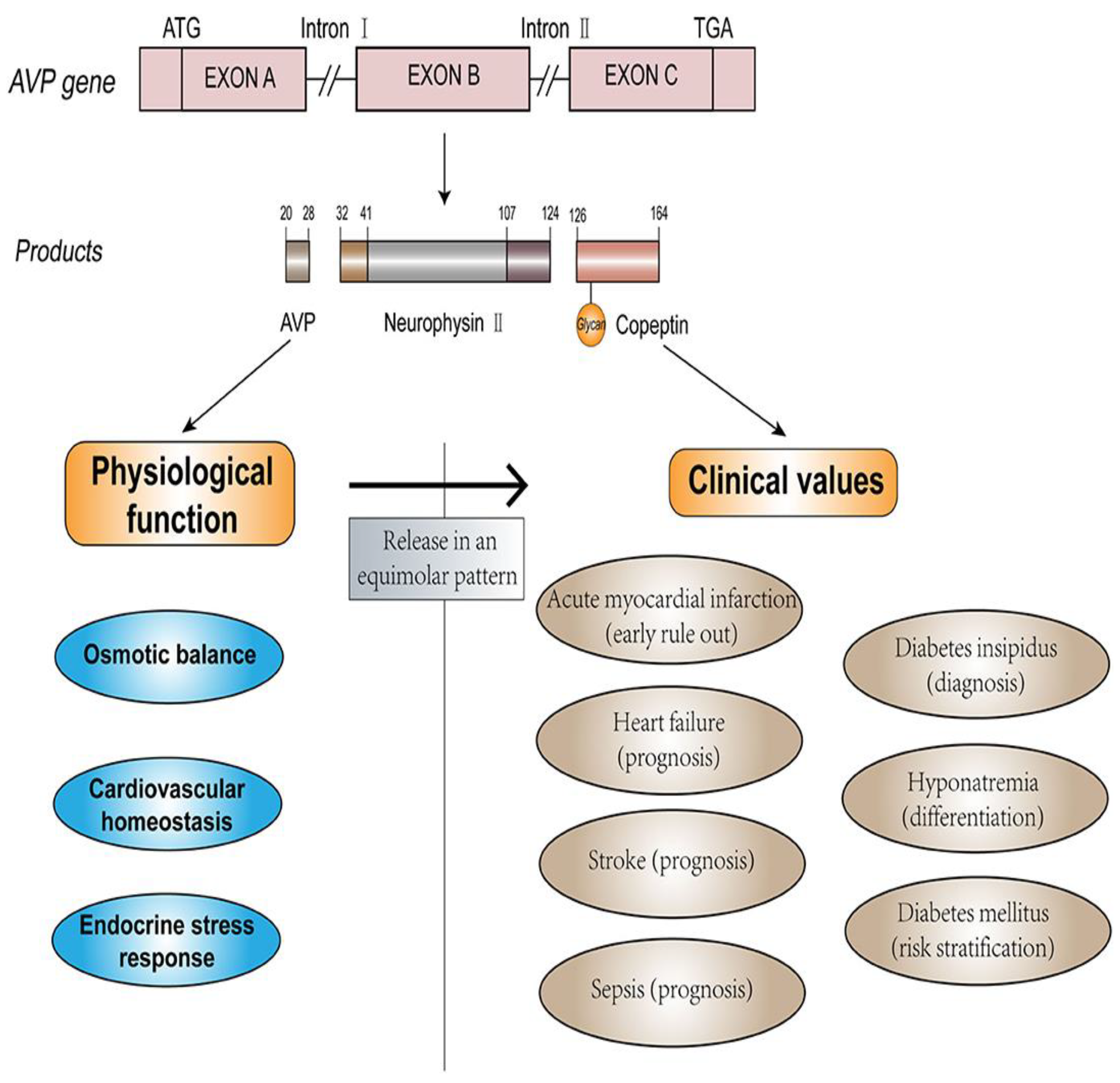
4.26. Copeptin
4.27. Galectin-3 (Gal-3)
4.28. Trimethylamine n-Oxide (TMAO)
4.29. MicroRNAs and Long Non-Coding RNAs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaziano, T.A. Cardiovascular diseases worldwide. Public Health Approach Cardiovasc. Dis. Prev. Manag. 2022, 1, 8–18. [Google Scholar]
- Wang, Y.; Wang, X.; Wang, C.; Zhou, J. Global, Regional, and National Burden of Cardiovascular Disease, 1990–2021: Results From the 2021 Global Burden of Disease Study. Cureus 2024, 16, e74333. [Google Scholar] [PubMed]
- Sun, B.; Wang, Z. A Short Review on Advances in Early Diagnosis and Treatment of Ischemic Stroke. Galen Med. J. 2023, 12, e2993. [Google Scholar] [PubMed]
- Ahmad, S.; Kumar, R. An update of new/potential cardiovascular markers: A narrative review. Mol. Biol. Rep. 2024, 51, 179. [Google Scholar] [CrossRef]
- Vasan, R.S. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation 2006, 113, 2335–2362. [Google Scholar]
- Shao, C.; Wang, J.; Tian, J.; Tang, Y.D. Coronary Artery Disease: From Mechanism to Clinical Practice. In Coronary Artery Disease: Therapeutics and Drug Discovery; Wang, M., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1177. [Google Scholar] [CrossRef]
- Morley, R.L.; Sharma, A.; Horsch, A.D.; Hinchliffe, R.J. Peripheral artery disease. BMJ 2018, 360, j5842. [Google Scholar]
- Goldhaber, S.Z.; Bounameaux, H. Pulmonary embolism and deep vein thrombosis. Lancet 2012, 379, 1835–1846. [Google Scholar]
- Marijon, E.; Mirabel, M.; Celermajer, D.S.; Jouven, X. Rheumatic heart disease. Lancet 2012, 379, 953–964. [Google Scholar]
- Sun, R.; Liu, M.; Lu, L.; Zheng, Y.; Zhang, P. Congenital heart disease: Causes, diagnosis, symptoms, and treatments. Cell Biochem. Biophys. 2015, 72, 857–860. [Google Scholar]
- Netala, V.R.; Teertam, S.K.; Li, H.; Zhang, Z. A Comprehensive Review of Cardiovascular Disease Management: Cardiac Biomarkers, Imaging Modalities, Pharmacotherapy, Surgical Interventions, and Herbal Remedies. Cells 2024, 13, 1471. [Google Scholar] [CrossRef]
- Restrepo Tique, M.; Araque, O.; Sanchez-Echeverri, L.A. Technological Advances in the Diagnosis of Cardiovascular Disease: A Public Health Strategy. Int. J. Environ. Res. Public Health 2024, 21, 1083. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.; Khan, M. Cardiac Biomarkers: What Is and What Can Be. Indian J. Cardiovasc. Dis. Women WINCARS 2018, 3, 240–244. [Google Scholar] [CrossRef]
- Thupakula, S.; Nimmala, S.S.R.; Ravula, H.; Chekuri, S.; Padiya, R. Emerging biomarkers for the detection of cardiovascular diseases. Egypt Heart J. 2022, 74, 77. [Google Scholar] [CrossRef]
- Ladue, J.S.; Wroblewski, F.; Karmen, A. Serum glutamic oxaloacetic transaminase activity in human acute transmural myocardial infarction. Science 1954, 120, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, I.; Yardim, M. Biomarkers in acute myocardial infarction: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, F.; Ladue, J.S. Lactic dehydrogenase activity in blood. Proc. Soc. Exp. Biol. Med. 1955, 90, 210–213. [Google Scholar] [CrossRef]
- Feng, H.Z.; Jin, J.P. Transgenic expression of carbonic anhydrase III in cardiac muscle demonstrates a mechanism to tolerate acidosis. Am. J. Physiol. Cell Physiol. 2019, 317, C922–C931. [Google Scholar]
- Fliegel, L. Molecular biology of the myocardial Na+/H+ exchanger. J. Mol. Cell Cardiol. 2008, 44, 228–237. [Google Scholar] [CrossRef]
- Fliegel, L. Regulation of the Na+/H+ exchanger in the healthy and diseased myocardium. Exp. Opin. Ther. Targ. 2009, 13, 55–68. [Google Scholar]
- Otaki, Y.; Watanabe, T.; Kubota, I. Heart-type fatty acid-binding protein in cardiovascular disease: A systemic review. Clin. Chim. Acta 2017, 474, 44–53. [Google Scholar]
- Ye, X.D.; He, Y.; Wang, S.; Wong, G.T.; Irwin, M.G.; Xia, Z. Heart-type fatty acid binding protein (H-FABP) as a biomarker for acute myocardial injury and long-term post-ischemic prognosis. Acta Pharmacol. Sin. 2018, 39, 1155–1163. [Google Scholar] [CrossRef]
- Rezar, R.; Jirak, P.; Gschwandtner, M.; Derler, R.; Felder, T.K.; Haslinger, M.; Kopp, K.; Seelmaier, C.; Granitz, C.; Hoppe, U.C.; et al. Heart-Type Fatty Acid-Binding Protein (H-FABP) and its Role as a Biomarker in Heart Failure: What Do We Know So Far? J. Clin. Med. 2020, 9, 164. [Google Scholar] [CrossRef]
