Thymoquinone Attenuates NF-κβ Signalling Activation in Retinal Pigment Epithelium Cells Under AMD-Mimicking Conditions
Abstract
1. Introduction
2. Results
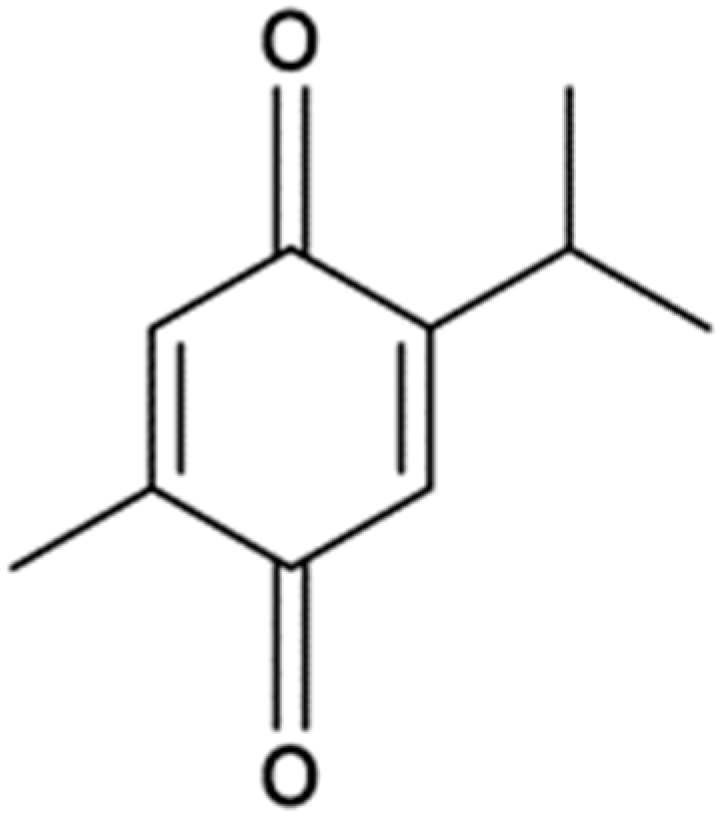
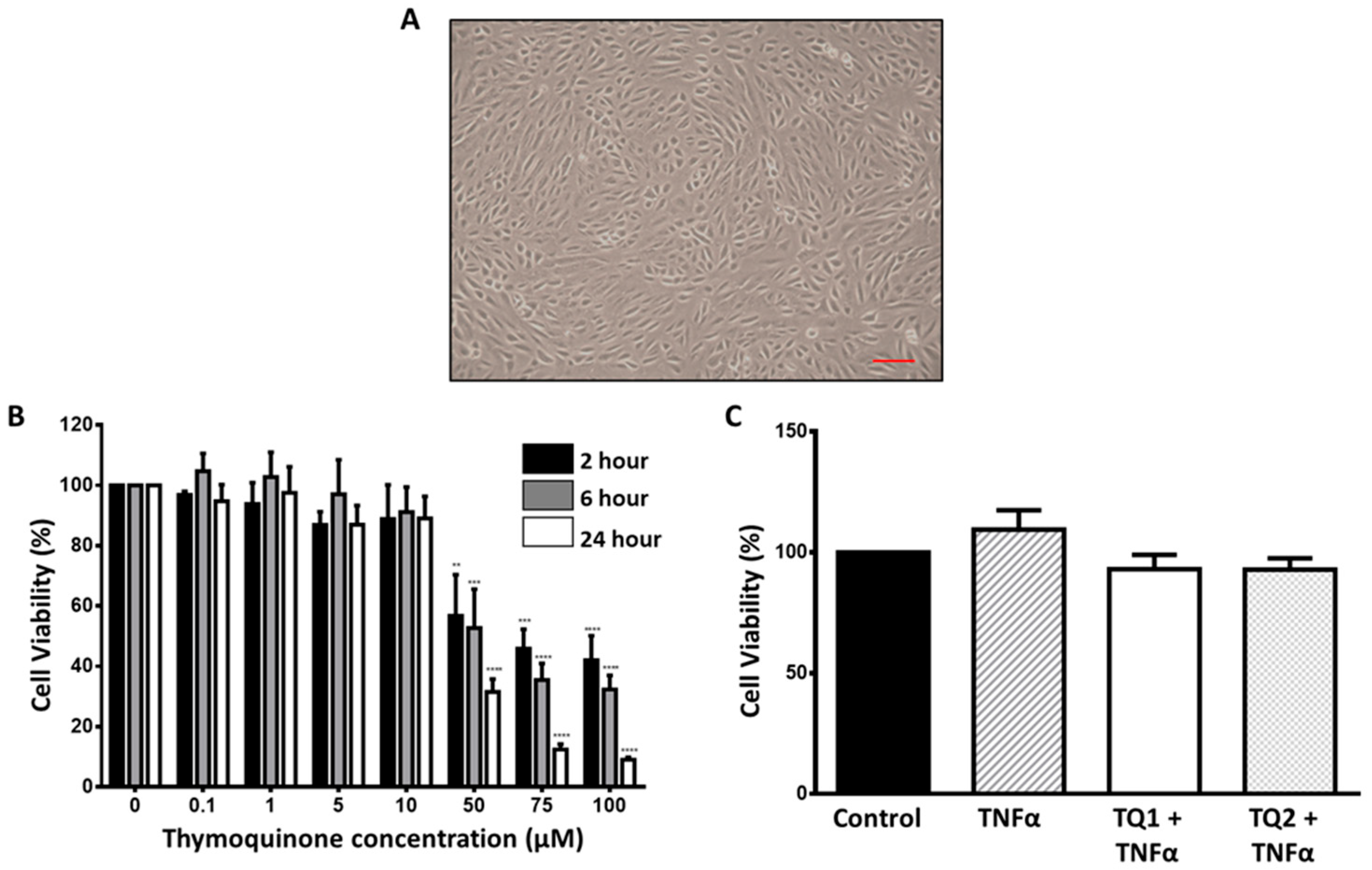
2.1. Dose–Response Effect of Thymoquinone on ARPE-19 Cells
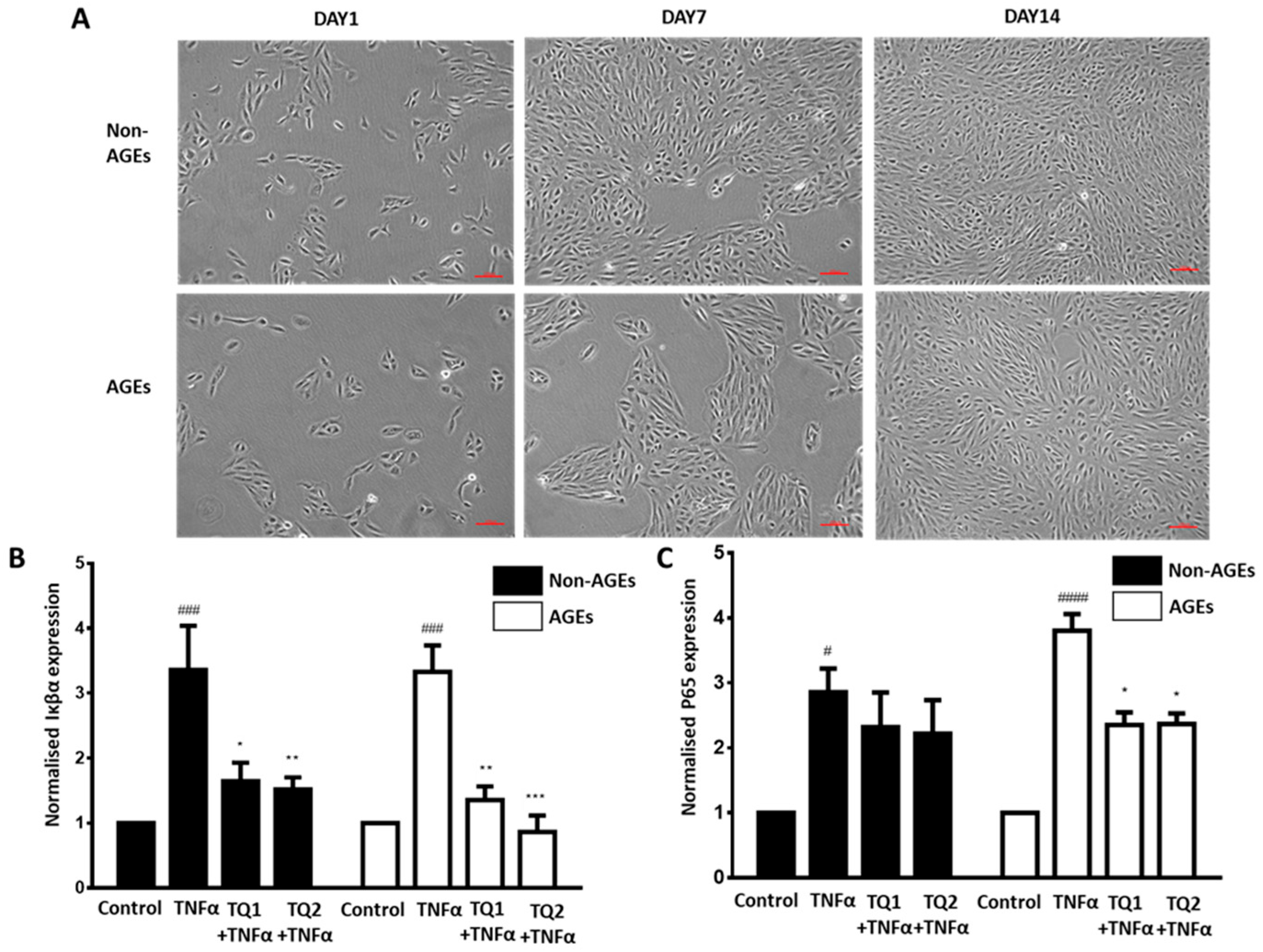
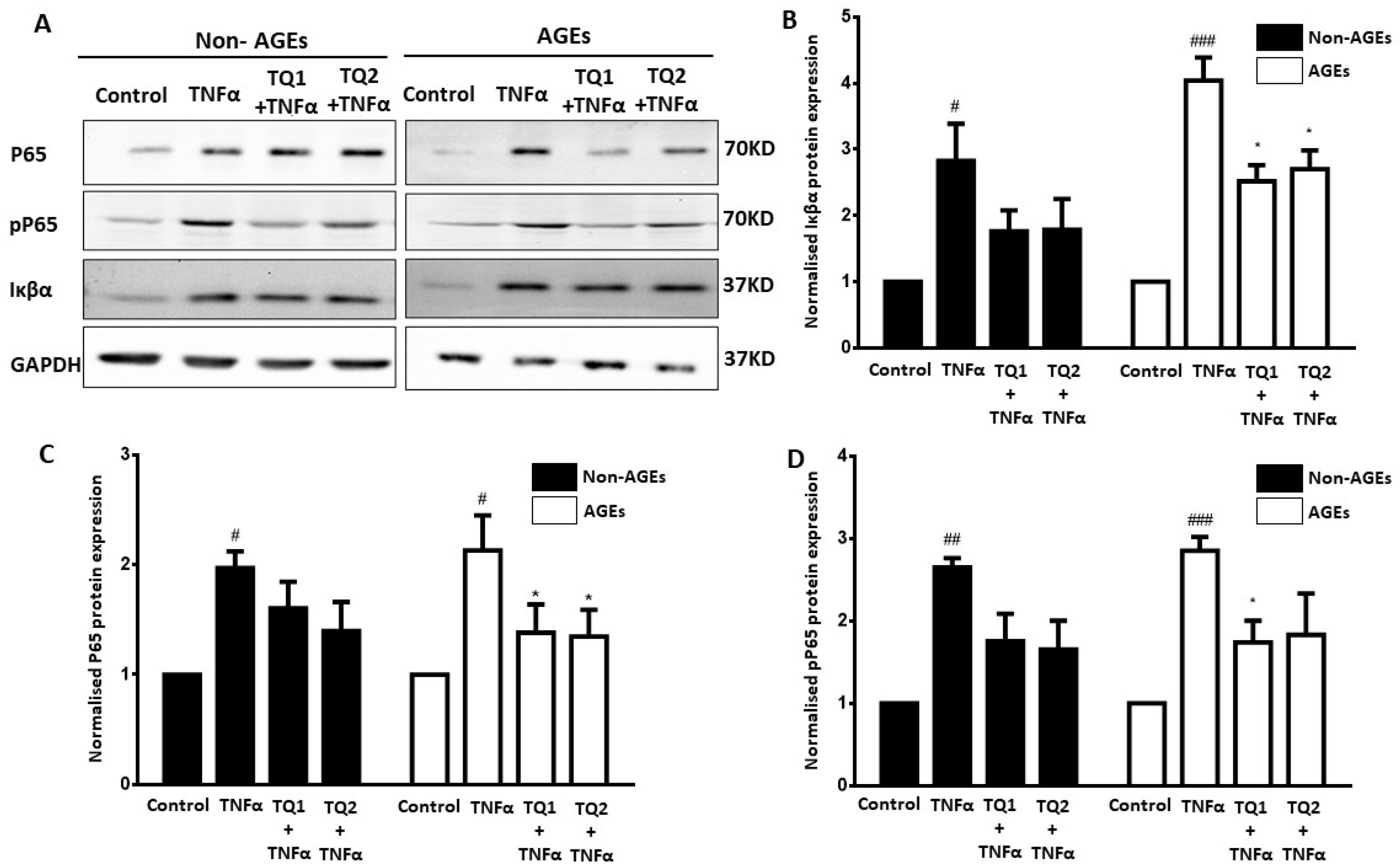
2.2. Effect of Thymoquinone Against TNFα-Induced Oxidative Stress in Ageing ARPE-19 Cells via Modulation of the NF-κβ Pathway
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. RPE Cell Culture and AGE Modification of Extracellular Matrix (ECM)
4.3. Thymoquinone Treatment
4.4. TNFα Treatment
4.5. Cell Viability Assay
4.6. Gene Expression Analysis by Real-Time PCR
4.7. Protein Detection by Western Blotting
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer Disease |
| AGEs | Advanced Glycation Endproducts |
| AMD | Age-related Macular Degeneration |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PD | Parkinson Disease |
| RPE | Retinal Pigment Epithelium |
| SEM | Standard Error of the Mean |
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Flores, R.; Carneiro, Â.; Vieira, M.; Tenreiro, S.; Seabra, M.C. Age-related macular degeneration: Pathophysiology, management, and future perspectives. Ophthalmologica 2021, 244, 495–511. [Google Scholar] [CrossRef]
- Kawasaki, R.; Yasuda, M.; Song, S.J.; Chen, S.-J.; Jonas, J.B.; Wang, J.J.; Mitchell, P.; Wong, T.Y. The prevalence of age-related macular degeneration in Asians: A systematic review and meta-analysis. Ophthalmology 2010, 117, 921–927. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and diseases of the retinal pigment epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- BDomènech, E.; Marfany, G. The relevance of oxidative stress in the pathogenesis and therapy of retinal dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal pigment epithelium and age-related macular degeneration: A review of major disease mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef]
- Stern, J.; Temple, S. Retinal pigment epithelial cell proliferation. Exp. Biol. Med. 2015, 240, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Nociari, M.M.; Kiss, S.; Rodriguez-Boulan, E. Lipofuscin accumulation into and clearance from retinal pigment epithelium lysosomes: Physiopathology and emerging therapeutics. In Lysosomes-Associated Diseases and Methods to Study Their Function; IntechOpen: London, UK, 2017; Volume 28. [Google Scholar]
