Melatonin Rescues Heat Stress-Induced Suppression of TCA Cycle and Mitochondrial Damage in Goat Sertoli Cells
Abstract
1. Introduction
2. Results
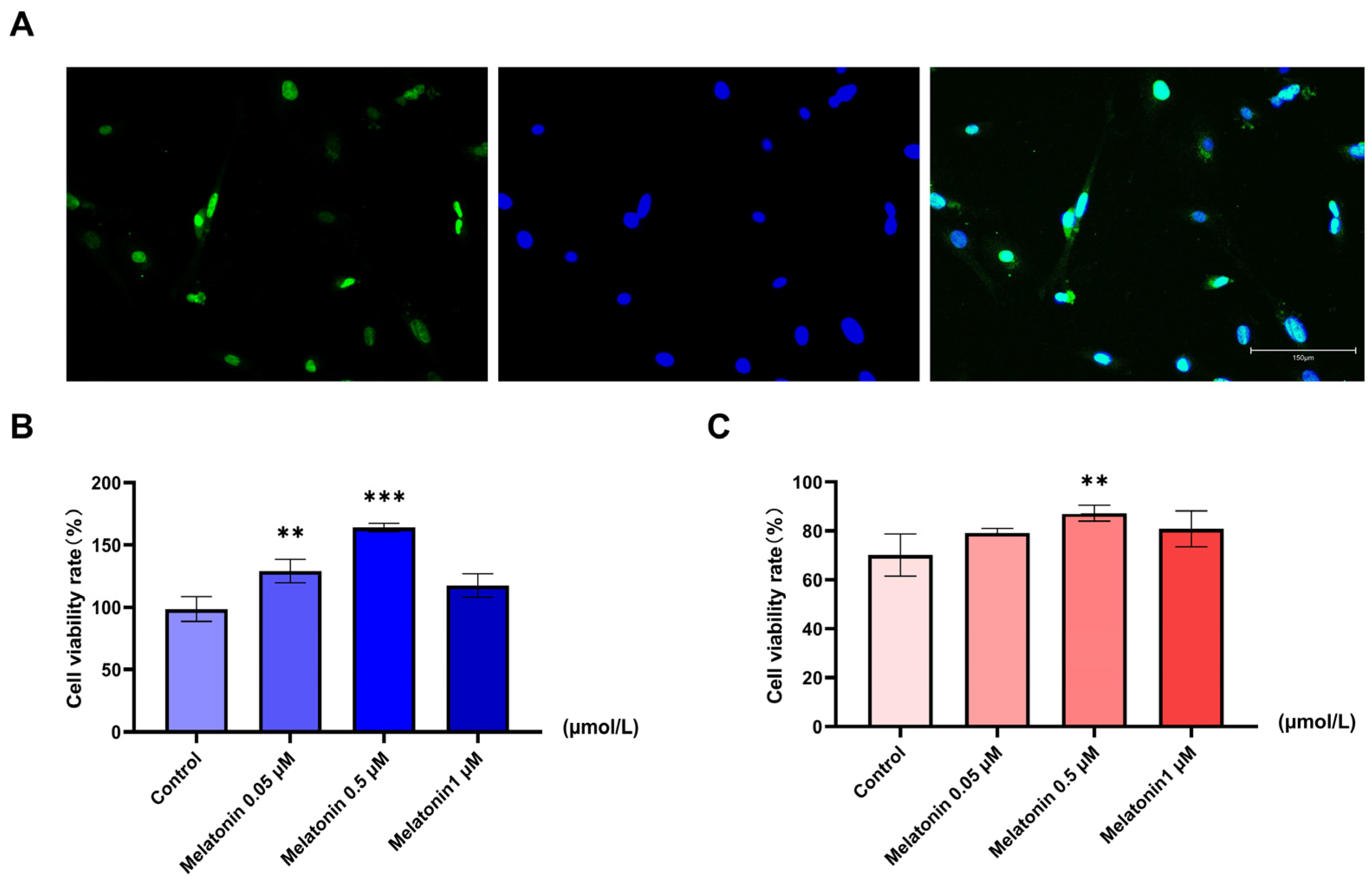
2.1. Melatonin Preserves Viability of Heat-Stressed Goat Sertoli Cells
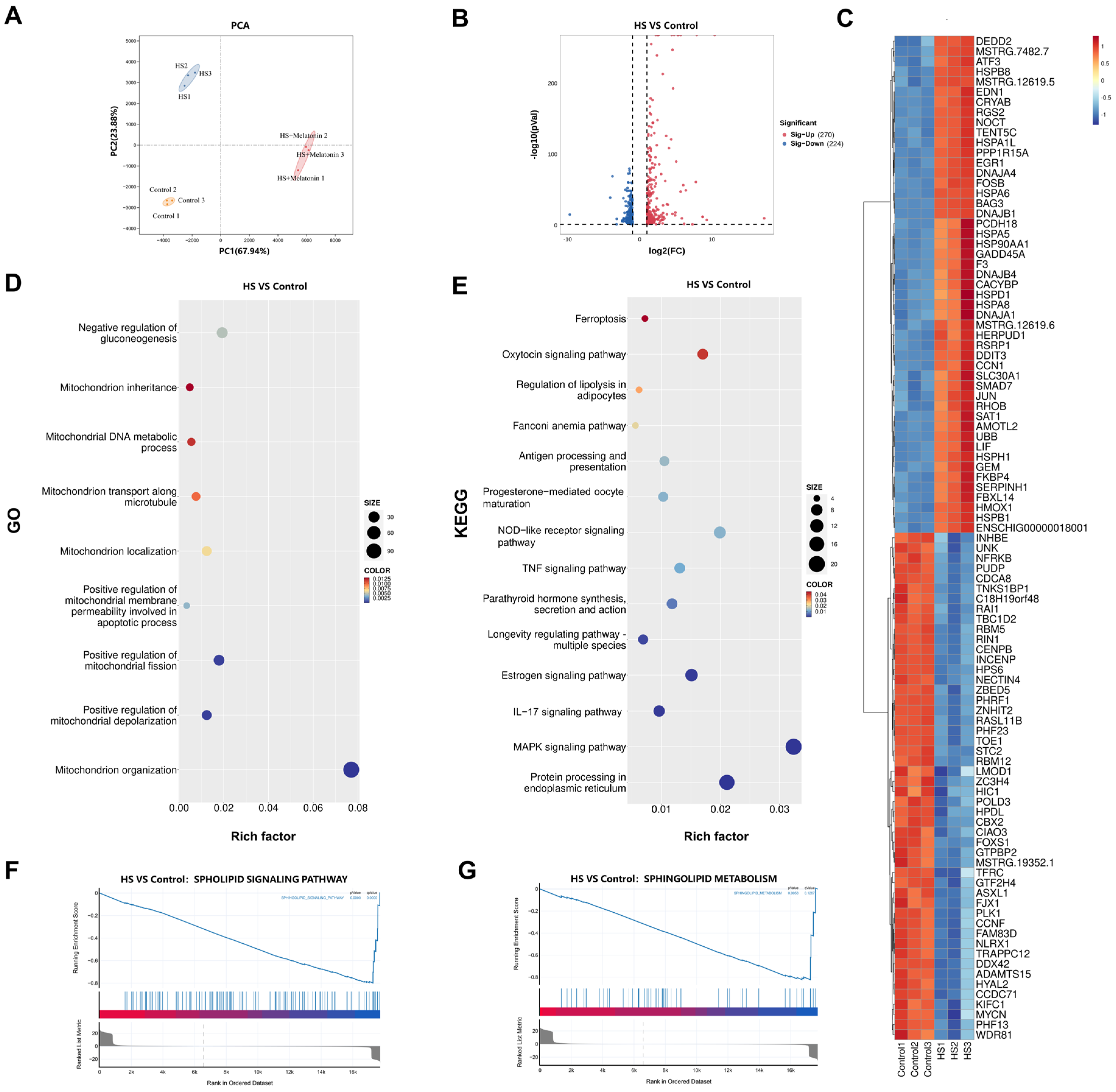
2.2. Transcriptomic Profiling of Heat-Stressed SCs
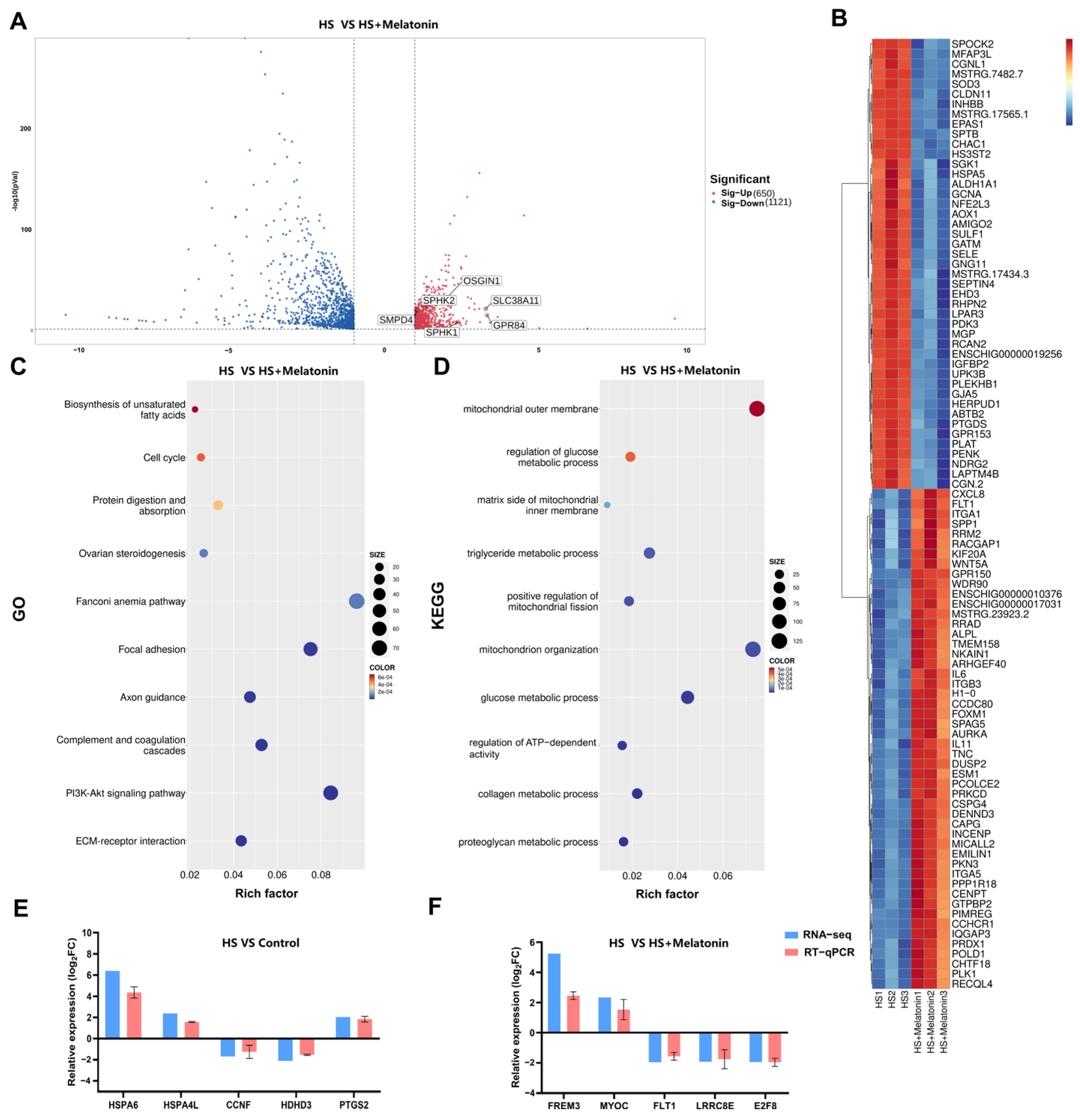
2.3. Melatonin Reverses Heat Stress-Induced Transcriptional Alterations in Metabolic Pathways
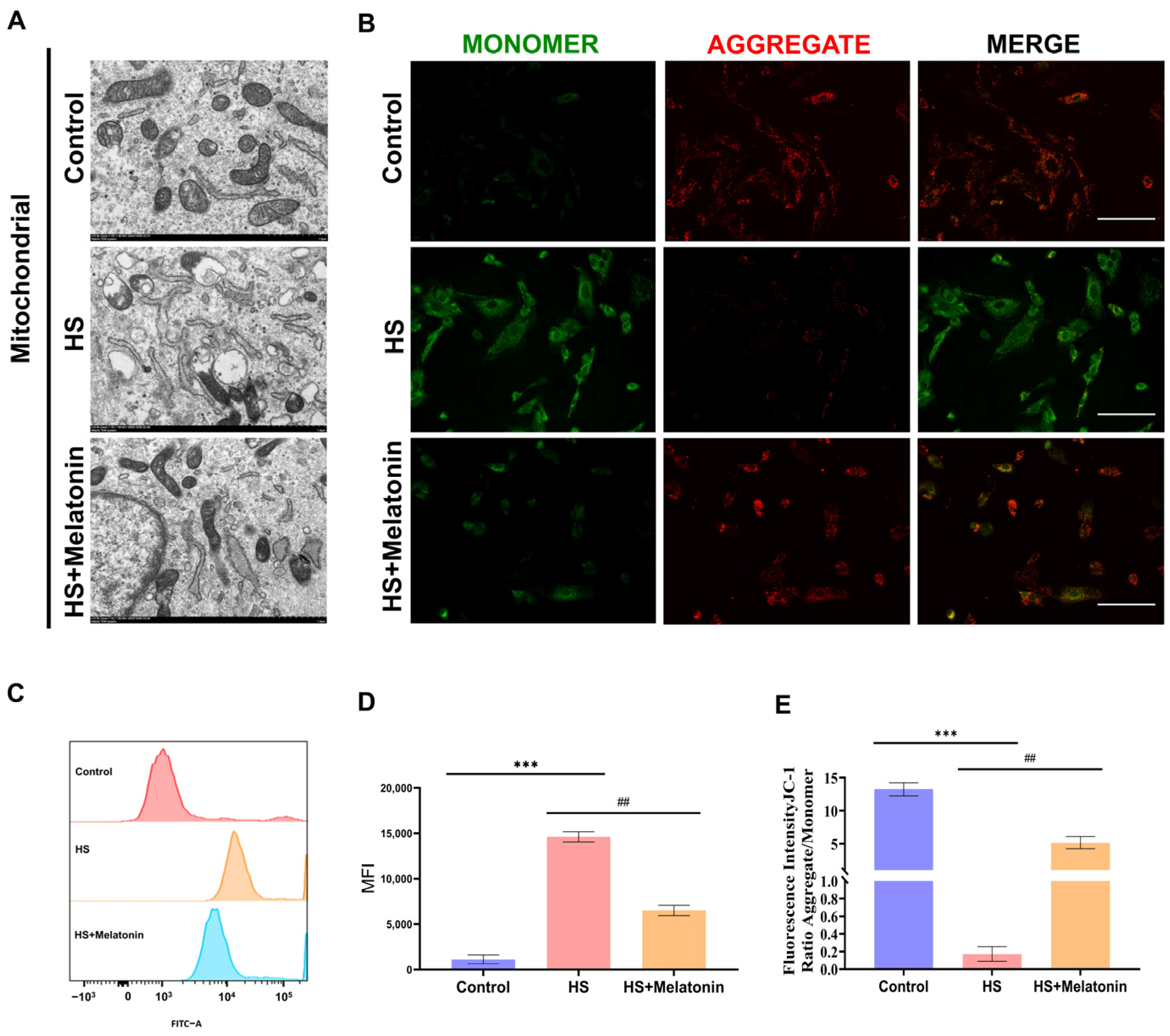
2.4. Melatonin Preserves Mitochondrial Ultrastructure and Function in Heat-Stressed SCs
2.5. Melatonin Orchestrates a Protective Metabolic Reprogramming Beyond Simple Reversal of Heat Stress Effects
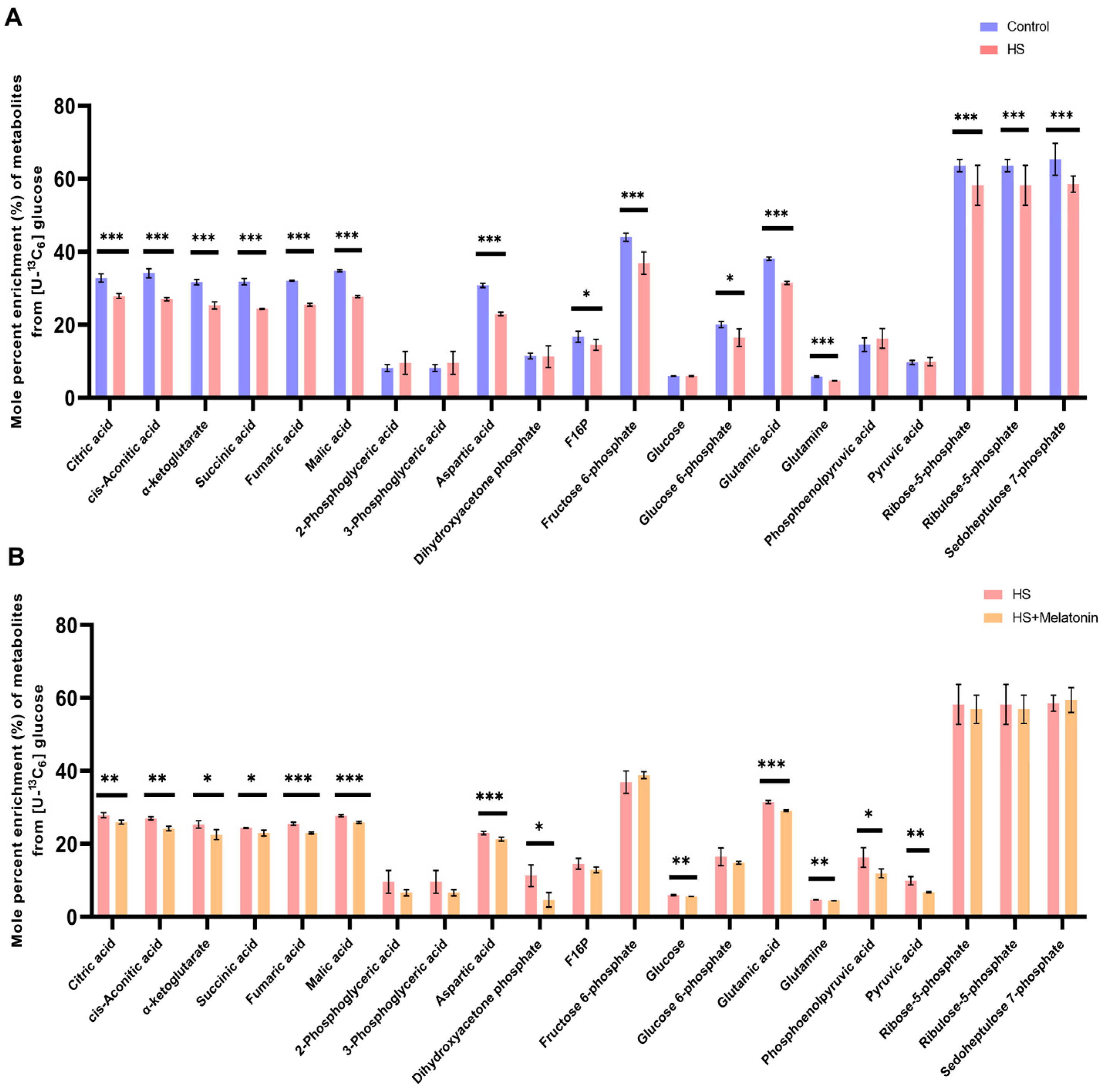
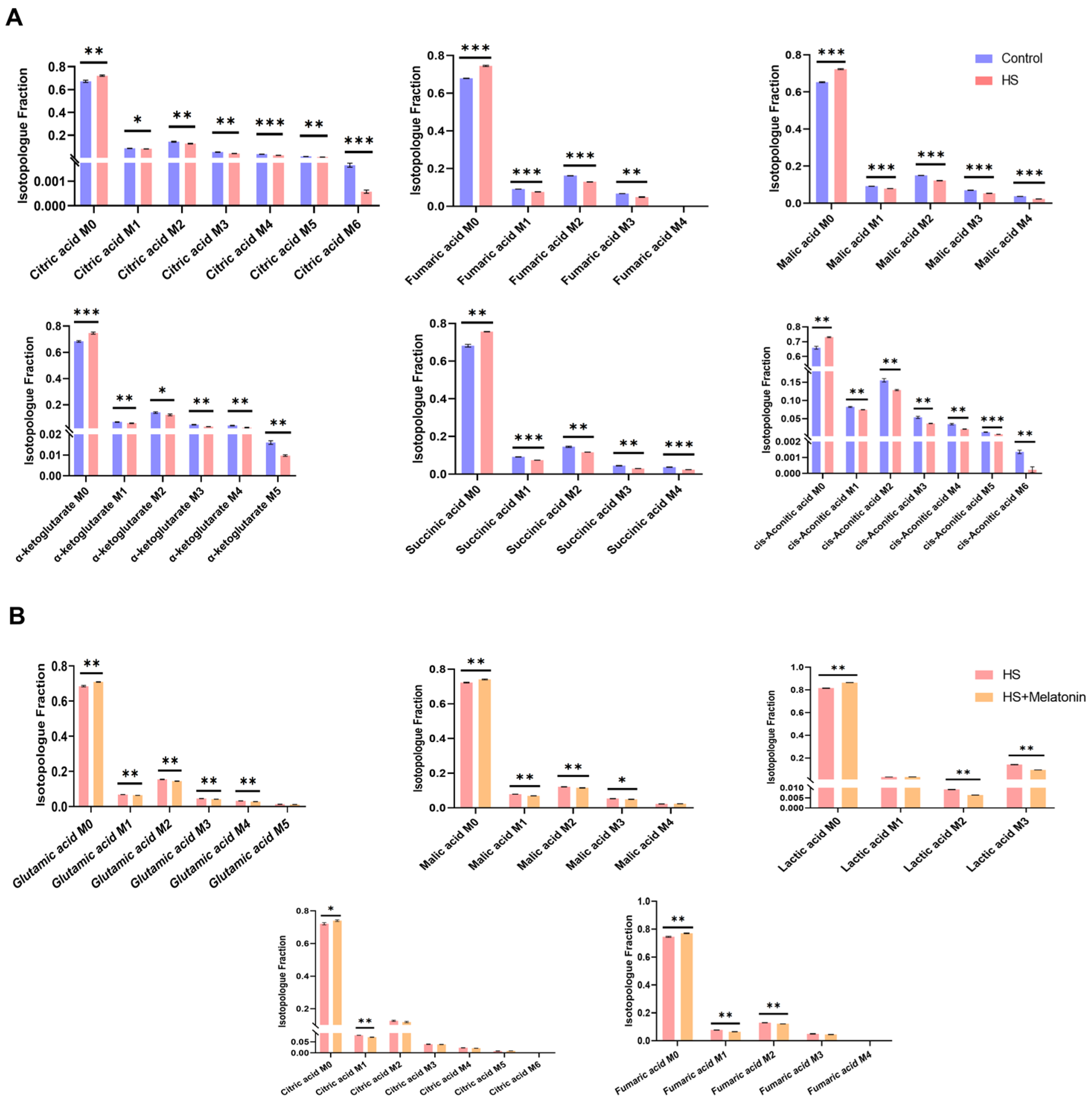
2.6. Melatonin Further Attenuates Glucose-Derived Carbon Flux into the TCA Cycle Under Heat Stress
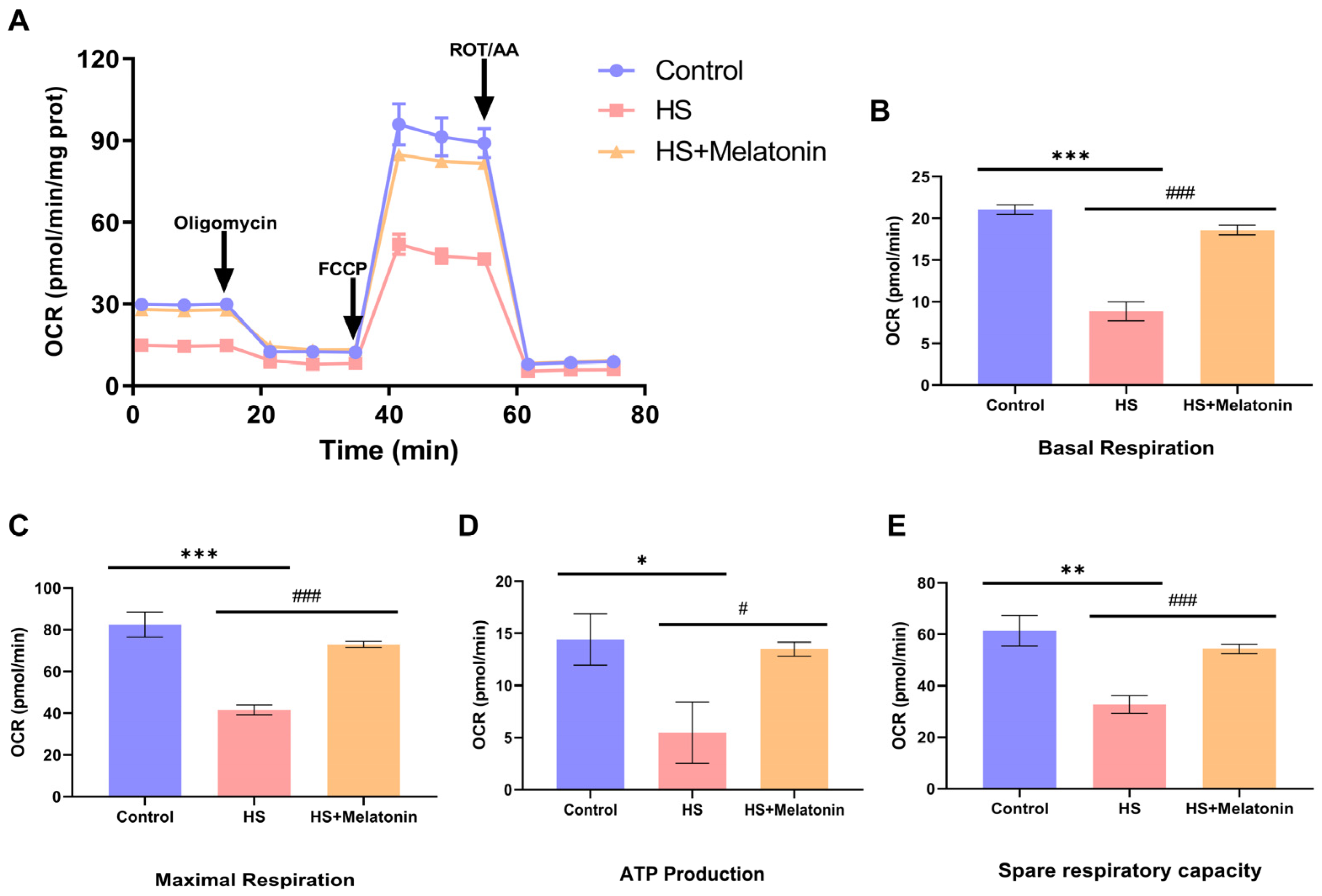
2.7. Melatonin Restores Mitochondrial Respiratory Function Despite Reduced Glucose Oxidation
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. Isolation, Purification, and Identification of SCs
4.3. Cell Viability Assay
4.4. RNA-seq Analysis
4.5. RT-qPCR
4.6. Transmission Electron Microscopy
4.7. Measurement of Mitochondrial Membrane Potential
4.8. Measurement of Mitochondrial Superoxide by Flow Cytometry
4.9. Metabolic Flux Analysis
4.10. Measurement of Mitochondrial Respiration
4.11. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quilcaille, Y.; Gudmundsson, L.; Schumacher, D.L.; Gasser, T.; Heede, R.; Heri, C.; Lejeune, Q.; Nath, S.; Naveau, P.; Thiery, W.; et al. Systematic attribution of heatwaves to the emissions of carbon majors. Nature 2025, 645, 392–398. [Google Scholar] [CrossRef]
- V, R.C.; Johnvictor, A.C.; N, P.S. Comparative analysis of machine learning approaches for heatwave event prediction in India. Sci. Rep. 2025, 15, 22431. [Google Scholar] [CrossRef]
- Wen, F.; Gao, J.; Zhang, G.; Guo, S.; Zhang, X.; Han, S.; Feng, X.; Chen, X.; Hu, J. ROS-DRP1-mediated excessive mitochondrial fission and autophagic flux inhibition contribute to heat stress-induced apoptosis in goat Sertoli cells. J. Anim. Sci. Biotechnol. 2025, 16, 58. [Google Scholar] [CrossRef]
