Glycine soja Leaf and Stem Extract Ameliorates Atopic Dermatitis-like Skin Inflammation by Inhibiting JAK/STAT Signaling
Abstract
1. Introduction
2. Results
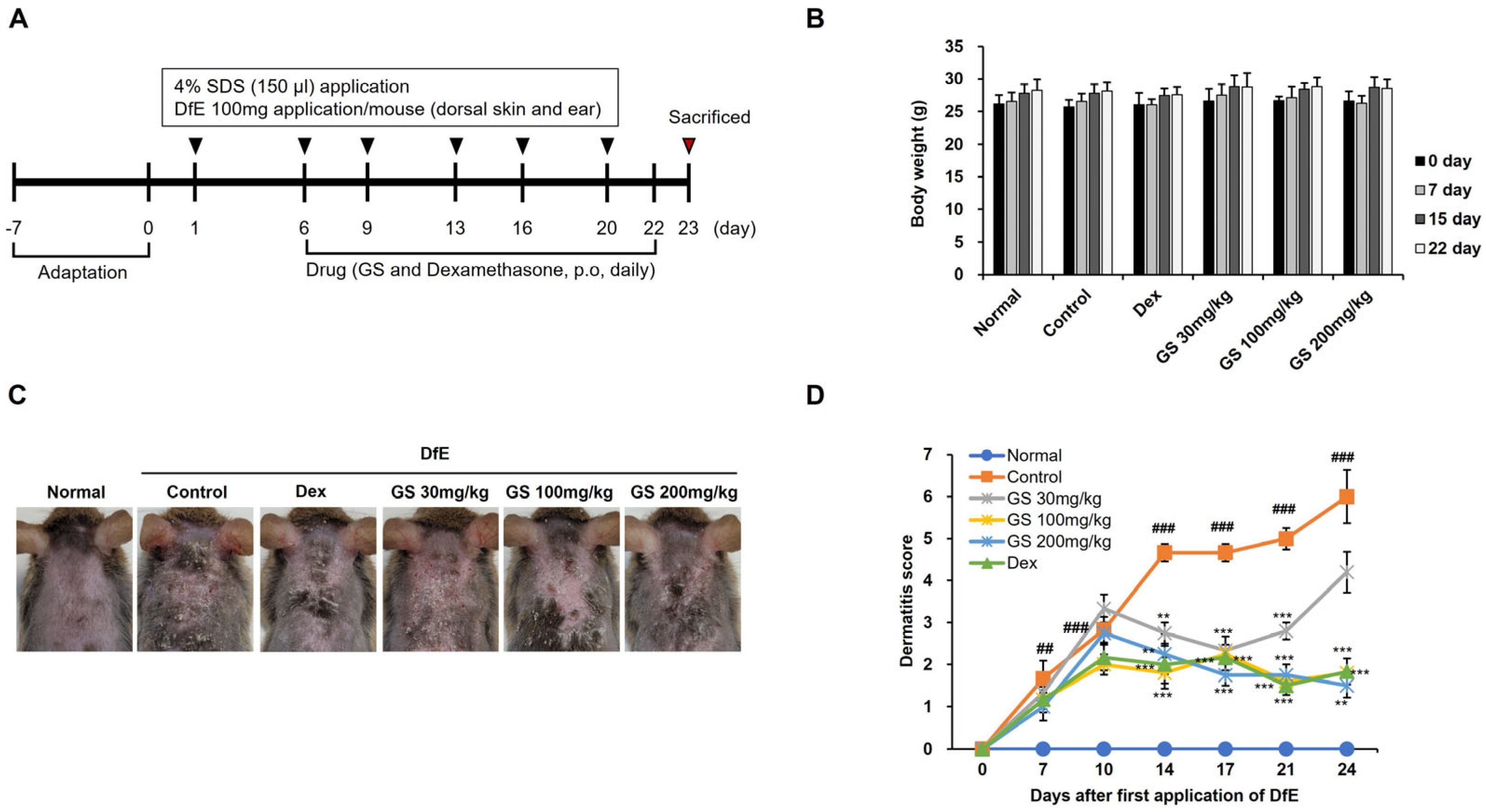
2.1. AD Symptoms
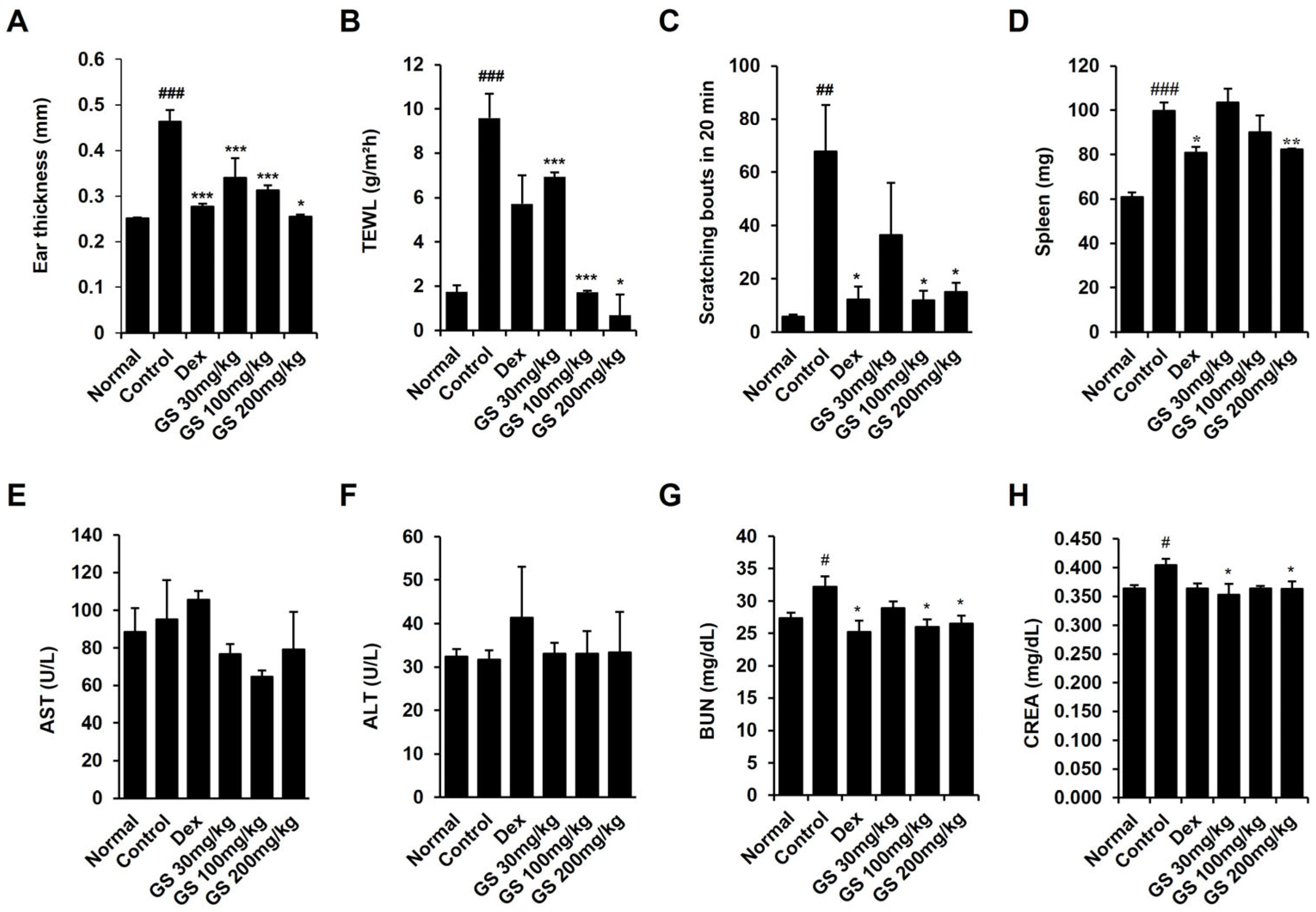
2.2. Effect of GS on Epidermal Thickness, Scratching, Transepidermal Water Loss (TEWL), and Spleen Weight
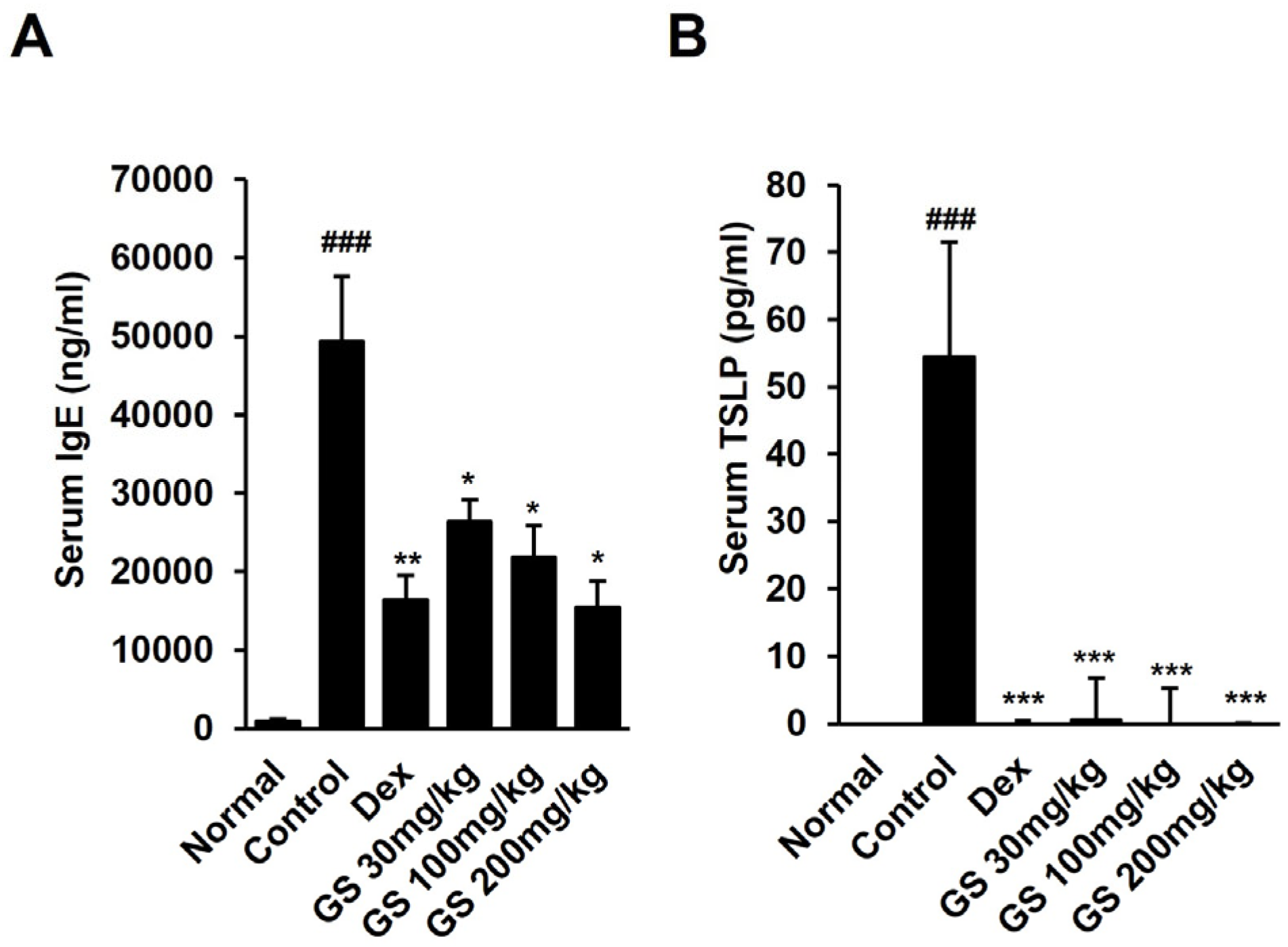
2.3. Effect of GS on AST, ALT, BUN, Creatinine, TSLP, and IgE Levels in Mouse Serum
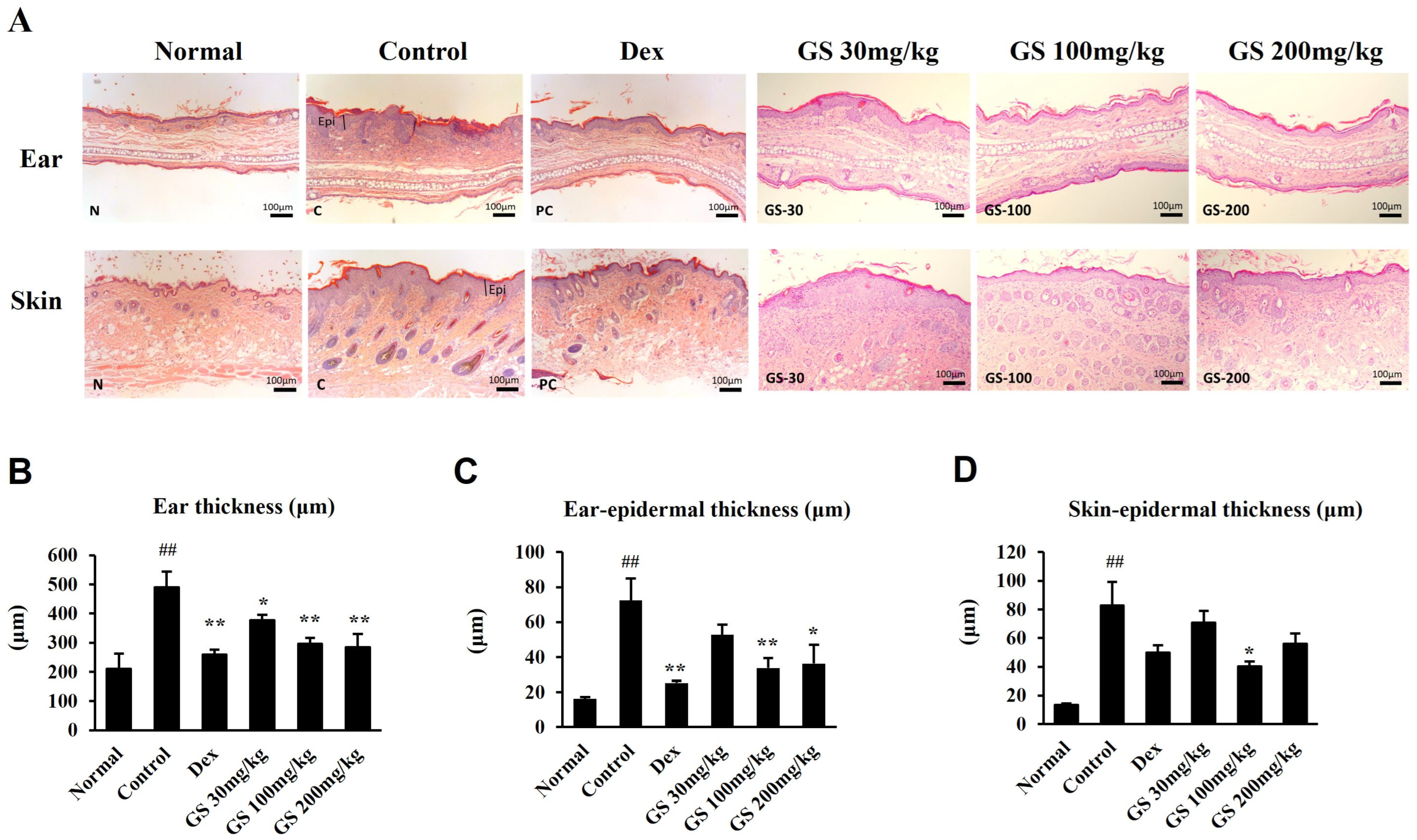
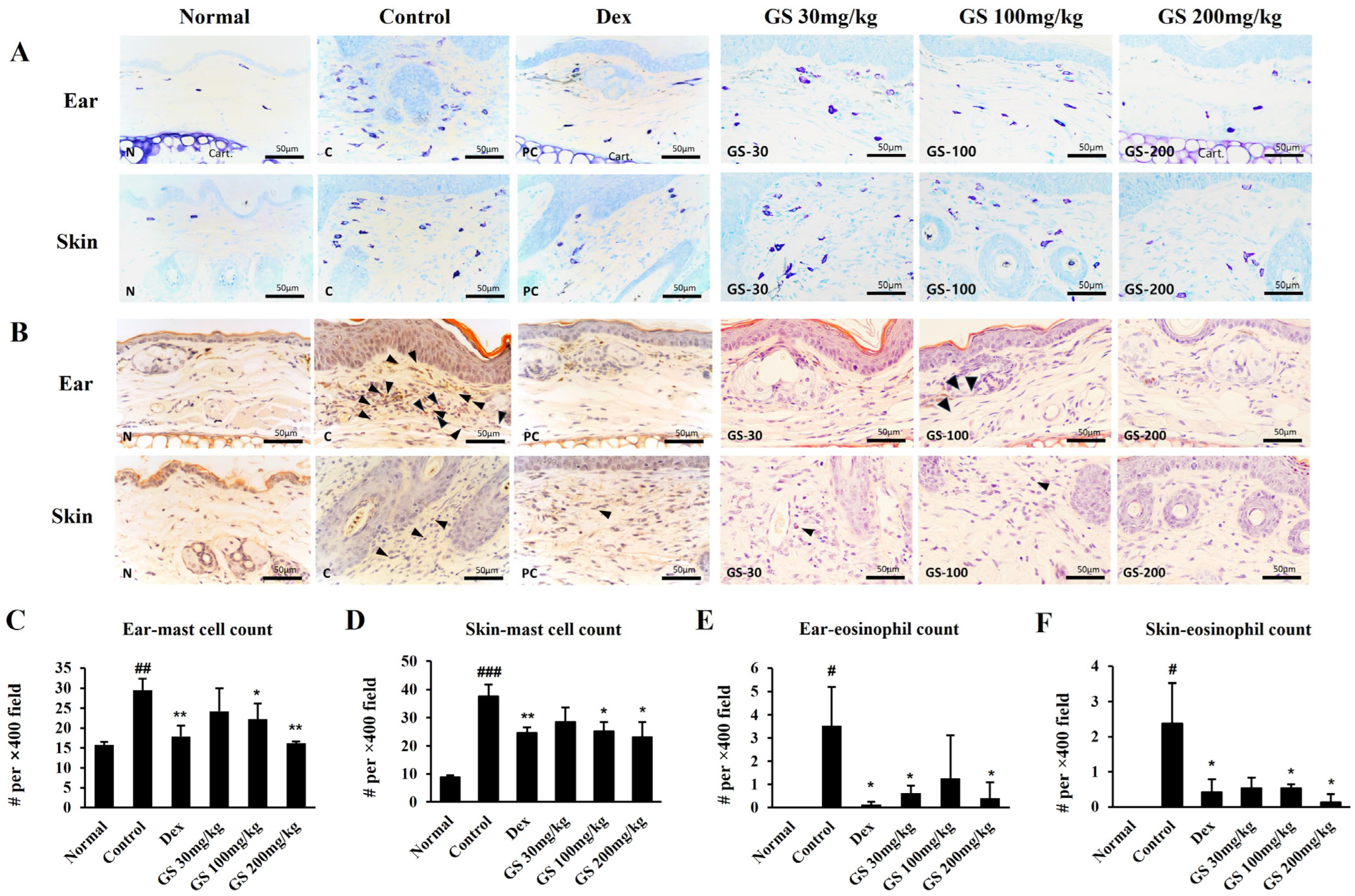
2.4. Skin Histopathology
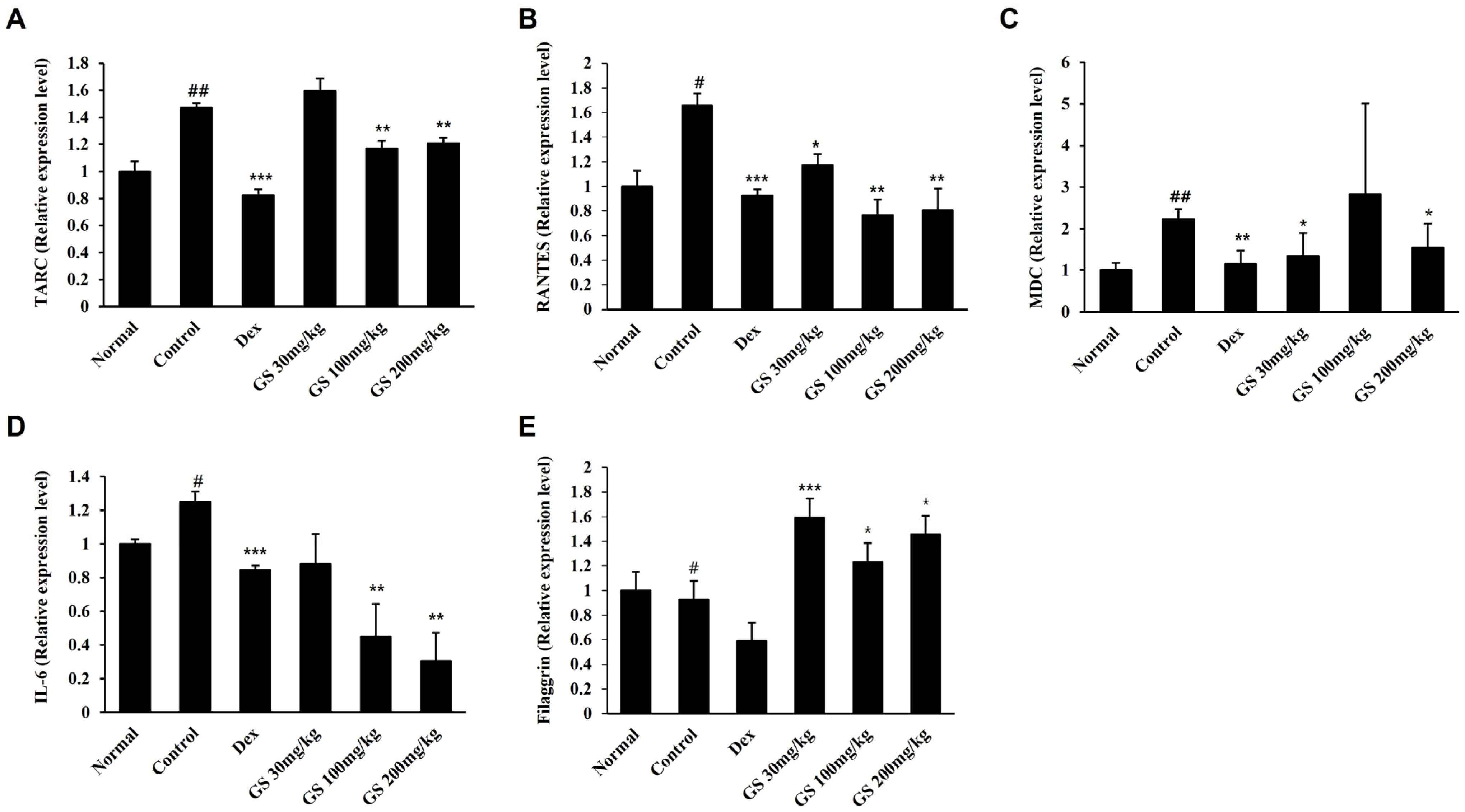
2.5. AD-Related Gene Expression in the Skin
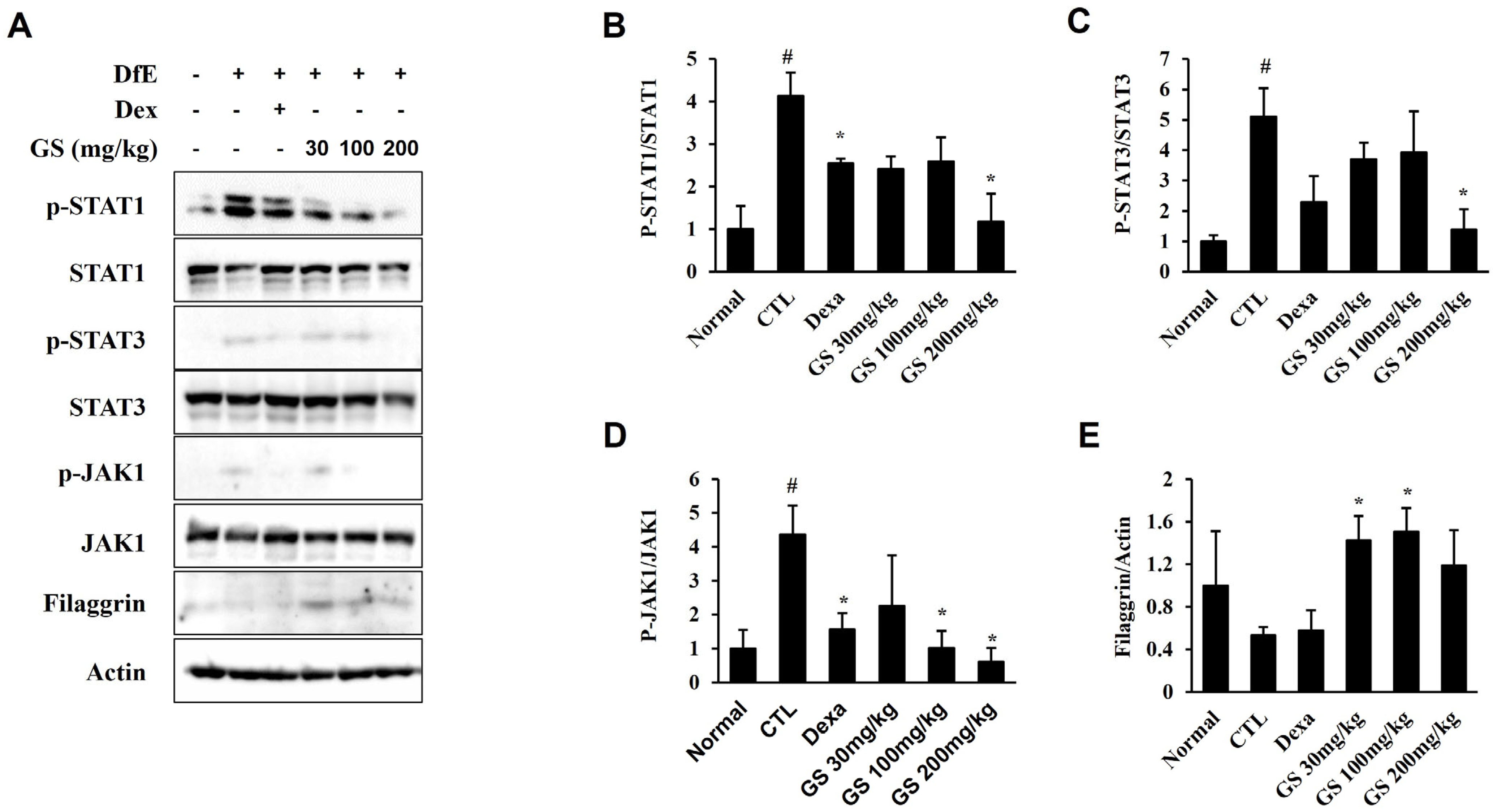
2.6. Effect of GS on Signaling Pathways in the Skin
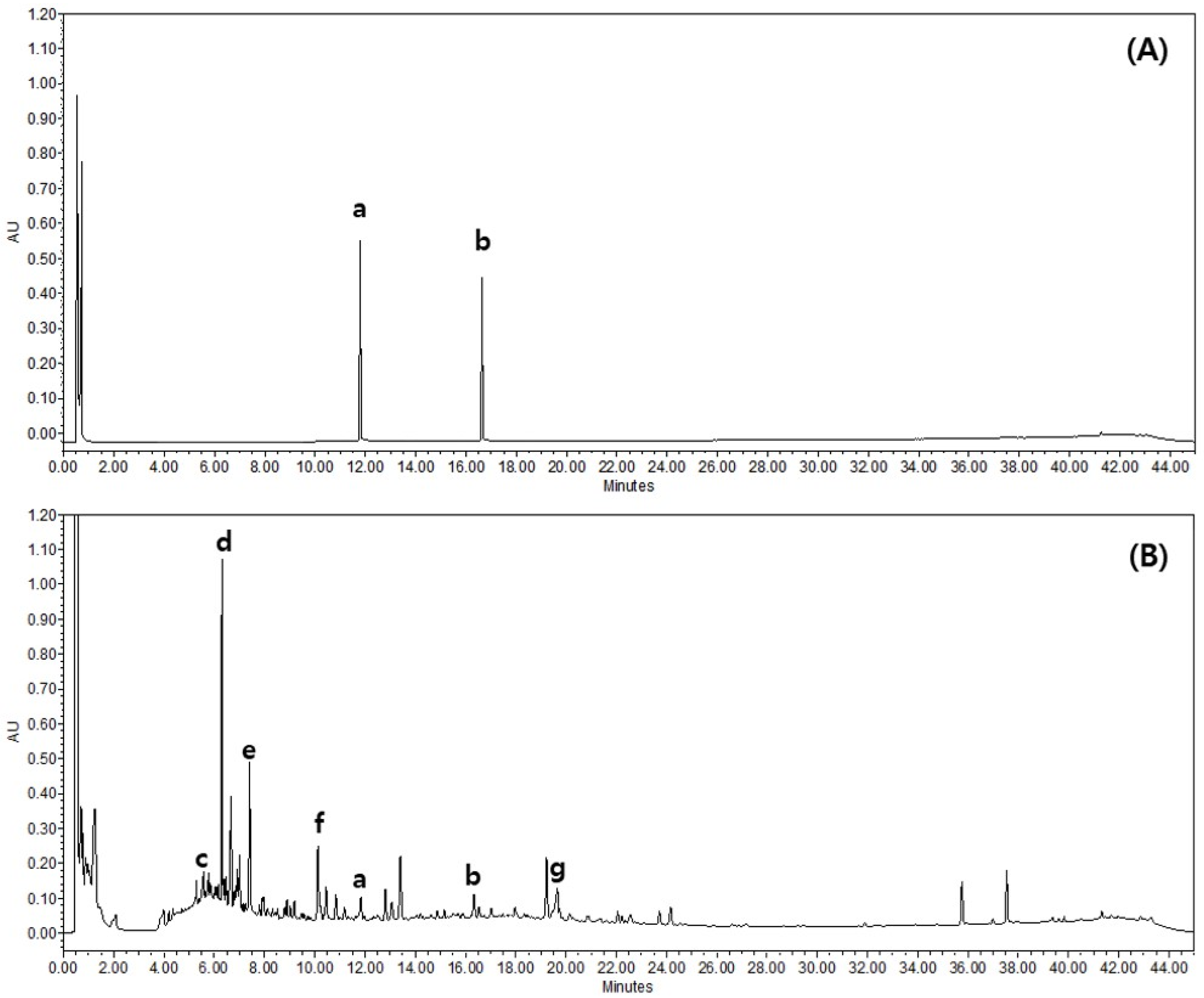
2.7. Quantitative Analysis of GS
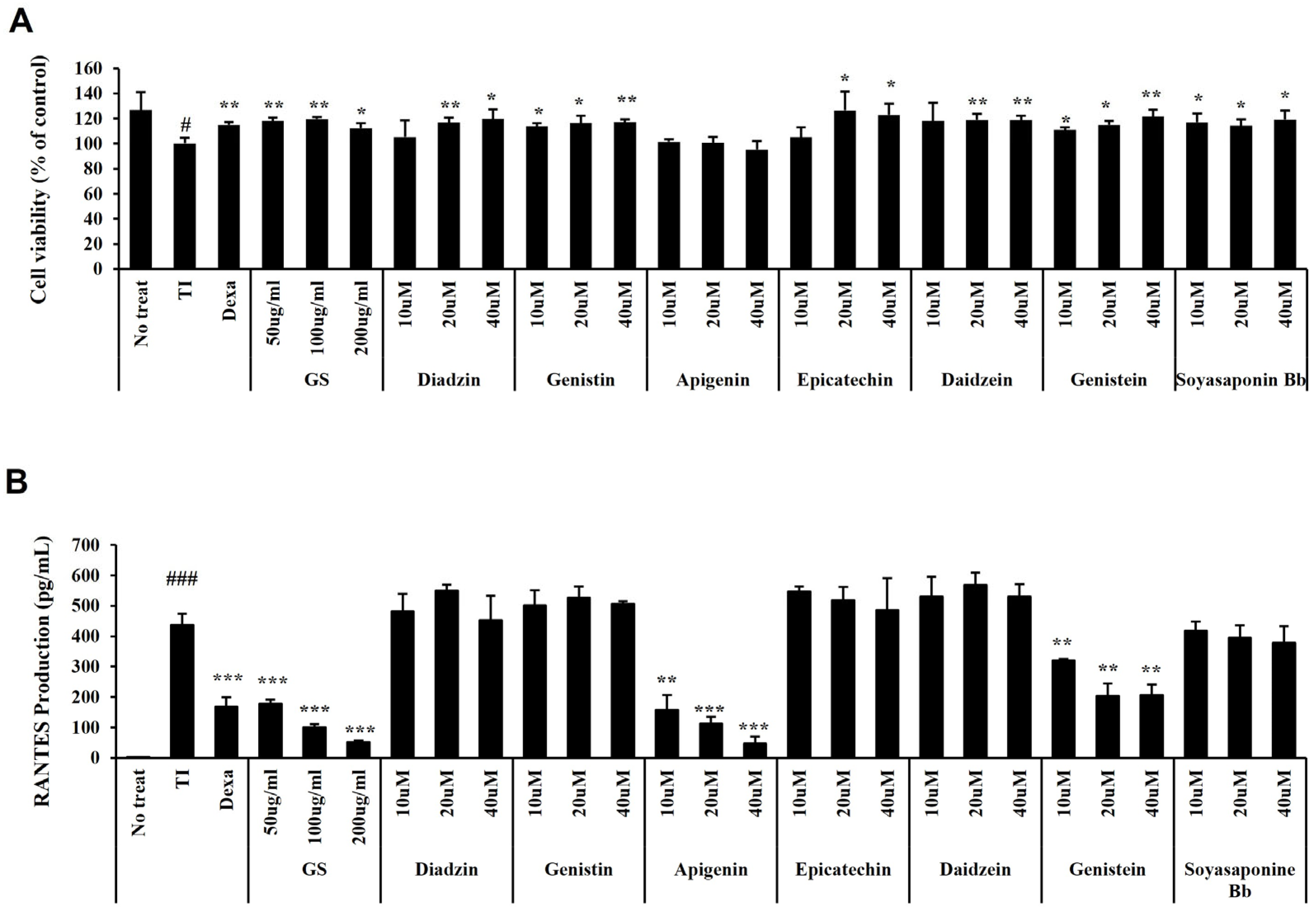
2.8. Effect of GS and Its Components on RANTES Secretion in HaCaT Cells
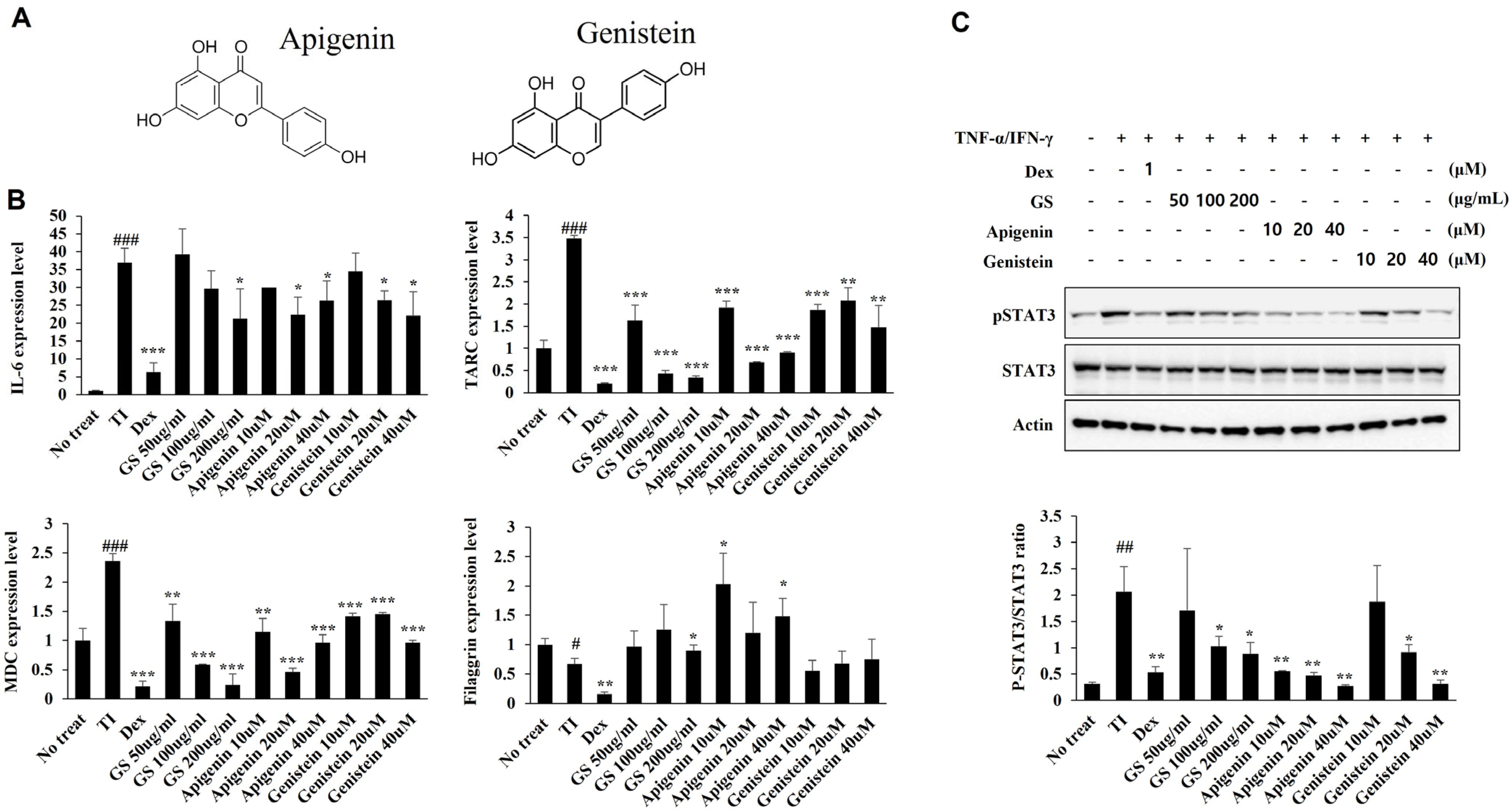
2.9. Effect of GS and Its Components on mRNA Expression and STAT Pathways in HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Extraction of GS
4.2. Application of DfE and GS
4.3. Assessment of Ear Thickness and Dermatitis Scores
4.4. Determination of TSLP and IgE in Mouse Serum
4.5. Skin Barrier Assessment
4.6. Evaluation of Scratching Behavior
4.7. Measurement of Biomarkers for Liver and Kidney Function
4.8. Histopathological Analysis
4.9. Analytical Conditions
4.10. HaCaT Cell Culture