- Schutt, F.; Bergmann, M.; Holz, F.G.; Kopitz, J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3663–3668. [Google Scholar] [CrossRef]
- Faber, C.; Jehs, T.; Juel, H.B.; Singh, A.; Falk, M.K.; Sørensen, T.L.; Nissen, M.H. Early and exudative age-related macular degeneration is associated with increased plasma levels of soluble TNF receptor II. Acta Ophthalmol. 2015, 93, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Murata, T.; Hangai, M.; Nagai, R.; Horiuchi, S.; Lopez, P.F.; Hinton, D.R.; Ryan, S.J. Advanced glycation end products in age-related macular degeneration. Arch. Ophthalmol. 1998, 116, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Rumble, J.R.; Cooper, M.E.; Soulis, T.; Cox, A.; Wu, L.; Youssef, S.; Jasik, M.; Jerums, G.; Gilbert, R.E. Vascular hypertrophy in experimental diabetes. Role of advanced glycation end products. J. Clin. Investig. 1997, 99, 1016–1027. [Google Scholar] [CrossRef]
- Handa, J.T.; Reiser, K.M.; Matsunaga, H.; Hjelmeland, L.M. The advanced glycation endproduct pentosidine induces the expression of PDGF-B in human retinal pigment epithelial cells. Exp. Eye Res. 1998, 66, 411–419. [Google Scholar] [CrossRef]
- Sun, L.; Huang, T.; Xu, W.; Sun, J.; Lv, Y.; Wang, Y. Advanced glycation end products promote VEGF expression and thus choroidal neovascularization via Cyr61-PI3K/AKT signaling pathway. Sci. Rep. 2017, 7, 14925. [Google Scholar] [CrossRef]
- Schütze, S.; Wiegmann, K.; Machleidt, T.; Krönke, M. TNF-induced activation of NF-κB. Immunobiology 1995, 193, 193–203. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2014; pp. 253–266. [Google Scholar]
- Lin, T.; Walker, G.B.; Kurji, K.; Fang, E.; Law, G.; Prasad, S.S.; Kojic, L.; Cao, S.; White, V.; Cui, J.Z. Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: Implications for age-related degenerative diseases of the eye. Cytokine 2013, 62, 369–381. [Google Scholar] [CrossRef]
- Sharif, U.; Mahmud, N.M.; Kay, P.; Yang, Y.C.; Harding, S.P.; Grierson, I.; Kamalden, T.A.; Jackson, M.J.; Paraoan, L. Advanced glycation end products-related modulation of cathepsin L and NF-κB signalling effectors in retinal pigment epithelium lead to augmented response to TNFα. J. Cell. Mol. Med. 2019, 23, 405–416. [Google Scholar] [CrossRef]
- Han, X.; Gharahkhani, P.; Mitchell, P.; Liew, G.; Hewitt, A.W.; MacGregor, S. Genome-wide meta-analysis identifies novel loci associated with age-related macular degeneration. J. Hum. Genet. 2020, 65, 657–665. [Google Scholar] [CrossRef]