- El-Sherbiny, H.R.; Hashem, N.M.; Abdelnaby, E.A. Coat color affects the resilience against heat stress impacts on testicular hemodynamics, reproductive hormones, and semen quality in Baladi goats. BMC Vet. Res. 2023, 19, 107. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Lv, Y.; Li, F.; Su, L.; Qin, Y.; Zeng, W. Melatonin protects the mouse testis against heat-induced damage. Mol. Hum. Reprod. 2020, 26, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Aldahhan, R.A.; Stanton, P.G. Heat stress response of somatic cells in the testis. Mol. Cell Endocrinol. 2021, 527, 111216. [Google Scholar] [CrossRef] [PubMed]
- Kastelic, J.P.; Rizzoto, G.; Thundathil, J. Review: Testicular vascular cone development and its association with scrotal thermoregulation, semen quality and sperm production in bulls. Animal 2018, 12, s133–s141. [Google Scholar] [CrossRef]
- Shahat, A.M.; Rizzoto, G.; Kastelic, J.P. Amelioration of heat stress-induced damage to testes and sperm quality. Theriogenology 2020, 158, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Amitha, J.P.; Krishnan, G.; Bagath, M.; Sejian, V.; Bhatta, R. Heat stress impact on the expression patterns of different reproduction related genes in Malabari goats. Theriogenology 2019, 131, 169–176. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Li, X.; Li, J.H.; Zhou, Y.; Lei, M.K.; Yin, W.Q.; Ren, Y.S.; Yang, C.H.; Zhang, C.X. Melatonin Improves Semen Quality by Modulating Oxidative Stress, Endocrine Hormones, and Tryptophan Metabolism of Hu Rams Under Summer Heat Stress and the Non-Reproductive Season. Antioxidants 2025, 14, 630. [Google Scholar] [CrossRef]
- Heinrich, A.; Potter, S.J.; Guo, L.; Ratner, N.; DeFalco, T. Distinct Roles for Rac1 in Sertoli Cell Function during Testicular Development and Spermatogenesis. Cell Rep. 2020, 31, 107513. [Google Scholar] [CrossRef]
- Wu, S.; Yan, M.; Ge, R.; Cheng, C.Y. Crosstalk between Sertoli and Germ Cells in Male Fertility. Trends Mol. Med. 2020, 26, 215–231. [Google Scholar] [CrossRef]