4.11. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
4.12. Immunoblot
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| RANTES | Regulated on activation, normal T cell expressed and secreted chemokine |
| TARC | Thymus- and activation-regulated chemokine |
| MDC | Macrophage-derived chemokine |
| TSLP | Thymic stromal lymphopoietin |
| IL | Interleukin |
| IgE | Immunoglobulin E |
| JAK | Janus kinase |
| STAT | Signal transducer and activator of transcription |
| IFN-γ | Interferon-gamma |
| TNF-α | Tumor necrosis factor-alpha |
| GS | Glycine soja |
| DfE | Dermatophargoides farinae extract |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| BUN | Blood urea nitrogen |
| ELISA | Enzyme-linked immunosorbent assay |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| TEWL | Transepidermal water loss |
References
- Afshari, M.; Kolackova, M.; Rosecka, M.; Čelakovská, J.; Krejsek, J. Unraveling the skin; a comprehensive review of atopic dermatitis, current understanding, and approaches. Front. Immunol. 2024, 15, 1361005. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.M.; Caubet, J.C.; Boguniewicz, M.; Eigenmann, P.A. Evaluation of food allergy in patients with atopic dermatitis. J. Allergy Clin. Immunol. Pract. 2013, 1, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Katta, R.; Schlichte, M. Diet and dermatitis: Food triggers. J. Clin. Aesthet. Dermatol. 2014, 7, 30–36. [Google Scholar]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.H.; Oh, J.H.; Chung, J.H. Skullcapflavone II suppresses TNF-α/IFN-γ-induced TARC, MDC, and CTSS production in HaCaT Cells. Int. J. Mol. Sci. 2021, 22, 6428. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.H.; Chung, W.H.; Wu, P.C.; Chen, C.B. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front. Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Sokolowska-Wojdylo, M.; Ruckemann-Dziurdzinska, K.; Roszkiewicz, J.; Nowicki, R.J. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Adv. Dermatol. Allergol. 2014, 31, 84–91. [Google Scholar] [CrossRef]
- Kofsky, J.; Zhang, H.; Song, B.H. The untapped genetic reservoir: The past, current, and future applications of the wild soybean (Glycine soja). Front. Plant Sci. 2018, 9, 949. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the use of botanicals in anti-aging cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef]
- Jing, C.; Wen, Z.; Zou, P.; Yuan, Y.; Jing, W.; Li, Y.; Zhang, C. Consumption of Black legumes Glycine soja and Glycine max lowers serum lipids and alters the gut microbiome profile in mice fed a high-fat diet. J. Agric. Food Chem. 2018, 66, 7367–7375. [Google Scholar] [CrossRef]
- Kargozar, R.; Azizi, H.; Salari, R. A review of effective herbal medicines in controlling menopausal symptoms. Electron. Physician 2017, 9, 5826–5833. [Google Scholar] [CrossRef] [PubMed]
- Chuarienthong, P.; Lourith, N.; Leelapornpisid, P. Clinical efficacy comparison of anti-wrinkle cosmetics containing herbal flavonoids. Int. J. Cosmet. Sci. 2010, 32, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Son, E.; Kim, S.H.; Kim, D.S. Protective effects of Glycine soja leaf and stem extract against chondrocyte inflammation and osteoarthritis. Int. J. Mol. Sci. 2023, 24, 4829. [Google Scholar] [CrossRef] [PubMed]
- Oshio, T.; Sasaki, Y.; Funakoshi-Tago, M.; Aizu-Yokota, E.; Sonoda, Y.; Matsuoka, H.; Kasahara, T. Dermatophagoides farinae extract induces severe atopic dermatitis in NC/Nga mice, which is effectively suppressed by the administration of tacrolimus ointment. Int. Immunopharmacol. 2009, 9, 403–411. [Google Scholar] [CrossRef]
- Kim, M.; Yuk, H.J.; Min, Y.; Kim, D.S.; Sung, Y.Y. Securinega suffruticosa extract alleviates atopy-like lesions in NC/Nga mice via inhibition of the JAK1-STAT1/3 pathway. Biomed. Pharmacother. 2023, 169, 115903. [Google Scholar] [CrossRef]
- Liu, F.T.; Goodarzi, H.; Chen, H.Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef]
- Beck, L.A.; Cork, M.J.; Amagai, M.; De Benedetto, A.; Kabashima, K.; Hamilton, J.D.; Rossi, A.B. Type 2 inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innov. 2022, 2, 100131. [Google Scholar] [CrossRef]
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The translational revolution in atopic dermatitis: The paradigm shift from pathogenesis to treatment. Cell. Mol. Immunol. 2023, 20, 448–474. [Google Scholar] [CrossRef]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef]
