Protective Effects of Lindera obtusiloba Leaf Extract on Osteoarthritis in Mouse Primary Chondrocytes and a Medial Meniscus Destabilization Model
Abstract
1. Introduction
2. Results
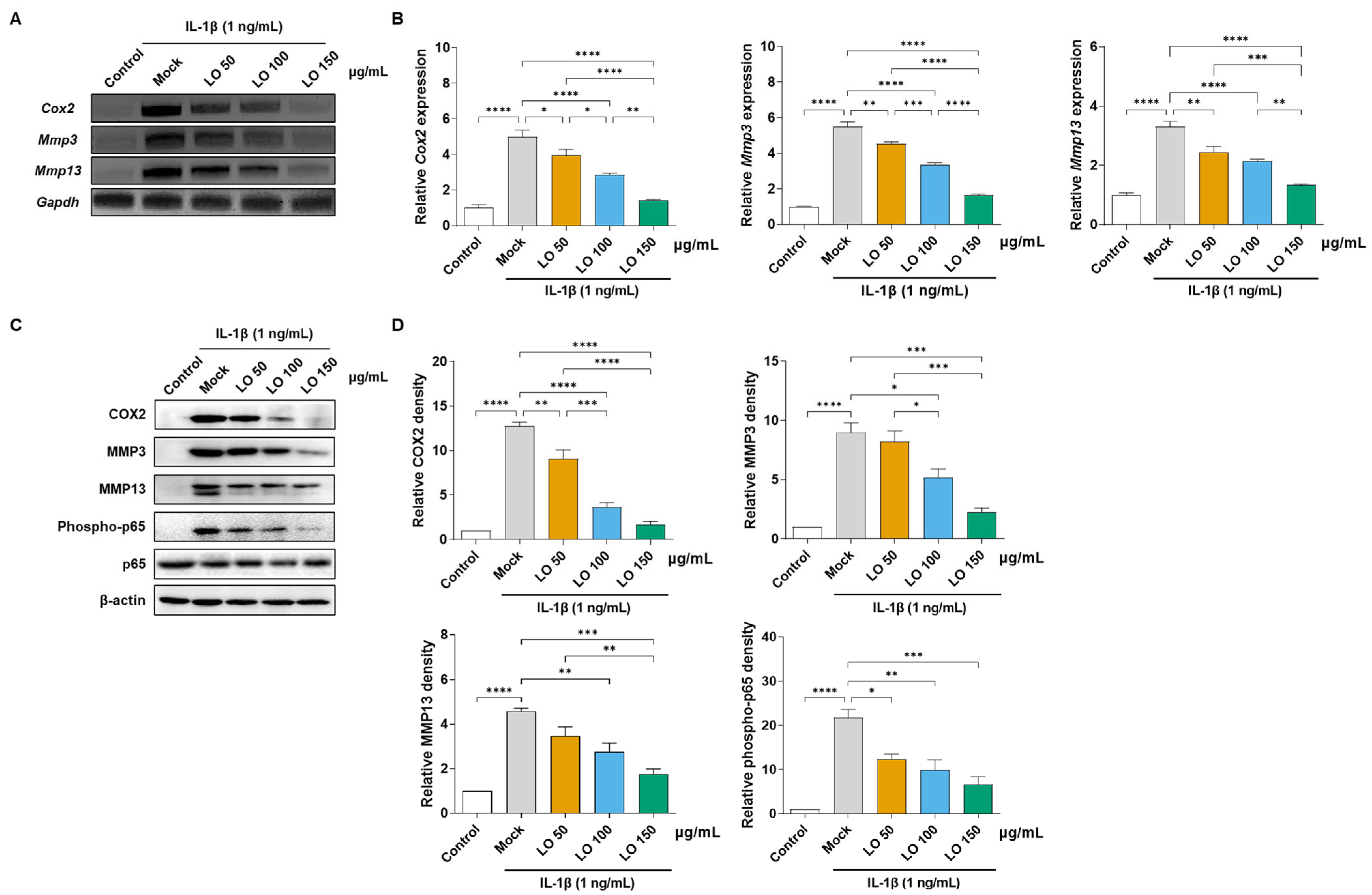
2.1. LO Leaf Extract Attenuates IL-1β-Induced Inflammatory Responses
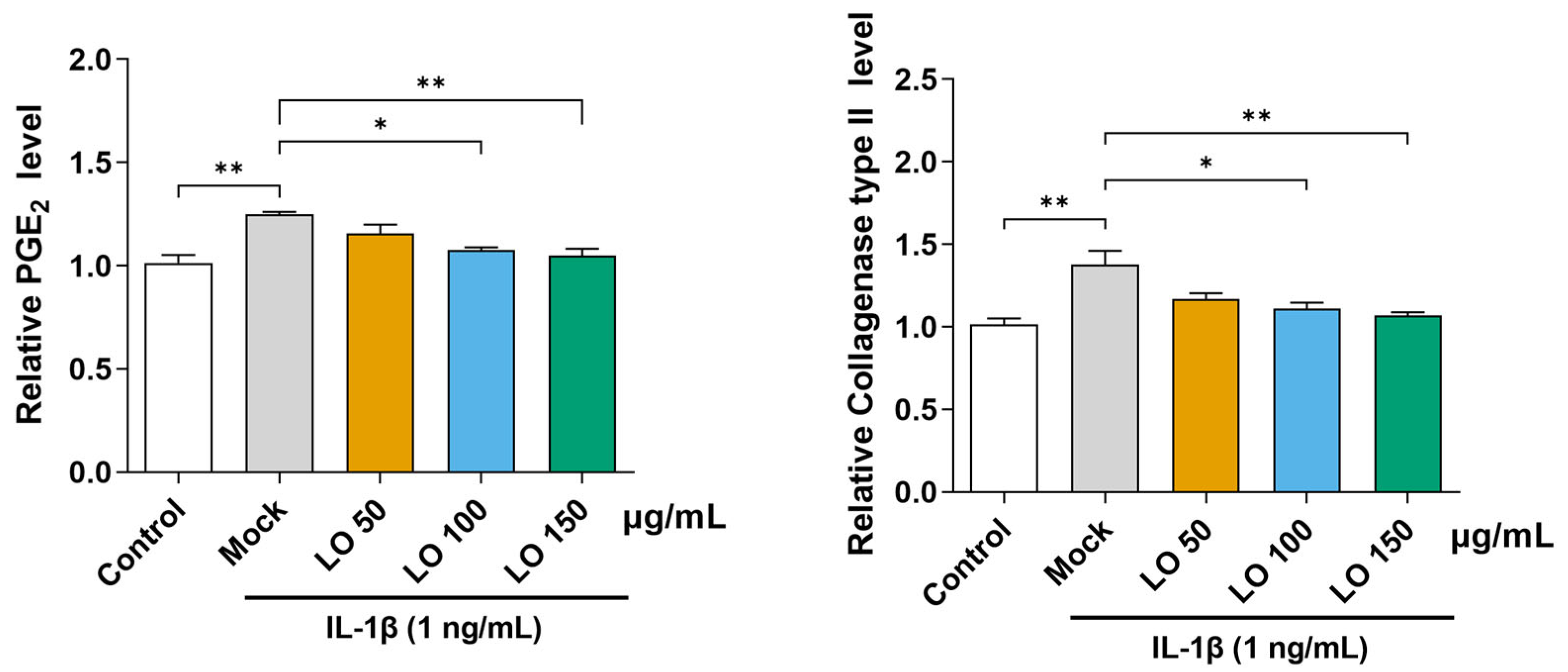
2.2. LO Leaf Extract Reduces IL-1β-Induced Secretion of PGE2 and Collagenase in Primary Chondrocytes
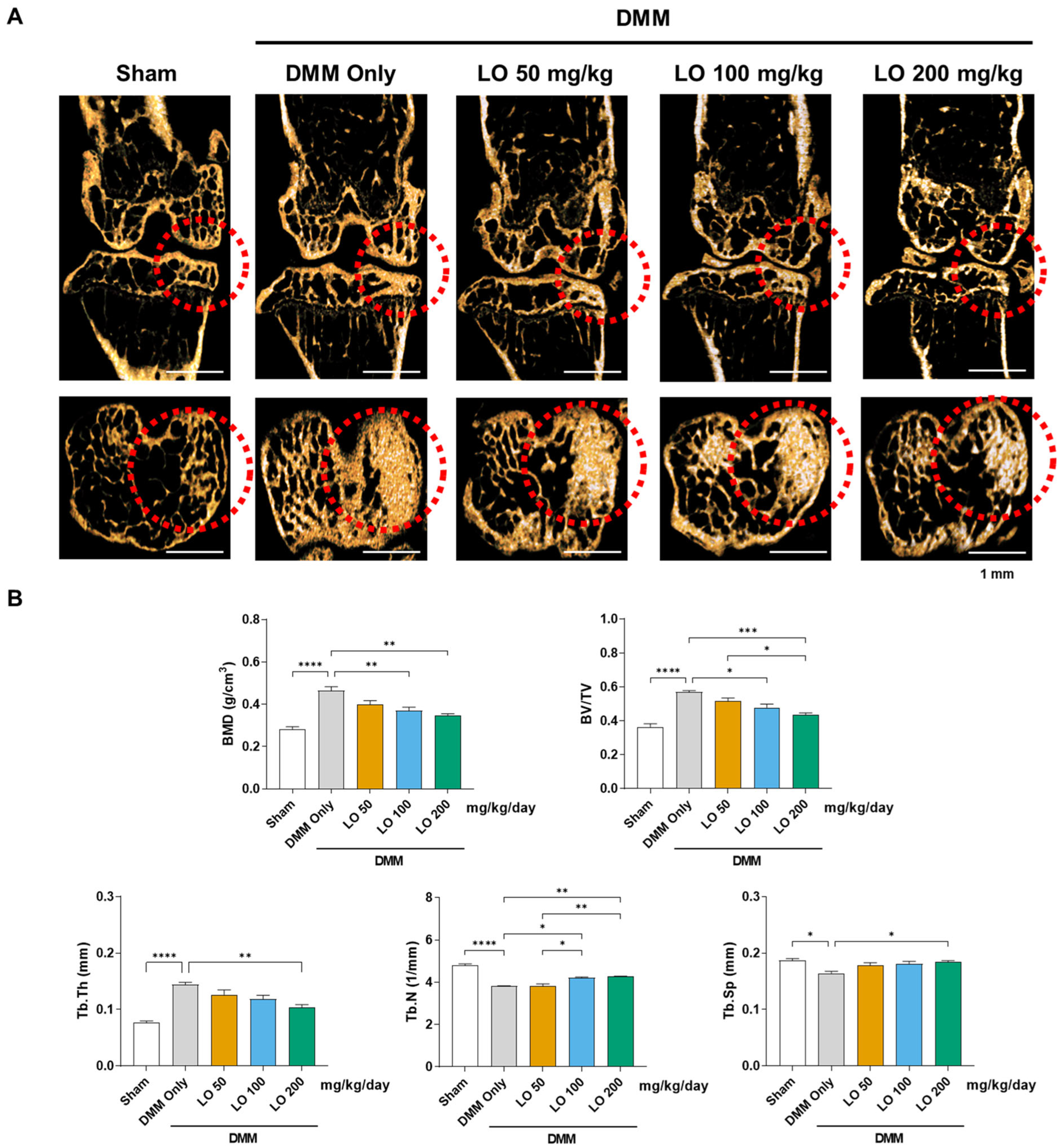
2.3. LO Leaf Extract Reduces Subchondral Sclerosis in a DMM-Induced OA Mouse Model
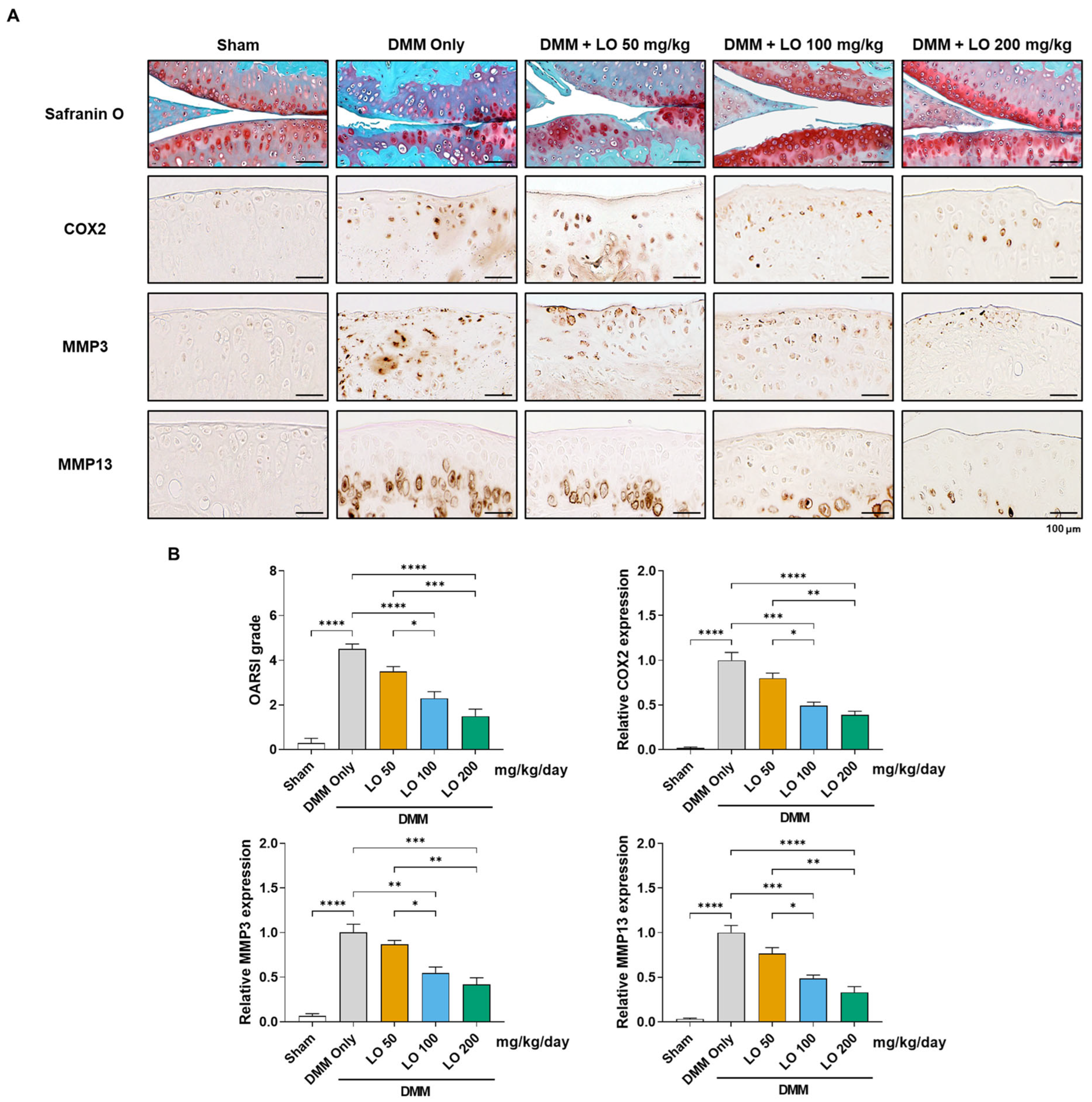
2.4. Histological Assessment of Cartilage Protection by LO Leaf Extract in a DMM-Induced OA Mouse Model
2.5. Modulatory Effect of LO Leaf Extract on Systemic Inflammatory Cytokines in a DMM-Induced OA Mouse Model
3. Discussion
4. Materials and Methods
4.1. Extraction and Identification of Quercitrin from LO Leaf Extract
4.2. Primary Culture of Chondrocytes from Mouse Knee Cartilage
4.3. Water-Soluble Tetrazolium Salt Assay
4.4. Quantitative Real-Time Polymerase Chain Reaction Analysis
4.5. Western Blot Analysis
4.6. PGE2 and Collagenase Quantification
4.7. DMM Mouse Model
4.8. Micro-CT Analysis
4.9. Histology (Safranin O Staining and Immunohistochemistry)
4.10. Cytokine Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMD | bone mineral density |
| COX2 | cyclooxygenase II |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMM | destabilization of medial meniscus |
| EDTA | ethylenediaminetetraacetic acid |
| IκB | inhibitory kappa B |
| IL | interleukin |
| IFN-γ | interferon gamma |
| LO | Lindera obtusiloba |
| MAPK | mitogen-activated protein kinase |
| micro-CT | micro-computed tomography |
| MMPs | metalloproteinases |