- Wen, Q.; Li, N.; Xiao, X.; Lui, W.Y.; Chu, D.S.; Wong, C.K.C.; Lian, Q.; Ge, R.; Lee, W.M.; Silvestrini, B.; et al. Actin nucleator Spire 1 is a regulator of ectoplasmic specialization in the testis. Cell Death Dis. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, A.; Bhandary, B.; Potter, S.J.; Ratner, N.; DeFalco, T. Cdc42 activity in Sertoli cells is essential for maintenance of spermatogenesis. Cell Rep. 2021, 37, 109885. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, S.A.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. J. Biol. Chem. 2017, 292, 7189–7207. [Google Scholar] [CrossRef]
- Yan, L.; Raj, P.; Yao, W.; Ying, H. Glucose Metabolism in Pancreatic Cancer. Cancers 2019, 11, 1460. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, N.J.; Gan, L.; Xue, H.Y.; Luo, K.Y.; Zhang, J.J.; Wang, X.Z. Heat stress upregulates arachidonic acid to trigger autophagy in sertoli cells via dysfunctional mitochondrial respiratory chain function. J. Transl. Med. 2024, 22, 501. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.C.; Zhang, J.P.; Huo, Y.N.; Xue, H.Y.; Wang, W.; Zhang, J.J.; Wang, X.Z. Melatonin alleviates the heat stress-induced impairment of Sertoli cells by reprogramming glucose metabolism. J. Pineal Res. 2022, 73, e12819. [Google Scholar] [CrossRef]
- Sun, J.; Yin, B.; Tang, S.; Zhang, X.; Xu, J.; Bao, E. Vitamin C mitigates heat damage by reducing oxidative stress, inducing HSP expression in TM4 Sertoli cells. Mol. Reprod. Dev. 2019, 86, 673–685. [Google Scholar] [CrossRef]
- Cai, X.Q.; Yang, H.; Liang, B.Q.; Deng, C.C.; Xue, H.Y.; Zhang, J.J.; Wang, X.Z. Glutamate rescues heat stress-induced apoptosis of Sertoli cells by enhancing the activity of antioxidant enzymes and activating the Trx1-Akt pathway in vitro. Theriogenology 2024, 223, 1–10. [Google Scholar] [CrossRef]
- Liu, D.L.; Liu, S.J.; Hu, S.Q.; Chen, Y.C.; Guo, J. Probing the Potential Mechanism of Quercetin and Kaempferol against Heat Stress-Induced Sertoli Cell Injury: Through Integrating Network Pharmacology and Experimental Validation. Int. J. Mol. Sci. 2022, 23, 11163. [Google Scholar] [CrossRef]
