Biological Activities Underlying the Cardiovascular Benefits of Olive Oil Polyphenols: Focus on Antioxidant, Anti-Inflammatory, and Anti-Atherogenic Effects
Abstract
1. Introduction
2. Results
2.1. Total Phenolic Content and Fatty Acid Composition
2.2. Cell Viability Measurement
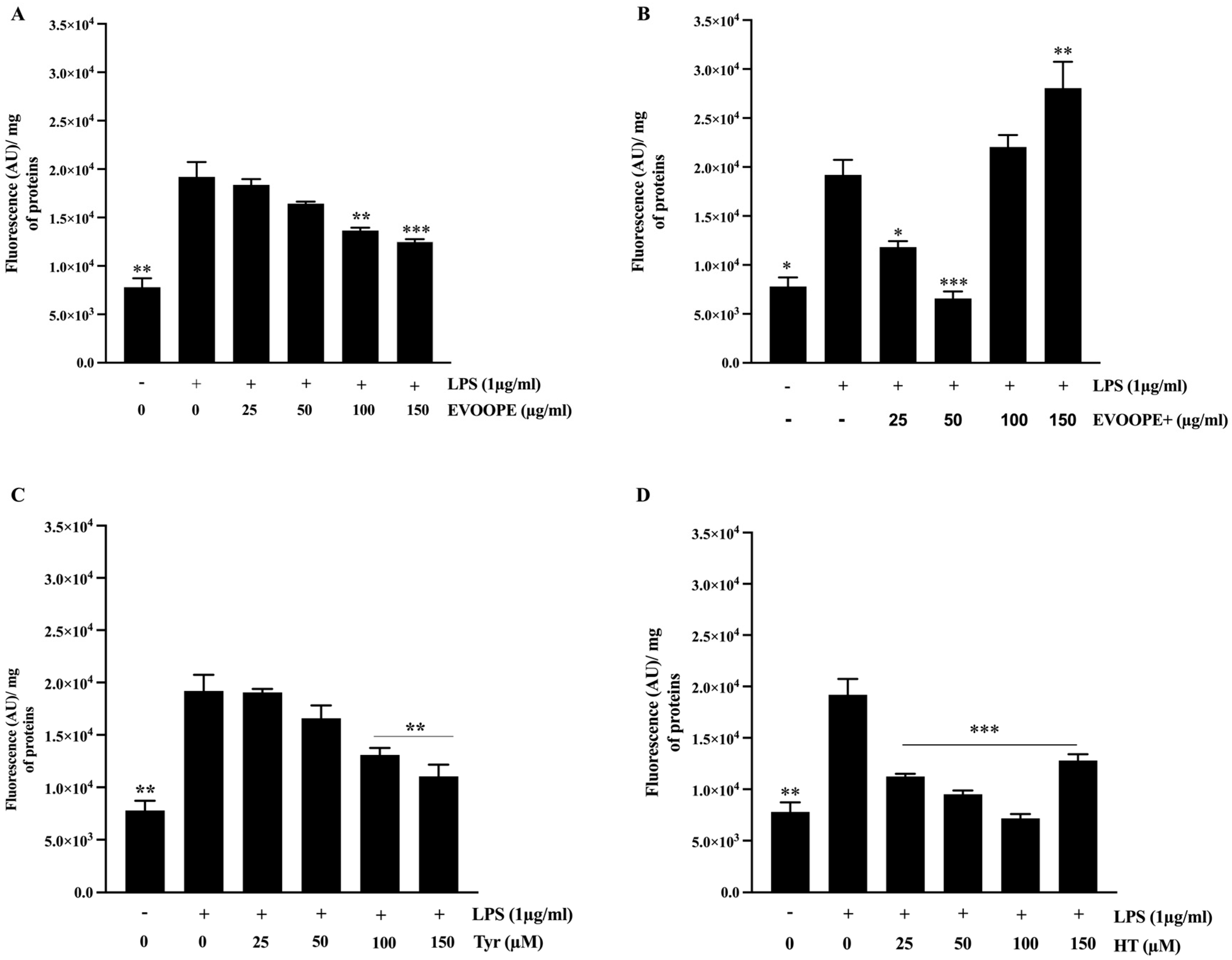
2.3. Antioxidant Activity
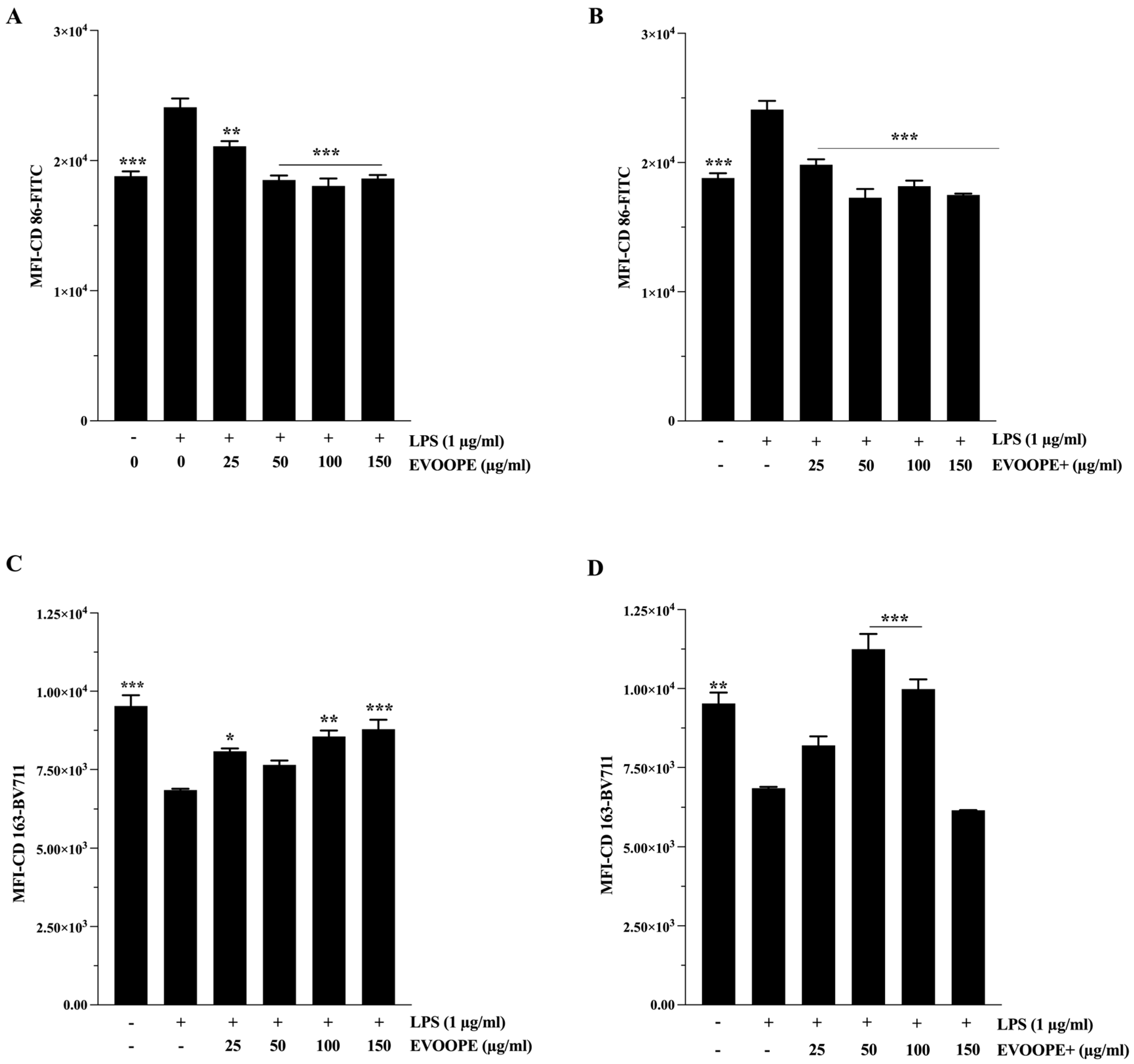
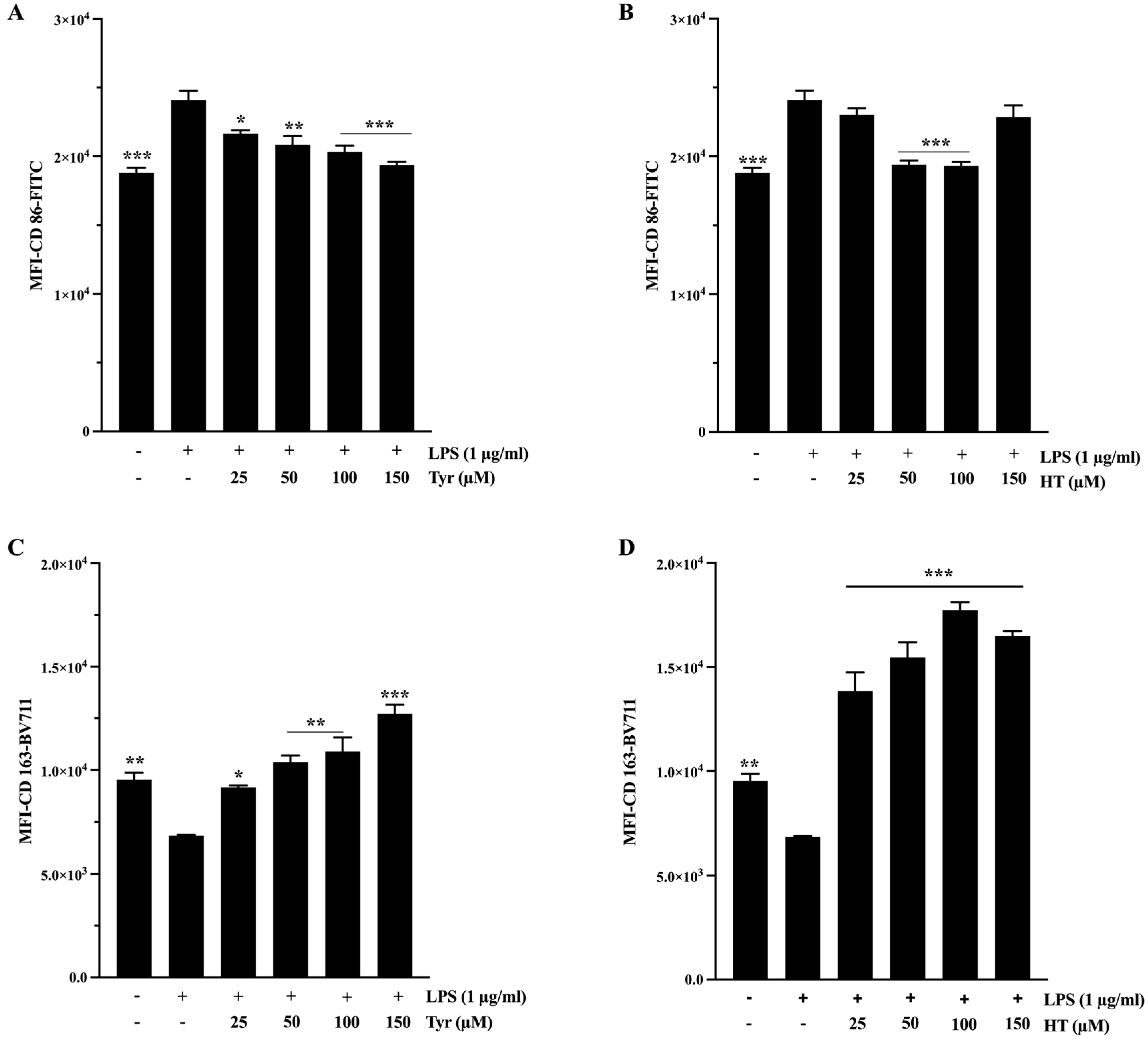
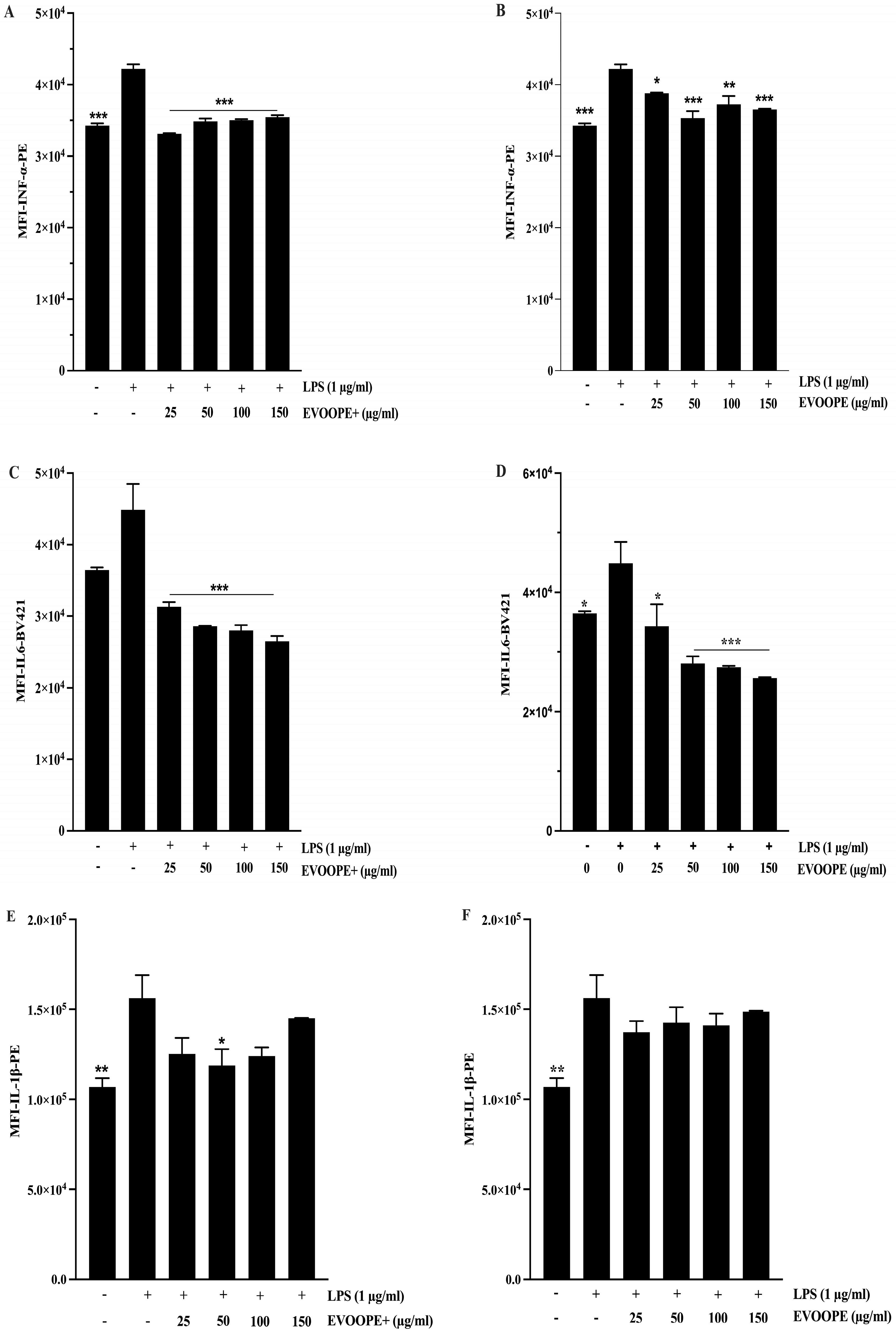
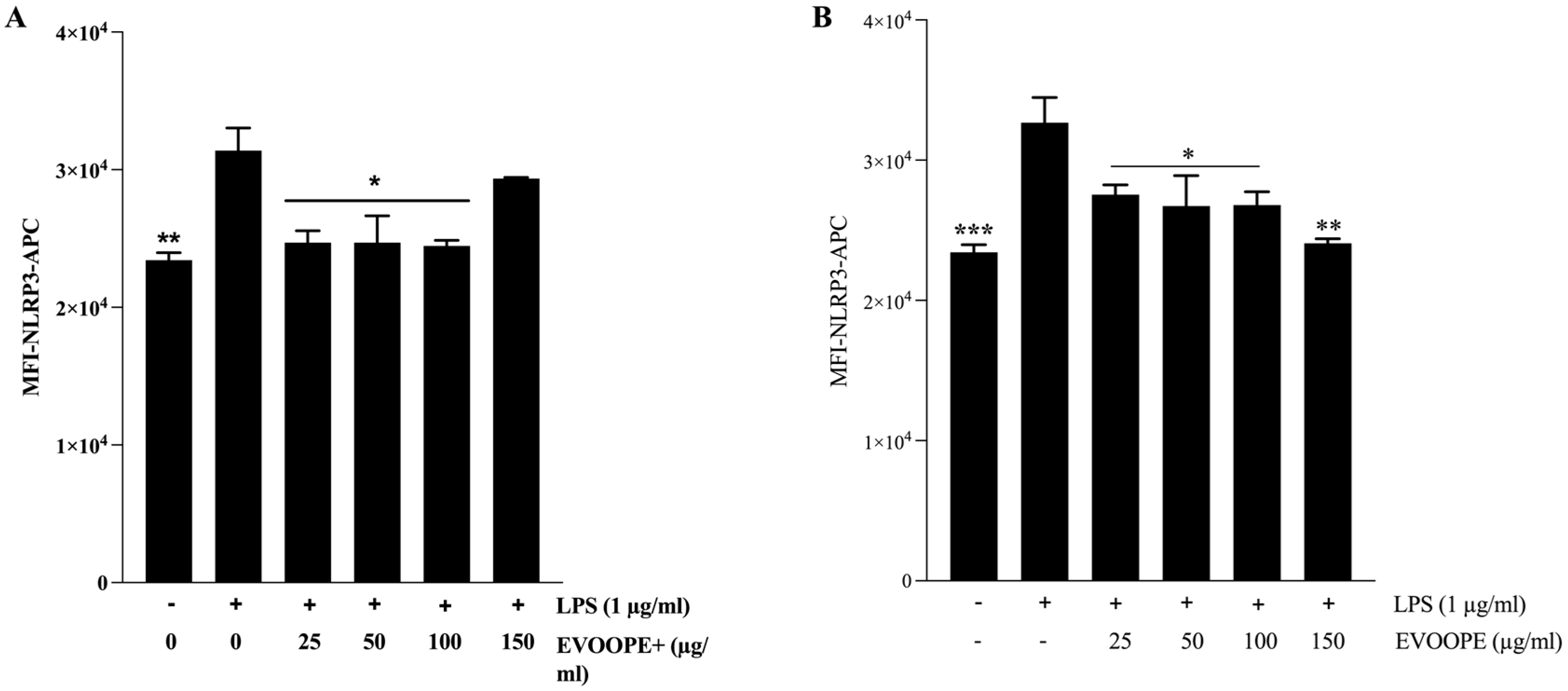
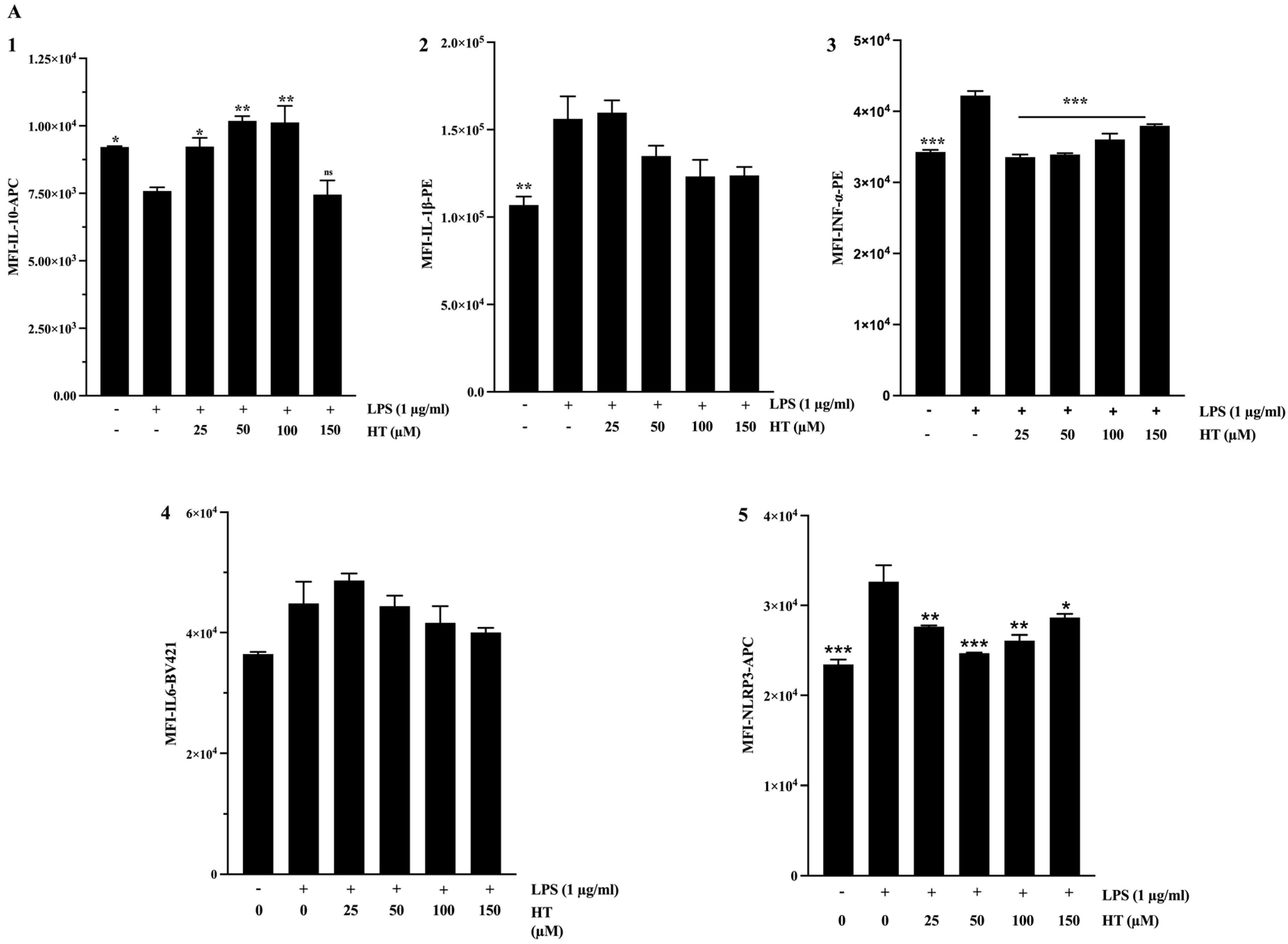
2.4. Anti-Inflammatory Activity
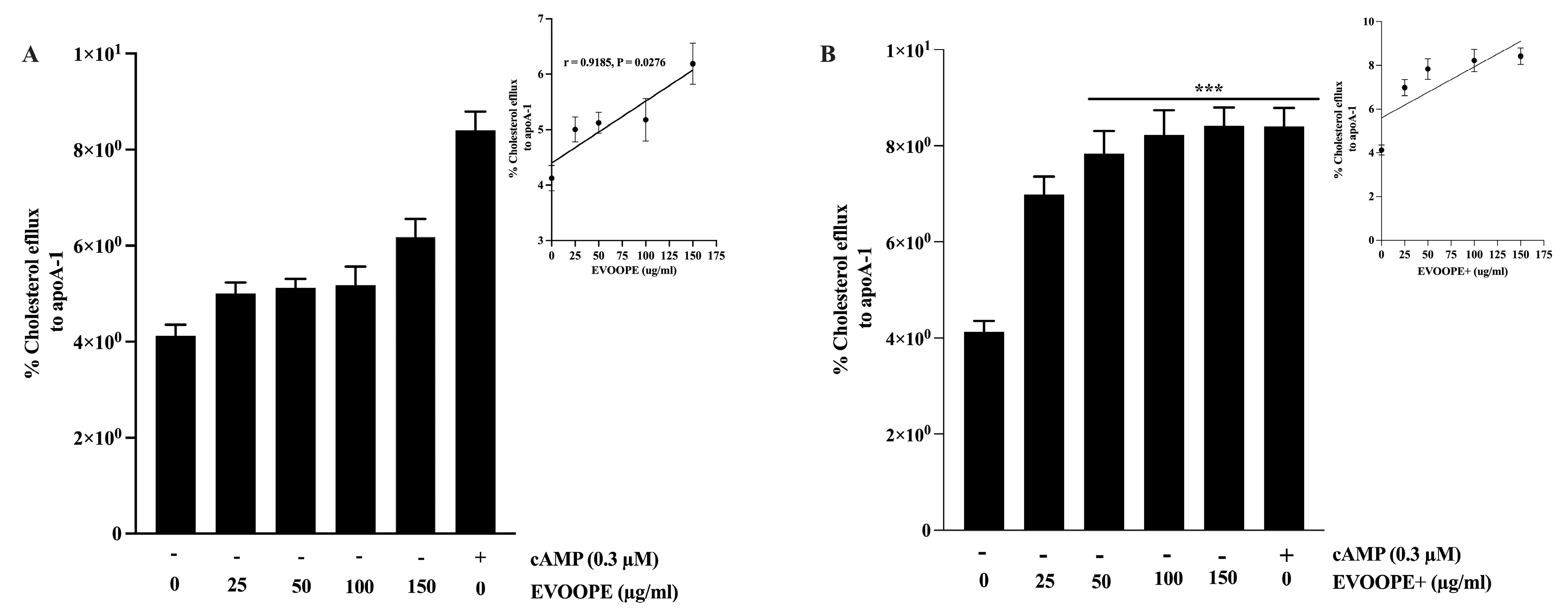
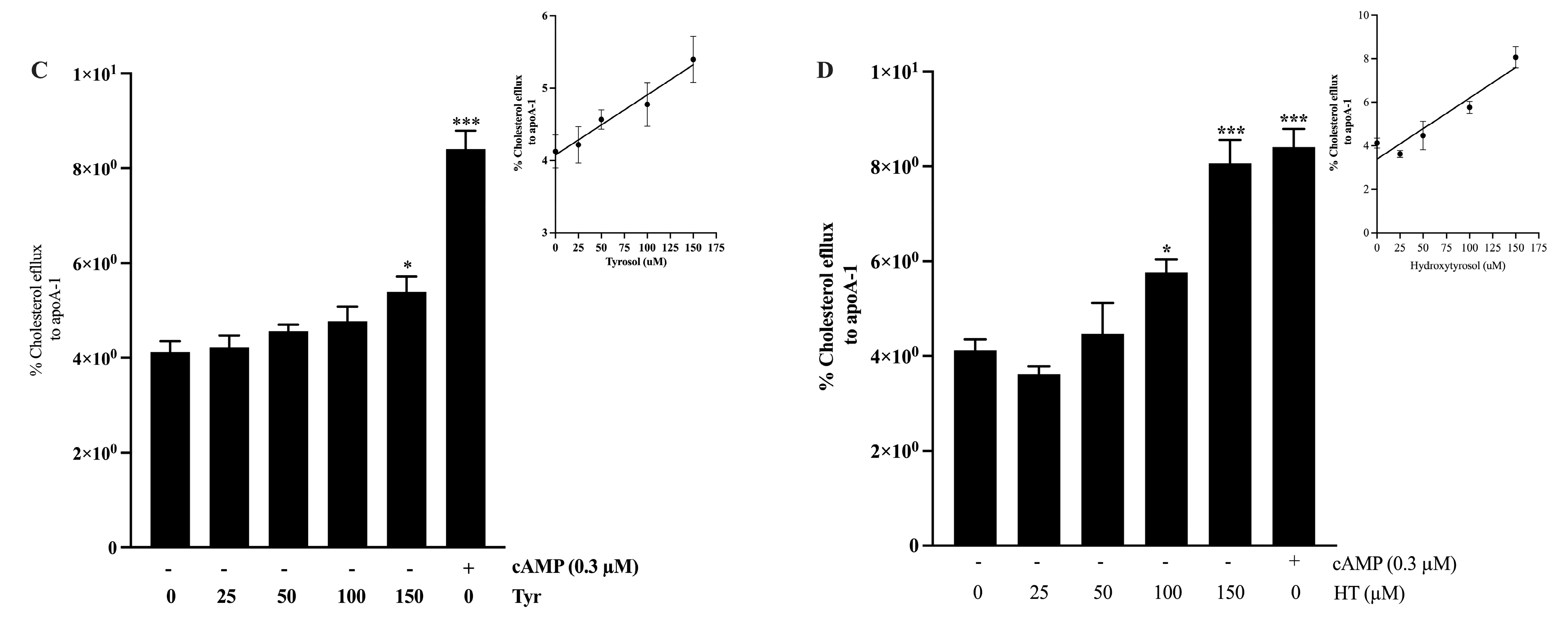
2.5. Cholesterol Efflux Measurement in J774 Macrophages
3. Discussion
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
3.3. Anti-Atherogenic Activity
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Extraction of the Phenolic Fraction
4.4. Total Phenolic Content
4.5. Cell Culture
4.6. Cell Viability Measurement
4.7. Measurement of Intracellular ROS
4.8. Lipid Peroxidation Assay
4.9. M1/M2 Polarization of THP1 Macrophages
4.10. Intracellular Inflammatory Markers Measurement
4.11. Cholesterol Efflux Measurement in J774 Macrophages
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-Binding Cassette Transporter A1 |
| ABCG1 | ATP-Binding Cassette Transporter G1 |
| Apo A1 | Apolipoprotein A1 |