- Kakinuma, T.; Nakamura, K.; Wakugawa, M.; Mitsui, H.; Tada, Y.; Saeki, H.; Torii, H.; Komine, M.; Asahina, A.; Tamaki, K. Serum macrophage-derived chemokine (MDC) levels are closely related with the disease activity of atopic dermatitis. Clin. Exp. Immunol. 2002, 127, 270–273. [Google Scholar] [CrossRef]
- Yamamura, Y.; Nakashima, C.; Otsuka, A. Interplay of cytokines in the pathophysiology of atopic dermatitis: Insights from murine models and human. Front. Med. 2024, 11, 1342176. [Google Scholar] [CrossRef] [PubMed]
- Maskey, A.R.; Mo, X.; Li, X.M. Preclinical Models of Atopic Dermatitis Suitable for Mechanistic and Therapeutic Investigations. J. Inflamm. Res. 2024, 17, 6955–6970. [Google Scholar] [CrossRef]
- Hatano, Y.; Elias, P.M. “Outside-to-inside”, “inside-to-outside”, and “intrinsic” endogenous pathogenic mechanisms in atopic dermatitis: Keratinocytes as the key functional cells involved in both permeability barrier dysfunction and immunological alterations. Front. Immunol. 2023, 14, 1239251. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Irvine, A.D.; Brunner, P.M.; Kim, B.S.; Boguniewicz, M.; Parmentier, J.; Platt, A.M.; Kabashima, K. The role of Janus kinase signaling in the pathology of atopic dermatitis. J. Allergy Clin. Immunol. 2023, 152, 1394–1404. [Google Scholar] [CrossRef]
- Cabanillas, B.; Novak, N. Atopic dermatitis and filaggrin. Curr. Opin. Immunol. 2016, 42, 1–8. [Google Scholar] [CrossRef]
- Shim, K.S.; Song, H.K.; Park, M.; Kim, H.J.; Jang, S.; Kim, T.; Kim, K.M. Reynoutria japonica consisted of emodin-8-β-D-glucoside ameliorates Dermatophagoides farinae extract-induced atopic dermatitis-like skin inflammation in mice by inhibiting JAK/STAT signaling. Biomed. Pharmacother. 2024, 176, 116765. [Google Scholar] [CrossRef]
