The Involvement of Ceramide, Sphingosine-1-Phosphate and Ganglioside GM1 in Regulating Some Nervous System Functions
Abstract
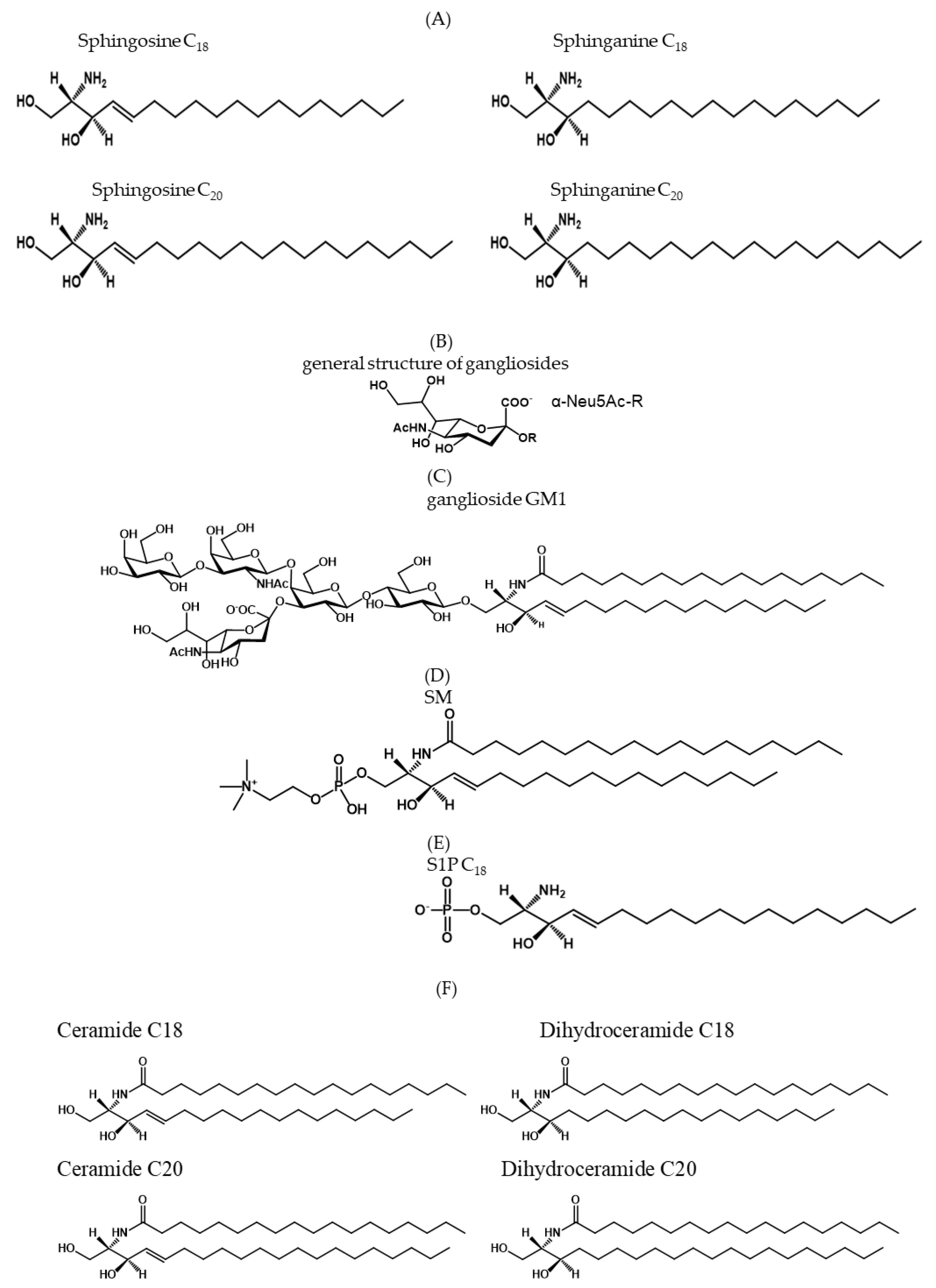
1. Sphingolipid Structure
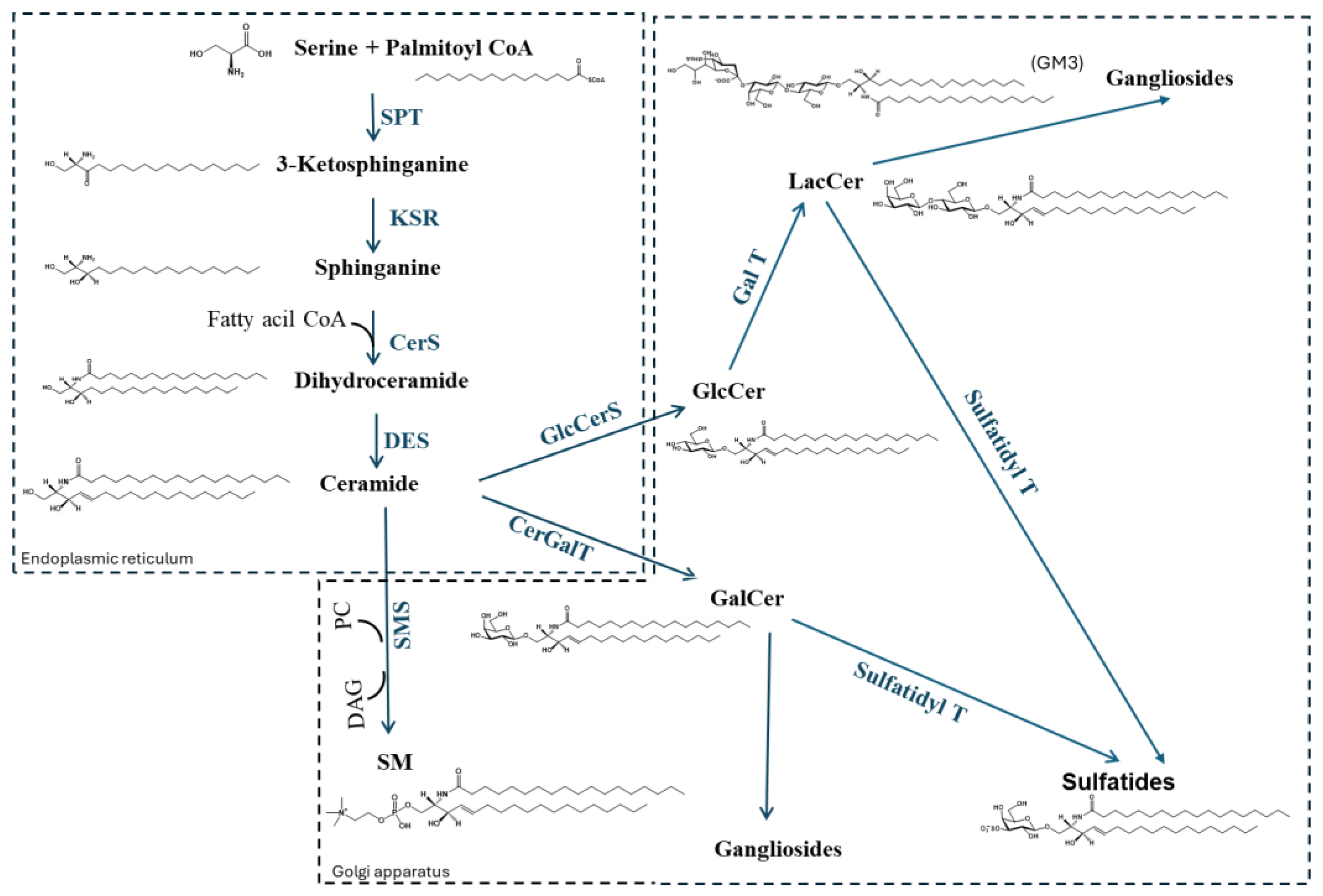
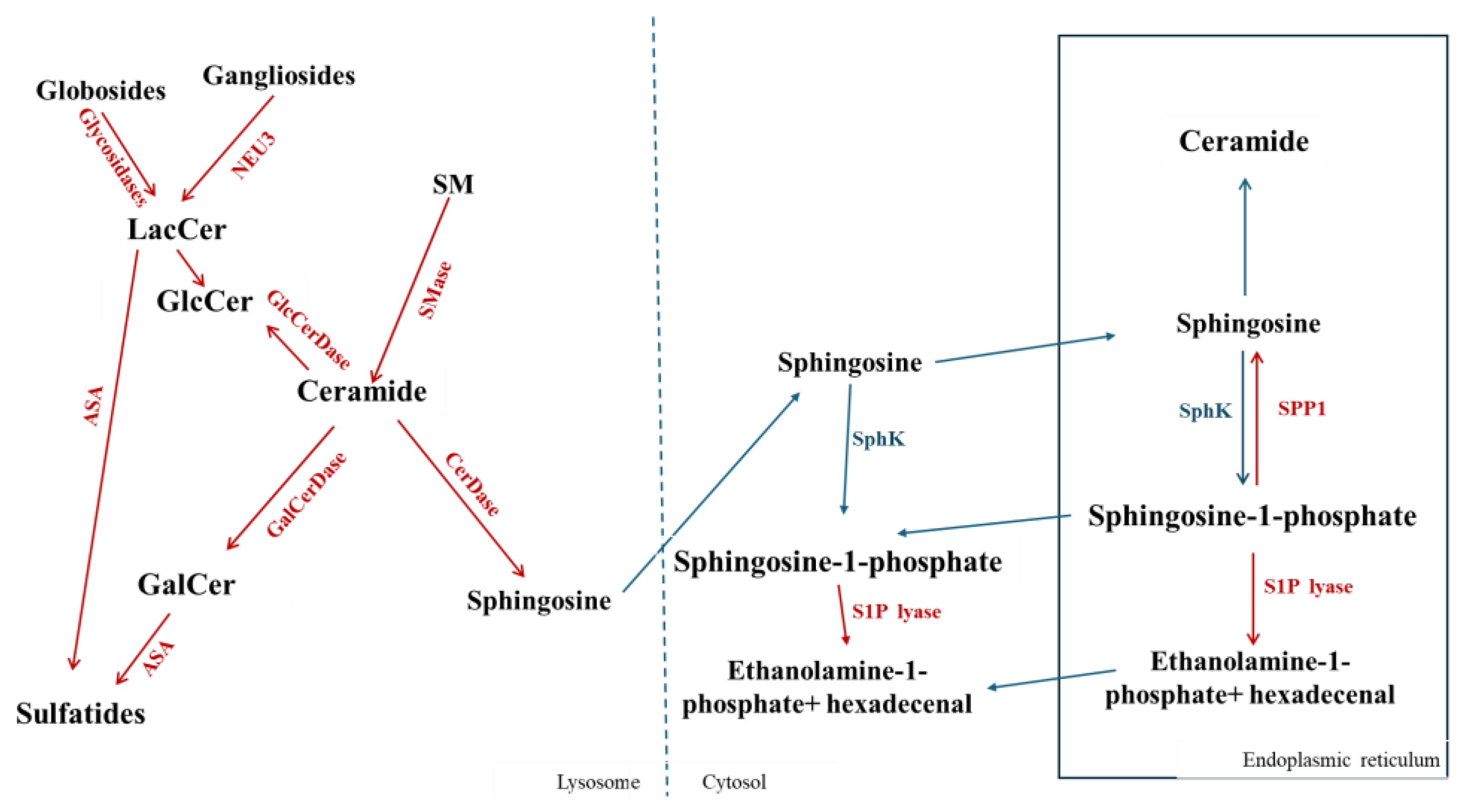
2. Sphingolipid Metabolism
3. The Hydrophobic Sphingolipids
3.1. Ceramide and Ceramide Properties
3.2. Sphingosine-1-Phosphate and Its Properties
4. The Amphiphilic Gangliosides
Ganglioside GM1 and Its Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muralidharan, S.; Shimobayashi, M.; Ji, S.; Burla, B.; Hall, M.N.; Wenk, M.R.; Torta, F. A reference map of sphingolipids in murine tissues. Cell Rep. 2021, 35, 109250. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Tettamanti, G.; Bassi, R.; Viani, P.; Riboni, L. Salvage pathways in glycosphingolipid metabolism. Biochimie 2003, 85, 423–437. [Google Scholar] [CrossRef]
- Rufail, M.L.; Bassi, R.; Giussani, P. Sphingosine-1-Phosphate Metabolic Pathway in Cancer: Implications for Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 1056. [Google Scholar] [CrossRef]
- Tringali, C.; Giussani, P. Ceramide and Sphingosine-1-Phosphate in Neurodegenerative Disorders and Their Potential Involvement in Therapy. Int. J. Mol. Sci. 2022, 23, 7806. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Farooqui, T. Phospholipids, Sphingolipids, and Cholesterol-Derived Lipid Mediators and Their Role in Neurological Disorders. Int. J. Mol. Sci. 2024, 25, 10672. [Google Scholar] [CrossRef]
- Mei, M.; Liu, M.; Mei, Y.; Zhao, J.; Li, Y. Sphingolipid metabolism in brain insulin resistance and neurological diseases. Front. Endocrinol. 2023, 14, 1243132. [Google Scholar] [CrossRef]
- Trayssac, M.; Hannun, Y.A.; Obeid, L.M. Role of sphingolipids in senescence: Implication in aging and age-related diseases. J. Clin. Investig. 2018, 128, 2702–2712. [Google Scholar] [CrossRef]