- Lei, Y.P.; Wang, J.; Yin, P.L.; Jia, H.; Ma, W.Z. Melatonin ameliorates heat stress-induced oxidative apoptosis in mouse spermatocytes via autophagy and ferroptosis pathways. Cell Stress Chaperones 2025, 30, 100078, Erratum in Cell Stress Chaperones 2025, 30, 100078. [Google Scholar] [CrossRef]
- Wu, H.; Qin, B.; Yang, G.; Ji, P.; Gao, Y.; Zhang, L.; Wang, B.; Liu, G. The Protective Effects of Melatonin on Hainan Black Goats Under Heat Stress: Understanding Its Actions and Mechanisms. Antioxidants 2025, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.S.; Rocha-Frigoni, N.A.S.; Mingoti, G.Z.; Roth, Z.; Hansen, P.J. Modification of embryonic resistance to heat shock in cattle by melatonin and genetic variation in HSPA1L. J. Dairy Sci. 2016, 99, 9152–9164. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhu, H.; Zhou, M.; Zhang, H.; Xiao, Q.; Yuan, Q.; Sun, G.; Zhang, Z.; Chu, H. OSGIN1 regulates PM(2.5)-induced fibrosis via mediating autophagy in an in vitro model of COPD. Toxicol. Lett. 2024, 401, 35–43. [Google Scholar] [CrossRef]
- Khoi, C.S.; Xiao, C.Q.; Hung, K.Y.; Lin, T.Y.; Chiang, C.K. Oxidative Stress-Induced Growth Inhibitor (OSGIN1), a Target of X-Box-Binding Protein 1, Protects Palmitic Acid-Induced Vascular Lipotoxicity through Maintaining Autophagy. Biomedicines 2022, 10, 992. [Google Scholar] [CrossRef]
- Kao, W.H.; Liao, L.Z.; Chen, Y.A.; Lo, U.G.; Pong, R.C.; Hernandez, E.; Chen, M.C.; Teng, C.J.; Wang, H.Y.; Tsai, S.C.; et al. SPHK1 promotes bladder cancer metastasis via PD-L2/c-Src/FAK signaling cascade. Cell Death Dis. 2024, 15, 678. [Google Scholar] [CrossRef]
- Arora, S.; Singh, P.; Tabassum, G.; Dohare, R.; Syed, M.A. miR-495-3p regulates sphingolipid metabolic reprogramming to induce Sphk1/ceramide mediated mitophagy and apoptosis in NSCLC. Free Radic. Biol. Med. 2022, 189, 71–84. [Google Scholar] [CrossRef]
- Luo, Y.; Xue, H.; Gao, Y.; Ji, G.; Wu, T. Sphingosine kinase 2 in cancer: A review of its expression, function, and inhibitor development. Int. J. Biol. Macromol. 2025, 306, 141392. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, W.; Xia, M.F.; Lu, Y.L.; Bian, H.; Yu, C.; Li, X.Y.; Vadas, M.A.; Gao, X.; Lin, H.D.; et al. Identification of circulating sphingosine kinase-related metabolites for prediction of type 2 diabetes. J. Transl. Med. 2021, 19, 393. [Google Scholar] [CrossRef]