- Jin, S.E.; Seo, C.S.; Jeon, W.Y.; Oh, Y.J.; Shin, H.K.; Jeong, H.G.; Ha, H. Evodiae Fructus extract suppresses inflammatory response in HaCaT cells and improves house dust mite-induced atopic dermatitis in NC/Nga mice. Sci. Rep. 2024, 14, 472. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, M.; Jang, S.; Song, H.K.; Lee, S.K.; Kim, T. Pulsatilla koreana Nakai Extract Attenuates Atopic Dermatitis-like Symptoms by Regulating Skin Barrier Factors and Inhibiting the JAK/STAT Pathway. Int. J. Mol. Sci. 2025, 26, 2994. [Google Scholar] [CrossRef]
- Ren, Z.; Yin, X.; Liu, L.; Zhang, L.; Shen, W.; Fang, Z.; Yu, Q.; Qin, L.; Chen, L.; Jia, R.; et al. Localization in soybean seeds: Comparative analysis of wild (Glycine soja) and cultivated (Glycine max) varieties. Food Chem. 2024, 456, 139883. [Google Scholar] [CrossRef]
- Wang, A.; Wei, J.; Lu, C.; Chen, H.; Zhong, X.; Lu, Y.; Li, L.H.; Huang, H.; Dai, Z.; Han, L. Genistein suppresses psoriasis-related inflammation through a STAT3-NF-κB-dependent mechanism in keratinocytes. Int. Immunopharmacol. 2019, 69, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kogiso, M.; Mitsuya, K.; Komatsu, T.; Yamamoto, S. Genistein suppresses development of spontaneous atopic-like dermatitis in NC/Nga mice. J. Nutr. Sci. Vitaminol. 2006, 52, 293–296. [Google Scholar] [CrossRef]
