Bacillus-Based Biocontrol Agents Mediate Pathogen Killing by Biodegradable Antimicrobials from Macrolactin Family
Abstract
1. Introduction
2. Results
2.1. Cultivation of the Strain and Isolation and Identification of Metabolites with Antibiotic Activity
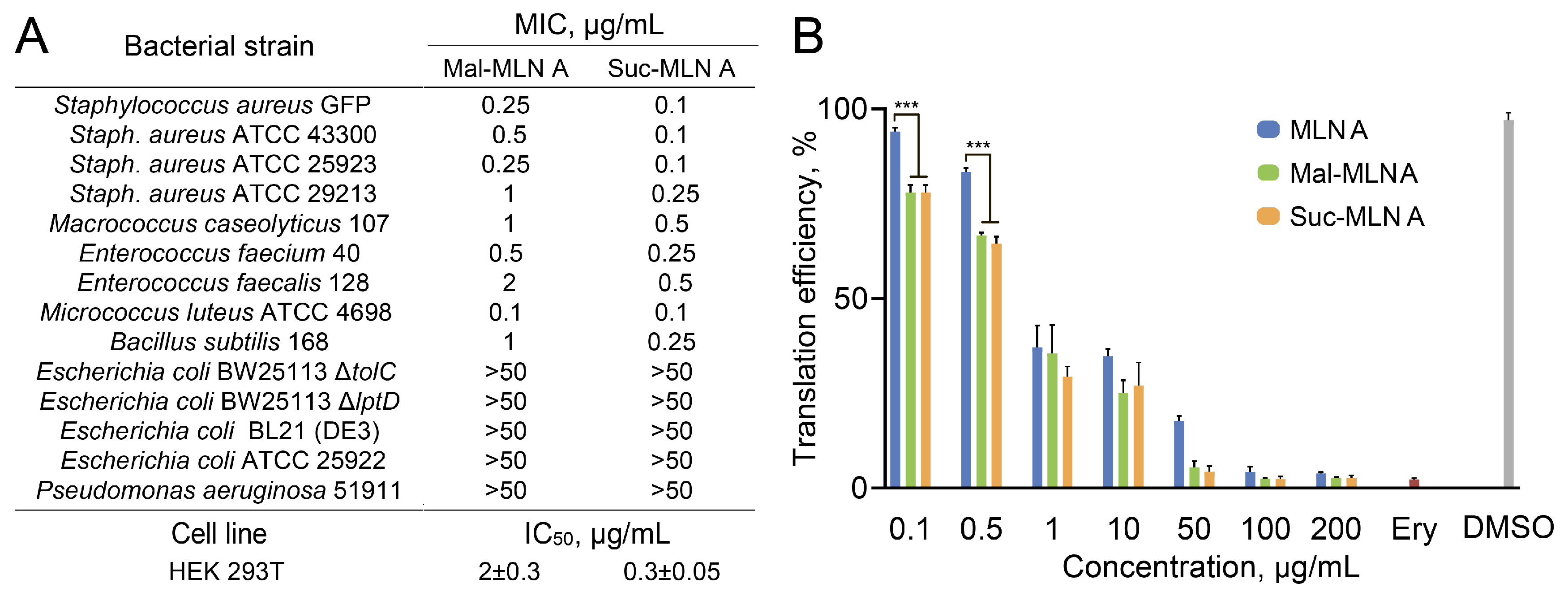
2.2. Bacterial Susceptibility and Cytotoxicity of the Macrolactins
2.3. Inhibition of Bacterial Protein Synthesis In Vitro by the Macrolactins
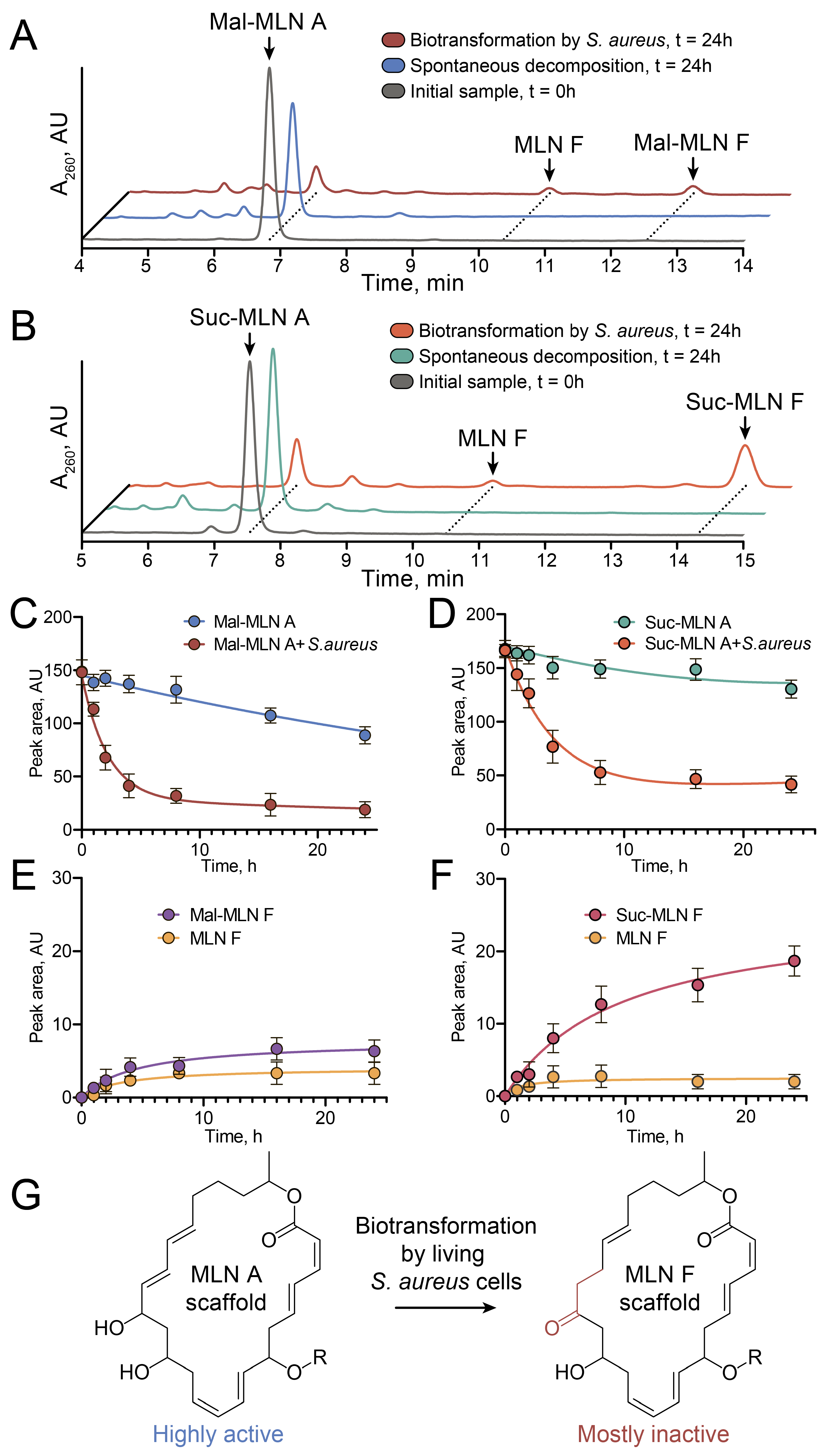
2.4. Transformation of Acylated Macrolactins in Staphylococcus aureus Cells
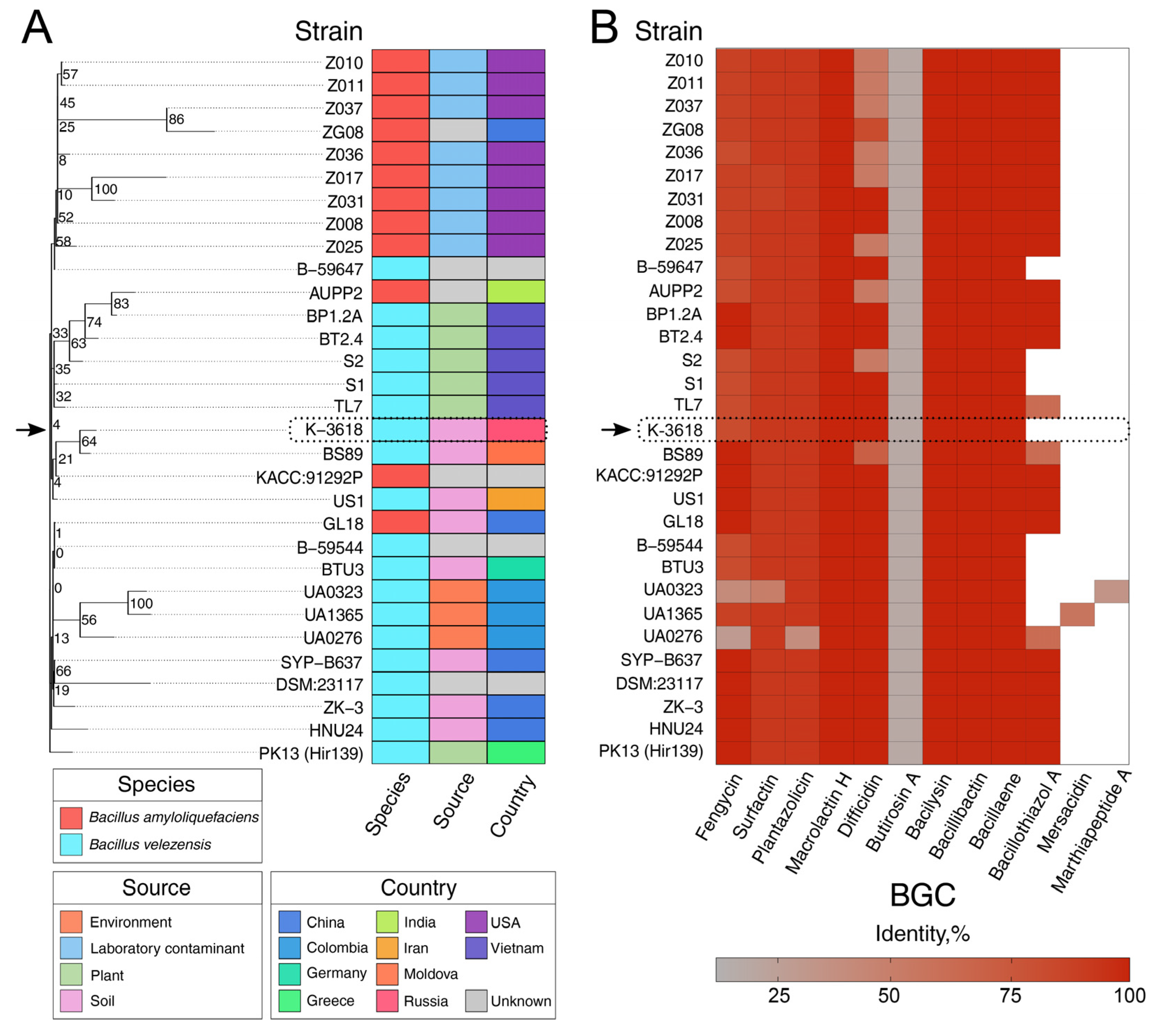
2.5. MLN Biosynthetic Gene Cluster Is Highly Abundant in Bacillus velezensis and Bacillus amyloliquefaciens Species
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Cell Lines
4.2. Genome Sequencing, Assembly, and Annotation
4.3. Phylogeny Reconstruction
4.4. Cultivation, Isolation, and Purification of Macrolactins
4.5. LC-MS Analysis
4.6. NMR
4.7. Antibacterial Activity Assessment
4.8. Cytotoxicity Assessment
4.9. Inhibition Assays in Cell-Free Translation System
4.10. Biotransformation of suc-MLN A and mal-MLN A by Staphylococcus aureus
4.11. Analysis of Reaction Broth and Isolation and Purification of Target Compounds After Incubation of the Macrolactins with Staphylococcus aureus Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, B.P.; Green, T.A.; Loker, A.J. Biological Control and Integrated Pest Management in Organic and Conventional Systems. Biol. Control 2020, 140, 104095. [Google Scholar] [CrossRef]
- Zhong, X.; Jin, Y.; Ren, H.; Hong, T.; Zheng, J.; Fan, W.; Hong, J.; Chen, Z.; Wang, A.; Lu, H.; et al. Research Progress of Bacillus Velezensis in Plant Disease Resistance and Growth Promotion. Front. Ind. Microbiol. 2024, 2, 1442980. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Hwang, B.-S.; Baek, K.-H. Bacillus velezensis: A Beneficial Biocontrol Agent or Facultative Phytopathogen for Sustainable Agriculture. Agronomy 2023, 13, 840. [Google Scholar] [CrossRef]