- Armento, A.; Schmidt, T.L.; Sonntag, I.; Merle, D.A.; Jarboui, M.A.; Kilger, E.; Clark, S.J.; Ueffing, M. CFH loss in human RPE cells leads to inflammation and complement system dysregulation via the NF-κB pathway. Int. J. Mol. Sci. 2021, 22, 8727. [Google Scholar] [CrossRef]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg Jr, P.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Koskela, A.; Kaarniranta, K.; Blasiak, J. Dietary Polyphenols in Age-Related Macular Degeneration: Protection against Oxidative Stress and Beyond. Oxid. Med. Cell Longev. 2019, 2019, 9682318. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yu, T.; Zhao, J.; Zhu, X.; Xin, W.; Zhang, F.; Zhang, L. Role of flavonoids in age-related macular degeneration. Biomed. Pharmacother. 2023, 159, 114259. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Rosli, N.; Ichwan, S.J.A.; Mishra, P. Thymoquinone and its pharmacological perspective: A review. Pharmacol. Res.-Mod. Chin. Med. 2021, 1, 100020. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Shahri, A.M.P.; Samini, F. The neuroprotective effects of thymoquinone: A review. Dose-Response 2018, 16, 1559325818761455. [Google Scholar] [CrossRef]
- Siriwattanasatorn, M.; Itharat, A.; Thongdeeying, P.; Ooraikul, B. In vitro wound healing activities of three most commonly used thai medicinal plants and their three markers. Evid.-Based Complement. Altern. Med. 2020, 2020, 6795383. [Google Scholar] [CrossRef]
- Ammar, E.-S.M.; Gameil, N.M.; Shawky, N.M.; Nader, M.A. Comparative evaluation of anti-inflammatory properties of thymoquinone and curcumin using an asthmatic murine model. Int. Immunopharmacol. 2011, 11, 2232–2236. [Google Scholar] [CrossRef]
- Ebrahimi, S.S.; Oryan, S.; Izadpanah, E.; Hassanzadeh, K. Thymoquinone exerts neuroprotective effect in animal model of Parkinson’s disease. Toxicol. Lett. 2017, 276, 108–114. [Google Scholar] [CrossRef]
- Elibol, B.; Beker, M.; Terzioglu-Usak, S.; Dalli, T.; Kilic, U. Thymoquinone administration ameliorates Alzheimer’s disease-like phenotype by promoting cell survival in the hippocampus of amyloid beta1–42 infused rat model. Phytomedicine 2020, 79, 153324. [Google Scholar] [CrossRef]
- Pop, R.M.; Sabin, O.; Suciu, Ș.; Vesa, S.C.; Socaci, S.A.; Chedea, V.S.; Bocsan, I.C.; Buzoianu, A.D. Nigella sativa’s anti-inflammatory and antioxidative effects in experimental inflammation. Antioxidants 2020, 9, 921. [Google Scholar] [CrossRef]