- Amezcua-Castillo, E.; González-Pacheco, H.; Sáenz-San Martín, A.; Méndez-Ocampo, P.; Gutierrez-Moctezuma, I.; Massó, F.; Sierra-Lara, D.; Springall, R.; Rodríguez, E.; Arias-Mendoza, A.; et al. C-Reactive Protein: The Quintessential Marker of Systemic Inflammation in Coronary Artery Disease-Advancing toward Precision Medicine. Biomedicines 2023, 11, 2444. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Buring, J.E.; Ridker, P.M. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann. Intern. Med. 2006, 145, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Frangie, C.; Daher, J. Role of myeloperoxidase in inflammation and atherosclerosis (Review). Biomed. Rep. 2022, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Anatoliotakis, N.; Deftereos, S.; Bouras, G.; Giannopoulos, G.; Tsounis, D.; Angelidis, C.; Kaoukis, A.; Stefanadis, C. Myeloperoxidase: Expressing inflammation and oxidative stress in cardiovascular disease. Curr. Top. Med. Chem. 2013, 13, 115–138. [Google Scholar] [CrossRef]
- Park, K.C.; Gaze, D.C.; Collinson, P.O.; Marber, M.S. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc. Res. 2017, 113, 1708–1718. [Google Scholar] [CrossRef]
- Kaier, T.E.; Alaour, B.; Marber, M. Cardiac troponin and defining myocardial infarction. Cardiovasc. Res. 2021, 117, 2203–2215. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Sun, Y.; Zhao, Y.B.; Ma, D. α-HBDH is a superior to LDH in predicting major adverse cardiovascular events in patients with acute aortic dissection. Heliyon 2024, 10, e29155. [Google Scholar] [CrossRef]
- Lee, S.; Koppensteiner, R.; Kopp, C.W.; Gremmel, T. α-Hydroxybutyrate dehydrogenase is associated with atherothrombotic events following infrainguinal angioplasty and stenting. Sci. Rep. 2019, 9, 18200. [Google Scholar]
- Molière, S.; Jaulin, A.; Tomasetto, C.-L.; Dali-Youcef, N. Roles of Matrix Metalloproteinases and Their Natural Inhibitors in Metabolism: Insights into Health and Disease. Int. J. Mol. Sci. 2023, 24, 10649. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zheng, Y.J.; Gu, S.S.; Tan, J.L.; Paul, C.; Wang, Y.G.; Yang, H.T. Degradation of cardiac myosin light chain kinase by matrix metalloproteinase-2 contributes to myocardial contractile dysfunction during ischemia/reperfusion. J. Mol. Cell. Cardiol. 2014, 77, 102–112. [Google Scholar] [CrossRef] [PubMed]
- De Coux, A.; Lindsey, M.L.; Villarreal, F.; Garcia, R.A.; Schulz, R. Myocardial matrix metalloproteinase-2: Inside out and upside down. J. Mol. Cell. Cardiol. 2014, 77, 64–72. [Google Scholar] [CrossRef]
- Alp, E.; Yilmaz, A.; Tulmac, M.; Dikmen, A.U.; Cengel, A.; Yalcin, R.; Menevse, E.S. Analysis of MMP-7 and TIMP-2 gene polymorphisms in coronary artery disease and myocardial infarction: A Turkish case-control study. Kaohsiung J. Med. Sci. 2017, 33, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Macrae, F.L.; Swieringa, F.; Heemskerk, J.W.M.; Ariëns, R.A.S. High fibrinogen γ’ levels in patient plasma increase clot formation at arterial and venous shear. Blood Adv. 2021, 5, 3468–3477. [Google Scholar] [CrossRef]
- Appiah, D.; Schreiner, J.P.; MacLehose, R.F.; Folsom, A.R. Association of plasma γ’ fibrinogen with incident cardiovascular disease: The atherosclerosis risk in communities (ARIC) study. Arter. Thromb. Vasc. Biol. 2015, 35, 2700–2706. [Google Scholar] [CrossRef]
- Vaidya, H.C. Myoglobin: An early biochemical marker for the diagnosis of acute myocardial infarction. J. Clin. Immunoass. 1994, 17, 35–39. [Google Scholar]
- Gibler, W.B.; Gibler, C.D.; Weinshenker, E.; Abbottsmith, C.; Hedges, J.R.; Barsan, W.G.; Sperling, M.; Chen, I.W.; Embry, S.; Kereiakes, D. Myoglobin as an early indicator of acute myocardial infarction. Ann. Emerg. Med. 1987, 16, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Mair, J.; Morandell, D.; Genser, N.; Lechleitner, P.; Dienstl, F.; Puschendorf, B. Equivalent early sensitivities of myoglobin, creatine kinase MB mass, creatine kinase isoform ratios, and cardiac troponins I and T for acute myocardial infarction. Clin. Chem. 1995, 41, 1266–1272. [Google Scholar] [CrossRef]
- Abadie, J.M.; Blassingame, C.L.; Bankson, D.D. Albumin cobalt binding assay to rule out acute coronary syndrome. Ann. Clin. Lab. Sci. 2005, 35, 66–72. [Google Scholar] [PubMed]
- Bhakthavatsala Reddy, C.; Cyriac, C.; Desle, H.B. Role of “Ischemia Modified Albumin” (IMA) in acute coronary syndromes. Indian Heart J. 2014, 66, 656–662. [Google Scholar] [CrossRef]
- Jawade, P.; Khillare, K.M.; Mangudkar, S.; Palange, A.; Dhadwad, J.; Deshmukh, M. A Comparative Study of Ischemia-Modified Albumin: A Promising Biomarker for Early Detection of Acute Coronary Syndrome (ACS). Cureus 2023, 15, e44357. [Google Scholar] [CrossRef]