| EVOO | Extra Virgin Olive Oil |
| EVOOPE | Extra Virgin Olive Oil Phenolic Extract |
| EVOOPE+ | High-polyphenol Extra Virgin Olive Oil Phenolic Extract |
| FA | Fatty Acids |
| HDL | High-Density Lipoprotein |
| HT | Hydroxytyrosol |
| LXR | Liver X Receptor |
| MDA | Malondialdehyd |
| OOPC | Olive Oil Phenolic Compounds |
| OOPE | Olive Oil Polyphenol Extract |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| RCT | Reverse Cholesterol Transport |
| Tyr | Tyrosol |
| VOO | Virgin Olive Oil |
References
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The secrets of the mediterranean diet. Does [only] olive oil matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef]
- Serreli, G.; Boronat, A.; De la Torre, R.; Rodriguez-Moratò, J.; Deiana, M. Cardiovascular and Metabolic Benefits of Extra Virgin Olive Oil Phenolic Compounds: Mechanistic Insights from In Vivo Studies. Cells 2024, 13, 1555. [Google Scholar] [CrossRef]
- Boumezough, K.; Alami, M.; Oubaouz, J.; Morvaridzadeh, M.; Zoubdane, N.; Khalil, A.; Ramchoun, M.H.; Zahir, I.; Ramassamy, C.; Fulop, T.; et al. Potential health benefits of olive oil polyphenols in metabolic disorders management. PharmaNutrition 2024, 31, 100428. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, A.; Martínez-Ortega, A.J.; Remón-Ruiz, P.J.; Piñar-Gutiérrez, A.; Pereira-Cunill, J.L.; García-Luna, P.P. Therapeutic Properties and Use of Extra Virgin Olive Oil in Clinical Nutrition: A Narrative Review and Literature Update. Nutrients 2022, 14, 1440. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Milena, E.; Maurizio, M. Exploring the Cardiovascular Benefits of Extra Virgin Olive Oil: Insights into Mechanisms and Therapeutic Potential. Biomolecules 2025, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Alami, M.; Zoubdane, N.; Sidibé, H.; Berrougui, H.; Fülöp, T.; Nguyen, M.; Khalil, A. High-Tyrosol/Hydroxytyrosol Extra Virgin Olive Oil Enhances Antioxidant Activity in Elderly Post-Myocardial Infarction Patients. Antioxidants 2025, 14, 867. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors of Reducing Species: Relationship with Human Antioxidant Metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Martin, K.R. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet Suppl. 2009, 1, 1–12. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols From Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 750508. [Google Scholar] [CrossRef]
- Bayır, A.G.; Aksoy, A.N.; Koçyiğit, A. The Importance of Polyphenols as Functional Food in Health. Bezmialem Sci. 2019, 7, 157–163. [Google Scholar] [CrossRef]
- Kanner, J. Polyphenols by generating H2O2, affect cell redox signaling, inhibit PTPs and activate Nrf2 axis for adaptation and cell surviving: In vitro, in vivo and human health. Antioxidants 2020, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Kouka, P.; Tekos, F.; Papoutsaki, Z.; Stathopoulos, P.; Halabalaki, M.; Tsantarliotou, M.; Zervos, I.; Nepka, C.; Liesivuori, J.; Rakitskii, V.N.; et al. Olive oil with high polyphenolic content induces both beneficial and harmful alterations on rat redox status depending on the tissue. Toxicol. Rep. 2020, 7, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Al Fazazi, S.; Casuso, R.A.; Aragón-Vela, J.; Casals, C.; Huertas, J.R. Effects of hydroxytyrosol dose on the redox status of exercised rats: The role of hydroxytyrosol in exercise performance. J. Int. Soc. Sports Nutr. 2018, 15, 20. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; Liu, J.; Ye, J.; Xu, Y.; Wang, Z.; Yu, J.; Ye, D.; Zhao, M.; Feng, Y.; et al. The role of interleukin-10 family members in cardiovascular diseases. Int. Immunopharmacol. 2021, 94, 107475. [Google Scholar] [CrossRef]
- Muffova, B.; Kauerova, S.; Bartuskova, H.; Paukner, K.; Janousek, L.; Cermakova, H.; Fronek, J.; Kollar, M.; Petras, M.; Pitha, J.; et al. Phenotypic Shifts in Macrophages Within Advanced Atherosclerotic Plaques in Humans. FASEB Bioadv. 2025, 7, e70017. [Google Scholar] [CrossRef]