- Park, C.H.; Min, S.Y.; Yu, H.W.; Kim, K.; Kim, S.; Lee, H.J.; Kim, J.H.; Park, Y.J. Effects of apigenin on RBL-2H3, RAW264.7, and HaCaT cells: Anti-allergic, anti-inflammatory, and skin-protective activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Singh, V.K.; Sahoo, D.; Agrahari, K.; Khan, A.; Mukhopadhyay, P.; Chanda, D.; Yadav, N.P. Anti-inflammatory, anti-proliferative and anti-psoriatic potential of apigenin in RAW 264.7 cells, HaCaT cells and psoriasis like dermatitis in BALB/c mice. Life Sci. 2023, 328, 121909. [Google Scholar] [CrossRef] [PubMed]
- Man, M.Q.; Hupe, M.; Sun, R.; Man, G.; Mauro, T.M.; Elias, P.M. Topical apigenin alleviates cutaneous inflammation in murine models. Evid. Based Complement. Altern. Med. 2012, 2012, 912028. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Kim, J.S.; Oh, H.; Kim, Y.S.; Jang, S.I. Apigenin inhibits IL-31 cytokine in human mast cell and mouse skin tissues. Molecules 2019, 24, 1290. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef]
- Alessandrello, C.; Sanfilippo, S.; Minciullo, P.L.; Gangemi, S. An overview on atopic dermatitis, oxidative stress, and psychological stress: Possible role of nutraceuticals as an additional therapeutic strategy. Int. J. Mol. Sci. 2024, 25, 5020. [Google Scholar] [CrossRef]
- Haag, C.; Alexis, A.; Aoki, V.; Bissonnette, R.; Blauvelt, A.; Chovatiya, R.; Cork, M.J.; Danby, S.G.; Eichenfield, L.F.; Eyerich, K.; et al. A practical guide to using oral Janus kinase inhibitors for atopic dermatitis from the International Eczema Council. Br. J. Dermatol. 