- Rabitzsch, G.; Mair, J.; Lechleitner, P.; Noll, F.; Hofmann, U.; Krause, E.G.; Dienstl, F.; Puschendorf, B. Immunoenzymometric assay of human glycogen phosphorylase isoenzyme BB in diagnosis of ischemic myocardial injury. Clin. Chem. 1995, 41, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Krause, E.G.; Rabitzsch, G.; Noll, F.; Mair, J.; Puschendorf, B. Glycogen phosphorylase isoenzyme BB in diagnosis of myocardial ischaemic injury and infarction. Mol. Cell Biochem. 1996, 60, 289–295. [Google Scholar] [CrossRef]
- Ghimire, A.; Giri, S.; Khanal, N.; Rayamajhi, S.; Thapa, A.; Bist, A.; Devkota, S. Diagnostic accuracy of glycogen phosphorylase BB for myocardial infarction: A systematic review and meta-analysis. J. Clin. Lab. Anal. 2022, 36, e24368. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, M.A. Role of oxylipins in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1142–1154. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Tellis, C.C.; Tselepis, A.D. Pathophysiological role and clinical significance of lipoprotein-associated phospholipase A2 (Lp-PLA2) bound to LDL and HDL. Curr. Pharm. Des. 2014, 20, 6256–6269. [Google Scholar] [CrossRef]
- Munzel, T.; Gori, T. Lipoprotein-associated phospholipase A(2), a marker of vascular inflammation and systemic vulnerability. Eur. Heart J. 2009, 30, 2829–2831. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, A.; Macphee, C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: Biology, epidemiology, and possible therapeutic target. Arter. Thromb. Vasc. Biol. 2005, 25, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; De Bold, M.K.; De Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Călburean, P.A.; Lupu, S.; Huțanu, A.; Oprica, M.; Opriș, D.R.; Stan, A.; Scurtu, A.C.; Aniței, D.; Harpa, M.; Brînzaniuc, K.; et al. Natriuretic peptides and soluble ST2 improves echocardiographic diagnosis of elevated left ventricular filling pressures. Sci. Rep. 2024, 14, 22171. [Google Scholar] [CrossRef]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 2006, 92, 843–849. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef]
- Gohar, A.; Rutten, F.H.; den Ruijter, H.; Kelder, J.C.; von Haehling, S.; Anker, S.D.; Möckel, M.; Hoes, A.W. Mid-regional pro-atrial natriuretic peptide for the early detection of non-acute heart failure. Eur. J. Heart Fail. 2019, 21, 1219–1227. [Google Scholar] [CrossRef]
- Chioncel, O.; Butler, J. Mid-regional pro-atrial natriuretic peptide for diagnosis of heart failure in non-acute settings: Biomarkers plus clinical sense make good sense. Eur. J. Heart Fail. 2019, 21, 1228–1230. [Google Scholar]
- Potocki, M.; Ziller, R.; Mueller, C. Mid-Regional Pro-Adrenomedullin in Acute Heart Failure: A Better Biomarker or Just Another Biomarker? Curr. Heart Fail. Rep. 2012, 9, 244–251. [Google Scholar] [CrossRef]
- Khan, S.Q.; O’Brien, R.J.; Struck, J.; Quinn, P.; Morgenthaler, N.; Squire, I.; Davies, J.; Bergmann, A.; Ng, L.L. Prognostic value of midregional pro-adrenomedullin in patients with acute myocardial infarction: The lamp (leicester acute myocardial infarction peptide) study. J. Am. Coll. Cardiol. 2007, 49, 1525–1532. [Google Scholar]
- Dmour, B.A.; Costache, A.D.; Dmour, A.; Huzum, B.; Duca, Ș.T.; Chetran, A.; Miftode, R.Ș.; Afrăsânie, I.; Tuchiluș, C.; Cianga, C.M.; et al. Could Endothelin-1 Be a Promising Neurohormonal Biomarker in Acute Heart Failure? Diagnostics 2023, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Dhaun, N.; Webb, D.J. Endothelins in cardiovascular biology and therapeutics. Nat. Rev. Cardiol. 2019, 16, 491–502. [Google Scholar] [CrossRef]
- Nishiyama, S.K.; Zhao, J.; Wray, D.W.; Richardson, R.S. Vascular function and endothelin-1: Tipping the balance between vasodilation and vasoconstriction. J. Appl. Physiol. 2017, 122, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.M.; Naga Prasad, S.V. Tumor Necrosis Factor-α in Heart Failure: An Updated Review. Curr. Cardiol. Rep. 2018, 20, 117. [Google Scholar] [CrossRef]
- Urschel, K.; Cicha, I. TNF-α in the cardiovascular system: From physiology to therapy. Int. J. Interferon Cytokine Mediat. Res. 2015, 7, 9–25. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Weston, S.A.; Redfield, M.M.; Killian, J.M.; Roger, V.L. Tumor necrosis factor-alpha and mortality in heart failure: A community study. Circulation 2008, 118, 625–631. [Google Scholar] [CrossRef]
- Rolski, F.; Błyszczuk, P. Complexity of TNF-? Signaling in Heart Disease. J. Clin. Med. 2020, 9, 3267. [Google Scholar] [CrossRef]