| NF-κB | nuclear factor-kappa B |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| OA | osteoarthritis |
| OARSI | Osteoarthritis Research Society International |
| PGE2 | prostaglandin E2 |
| TNF-α | tumor necrosis factor-α |
References
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain. Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Kouki, I.; Soliman, N.; Mathieu, S.; Sellam, J. Osteoarthritis year in review 2024: Epidemiology and therapy. Osteoarthr. Cartil. 2024, 32, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Abulhasan, J.; Grey, M. Anatomy and Physiology of Knee Stability. J. Funct. Morphol. Kinesiol. 2017, 2, 34. [Google Scholar] [CrossRef]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef]
- Blalock, D.; Miller, A.; Tilley, M.; Wang, J. Joint instability and osteoarthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015, 8, 15–23. [Google Scholar] [CrossRef]
- Twomey-Kozak, J.; Jayasuriya, C.T. Meniscus Repair and Regeneration: A Systematic Review from a Basic and Translational Science Perspective. Clin. Sports Med. 2020, 39, 125–163. [Google Scholar] [CrossRef]
- Hassebrock, J.D.; Gulbrandsen, M.T.; Asprey, W.L.; Makovicka, J.L.; Chhabra, A. Knee Ligament Anatomy and Biomechanics. Sports Med. Arthrosc. Rev. 2020, 28, 80–86. [Google Scholar] [CrossRef]
- Kittl, C.; Inderhaug, E.; Williams, A.; Amis, A.A. Biomechanics of the Anterolateral Structures of the Knee. Clin. Sports Med. 2018, 37, 21–31. [Google Scholar] [CrossRef]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.G.; Seale, P.; Furman, D. The infrapatellar fat pad in inflammaging, knee joint health, and osteoarthritis. npj Aging 2024, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Anderson, D.D.; Brown, T.D.; Tochigi, Y.; Martin, J.A. The Roles of Mechanical Stresses in the Pathogenesis of Osteoarthritis: Implications for Treatment of Joint Injuries. Cartilage 2013, 4, 286–294. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1beta signaling in osteoarthritis—Chondrocytes in focus. Cell Signal 2019, 53, 212–223. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Clockaerts, S.; Bastiaansen-Jenniskens, Y.M.; Runhaar, J.; Van Osch, G.J.; Van Offel, J.; Verhaar, J.; De Clerck, L.; Somville, J. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: A narrative review. Osteoarthr. Cartil. 2010, 18, 876–882. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The family of the interleukin-1 receptors. Immunol. Rev. 2018, 281, 197–232. [Google Scholar] [CrossRef]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-κB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Chae, S.W. Function and Activation of NF-kappa B in Immune System. Korean J. Otorhinolaryngol.-Head. Neck Surg. 2005, 48, 284–288. [Google Scholar]
- Nennig, S.E.; Schank, J.R. The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol. Alcohol. 2017, 52, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, A.; Yang, M.; Chen, S.; Deng, Z.; Li, L. p38MAPK Signaling Pathway in Osteoarthritis: Pathological and Therapeutic Aspects. J. Inflamm. Res. 2022, 15, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, D.; Hu, Z.; Xuan, J.; Ding, X.; Zheng, G.; Feng, Z.; Ni, W.; Wu, A. The Protective Effect of Ligustilide in Osteoarthritis: An in Vitro and in Vivo Study. Cell Physiol. Biochem. 2018, 48, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Wang, C.; Meng, Q. Global Research Trends of Herbal Medicine for Pain in Three Decades (1990–2019): A Bibliometric Analysis. J. Pain. Res. 2021, 14, 1611–1626. [Google Scholar] [CrossRef]