- Hu, X.; Liang, Y.; Zhao, B.; Wang, Y. Thymoquinone protects human retinal pigment epithelial cells against hydrogen peroxide induced oxidative stress and apoptosis. J. Cell. Biochem. 2019, 120, 4514–4522. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, X.; Wang, S.; Xu, C.; Gao, M.; Liu, S.; Li, X.; Cheng, N.; Han, Y.; Wang, X. Thymoquinone prevents dopaminergic neurodegeneration by attenuating oxidative stress via the Nrf2/ARE pathway. Front. Pharmacol. 2021, 11, 615598. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Ahmad, A.; Siddiquei, M.M.; Ghulam, M.; El-Asrar, A.A. Thymoquinone attenuates retinal expression of mediators and markers of neurodegeneration in a diabetic animal model. Curr. Mol. Pharmacol. 2023, 16, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kalamegam, G.; Alfakeeh, S.M.; Bahmaid, A.O.; AlHuwait, E.A.; Gari, M.A.; Abbas, M.M.; Ahmed, F.; Abu-Elmagd, M.; Pushparaj, P.N. In vitro evaluation of the anti-inflammatory effects of thymoquinone in osteoarthritis and in silico analysis of inter-related pathways in age-related degenerative diseases. Front. Cell Dev. Biol. 2020, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- Salama, B.; Alzahrani, K.J.; Alghamdi, K.S.; Al-Amer, O.; Hassan, K.E.; Elhefny, M.A.; Albarakati, A.J.A.; Alharthi, F.; Althagafi, H.A.; Al Sberi, H. Silver nanoparticles enhance oxidative stress, inflammation, and apoptosis in liver and kidney tissues: Potential protective role of thymoquinone. Biol. Trace Elem. Res. 2023, 201, 2942–2954. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Targeting nuclear factor-κB activation pathway by thymoquinone: Role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol. Cancer Res. 2008, 6, 1059–1070, Erratum in Mol Cancer Res. 2018, 16, 1441. [Google Scholar] [CrossRef] [PubMed]
- Usta, A.; Dede, S. The effect of thymoquinone on nuclear factor kappa B levels and oxidative DNA damage on experimental diabetic rats. Pharmacogn. Mag. 2017, 13 (Suppl. S3), S458. [Google Scholar] [CrossRef]
- Savran, M.; Ascı, H.; Armagan, İ.; Erzurumlu, Y.; Azırak, S.; Kaya Ozer, M.; Bilgic, S.; Korkmaz, D.T. Thymoquinone could be protective against valproic acid-induced testicular toxicity by antioxidant and anti-inflammatory mechanisms. Andrologia 2020, 52, e13623. [Google Scholar] [CrossRef]