- Penna, C.; Pagliaro, P. Endothelial Dysfunction: Redox Imbalance, NLRP3 Inflammasome, and Inflammatory Responses in Cardiovascular Diseases. Antioxidants 2025, 14, 256. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive polyphenols: Antioxidant and anti-inflammatory properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Costa, V.; Costa, M.; Videira, R.A.; Andrade, P.B.; Paiva-Martins, F. Anti-Inflammatory Activity of Olive Oil Polyphenols—The Role of Oleacein and Its Metabolites. Biomedicines 2022, 10, 2990. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Russo, C.; Musumeci, L.; Navarra, M.; Lombardo, G.E. Oleacein Attenuates Lipopolysaccharide-Induced Inflammation in THP-1-Derived Macrophages by the Inhibition of TLR4/MyD88/NF-κB Pathway. Int. J. Mol. Sci. 2022, 23, 1206. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Orecchioni, M. Ley, How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Wenzel, U.; Piccoli, T.; Jacob, U.M.; Nicolosi, L.; Fazzolari, G.; Failla, G.; Fritsch, T.; Osakabe, N.; Calabrese, E.J. Investigating hormesis, aging, and neurodegeneration: From bench to clinics. Open Med. 2024, 19, 20240986. [Google Scholar] [CrossRef]
- Franceschelli, S.; De Cecco, F.; Pesce, M.; Ripari, P.; Guagnano, M.T.; Nuevo, A.B.; Grilli, A.; Sancilio, S.; Speranza, L. Hydroxytyrosol Reduces Foam Cell Formation and Endothelial Inflammation Regulating the PPARγ/LXRα/ABCA1 Pathway. Int. J. Mol. Sci. 2023, 24, 2057. [Google Scholar] [CrossRef] [PubMed]