- Van Echten, G.; Sandhoff, K. Ganglioside metabolism. Enzymology, Topology, and regulation. J. Biol. Chem. 1993, 268, 5341–5344. [Google Scholar] [CrossRef]
- Kolter, T.; Proia, R.L.; Sandhoff, K. Combinatorial ganglioside biosynthesis. J. Biol. Chem. 2002, 277, 25859–25862. [Google Scholar] [CrossRef]
- Tettamanti, G. Ganglioside/glycosphingolipid turnover: New concepts. Glycoconj. J. 2004, 20, 301–317. [Google Scholar] [CrossRef]
- Hanada, K.; Kumagai, K.; Yasuda, S.; Miura, Y.; Kawano, M.; Fukasawa, M.; Nishijima, M. Molecular machinery for non-vesicular trafficking of ceramide. Nature 2003, 426, 803–809. [Google Scholar] [CrossRef]
- Hanada, K.; Kumagai, K.; Tomishige, N.; Yamaji, T. CERT-mediated trafficking of ceramide. Biochim. Biophys. Acta 2009, 1791, 684–691. [Google Scholar] [CrossRef]
- Hanada, K. Intracellular trafficking of ceramide by ceramide transfer protein. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 426–437. [Google Scholar] [CrossRef]
- Giussani, P.; Colleoni, T.; Brioschi, L.; Bassi, R.; Hanada, K.; Tettamanti, G.; Riboni, L.; Viani, P. Ceramide traffic in C6 glioma cells: Evidence for CERT-dependent and independent transport from ER to the Golgi apparatus. Biochim. Biophys. Acta 2008, 1781, 40–51. [Google Scholar] [CrossRef]
- Becker, I.; Wang-Eckhardt, L.; Yaghootfam, A.; Gieselmann, V.; Eckhardt, M. Differential expression of (dihydro)ceramide synthases in mouse brain: Oligodendrocyte-specific expression of CerS2/Lass2. Histochem. Cell Biol. 2008, 129, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Le, H.H.; Besler, K.R.; Johnson, E.L. Identification and characterization of 3-ketosphinganine reductase activity encoded at the BT_0972 locus in Bacteroides thetaiotaomicron. J. Lipid Res. 2022, 63, 100236. [Google Scholar] [CrossRef]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef]