- Su, J.H.; Luo, M.Y.; Liang, N.; Gong, S.X.; Chen, W.; Huang, W.Q.; Tian, Y.; Wang, A.P. Interleukin-6: A Novel Target for Cardio-Cerebrovascular Diseases. Front. Pharmacol. 2021, 12, 745061. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Mossmann, M.; Wainstein, M.V.; Mariani, S.; Machado, G.P.; de Araújo, G.N.; Andrades, M.; Gonçalves, S.C. Increased serum IL-6 is predictive of long-term cardiovascular events in high-risk patients submitted to coronary angiography: An observational study. Diabetol. Metab. Syndr. 2022, 14, 125. [Google Scholar] [CrossRef]
- Feng, Y.; Ye, D.; Wang, Z.; Pan, H.; Lu, X.; Wang, M.; Xu, Y.; Yu, J.; Zhang, J.; Zhao, M.; et al. The Role of Interleukin-6 Family Members in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 818890. [Google Scholar] [CrossRef]
- May, B.M.; Pimentel, M.; Zimerman, L.I.; Rohde, L.E. GDF-15 as a Biomarker in Cardiovascular Disease. Arq. Bras. Cardiol. 2021, 116, 494–500. [Google Scholar]
- Li, J.; Hu, X.; Xie, Z.; Li, J.; Huang, C.; Huang, Y. Overview of growth differentiation factor 15 (GDF15) in metabolic diseases. Biomed. Pharmacother. 2024, 176, 116809. [Google Scholar]
- Nyárády, B.B.; Kiss, L.Z.; Bagyura, Z.; Merkely, B.; Dósa, E.; Láng, O.; Kőhidai, L.; Pállinger, É. Growth and differentiation factor-15: A link between inflammaging and cardiovascular disease. Biomed. Pharmacother. 2024, 174, 116475. [Google Scholar]
- Di Candia, A.M.; De Avila, D.X.; Moreira, G.R.; Villacorta, H.; Maisel, A.S. Growth differentiation factor-15, a novel systemic biomarker of oxidative stress, inflammation, and cellular aging: Potential role in cardiovascular diseases. Am. Heart J. Plus Cardiol. Res. Pract. 2021, 9, 100046. [Google Scholar]
- Aimo, A.; Januzzi, J.L.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and prognostic significance of sST2 in heart failure: JACC review topic of the week. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar]
- Jin, M.; Wei, S.; Gao, R.; Wang, K.; Xu, X.; Yao, W.; Zhang, H.; Zhou, Y.; Xu, D.; Zhou, F.; et al. Predictors of Long-Term Mortality in Patients With Acute Heart Failure. Int. Heart. J. 2017, 58, 409–415. [Google Scholar]
- Manzano-Fernández, S.; Januzzi, J.L.; Pastor-Pérez, F.J.; Bonaque-González, J.C.; Boronat-Garcia, M.; Pascual-Figal, D.A.; Montalban-Larrea, S.; Navarro-Peñalver, M.; Andreu-Cayuelas, J.M.; Valdés, M. Serial monitoring of soluble interleukin family member ST2 in patients with acutely decompensated heart failure. Cardiology 2012, 122, 158–166. [Google Scholar]
- Gul, I.; Yucel, O.; Zararsiz, A.; Demirpence, O.; Yucel, H.; Zorlu, A.; Yilmaz, M.B. Prognostic role of soluble suppression of tumorigenicity-2 on cardiovascular mortality in outpatients with heart failure. Anatol. J. Cardiol. 2017, 18, 200–205. [Google Scholar]
- Pfetsch, V.; Sanin, V.; Jaensch, A.; Dallmeier, D.; Mons, U.; Brenner, H.; Koenig, W.; Rothenbacher, D. Increased Plasma Concentrations of Soluble ST2 Independently Predict Mortality but not Cardiovascular Events in Stable Coronary Heart Disease Patients: 13-Year Follow-up of the KAROLA Study. Cardiovasc. Drugs Ther. 2017, 31, 167–177. [Google Scholar]
- Inoue, K.; Kodama, T.; Daida, H. Pentraxin 3: A novel biomarker for inflammatory cardiovascular disease. Int. J. Vasc. Med. 2012, 2012, 657025. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, Z.; Lei, W.; Shen, M.; Tang, J.; Xu, X.; Yang, Y.; Zhang, H. Pentraxin 3: A promising Therapeutic Target for Cardiovascular Diseases. Ageing Res. Rev. 2024, 93, 102163. [Google Scholar]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef]
- Fornai, F.; Carrizzo, A.; Forte, M.; Ambrosio, M.; Damato, A.; Ferrucci, M.; Biagioni, F.; Busceti, C.; Puca, A.A. The inflammatory protein Pentraxin 3 in cardiovascular disease. Immun. Ageing 2016, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Conover, C.A.; Oxvig, C. The Pregnancy-Associated Plasma Protein-A (PAPP-A) Story. Endocr. Rev. 2023, 44, 1012–1028. [Google Scholar] [CrossRef] [PubMed]
- Ziviello, F.; Conte, S.; Cimmino, G.; Carlo Sasso, F.; Trimarco, B.; Cirillo, P. Pregnancy-associated plasma protein-A and its role in cardiovascular disease. Biology, experimental/clinical evidences and potential therapeutic approaches. Curr. Vasc. Pharmacol. 2017, 15, 197–206. [Google Scholar]
- Wang, J.; Tan, G.J.; Han, L.N.; Bai, Y.Y.; He, M.; Liu, H.B. Novel biomarkers for cardiovascular risk prediction. J. Geriatr. Cardiol. JGC 2017, 14, 135. [Google Scholar]
- Gururajan, P.; Gurumurthy, P.; Nayar, P.; Rao, G.S.N.; Babu, R.S.; Sarasabharati, A.; Cherian, K.M. Pregnancy associated plasma protein-A (PAPP-A) as an early marker for the diagnosis of acute coronary syndrome. Indian Heart J. 2012, 64, 141–145. [Google Scholar]