- Berrougui, H.; Ikhlef, S.; Khalil, A. Extra Virgin Olive Oil Polyphenols Promote Cholesterol Efflux and Improve HDL Functionality. Evidence-Based Complement. Altern. Med. 2015, 2015, 208062. [Google Scholar] [CrossRef] [PubMed]
- de las Hazas, M.C.L.; del Saz-Lara, A.; Cedó, L.; Crespo, M.C.; Tomé-Carneiro, J.; Chapado, L.A.; Macià, A.; Visioli, F.; Escola-Gil, J.C.; Dávalos, A. Hydroxytyrosol Induces Dyslipidemia in an ApoB100 Humanized Mouse Model. Mol. Nutr. Food Res. 2024, 68, e2300508. [Google Scholar] [CrossRef]
- Acín, S.; Navarro, M.A.; Arbonés-Mainar, J.M.; Guillén, N.; Sarría, A.J.; Carnicer, R.; Surra, J.C.; Orman, I.; Segovia, J.C.; De La Torre, R.; et al. Hydroxytyrosol administration enhances atherosclerotic lesion development in Apo E deficient mice. J. Biochem. 2006, 140, 383–391. [Google Scholar] [CrossRef]
- González-Santiago, M.; Martín-Bautista, E.; Carrero, J.J.; Fonollá, J.; Baró, L.; Bartolomé, M.V.; Gil-Loyzaga, P.; López-Huertas, E. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 2006, 188, 35–42. [Google Scholar] [CrossRef]
- Pirisi, F.M.; Cabras, P.; Cao, C.F.; Migliorini, M.; Muggelli, M. Phenolic compounds in virgin olive oil. 2. Reappraisal of the extraction, HPLC separation, and quantification procedures. J. Agric. Food Chem. 2000, 48, 1191–1196. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–777. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xue, X. Detection of Total Reactive Oxygen Species in Adherent Cells by 2’,7’-Dichlorodihydrofluorescein Diacetate Staining. J. Vis. Exp. 2020. [Google Scholar]