- Majka, S.; McGuire, P.G.; Das, A. Regulation of matrix metalloproteinase expression by tumor necrosis factor in a murine model of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2002, 43, 260–266. [Google Scholar]
- Al-Gayyar, M.; Elsherbiny, N. Contribution of TNF-α to the development of retinal neurodegenerative disorders. Eur. Cytokine Netw. 2013, 24, 27–36. [Google Scholar] [CrossRef]
- Chernykh, V.; Shevchenko, A.; Konenkov, V.; Prokofiev, V.; Eremina, A.; Trunov, A. TNF-α gene polymorphisms: Association with age-related macular degeneration in Russian population. Int. J. Ophthalmol. 2019, 12, 25. [Google Scholar]
- Brasier, A.R. The NF-κB regulatory network. Cardiovasc. Toxicol. 2006, 6, 111–130. [Google Scholar] [CrossRef]
- Gao, J.; Cui, J.Z.; To, E.; Cao, S.; Matsubara, J.A. Evidence for the activation of pyroptotic and apoptotic pathways in RPE cells associated with NLRP3 inflammasome in the rodent eye. J. Neuroinflamm. 2018, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Muangnoi, C.; Phumsuay, R.; Jongjitphisut, N.; Waikasikorn, P.; Sangsawat, M.; Rashatasakhon, P.; Paraoan, L.; Rojsitthisak, P. Protective effects of a lutein ester prodrug, lutein diglutaric acid, against H2O2-induced oxidative stress in human retinal pigment epithelial cells. Int. J. Mol. Sci. 2021, 22, 4722. [Google Scholar] [CrossRef] [PubMed]
- Muangnoi, C.; Sharif, U.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Paraoan, L. Protective effects of curcumin ester prodrug, curcumin diethyl disuccinate against H2O2-induced oxidative stress in human retinal pigment epithelial cells: Potential therapeutic avenues for age-related macular degeneration. Int. J. Mol. Sci. 2019, 20, 3367. [Google Scholar] [CrossRef] [PubMed]
- Hytti, M.; Piippo, N.; Korhonen, E.; Honkakoski, P.; Kaarniranta, K.; Kauppinen, A. Fisetin and luteolin protect human retinal pigment epithelial cells from oxidative stress-induced cell death and regulate inflammation. Sci. Rep. 2015, 5, 17645. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Huang, W.-C.; S Pang, J.-H.; Wu, Y.-H.; Cheng, C.-Y. Quercetin inhibits the production of IL-1β-induced inflammatory cytokines and chemokines in ARPE-19 cells via the MAPK and NF-κB signaling pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.J.; Lee, E.K.; Park, S.W.; Yu, H.G. Antioxidative effects of ascorbic acid and astaxanthin on arpe-19 cells in an oxidative stress model. Antioxidants 2020, 9, 833. [Google Scholar] [CrossRef]