- Panickar, K.S. Beneficial effects of herbs, spices and medicinal plants on the metabolic syndrome, brain and cognitive function. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 13–29. [Google Scholar] [CrossRef]
- Cao, Y.; Xuan, B.; Peng, B.; Li, C.; Chai, X.; Tu, P. The genus Lindera: A source of structurally diverse molecules having pharmacological significance. Phytochem. Rev. 2015, 15, 869–906. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Kang, S.; Moon, H.; Chung, K.H.; Kim, K.R. Antiplatelet and Antithrombotic Effects of the Extract of Lindera obtusiloba Leaves. Biomol. Ther. 2016, 24, 659–664. [Google Scholar] [CrossRef]
- Hong, C.O.; Rhee, C.H.; Won, N.H.; Choi, H.D.; Lee, K.W. Protective effect of 70% ethanolic extract of Lindera obtusiloba Blume on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem. Toxicol. 2013, 53, 214–220. [Google Scholar] [CrossRef]
- Freise, C.; Erben, U.; Neuman, U.; Kim, K.; Zeitz, M.; Somasundaram, R.; Ruehl, M. An active extract of Lindera obtusiloba inhibits adipogenesis via sustained Wnt signaling and exerts anti-inflammatory effects in the 3T3-L1 preadipocytes. J. Nutr. Biochem. 2010, 21, 1170–1177. [Google Scholar] [CrossRef]
- Kim, S.H.; Son, J.H.; Lee, S.H. Inhibitory effects of water extract of Lindera obtusiloba on the mast cell-mediated allergic inflammation. Korean J. Pharmacogn. 2009, 40, 233–237. [Google Scholar]