- Alami, M.; Boumezough, K.; Zerif, E.; Zoubdane, N.; Khalil, A.; Bunt, T.; Laurent, B.; Witkowski, J.M.; Ramassamy, C.; Boulbaroud, S.; et al. In Vitro Assessment of the Neuroprotective Effects of Pomegranate (Punica granatum L.) Polyphenols Against Tau Phosphorylation, Neuroinflammation, and Oxidative Stress. Nutrients 2024, 16, 3667. [Google Scholar] [CrossRef]



| Extract | TPC (mg GAE/Kg of Oil) |
|---|---|
| EVOOPE+ | 1525.13 ± 21.21 *** |
| EVOOPE | 715.6 ± 8.61 |
| 0 (µg/mL) | 25 (µg/mL) | 50 (µg/mL) | 100 (µg/mL) | 150 (µg/mL) | 25 (µM) | 50 (µM) | 100 (µM) | 150 (µM) | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 100 ± 2.70% | - | - | - | - | - | - | - | - |
| EVOOPE+ | - | 129.68 ± 4.25% *** | 122.36 ± 0.17% ** | 107.85 ± 1.93% | 78.77 ± 2.14% ** | - | - | - | - |
| EVOOPE | - | 117.64 ± 2.37% | 107.69 ± 1.68% | 112.23 ± 2.92% | 121.28 ± 3.37% ** | - | - | - | - |
| HT | - | - | - | - | - | 120.07 ± 1.76% *** | 125.72 ± 0.37% *** | 115.18 ± 1.89% ** | 99.13 ± 1.47% |
| Tyr | - | - | - | - | - | 121.28 ± 3.37% ** | 117.47 ± 2.06% * | 109.29 ± 1.62% | 106.83 ± 3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boumezough, K.; Alami, M.; Fulop, T.; Zoubdane, N.; Salih, I.; Ramchoun, M.; Khalil, A.; Berrougui, H. Biological