- Mu, D.; Cheng, J.; Qiu, L.; Cheng, X. Copeptin as a Diagnostic and Prognostic Biomarker in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 901990. [Google Scholar] [CrossRef]
- Łukaszyk, E.; Małyszko, J. Copeptin: Pathophysiology and potential clinical impact. Adv. Med. Sci. 2015, 60, 335–341. [Google Scholar]
- Morgenthaler, N.G. Copeptin: A biomarker of cardiovascular and renal function. Congest. Heart Fail. 2010, 16, S37–S44. [Google Scholar] [CrossRef] [PubMed]
- Zaborska, B.; Sikora-Frąc, M.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A.; Sitkiewicz, D.; Sygitowicz, G. The Role of Galectin-3 in Heart Failure-The Diagnostic, Prognostic and Therapeutic Potential-Where Do We Stand? Int. J. Mol. Sci. 2023, 24, 13111. [Google Scholar] [CrossRef]
- Zaborska, B.; Sygitowicz, G.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A. Galectin-3 is related to right ventricular dysfunction in heart failure patients with reduced ejection fraction and may affect exercise capacity. Sci. Rep. 2020, 10, 16682. [Google Scholar] [CrossRef]
- Amin, H.Z.; Amin, L.Z.; Wijaya, I.P. Galectin-3: A novel biomarker for the prognosis of heart failure. Clujul Med. 2017, 90, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Filipe, M.D.; Meijers, W.C.; Van Der Velde, A.R.; De Boer, R.A. Galectin-3 and heart failure: Prognosis, prediction & clinical utility. Clin. Chim. Acta 2015, 443, 48–56. [Google Scholar] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211. [Google Scholar] [CrossRef]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef]
- Kanitsoraphan, C.; Rattanawong, P.; Charoensri, S.; Senthong, V. Trimethylamine N-Oxide and Risk of Cardiovascular Disease and Mortality. Curr. Nutr. Rep. 2018, 7, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Romaine, S.P.; Tomaszewski, M.; Condorelli, G.; Samani, N.J. MicroRNAs in cardiovascular disease: An introduction for clinicians. Heart 2015, 101, 921–928. [Google Scholar] [CrossRef]
- Searles, C.D. MicroRNAs and cardiovascular disease risk. Curr. Cardiol. Rep. 2024, 26, 51–60. [Google Scholar] [PubMed]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef] [PubMed]

| Feature | Details |
|---|---|
| Biochemical role | Member of the phospholipase A2 superfamily; hydrolyzes oxidized phospholipids within lipoproteins, leading to the generation of proinflammatory mediators. |
| Primary source | Synthesized predominantly by inflammatory cells within atherosclerotic plaques. |
| Lipoprotein binding | Strong affinity for lipoprotein fractions, including HDL, LDL, and VLDL. This association highlights its role in lipid metabolism and atherogenesis. |
| Initial identification | Initially recognized as plasma platelet-activating factor acetylhydrolase (pPAF-AH) due to its ability to hydrolyze platelet-activating factor (PAF), a potent proinflammatory mediator. |
| Role in atherosclerosis | Contributes to vascular inflammation by hydrolyzing oxidized phospholipids in LDL, generating lysophosphatidylcholine and oxidized fatty acids, which promote plaque progression and instability. |
| Clinical significance | Elevated Lp-PLA2 levels are strongly associated with increased risk of cardiovascular diseases (CVDs), such as coronary heart disease (CHD), atherosclerosis, and ischemic stroke. |
| Diagnostic utility | Provides high specificity for vascular inflammation with minimal biological variability, making it a robust biomarker for assessing cardiovascular risk. |
| Prognostic implications | Elevated levels correlate with higher risks of cardiovascular events, plaque rupture, and future adverse outcomes. |
| Therapeutic potential | A potential therapeutic target due to its role in vascular inflammation and atherogenesis. Inhibitors of Lp-PLA2 are being investigated as possible interventions for reducing cardiovascular risk. |
| Inflammatory role | Mediates vascular inflammation through its regulatory effects on lipid metabolism and its proinflammatory activity within atherosclerotic plaques. |
| Utility in CVDs | Helps in diagnosing, prognosticating, and managing vascular diseases, particularly in patients predisposed to atherosclerosis, CHD, and ischemic stroke. |
| Advantages | High specificity for vascular inflammation; reflects the inflammatory state of atherosclerotic plaques; minimal biological variability. |
| Limitations | Elevated levels may reflect an inflammatory environment without necessarily pinpointing specific events, requiring integration with other clinical parameters for comprehensive assessment. |
| Feature | BNP | NT-proBNP |
|---|---|---|
| Source | Secreted by cardiac ventricles in response to stretch | Cleaved from proBNP during BNP synthesis |