- Smits, D.J.; Schot, R.; Krusy, N.; Wiegmann, K.; Utermöhlen, O.; Mulder, M.T.; den Hoedt, S.; Yoon, G.; Deshwar, A.R.; Kresge, C.; et al. SMPD4 regulates mitotic nuclear envelope dynamics and its loss causes microcephaly and diabetes. Brain 2023, 146, 3528–3541. [Google Scholar] [CrossRef]
- Du Toit, E.; Browne, L.; Irving-Rodgers, H.; Massa, H.M.; Fozzard, N.; Jennings, M.P.; Peak, I.R. Effect of GPR84 deletion on obesity and diabetes development in mice fed long chain or medium chain fatty acid rich diets. Eur. J. Nutr. 2018, 57, 1737–1746. [Google Scholar] [CrossRef]
- Hellsten, S.V.; Tripathi, R.; Ceder, M.M.; Fredriksson, R. Nutritional Stress Induced by Amino Acid Starvation Results in Changes for Slc38 Transporters in Immortalized Hypothalamic Neuronal Cells and Primary Cortex Cells. Front. Mol. Biosci. 2018, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, Z.; Shi, L.; Wei, Z.; Shangguan, J.; Shi, L.; Zhao, M. Spermidine enhances the heat tolerance of Ganoderma lucidum by promoting mitochondrial respiration driven by fatty acid β-oxidation. Appl. Environ. Microbiol. 2025, 91, e0097924. [Google Scholar] [CrossRef]
- Li, H.; Spade, D.J. REPRODUCTIVE TOXICOLOGY: Environmental exposures, fetal testis development and function: Phthalates and beyond. Reproduction 2021, 162, F147–F167. [Google Scholar] [CrossRef] [PubMed]
- Sakib, S.; Uchida, A.; Valenzuela-Leon, P.; Yu, Y.; Valli-Pulaski, H.; Orwig, K.; Ungrin, M.; Dobrinski, I. Formation of organotypic testicular organoids in microwell culture†. Biol. Reprod. 2019, 100, 1648–1660. [Google Scholar] [CrossRef]
- Guo, X.; Xu, J.; Zhao, Y.; Wang, J.; Fu, T.; Richard, M.L.; Sokol, H.; Wang, M.; Li, Y.; Liu, Y.; et al. Melatonin alleviates heat stress-induced spermatogenesis dysfunction in male dairy goats by regulating arachidonic acid metabolism mediated by remodeling the gut microbiota. Microbiome 2024, 12, 233. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Xu, Q.; Li, H.; Liu, R.; Chen, J.; Jiang, X.; Sun, J.; Lai, C.; Bai, W. Cyanidin-3-O-glucoside and protocatechuic acid alleviate heat stress-induced testicular damage. Food Funct. 2023, 14, 2200–2211. [Google Scholar] [CrossRef]