- Tran, C.; Cock, I.E.; Chen, X.; Feng, Y. Antimicrobial Bacillus: Metabolites and Their Mode of Action. Antibiotics 2022, 11, 88. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Liu, Y. Contribution of Macrolactin in Bacillus velezensis CLA178 to the Antagonistic Activities against Agrobacterium tumefaciens C58. Arch. Microbiol. 2021, 203, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, B.; Zhang, N.; Waseem, R.; Shen, Q.; Huang, Q. Production of Bacillomycin- and Macrolactin-Type Antibiotics by Bacillus Amyloliquefaciens NJN-6 for Suppressing Soilborne Plant Pathogens. J. Agric. Food Chem. 2012, 60, 2976–2981. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Y.; Xie, S.; Yu, X.; Bian, C.; Wu, H.; Wang, Y.; Ding, T. Biocontrol and Molecular Characterization of Bacillus velezensis D against Tobacco Bacterial Wilt. Phytopathol. Res. 2023, 5, 50. [Google Scholar] [CrossRef]
- Yousfi, S.; Krier, F.; Deracinois, B.; Steels, S.; Coutte, F.; Frikha-Gargouri, O. Characterization of Bacillus Velezensis 32a Metabolites and Their Synergistic Bioactivity against Crown Gall Disease. Microbiol. Res. 2024, 280, 127569. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xu, Q.; Liu, X.; Cao, X.; Ni, K.; Jiao, B. Marine Drugs–Macrolactins. Chem. Biodivers. 2008, 5, 1669–1674. [Google Scholar] [CrossRef]
- Wu, T.; Xiao, F.; Li, W. Macrolactins: Biological Activity and Biosynthesis. Mar. Life Sci. Technol. 2021, 3, 62–68. [Google Scholar] [CrossRef]
- Gustafson, K.; Roman, M.; Fenical, W. The Macrolactins, a Novel Class of Antiviral and Cytotoxic Macrolides from a Deep-Sea Marine Bacterium. J. Am. Chem. Soc. 1989, 111, 7519–7524. [Google Scholar] [CrossRef]
- Xu, Y.; Song, Y.; Ning, Y.; Li, S.; Qu, Y.; Jiao, B.; Lu, X. Macrolactin XY, a Macrolactin Antibiotic from Marine-Derived Bacillus subtilis sp. 18. Mar. Drugs 2024, 22, 331. [Google Scholar] [CrossRef]
- Jaruchoktaweechai, C.; Suwanborirux, K.; Tanasupawatt, S.; Kittakoop, P.; Menasveta, P. New Macrolactins from a Marine Bacillus sp. Sc026. J. Nat. Prod. 2000, 63, 984–986. [Google Scholar] [CrossRef]