| Molecular structure | Active hormone | Inactive fragment |
| Half-life | ~20 min | ~60–120 min |
| Stability | Less stable in blood samples | More stable in blood samples |
| Diagnostic role | Heart failure (HF) diagnosis, prognosis, and treatment monitoring | Heart failure diagnosis, prognosis, and treatment monitoring |
| Age impact | Less affected by age | Levels increase significantly with age |
| Renal impact | Moderately influenced by renal dysfunction | More significantly influenced by renal dysfunction |
| Reference ranges | <100 pg/mL typically indicates no HF | Age-specific cutoffs; <300 pg/mL often indicates no HF |
| Clinical use | Acute and chronic HF management | Acute and chronic HF management |
| Advantages | Directly reflects cardiac activity | Higher stability; useful for longer sample transport |
| Disadvantages | Short half-life, less stable | Age- and renal-dependent levels |
| Feature | MR-proANP | MR-proADM |
|---|---|---|
| Biochemical source | Derived from the stable N-terminal portion of pro-atrial natriuretic peptide (proANP). | A stable fragment of adrenomedullin, a peptide involved in vasodilation, natriuresis, and diuresis. |
| Primary role | Reflects atrial stretch and fluid overload, primarily linked to heart failure (HF). | Reflects vascular stress, endothelial dysfunction, and cardiovascular stress. |
| Stability | Highly stable in circulation due to its longer half-life compared to atrial natriuretic peptide (ANP). | Highly stable, offering reliable measurement and prognostic insights. |
| Diagnostic utility | Effective for diagnosing heart failure (HF), although slightly less sensitive than BNP and NT-proBNP. | Useful in acute and chronic HF, acute coronary syndrome (ACS), and acute myocardial infarction (AMI). Adds diagnostic value beyond natriuretic peptides. |
| Prognostic utility | Excels in long-term prognostic value, particularly in predicting mortality over five years in chronic HF. | Superior prognostic value in predicting mortality and cardiovascular events in HF, ACS, and AMI. Outperforms natriuretic peptides in risk stratification for mild-to-moderate HF. |
| Heart failure (HF) | Strongly associated with disease severity and mortality in chronic HF; valuable for monitoring disease progression and treatment efficacy. | Levels correlate with HF severity and NYHA class; provides predictive value for long-term outcomes and mortality. |
| Acute coronary syndrome (ACS) and AMI | Limited data on utility in ACS and AMI. | Independently associated with fatal and nonfatal cardiovascular events in ACS and AMI. Enhances risk stratification beyond traditional models and natriuretic peptides. |
| Screening potential | Effective for screening atrial fibrillation (AF), particularly in community populations, and identifying individuals at risk of developing AF. | Primarily used for prognostic and risk stratification purposes; limited use in AF screening. |
| Influencing factors | Levels influenced by age, BMI, race, and sex; despite variability, it remains reliable due to its stability. | Levels independent of renal function, age, and systolic blood pressure; remains highly predictive despite other clinical variations. |
| Advantages | Long half-life, stable, and highly prognostic for HF and atrial fibrillation risk. | Adds significant prognostic value to traditional risk models, particularly in ACS and AMI; strong correlation with cardiovascular stress and mortality. |
| Limitations | Diagnostic sensitivity slightly lower than BNP and NT-proBNP; levels vary with demographic and physiological factors. | Limited availability in routine clinical practice; specific role in AF screening not established. |
| Clinical utility | Valuable in chronic HF management, long-term mortality prediction, and AF screening. | Crucial for prognosticating HF, ACS, and AMI outcomes, particularly in mild-to-moderate HF; enhances risk models for predicting mortality and cardiovascular events. |
| Biomarker | Source | Half-Life | Detection Time Window | Specificity and Sensitivity | Factors Affecting Interpretation |
|---|---|---|---|---|---|
| Aspartate aminotransferase (AST) | Cardiac muscle, liver, and skeletal muscle | 17 ± 5 h | Rises in 6–12 h, peaks at 24–36 h, and normalizes in 3–7 days | Moderate sensitivity; low specificity for MI | Elevated in liver disease, muscle injury, and hemolysis |
| Creatine kinase MB (CK-MB) | Predominantly cardiac muscle and minor in skeletal muscle | ~12–24 h | Rises in 3–6 h, peaks at 18–24 h, and normalizes in 48–72 h | High specificity for MI; lower sensitivity than troponins | Skeletal muscle injury, renal failure, and chronic muscle diseases |