- Yang, C.X.; Chen, L.; Yang, Y.W.; Mou, Q.; Du, Z.Q. Acute heat stress reduces viability but increases lactate secretion of porcine immature Sertoli cells through transcriptome reprogramming. Theriogenology 2021, 173, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.R.; Li, B.B.; Hu, Y.; Zhang, L.; Wang, X.Z. Oxidative stress mediates heat-induced changes of tight junction proteins in porcine sertoli cells via inhibiting CaMKKbeta-AMPK pathway. Theriogenology 2020, 142, 104–113. [Google Scholar] [CrossRef]
- Huang, B.; Li, F.; You, D.; Deng, L.; Xu, T.; Lai, S.; Ai, Y.; Huang, J.; Zhou, Y.; Ge, L.; et al. Porcine reproductive and respiratory syndrome virus infects the reproductive system of male piglets and impairs development of the blood-testis barrier. Virulence 2024, 15, 2384564. [Google Scholar] [CrossRef]
- Jafari, N.; Drury, J.; Morris, A.J.; Onono, F.O.; Stevens, P.D.; Gao, T.; Liu, J.; Wang, C.; Lee, E.Y.; Weiss, H.L.; et al. De Novo Fatty Acid Synthesis-Driven Sphingolipid Metabolism Promotes Metastatic Potential of Colorectal Cancer. Mol. Cancer Res. 2019, 17, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bai, L.; Dai, X.; Ba, L.; Wan, J.; Liang, W.; Lin, H.; Fan, Z. Comparative transcriptomic analysis reveals the reactive oxygen species metabolism involving in melatonin-alleviated chilling injury in postharvest banana fruit. Plant Physiol. Biochem. 2025, 222, 109693. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, P.; Chen, X.; Peng, Y.; Cai, B.; Song, J.; Yin, G.; Jia, S.; Zhang, H. Melatonin alleviates cadmium toxicity and abiotic stress by promoting glandular trichome development and antioxidant capacity in Nicotiana tabacum. Ecotoxicol. Environ. Saf. 2022, 236, 113437. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Zhang, R.; Ma, B.; Ni, L.; Feng, H.; Liu, C. Melatonin attenuates high glucose-induced endothelial cell pyroptosis by activating the Nrf2 pathway to inhibit NLRP3 inflammasome activation. Mol. Med. Rep. 2023, 27, 71. [Google Scholar] [CrossRef]