- Romero-Tabarez, M.; Jansen, R.; Sylla, M.; Lünsdorf, H.; Häußler, S.; Santosa, D.A.; Timmis, K.N.; Molinari, G. 7-O-Malonyl Macrolactin A, a New Macrolactin Antibiotic from Bacillus Subtilis Active against Methicillin-Resistant Staphylococcus Aureus, Vancomycin-Resistant Enterococci, and a Small-Colony Variant of Burkholderia Cepacia. Antimicrob. Agents Chemother. 2006, 50, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Chen, X.-H.; Vater, J.; Franke, P.; Nicholson, G.; Borriss, R.; Süssmuth, R.D. Macrolactin Is the Polyketide Biosynthesis Product of the Pks2 Cluster of Bacillus Amyloliquefaciens FZB42. J. Nat. Prod. 2007, 70, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Dong, S.; Liu, Y.; Feng, Y.; Li, H.; Yun, C.-H.; Cui, Q.; Li, W. Structural Basis of Specificity for Carboxyl-Terminated Acyl Donors in a Bacterial Acyltransferase. J. Am. Chem. Soc. 2020, 142, 16031–16038. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Kwon, M.J.; Park, J.B.; Kim, D.; Kim, D.-H.; Kang, J.-S.; Kim, C.-G.; Oh, E.; Bae, S.K. Metabolic Drug-Drug Interaction Potential of Macrolactin A and 7-O-Succinyl Macrolactin A Assessed by Evaluating Cytochrome P450 Inhibition and Induction and UDP-Glucuronosyltransferase Inhibition In Vitro. Antimicrob. Agents Chemother. 2014, 58, 5036–5046. [Google Scholar] [CrossRef]
- Kim, J.M.; Jung, J.W.; Kim, D.H.; Kang, J.S.; Kim, C.G.; Kang, H.E. A Simple and Sensitive HPLC-UV Determination of 7-O-succinyl Macrolactin A in Rat Plasma and Urine and Its Application to a Pharmacokinetic Study. Biomed. Chromatogr. 2013, 27, 273–279. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, J.M.; Kwon, M.H.; Kim, D.H.; Kang, H.E. Pharmacokinetics of Macrolactin A and 7-O-Succinyl Macrolactin A in Mice. Xenobiotica 2014, 44, 547–554. [Google Scholar] [CrossRef]
- Salazar, F.; Ortiz, A.; Sansinenea, E. A Strong Antifungal Activity of 7-O-Succinyl Macrolactin A vs Macrolactin A from Bacillus Amyloliquefaciens ELI149. Curr. Microbiol. 2020, 77, 3409–3413. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, H.K.; Kim, K.M.; Kim, C.K.; Jeong, M.H.; Ko, C.Y.; Moon, K.H.; Kang, J.S. Antibacterial Activities of Macrolactin a and 7-O-Succinyl Macrolactin a from Bacillus Polyfermenticus KJS-2 against Vancomycin-Resistant Enterococci and Methicillin-Resistant Staphylococcus aureus. Arch. Pharm. Res. 2011, 34, 147–152. [Google Scholar] [CrossRef]