- Kocatürk, T.; Erkan, E.; Meteoğlu, İ.; Ekici, M.; Büyüköztürk, A.K.; Yavaşoğlu, İ.; Çakmak, H.; Dayanır, V.; Balkaya, M. Effects of Topical Thymoquinone in an Experimental Dry Eye Model. Turk. J. Ophthalmol. 2018, 48, 281. [Google Scholar] [CrossRef]
- Hayat, K.; Asim, M.R.; Nawaz, M.; Li, M.; Zhang, L.; Sun, N. Ameliorative effect of thymoquinone on ovalbumin-induced allergic conjunctivitis in Balb/c mice. Curr. Eye Res. 2011, 36, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.M.; Saad, E.A.E.-M.S.; Sabra, N.M.; El-Gohary, A.A.; Mohamed, F.F.; Gaber, M.H. Treatment merits of latanoprost/thymoquinone–encapsulated liposome for glaucomatus rabbits. Int. J. Pharm. 2018, 548, 597–608. [Google Scholar] [CrossRef]
- Mohamed, A.; Afridi, D.; Garani, O.; Tucci, M. Thymoquinone inhibits the activation of NF-kappaB in the brain and spinal cord of experimental autoimmune encephalomyelitis. Biomed. Sci. Instrum. 2005, 41, 388–393. [Google Scholar]
- Mahmud, N.M.; Paraoan, L.; Khaliddin, N.; Kamalden, T.A. Thymoquinone in ocular neurodegeneration: Modulation of pathological mechanisms via multiple pathways. Front. Cell. Neurosci. 2022, 16, 786926. [Google Scholar] [CrossRef]
- Xue, B.; DasGupta, D.; Alam, M.; Khan, M.S.; Wang, S.; Shamsi, A.; Islam, A.; Hassan, M.I. Investigating binding mechanism of thymoquinone to human transferrin, targeting Alzheimer’s disease therapy. J. Cell. Biochem. 2022, 123, 1381–1393. [Google Scholar] [CrossRef]
- Abo Mansour, H.E.; Elberri, A.I.; Ghoneim, M.E.-S.; Samman, W.A.; Alhaddad, A.A.; Abdallah, M.S.; El-Berri, E.I.; Salem, M.A.; Mosalam, E.M. The Potential Neuroprotective Effect of Thymoquinone on Scopolamine-Induced In Vivo Alzheimer’s Disease-like Condition: Mechanistic Insights. Molecules 2023, 28, 6566. [Google Scholar] [CrossRef]
- Ardah, M.T.; Merghani, M.M.; Haque, M.E. Thymoquinone prevents neurodegeneration against MPTP in vivo and modulates α-synuclein aggregation in vitro. Neurochem. Int. 2019, 128, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Alhebshi, A.; Gotoh, M.; Suzuki, I. Thymoquinone protects cultured rat primary neurons against amyloid β-induced neurotoxicity. Biochem. Biophys. Res. Commun. 2013, 433, 362–367. [Google Scholar] [CrossRef]
- Abulfadl, Y.; El-Maraghy, N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ-activated BV-2 microglia cells. J. Neuroimmunol. 2018, 320, 87–97. [Google Scholar] [CrossRef]