- Choi, E.J.; Lee, S.; Kim, H.H.; Singh, T.S.; Choi, J.K.; Choi, H.G.; Suh, W.M.; Lee, S.H.; Kim, S.H. Suppression of dust mite extract and 2,4-dinitrochlorobenzene-induced atopic dermatitis by the water extract of Lindera obtusiloba. J. Ethnopharmacol. 2011, 137, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhang, B.; Xiang, W.; Zheng, L.; Wang, X.; Li, S.; Zhang, T.; Feng, D.; Gong, Y.; Wu, J.; et al. Natural products in osteoarthritis treatment: Bridging basic research to clinical applications. Chin. Med. 2024, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Knights, A.J.; Redding, S.J.; Maerz, T. Inflammation in osteoarthritis: The latest progress and ongoing challenges. Curr. Opin. Rheumatol. 2023, 35, 128–134. [Google Scholar] [CrossRef]
- Geng, R.; Li, J.; Yu, C.; Zhang, C.; Chen, F.; Chen, J.; Ni, H.; Wang, J.; Kang, K.; Wei, Z.; et al. Knee osteoarthritis: Current status and research progress in treatment (Review). Exp. Ther. Med. 2023, 26, 481. [Google Scholar] [CrossRef] [PubMed]
- Salis, Z.; Sainsbury, A. Association of long-term use of non-steroidal anti-inflammatory drugs with knee osteoarthritis: A prospective multi-cohort study over 4-to-5 years. Sci. Rep. 2024, 14, 6593. [Google Scholar] [CrossRef] [PubMed]
- Samy, B.A.; Raman, K.; Velayutham, S.; Senthilkumar, N.; Thirumalaivasan, N.; Kanagaraj, K.; Pothu, R.; Boddula, R.; Radwan, A.B.; Al-Qahtani, N. Natural product extract fractions as potential arthritis treatments: A detailed analysis using in-silico, in-vivo, and in-vitro methods. Int. Immunopharmacol. 2025, 144, 113595. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kurth, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A.; Ossendorff, R. Ossendorff, TNFα-related chondrocyte inflammation models: A systematic review. Int. J. Mol. Sci. 2024, 25, 10805. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Z.; Shen, M.; Ma, Y.; Li, R.; Jin, X.; Gao, L.; Wang, Z. Changes of type II collagenase biomarkers on IL-1β-induced rat articular chondrocytes. Exp. Ther. Med. 2021, 21, 582. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the War Against Inflammation with Natural Products. Front. Pharmacol. 2018, 9, 976. [Google Scholar] [CrossRef]