Activities Underlying the Cardiovascular Benefits of Olive Oil Polyphenols: Focus on Antioxidant, Anti-Inflammatory, and Anti-Atherogenic Effects. Int. J. Mol. Sci. 2025, 26, 11165. https://doi.org/10.3390/ijms262211165
Boumezough K, Alami M, Fulop T, Zoubdane N, Salih I, Ramchoun M, Khalil A, Berrougui H. Biological Activities Underlying the Cardiovascular Benefits of Olive Oil Polyphenols: Focus on Antioxidant, Anti-Inflammatory, and Anti-Atherogenic Effects. International Journal of Molecular Sciences. 2025; 26(22):11165. https://doi.org/10.3390/ijms262211165
Chicago/Turabian StyleBoumezough, Kaoutar, Mehdi Alami, Tamas Fulop, Nada Zoubdane, Ikram Salih, Mhamed Ramchoun, Abdelouahed Khalil, and Hicham Berrougui. 2025. "Biological Activities Underlying the Cardiovascular Benefits of Olive Oil Polyphenols: Focus on Antioxidant, Anti-Inflammatory, and Anti-Atherogenic Effects" International Journal of Molecular Sciences 26, no. 22: 11165. https://doi.org/10.3390/ijms262211165
APA StyleBoumezough, K., Alami, M., Fulop, T., Zoubdane, N., Salih, I., Ramchoun, M., Khalil, A., & Berrougui, H. (2025). Biological Activities Underlying the Cardiovascular Benefits of Olive Oil Polyphenols: Focus on Antioxidant, Anti-Inflammatory, and Anti-Atherogenic Effects. International Journal of Molecular Sciences, 26(22), 11165. https://doi.org/10.3390/ijms262211165