| Lactate dehydrogenase (LDH) | Cardiac muscle and red blood cells (LDH1 > LDH2 ratio indicative of MI) | ~9 h | Rises in 12–24 h, peaks in 2–3 days, and normalizes in 10–14 days | Low specificity; moderate sensitivity | Liver disease, hemolysis, malignancies, and strenuous exercise |
| Carbonic anhydrase III (CA-III) | Cardiac muscle and skeletal muscle | Not well established | Altered levels in oxidative stress and ischemia | Emerging biomarker; specificity/sensitivity under study | Ischemia, oxidative stress, and metabolic disorders |
| Heart-type fatty acid-binding protein (H-FABP) | Cardiac muscle | ~1–2 h | Detectable within 90 min, peaks at 6–8 h, and normalizes in 24–36 h | High sensitivity for early MI detection; moderate specificity | Skeletal muscle injury and renal dysfunction |
| C-reactive protein (CRP) | Produced in response to cardiac inflammation, vascular endothelium | 19 h | Increases within 6–12 h, peaks at 24–48 h, and remains elevated for days | High sensitivity for inflammation; low specificity for cardiac events | Chronic inflammation, infections, and autoimmune diseases |
| Myeloperoxidase (MPO) | Secreted by neutrophils and macrophages in atherosclerotic plaques | ~12–24 h | Detectable early in ACS and remains elevated for several days | High sensitivity for oxidative stress; moderate specificity for MI | Infections, systemic inflammation, and autoimmune diseases |
| Cardiac troponins (cTnI, cTnT) | Cardiac myocytes (specific to heart muscle) | cTnI: 2 h–1 day, cTnT: 2–14 days | Rises in 2–4 h, peaks at 12–24 h, and persists for 7–14 days (cTnT) | Gold standard for MI; high specificity and sensitivity | Chronic renal disease, heart failure, sepsis, and strenuous exercise |
| Hydroxybutyrate dehydrogenase (HBDH) | Cardiac muscle and red blood cells | ~10 h | Rises in 6–12 h, peaks at 48–72 h, and normalizes in 7–10 days | Moderate specificity; lower sensitivity than troponins | Liver disease, hemolysis, and muscle injury |
| Matrix metalloproteinases (MMPs) and TIMPs | Cardiac extracellular matrix and vascular smooth muscle cells | Hours to days | Chronically elevated in heart failure and atherosclerosis | High specificity for ECM remodeling; moderate sensitivity | Inflammation, cancer, and chronic heart diseases |
| Fibrinogen | Liver | ~3–5 days | Elevated in the presence of inflammation or cardiovascular risk | Moderate specificity; increased in inflammatory states | Liver disease (synthesis affected), inflammation, and malignancies |
| Myoglobin | Skeletal and cardiac muscle | 2–4 h | Detectable within 1–3 h, peaks at 6–9 h, and returns to baseline in 24 h | High sensitivity; low specificity | Skeletal muscle injury, renal failure, and exercise |
| Ischemia-modified albumin (IMA) | Serum albumin (modified by ischemia) | ~6 min | Detectable within minutes and peaks within 3–6 h | Moderate sensitivity and specificity for ischemia | Liver disease, infections, and oxidative stress conditions |
| Glycogen phosphorylase isoenzyme BB (GPBB) | Cardiac and brain tissue | ~1–3 h | Detectable within 1–4 h, peaks at 6–12 h, and normalizes in 24 h | High sensitivity; moderate specificity | Pregnancy, brain injury, and liver dysfunction |
| Oxylipins | Endothelial cells and inflammatory cells | Variable (minutes to hours) | Depends on lipid subtype | High specificity for endothelial function | Dietary intake and genetic polymorphisms |
| Lipoprotein-associated phospholipase A2 (Lp-PLA2) | Circulating with LDL and HDL | ~6 days | Chronic marker | High specificity for vascular inflammation | Affected by lipid-lowering therapies and metabolic syndrome |
| B-type natriuretic peptide (BNP) and NT-proBNP | Cardiac myocytes | BNP: ~20 min; NT-proBNP: ~90 min | Detectable within hours and peaks at 24–48 h | High specificity and sensitivity for HF | Affected by age, renal function, and obesity |
| Mid-regional pro-atrial natriuretic peptide (MR-proANP) | Cardiac atria | ~60 min | Detectable within hours, peaks within 24 h | High specificity, moderate sensitivity | Affected by renal dysfunction and age |
| Mid-regional pro-adrenomedullin (MR-proADM) | Endothelium, adrenal glands | ~22 min | Detectable early in sepsis, HF | High sensitivity, moderate specificity | Affected by inflammation and sepsis |
| Endothelin-1 (ET-1) | Endothelium | ~4–7 min | Detectable early in HF and hypertension | High specificity for endothelial dysfunction | Affected by renal disease and inflammatory conditions |