- Ling, H.; Zeng, Q.; Ge, Q.; Chen, J.; Yuan, W.; Xu, R.; Shi, Z.; Xia, H.; Hu, S.; Jin, H.; et al. Osteoking Decelerates Cartilage Degeneration in DMM-Induced Osteoarthritic Mice Model Through TGF-β/smad-dependent Manner. Front. Pharmacol. 2021, 12, 678810. [Google Scholar] [CrossRef]
- Rösch, G.; El Bagdadi, K.; Muschter, D.; Taheri, S.; Dorn, C.; Meurer, A.; Straub, R.H.; Zaucke, F.; Schilling, A.F.; Grässel, S.; et al. Sympathectomy aggravates subchondral bone changes during osteoarthritis progression in mice without affecting cartilage degeneration or synovial inflammation. Osteoarthr. Cartil. 2022, 30, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Ziemian, S.N.; Witkowski, A.M.; Wright, T.M.; Otero, M.; van der Meulen, M.C.H. Early inhibition of subchondral bone remodeling slows load-induced posttraumatic osteoarthritis development in mice. J. Bone Miner. Res. 2021, 36, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Onuora, S. Bone-modifying drugs slow OA progression. Nat. Rev. Rheumatol. 2024, 20, 319. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, G.; Li, X.; Qiu, X.; Ouyang, J.; Dai, J.; Min, S. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiation. Front. Physiol. 2021, 12, 663978. [Google Scholar] [CrossRef]
- Lee, Y.T.; Yunus, M.H.M.; Ugusman, A.; Yazid, M.D. Natural Compounds Affecting Inflammatory Pathways of Osteoarthritis. Antioxidants 2022, 11, 1722. [Google Scholar] [CrossRef]