- Nagao, T.; Adachi, K.; Sakai, M.; Nishijima, M.; Sano, H. Novel Macrolactins as Antibiotic Lactones from a Marine Bacterium. J. Antibiot. 2001, 54, 333–339. [Google Scholar] [CrossRef]
- Vasilchenko, A.S.; Lukyanov, D.A.; Dilbaryan, D.S.; Usachev, K.S.; Poshvina, D.V.; Taldaev, A.K.; Nikandrova, A.A.; Imamutdinova, A.N.; Garaeva, N.S.; Bikmullin, A.G.; et al. Macrolactin a Is an Inhibitor of Protein Biosynthesis in Bacteria. Biochimie 2025, 232, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-S.; Zheng, C.-J.; Lee, S.; Kwak, J.-H.; Kim, W.-G. Macrolactin N, a New Peptide Deformylase Inhibitor Produced by Bacillus subtilis. Bioorg. Med. Chem. Lett. 2006, 16, 4889–4892. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.-J.; Zheng, C.-J.; Kim, W.-G. Macrolactin S, a New Antibacterial Agent with Fab G-Inhibitory Activity from Bacillus sp. AT28. J. Antibiot. 2008, 61, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Kalai, K.; Rufus, A.C.; Manz, A.M.; Elangovan, E. Unveiling the Antimicrobial Potential of 7-O-Succinyl Macrolactin F from Bacillus subtilis Group against HtsA Siderophore Receptor of Staphylococcus aureus: A Computational Exploration. Biomed. Biotechnol. Res. J. 2024, 8, 92–99. [Google Scholar] [CrossRef]
- Terekhov, S.S.; Smirnov, I.V.; Malakhova, M.V.; Samoilov, A.E.; Manolov, A.I.; Nazarov, A.S.; Danilov, D.V.; Dubiley, S.A.; Osterman, I.A.; Rubtsova, M.P.; et al. Ultrahigh-Throughput Functional Profiling of Microbiota Communities. Proc. Natl. Acad. Sci. USA 2018, 115, 9551–9556. [Google Scholar] [CrossRef]
- Duddeck, H. E. Pretsch, P. Bühlmann, C. Affolter. Structure Determination of Organic Compounds—Tables of Spectra Data. Springer, Berlin, 2000. 421 pp. plus CD-ROM. Price £ 40.39, DM 79.00. ISBN 3 540 67815 8. Magn. Reson. Chem. 2002, 40, 247. [Google Scholar] [CrossRef]