- Thomas, J.V.; Mohan, M.; Prabhakaran, P.; Maliakel, B.; Krishnakumar, I. A phase I clinical trial to evaluate the safety of thymoquinone-rich black cumin oil (BlaQmax®) on healthy subjects: Randomized, double-blinded, placebo-controlled prospective study. Toxicol. Rep. 2022, 9, 999–1007. [Google Scholar] [CrossRef]
- Badary, O.A.; Al-Shabanah, O.A.; Nagi, M.N.; Al-Bekairi, A.M.; Elmazar, M. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev. Res. 1998, 44, 56–61. [Google Scholar] [CrossRef]
- Mashayekhi-Sardoo, H.; Rezaee, R.; Karimi, G. An overview of in vivo toxicological profile of thymoquinone. Toxin Rev. 2020, 39, 115–122. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Mahdian, V.; Razavi, B.M.; Hosseinzadeh, H. Review on Clinical Trials of Black Seed (Nigella sativa ) and Its Active Constituent, Thymoquinone. J. Pharmacopunct. 2017, 20, 179–193. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Zhang, W.; Zhang, W.; Fang, L. Thymoquinone inhibits lipopolysaccharide-induced inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 2015, 26, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, R.; Kumar, A.; Bhatia, H.S.; El-Bakoush, A.; Lepiarz, I.; Fiebich, B.L.; Olajide, O.A. Inhibition of neuroinflammation by thymoquinone requires activation of Nrf2/ARE signalling. Int. Immunopharmacol. 2017, 48, 17–29. [Google Scholar] [CrossRef] [PubMed]
- El Gazzar, M.A.; El Mezayen, R.; Nicolls, M.R.; Dreskin, S.C. Thymoquinone attenuates proinflammatory responses in lipopolysaccharide-activated mast cells by modulating NF-kappaB nuclear transactivation. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2007, 1770, 556–564. [Google Scholar] [CrossRef]
- Al-Malki, A.L.; Sayed, A.A.R. Thymoquinone attenuates cisplatin-induced hepatotoxicity via nuclear factor kappa-β. BMC Complement. Altern. Med. 2014, 14, 282. [Google Scholar] [CrossRef]
- Nagineni, C.N.; Kommineni, V.K.; William, A.; Detrick, B.; Hooks, J.J. Regulation of VEGF expression in human retinal cells by cytokines: Implications for the role of inflammation in age-related macular degeneration. J. Cell. Physiol. 2012, 227, 116–126. [Google Scholar] [CrossRef]