- Yang, W.; Kang, X.; Qin, N.; Li, F.; Jin, X.; Ma, Z.; Qian, Z.; Wu, S. Melatonin protects chondrocytes from impairment induced by glucocorticoids via NAD(+)-dependent SIRT1. Steroids 2017, 126, 24–29. [Google Scholar] [CrossRef]
- Qin, D.Z.; Cai, H.; He, C.; Yang, D.H.; Sun, J.; He, W.L.; Li, B.L.; Hua, J.L.; Peng, S. Melatonin relieves heat-induced spermatocyte apoptosis in mouse testes by inhibition of ATF6 and PERK signaling pathways. Zool. Res. 2021, 42, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Madhu, V.; Hernandez-Meadows, M.; Boneski, P.K.; Qiu, Y.; Guntur, A.R.; Kurland, I.J.; Barve, R.A.; Risbud, M.V. The mitophagy receptor BNIP3 is critical for the regulation of metabolic homeostasis and mitochondrial function in the nucleus pulposus cells of the intervertebral disc. Autophagy 2023, 19, 1821–1843. [Google Scholar] [CrossRef]
- Li, T.; Pang, N.; He, L.; Xu, Y.; Fu, X.; Tang, Y.; Shachar-Hill, Y.; Chen, S. Re-Programing Glucose Catabolism in the Microalga Chlorella sorokiniana under Light Condition. Biomolecules 2022, 12, 939. [Google Scholar] [CrossRef]
- An, X.; Li, Q.; Chen, N.; Li, T.; Wang, H.; Su, M.; Shi, H.; Ma, Y. Effects of Pgam1-mediated glycolysis pathway in Sertoli cells on Spermatogonial stem cells based on transcriptomics and energy metabolomics. Front. Vet. Sci. 2022, 9, 992877. [Google Scholar] [CrossRef]
- Jing, J.; Ouyang, L.; Zhang, H.; Liang, K.; Ma, R.; Ge, X.; Tang, T.; Zhao, S.; Xue, T.; Shen, J.; et al. Omega-3 polyunsaturated fatty acids and its metabolite 12-HEPE rescue busulfan disrupted spermatogenesis via target to GPR120. Cell Prolif. 2024, 57, e13551. [Google Scholar] [CrossRef]
- Signorini, C.; Saso, L.; Ghareghomi, S.; Telkoparan-Akillilar, P.; Collodel, G.; Moretti, E. Redox Homeostasis and Nrf2-Regulated Mechanisms Are Relevant to Male Infertility. Antioxidants 2024, 13, 193. [Google Scholar] [CrossRef]
- Shehata, A.M.; Saadeldin, I.M.; Tukur, H.A.; Habashy, W.S. Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress. Animals 2020, 10, 2407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Linghu, T.; Wang, Q.; Qin, X.; Tian, J. Metabolic Flux Analysis Uncovers Substrate-Specific Reprogramming and ATP Deficit in CORT-Induced Depressive-like Astrocytes. J. Proteome Res. 2025, 24, 5894–5903. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-S.; Wu, W.-Z.; Liu, S.-B.; Ling-hu, T.; Zhao, Y.-H.; Gao, Y.; Qin, X.-M. Stable Isotope-Resolved Metabolomics Studies on Corticosteroid-Induced PC12 Cells: A Strategy for Evaluating Glucose Catabolism in an in Vitro Model of Depression. J. Proteome Res. 2022, 21, 788–797. [Google Scholar] [CrossRef]
- Zhang, H.; Badur, M.G.; Divakaruni, A.S.; Parker, S.J.; Jäger, C.; Hiller, K.; Murphy, A.N.; Metallo, C.M. Distinct Metabolic States Can Support Self-Renewal and Lipogenesis in Human Pluripotent Stem Cells under Different Culture Conditions. Cell Rep. 2016, 16, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, B.; Lin, L.; Zhang, H.; Luan, T. (13)C isotope-based metabolic flux analysis revealing cellular landscape of glucose metabolism in human liver cells exposed to perfluorooctanoic acid. Sci. Total Environ. 2021, 770, 145329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Shan, Y.; Zhang, Z.; Wu, H.; Lei, Y.; Sun, Y.; Ji, P.; Guoshi, L. Melatonin Rescues Heat Stress-Induced Suppression of TCA Cycle and Mitochondrial Damage in Goat Sertoli Cells. Int. J. Mol. Sci. 2025, 26, 11475. https://doi.org/10.3390/ijms262311475
Yang G, Shan Y, Zhang Z, Wu H, Lei Y, Sun Y, Ji P, Guoshi L. Melatonin Rescues Heat Stress-Induced Suppression of TCA Cycle and Mitochondrial Damage in Goat Sertoli Cells. International Journal of Molecular Sciences. 2025; 26(23):11475. https://doi.org/10.3390/ijms262311475
Chicago/Turabian StyleYang, Guang, Yilin Shan, Zhen Zhang, Hao Wu, Yuanyuan Lei, Yichong Sun, Pengyun Ji, and Liu Guoshi. 2025. "Melatonin Rescues Heat Stress-Induced Suppression of TCA Cycle and Mitochondrial Damage in Goat Sertoli Cells" International Journal of Molecular Sciences 26, no. 23: 11475. https://doi.org/10.3390/ijms262311475
APA StyleYang, G., Shan, Y., Zhang, Z., Wu, H., Lei, Y., Sun, Y., Ji, P., & Guoshi, L. (2025). Melatonin Rescues Heat Stress-Induced Suppression of TCA Cycle and Mitochondrial Damage in Goat Sertoli Cells. International Journal of Molecular Sciences, 26(23), 11475. https://doi.org/10.3390/ijms262311475