- Kuang, S.; Liu, L.; Hu, Z.; Luo, M.; Fu, X.; Lin, C.; He, Q. A review focusing on the benefits of plant-derived polysaccharides for osteoarthritis. Int. J. Biol. Macromol. 2023, 228, 582–593. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Zhang, H.; Wu, W.; Xue, S.; Zhu, Z.; Ding, C. Inflammatory Mechanisms Underlying Metabolic Syndrome-associated Osteoarthritis and Potential Treatments. Osteoarthr. Cartil. Open 2025, 7, 100614. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Bentley, E.R.; Little, S.R. Local delivery strategies to restore immune homeostasis in the context of inflammation. Adv. Drug Deliv. Rev. 2021, 178, 113971. [Google Scholar] [CrossRef]
- Moudgil, K.D.; Venkatesha, S.H. The Anti-Inflammatory and Immunomodulatory Activities of Natural Products to Control Autoimmune Inflammation. Int. J. Mol. Sci. 2022, 24, 95. [Google Scholar] [CrossRef] [PubMed]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a flavonoid with great pharmacological capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef]
- Mattiuzzo, E.; Faggian, A.; Venerando, R.; Benetti, A.; Belluzzi, E.; Abatangelo, G.; Ruggieri, P.; Brun, P. In vitro effects of low doses of β-caryophyllene, ascorbic acid and d-glucosamine on human chondrocyte viability and inflammation. Pharmaceuticals 2021, 14, 286. [Google Scholar] [CrossRef]
- Park, E.; Lee, C.G.; Yun, S.H.; Hwang, S.; Jeon, H.; Kim, J.; Yeo, S.; Jeong, H.; Yun, S.H.; Jeong, S.Y. Ameliorative Effects of Loganin on Arthritis in Chondrocytes and Destabilization of the Medial Meniscus-Induced Animal Model. Pharmaceuticals 2021, 14, 135. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.; Ostergaard, K.; Pelletier, J.-P.; Revell, P.; Salter, D.; Van den Berg, W. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| Gapdh | TCA CTG CCA CCC AGA C | TGT AGG CCA TGA GGT CCA C |