2024, 192, 135–143. [Google Scholar] [CrossRef]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J. Allergy Clin. Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xian, Y.F.; Hu, Z.; Loo, S.K.F.; Ip, S.P.; Chan, W.Y.; Lin, Z.X.; Wu, J.C.Y. Efficacy and action mechanisms of a Chinese herbal formula on experimental models of atopic dermatitis. J. Ethnopharmacol. 2021, 274, 114021. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, Y.-Y.; Kim, M.; Kim, D.-S.; Son, E. Glycine soja Leaf and Stem Extract Ameliorates Atopic Dermatitis-like Skin Inflammation by Inhibiting JAK/STAT Signaling. Int. J. Mol. Sci. 2025, 26, 4560. https://doi.org/10.3390/ijms26104560
Sung Y-Y, Kim M, Kim D-S, Son E. Glycine soja Leaf and Stem Extract Ameliorates Atopic Dermatitis-like Skin Inflammation by Inhibiting JAK/STAT Signaling. International Journal of Molecular Sciences. 2025; 26(10):4560. https://doi.org/10.3390/ijms26104560
Chicago/Turabian StyleSung, Yoon-Young, Misun Kim, Dong-Seon Kim, and Eunjung Son. 2025. "Glycine soja Leaf and Stem Extract Ameliorates Atopic Dermatitis-like Skin Inflammation by Inhibiting JAK/STAT Signaling" International Journal of Molecular Sciences 26, no. 10: 4560. https://doi.org/10.3390/ijms26104560
APA StyleSung, Y.-Y., Kim, M., Kim, D.-S., & Son, E. (2025). Glycine soja Leaf and Stem Extract Ameliorates Atopic Dermatitis-like Skin Inflammation by Inhibiting JAK/STAT Signaling. International Journal of Molecular Sciences, 26(10), 4560. https://doi.org/10.3390/ijms26104560