- Lamour, N.F.; Stahelin, R.V.; Wijesinghe, D.S.; Maceyka, M.; Wang, E.; Allegood, J.C.; Merrill, A.H., Jr.; Cho, W.; Chalfant, C.E. Ceramide kinase uses ceramide provided by ceramide transport protein: Localization to organelles of eicosanoid synthesis. J. Lipid Res. 2007, 48, 1293–1304. [Google Scholar] [CrossRef]
- D’Angelo, G.; Polishchuk, E.; Di Tullio, G.; Santoro, M.; Di Campli, A.; Godi, A.; West, G.; Bielawski, J.; Chuang, C.C.; van der Spoel, A.C.; et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature 2007, 449, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Simanshu, D.K.; Kamlekar, R.K.; Wijesinghe, D.S.; Zou, X.; Zhai, X.; Mishra, S.K.; Molotkovsky, J.G.; Malinina, L.; Hinchcliffe, E.H.; Chalfant, C.E.; et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature 2013, 500, 463–467. [Google Scholar] [CrossRef]
- Gao, Y.G.; Zhai, X.; Boldyrev, I.A.; Molotkovsky, J.G.; Patel, D.J.; Malinina, L.; Brown, R.E. Ceramide-1-phosphate transfer protein (CPTP) regulation by phosphoinositides. J. Biol. Chem. 2021, 296, 100600. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Gao, Y.G.; Mishra, S.K.; Simanshu, D.K.; Boldyrev, I.A.; Benson, L.M.; Bergen, H.R., 3rd; Malinina, L.; Mundy, J.; Molotkovsky, J.G.; et al. Phosphatidylserine Stimulates Ceramide 1-Phosphate (C1P) Intermembrane Transfer by C1P Transfer Proteins. J. Biol. Chem. 2017, 292, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Warnock, D.E.; Lutz, M.S.; Blackburn, W.A.; Young, W.W., Jr.; Baenziger, J.U. Transport of newly synthesized glucosylceramide to the plasma membrane by a non-Golgi pathway. Proc. Natl. Acad. Sci. USA 1994, 91, 2708–2712. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Körner, C.; Fröhlich, F. Compartmentation and functions of sphingolipids. Curr. Opin. Cell Biol. 2022, 74, 104–111. [Google Scholar] [CrossRef]