- Malfanova, N.; Shcherbakov, A.; Zaplatkin, A.; Zavalin, A.; Chebotar, V. Bacillus Subtilis CH13: A Highly Effective Biocontrol Agent for the Integrated Management of Plant Diseases. IOBC-WPRS Bull. 2016, 117, 62–66. [Google Scholar]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Gilchrist, C.L.M.; Chooi, Y.-H. Clinker & Clustermap.Js: Automatic Generation of Gene Cluster Comparison Figures. Bioinformatics 2021, 37, 2473–2475. [Google Scholar] [CrossRef]
- Zdouc, M.M.; Blin, K.; Louwen, N.L.L.; Navarro, J.; Loureiro, C.; Bader, C.D.; Bailey, C.B.; Barra, L.; Booth, T.J.; Bozhüyük, K.A.J.; et al. MIBiG 4.0: Advancing Biosynthetic Gene Cluster Curation through Global Collaboration. Nucleic Acids Res. 2025, 53, D678–D690. [Google Scholar] [CrossRef]
- Chen, X.-H.; Vater, J.; Piel, J.; Franke, P.; Scholz, R.; Schneider, K.; Koumoutsi, A.; Hitzeroth, G.; Grammel, N.; Strittmatter, A.W.; et al. Structural and Functional Characterization of Three Polyketide Synthase Gene Clusters in Bacillus amyloliquefaciens FZB 42. J. Bacteriol. 2006, 188, 4024–4036. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O.; et al. Comparative Analysis of the Complete Genome Sequence of the Plant Growth–Promoting Bacterium bacillus Amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, W.; Liu, Q.; Zhang, J.; Li, H.; Xu, S.; Ren, P.; Tian, L.; Li, W. Genome-wide Identification and Characterization of Macrolide Glycosyltransferases from a Marine-derived Bacillus Strain and Their Phylogenetic Distribution. Environ. Microbiol. 2016, 18, 4770–4781. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic Resistance in Staphylococcus aureus. Current Status and Future Prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kawabata, Y.; Okada, S.; Takahashi, J.; Hashimoto, K.; Nagai, Y.; Tatsuta, J.; Hatanaka, M. Enantioselective Microbial Oxidation of Allyl Alcohols. Chem. Lett. 2007, 36, 1428–1429. [Google Scholar] [CrossRef]
- Wilson, D.N. Ribosome-Targeting Antibiotics and Mechanisms of Bacterial Resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- ADH-Catalyzed Biooxidation of (Hetero)Aromatic sec-Alcohols to Ketones Employing Vinyl Acetate as Acetaldehyde Surrogate. ChemCatChem 2024, 16, e202400803. [CrossRef]