- Sayed, A.A.R.; Morcos, M. Thymoquinone decreases AGE-induced NF-κB activation in proximal tubular epithelial cells. Phytother. Res. 2007, 21, 898–899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bai, Y.; Yang, Y. Thymoquinone chemosensitizes colon cancer cells through inhibition of NF-κB. Oncol. Lett. 2016, 12, 2840–2845. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Yang, W.S.; Kim, D.; Aravinthan, A.; Kim, J.-H.; Cho, J.Y. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci. Rep. 2017, 7, 42995. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F. Thymoquinone inhibits bone metastasis of breast cancer cells through abrogation of the CXCR4 signaling axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- El Gazzar, M. Thymoquinone suppressses in vitro production of IL-5 and IL-13 by mast cells in response to lipopolysaccharide stimulation. Inflamm. Res. 2007, 56, 345–351. [Google Scholar] [CrossRef]
| Primer | Template | 5′→3′ Sequence |
|---|---|---|
| Iĸβα | Forward | GAAGTGATCCGCCAGGTGAA |
| Reverse | CTCACAGGCAAGGTGTAGGG | |
| p65 | Forward | CCAGACCAACAACAACCCCT |
| Reverse | TCACTCGGCAGATCTTGAGC | |
| β- actin | Forward | CACCATTGGCAATGAGCGGTTC |
| Reverse | AGGTCTTTGCGGATGTCCACGT | |
| β- tubulin | Forward | CTGGACCGCATCTCTGTGTACT |
| Reverse | GCCAAAAGGACCTGAGCGAACA |
| Antibody | Dilution |
|---|---|
| Anti NF-kB P65 | 1:500 |
| Anti Phospho-NF-kB P65 (Ser536) | 1:500 |
| Anti IKB-α | 1:500 |
| Anti GAPDH | 1:10,000 |
| Secondary horseradish peroxidase (HRP)-conjugated anti-rabbit | 1:1000 |
| Secondary horseradish peroxidase (HRP)- conjugated anti-mouse | 1:2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, N.M.; Paraoan, L.; Kamalden, T.A. Thymoquinone Attenuates NF-κβ Signalling Activation in Retinal Pigment Epithelium Cells Under AMD-Mimicking Conditions. Int. J. Mol. Sci. 2025, 26, 11473. https://doi.org/10.3390/ijms262311473
Mahmud NM, Paraoan L, Kamalden TA. Thymoquinone Attenuates NF-κβ Signalling Activation in Retinal Pigment Epithelium Cells Under AMD-Mimicking Conditions. International Journal of Molecular Sciences. 2025; 26(23):11473. https://doi.org/10.3390/ijms262311473
Chicago/Turabian StyleMahmud, Nur Musfirah, Luminita Paraoan, and Tengku Ain Kamalden. 2025. "Thymoquinone Attenuates NF-κβ Signalling Activation in Retinal Pigment Epithelium Cells Under AMD-Mimicking Conditions" International Journal of Molecular Sciences 26, no. 23: 11473. https://doi.org/10.3390/ijms262311473
APA StyleMahmud, N. M., Paraoan, L., & Kamalden, T. A. (2025). Thymoquinone Attenuates NF-κβ Signalling Activation in Retinal Pigment Epithelium Cells Under AMD-Mimicking Conditions. International Journal of Molecular Sciences, 26(23), 11473. https://doi.org/10.3390/ijms262311473