- D’Angelo, G.; Capasso, S.; Sticco, L.; Russo, D. Glycosphingolipids: Synthesis and functions. Febs J. 2013, 280, 6338–6353. [Google Scholar] [CrossRef]
- D’Angelo, G.; Uemura, T.; Chuang, C.C.; Polishchuk, E.; Santoro, M.; Ohvo-Rekilä, H.; Sato, T.; Di Tullio, G.; Varriale, A.; D’Auria, S.; et al. Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature 2013, 501, 116–120. [Google Scholar] [CrossRef]
- Veldman, R.J.; Klappe, K.; Hinrichs, J.; Hummel, I.; van der Schaaf, G.; Sietsma, H.; Kok, J.W. Altered sphingolipid metabolism in multidrug-resistant ovarian cancer cells is due to uncoupling of glycolipid biosynthesis in the Golgi apparatus. FASEB J. 2002, 16, 1111–1113. [Google Scholar] [CrossRef]
- Riboni, L.; Prinetti, A.; Bassi, R.; Tettamanti, G. Formation of bioactive sphingoid molecules from exogenous sphingomyelin in primary cultures of neurons and astrocytes. FEBS Lett. 1994, 352, 323–326. [Google Scholar] [CrossRef]
- Riboni, L.; Bassi, R.; Prinetti, A.; Tettamanti, G. Salvage of catabolic products in ganglioside metabolism: A study on rat cerebellar granule cells in culture. FEBS Lett. 1996, 391, 336–340. [Google Scholar] [CrossRef]
- Giussani, P.; Tringali, C.; Riboni, L.; Viani, P.; Venerando, B. Sphingolipids: Key regulators of apoptosis and pivotal players in cancer drug resistance. Int. J. Mol. Sci. 2014, 15, 4356–4392. [Google Scholar] [CrossRef]
- Pyne, S.; Adams, D.R.; Pyne, N.J. Sphingosine 1-phosphate and sphingosine kinases in health and disease: Recent advances. Prog. Lipid Res. 2016, 62, 93–106. [Google Scholar] [CrossRef]
- Goins, L.; Spassieva, S. Sphingoid bases and their involvement in neurodegenerative diseases. Adv. Biol. Regul. 2018, 70, 65–73. [Google Scholar] [CrossRef]