- Gandomkar, S.; Jost, E.; Loidolt, D.; Swoboda, A.; Pickl, M.; Elaily, W.; Daniel, B.; Fraaije, M.W.; Macheroux, P.; Kroutil, W. Biocatalytic Enantioselective Oxidation of Sec-Allylic Alcohols with Flavin-Dependent Oxidases. Adv. Synth. Catal. 2019, 361, 5264–5271. [Google Scholar] [CrossRef] [PubMed]
- Hahn, V.; Sünwoldt, K.; Mikolasch, A.; Schauer, F. Two Different Primary Oxidation Mechanisms during Biotransformation of Thymol by Gram-Positive Bacteria of the Genera Nocardia and Mycobacterium. Appl. Microbiol. Biotechnol. 2013, 97, 1289–1297. [Google Scholar] [CrossRef]
- Jehangir, M.; Iqbal, M.S.; Aftab, U. Biotransformation of Sumatriptan by Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and Salmonella enterica subsp. enterica. Molecules 2024, 29, 4226. [Google Scholar] [CrossRef]
- Bianchi, P.; Varela, R.F.; Bianchi, D.A.; Kemppainen, M.; Iribarren, A.M.; Lewkowicz, E. Selection of Microbial Biocatalysts for the Reduction of Cyclic and Heterocyclic Ketones. Process Biochem. 2017, 58, 137–144. [Google Scholar] [CrossRef]
- Babraham Bioinformatics—FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 16 May 2025).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) Database at NCBI: Current Status, Taxonomic Expansion, and Functional Annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High Throughput ANI Analysis of 90K Prokaryotic Genomes Reveals Clear Species Boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]
- Tonkin-Hill, G.; MacAlasdair, N.; Ruis, C.; Weimann, A.; Horesh, G.; Lees, J.A.; Gladstone, R.A.; Lo, S.; Beaudoin, C.; Floto, R.A.; et al. Producing Polished Prokaryotic Pangenomes with the Panaroo Pipeline. Genome Biol. 2020, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef]
- Page, A.J.; Taylor, B.; Delaney, A.J.; Soares, J.; Seemann, T.; Keane, J.A.; Harris, S.R. SNP-Sites: Rapid Efficient Extraction of SNPs from Multi-FASTA Alignments. Microb. Genom. 2016, 2, e000056. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A Fast, Scalable and User-Friendly Tool for Maximum Likelihood Phylogenetic Inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Ggplot2: Elegant Graphics for Data Analysis|SpringerLink. Available online: https://link.springer.com/book/10.1007/978-0-387-98141-3 (accessed on 16 May 2025).