| Tumor necrosis factor-alpha (TNFα) | Immune cells | ~30 min | Chronic marker | High sensitivity, low specificity | Elevated in systemic inflammation, and cancer, infections |
| Myoglobin | Skeletal and cardiac muscle | 2–4 h | Detectable within 1–3 h, peaks at 6–9 h, and returns to baseline in 24 h | High sensitivity, low specificity | Skeletal muscle injury, renal failure, exercise |
| Ischemia-modified albumin (IMA) | Serum albumin (modified by ischemia) | ~6 min | Detectable within minutes and peaks within 3–6 h | Moderate sensitivity and specificity for ischemia | Liver disease, infections, and oxidative stress conditions |
| Interleukin-6 (IL-6) | Immune cells and endothelial cells | ~15–20 h | Peaks within 1–2 h and remains elevated for days | High sensitivity for inflammation, but low specificity for CVD alone | Chronic inflammatory diseases, infections, autoimmune disorders, and obesity |
| Growth differentiation factor-15 (GDF-15) | Cardiomyocytes and macrophages | ~4–6 h | Elevated in hours to days during acute and chronic cardiovascular conditions | High sensitivity for heart failure; moderate specificity for CVD | Kidney disease, aging, cancer, and metabolic disorders |
| Suppression of tumorigenicity-2 (ST2) | Cardiac fibroblasts and immune cells | ~1.5 h | Elevated in hours to days in acute and chronic heart failure; linked to adverse outcomes | High prognostic value in heart failure; moderate specificity for CAD | Infection, inflammatory diseases, and renal dysfunction |
| Pentraxin 3 (PTX3) | Endothelial cells, neutrophils, and macrophages | ~12–48 h | Peaks within hours and stays elevated for up to several days during acute myocardial infarction | High sensitivity for acute inflammation; moderate specificity for CAD | Sepsis, autoimmune diseases, and infections |
| Pregnancy-associated plasma protein-A (PAPP-A) | Vascular smooth muscle cells and macrophages | ~4 h | Elevated within hours to days in acute coronary syndromes and atherosclerosis progression | High specificity for unstable plaques; useful in CVD risk stratification | Renal dysfunction, pregnancy, and chronic inflammation |
| Copeptin | Secreted in equimolar amounts with AVP, from the posterior pituitary gland | 1–2 h | Within hours of acute myocardial infarction (AMI) onset; peaks on 1st day | High sensitivity for early AMI detection; good negative predictive value | Kidney function, age, and comorbidities like diabetes may affect copeptin levels. |
| Galectin-3 (Gal-3) | Secreted by activated macrophages, linked to fibrosis, inflammation, and remodeling | ~1–2 days | Elevated in both acute and chronic heart failure (HF); often detectable early | Moderate specificity for HF; high sensitivity for fibrosis and inflammation | Renal dysfunction can elevate levels; interpretation may vary with concurrent inflammation. |
| Trimethylamine N-oxide (TMAO) | Produced by gut microbiota from l-carnitine and choline metabolism | Several hours to days | Can be detected after consumption of dietary choline or l-carnitine, early in atherosclerosis | Moderate sensitivity for cardiovascular risk; linked to poor prognosis in CVD | Dietary intake, gut microbiota composition, and kidney function can influence TMAO levels. |
| MicroRNAs (miRNAs) | Released from cardiomyocytes, endothelial cells, and other cells during cardiovascular stress | Varies (hours to days) | Can be detected within hours of AMI or heart failure onset | High specificity for distinct CVDs; good sensitivity for early detection | Stable in plasma, but affected by collection and processing methods. Interference from other conditions can complicate interpretation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netala, V.R.; Hou, T.; Wang, Y.; Zhang, Z.; Teertam, S.K. Cardiovascular Biomarkers: Tools for Precision Diagnosis and Prognosis. Int. J. Mol. Sci. 2025, 26, 3218. https://doi.org/10.3390/ijms26073218
Netala VR, Hou T, Wang Y, Zhang Z, Teertam SK. Cardiovascular Biomarkers: Tools for Precision Diagnosis and Prognosis. International Journal of Molecular Sciences. 2025; 26(7):3218. https://doi.org/10.3390/ijms26073218
Chicago/Turabian StyleNetala, Vasudeva Reddy, Tianyu Hou, Yanbo Wang, Zhijun Zhang, and Sireesh Kumar Teertam. 2025. "Cardiovascular Biomarkers: Tools for Precision Diagnosis and Prognosis" International Journal of Molecular Sciences 26, no. 7: 3218. https://doi.org/10.3390/ijms26073218
APA StyleNetala, V. R., Hou, T., Wang, Y., Zhang, Z., & Teertam, S. K. (2025). Cardiovascular Biomarkers: Tools for Precision Diagnosis and Prognosis. International Journal of Molecular Sciences, 26(7), 3218. https://doi.org/10.3390/ijms26073218