| Cox2 | GGT CTG GTG CCT GGT CTG ATG AT | GTC CTT TCA AGG AGA ATG GTG C |
| Mmp3 | CTG TGT GTG GTT GTG TGC TCA TCC TAC | GGC AAA TCC GGT GTA TAA TTC ACA ATC |
| Mmp13 | TGA TGG ACC TTC TGG TCT TCT GGC | CAT CCA CAT GGT TGG GAA GTT CTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, K.-I.; Bae, M.H.; Jeong, J.; Hwang, S.; Park, J.; Kwon, H.-W.; Park, E.; Jeong, S.-Y. Protective Effects of Lindera obtusiloba Leaf Extract on Osteoarthritis in Mouse Primary Chondrocytes and a Medial Meniscus Destabilization Model. Int. J. Mol. Sci. 2025, 26, 9877. https://doi.org/10.3390/ijms26209877
Oh K-I, Bae MH, Jeong J, Hwang S, Park J, Kwon H-W, Park E, Jeong S-Y. Protective Effects of Lindera obtusiloba Leaf Extract on Osteoarthritis in Mouse Primary Chondrocytes and a Medial Meniscus Destabilization Model. International Journal of Molecular Sciences. 2025; 26(20):9877. https://doi.org/10.3390/ijms26209877
Chicago/Turabian StyleOh, Kang-Il, Mun Hyoung Bae, Junhwan Jeong, Seokjin Hwang, Jonggyu Park, Hyun-Woo Kwon, Eunkuk Park, and Seon-Yong Jeong. 2025. "Protective Effects of Lindera obtusiloba Leaf Extract on Osteoarthritis in Mouse Primary Chondrocytes and a Medial Meniscus Destabilization Model" International Journal of Molecular Sciences 26, no. 20: 9877. https://doi.org/10.3390/ijms26209877
APA StyleOh, K.-I., Bae, M. H., Jeong, J., Hwang, S., Park, J., Kwon, H.-W., Park, E., & Jeong, S.-Y. (2025). Protective Effects of Lindera obtusiloba Leaf Extract on Osteoarthritis in Mouse Primary Chondrocytes and a Medial Meniscus Destabilization Model. International Journal of Molecular Sciences, 26(20), 9877. https://doi.org/10.3390/ijms26209877