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R Language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guglya, E.B.; Belozerova, O.A.; Shikov, A.E.; Alferova, V.A.; Romanenko, M.N.; Chebotar, V.K.; Gancheva, M.S.; Baganova, M.E.; Vinogradova, E.A.; Marenkova, E.A.; et al. Bacillus-Based Biocontrol Agents Mediate Pathogen Killing by Biodegradable Antimicrobials from Macrolactin Family. Int. J. Mol. Sci. 2025, 26, 11167. https://doi.org/10.3390/ijms262211167
Guglya EB, Belozerova OA, Shikov AE, Alferova VA, Romanenko MN, Chebotar VK, Gancheva MS, Baganova ME, Vinogradova EA, Marenkova EA, et al. Bacillus-Based Biocontrol Agents Mediate Pathogen Killing by Biodegradable Antimicrobials from Macrolactin Family. International Journal of Molecular Sciences. 2025; 26(22):11167. https://doi.org/10.3390/ijms262211167
Chicago/Turabian StyleGuglya, Elena B., Olga A. Belozerova, Anton E. Shikov, Vera A. Alferova, Maria N. Romanenko, Vladimir K. Chebotar, Maria S. Gancheva, Maria E. Baganova, Ekaterina A. Vinogradova, Elizaveta A. Marenkova, and et al. 2025. "Bacillus-Based Biocontrol Agents Mediate Pathogen Killing by Biodegradable Antimicrobials from Macrolactin Family" International Journal of Molecular Sciences 26, no. 22: 11167. https://doi.org/10.3390/ijms262211167
APA StyleGuglya, E. B., Belozerova, O. A., Shikov, A. E., Alferova, V. A., Romanenko, M. N., Chebotar, V. K., Gancheva, M. S., Baganova, M. E., Vinogradova, E. A., Marenkova, E. A., Lushpa, V. A., Baranova, A. A., Baranova, M. N., Shevtsova, O. A., Kudzhaev, A. M., Prokopenko, Y. A., Kovalchuk, S. I., Lukianov, D. A., Antonets, K. S., ... Terekhov, S. S. (2025). Bacillus-Based Biocontrol Agents Mediate Pathogen Killing by Biodegradable Antimicrobials from Macrolactin Family. International Journal of Molecular Sciences, 26(22), 11167. https://doi.org/10.3390/ijms262211167