- Le Stunff, H.; Milstien, S.; Spiegel, S. Generation and metabolism of bioactive sphingosine-1-phosphate. J. Cell Biochem. 2004, 92, 882–899. [Google Scholar] [CrossRef]
- Igarashi, N.; Okada, T.; Hayashi, S.; Fujita, T.; Jahangeer, S.; Nakamura, S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 2003, 278, 46832–46839. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Furuhata, M.; Kamiya, S.; Hisada, M.; Miyaji, H.; Magami, Y.; Yamamoto, K.; Fujiwara, H.; Mizuguchi, J. Positive modulation of IL-12 signaling by sphingosine kinase 2 associating with the IL-12 receptor beta 1 cytoplasmic region. J. Immunol. 2003, 171, 1352–1359. [Google Scholar] [CrossRef]
- Le Stunff, H.; Peterson, C.; Liu, H.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate and lipid phosphohydrolases. Biochim. Biophys. Acta 2002, 1582, 8–17. [Google Scholar] [CrossRef]
- Le Stunff, H.; Peterson, C.; Thornton, R.; Milstien, S.; Mandala, S.M.; Spiegel, S. Characterization of murine sphingosine-1-phosphate phosphohydrolase. J. Biol. Chem. 2002, 277, 8920–8927. [Google Scholar] [CrossRef]
- Le Stunff, H.; Galve-Roperh, I.; Peterson, C.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J. Cell Biol. 2002, 158, 1039–1049. [Google Scholar] [CrossRef]
- Brindley, D.N.; English, D.; Pilquil, C.; Buri, K.; Ling, Z.C. Lipid phosphate phosphatases regulate signal transduction through glycerolipids and sphingolipids. Biochim. Biophys. Acta 2002, 1582, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, C.; Kihara, A.; Gokoh, M.; Igarashi, Y. Identification and characterization of a novel human sphingosine-1-phosphate phosphohydrolase, hSPP2. J. Biol. Chem. 2003, 278, 1268–1272. [Google Scholar] [CrossRef]
- Zhou, J.; Saba, J.D. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem. Biophys. Res. Commun. 1998, 242, 502–507. [Google Scholar] [CrossRef]
- Van Veldhoven, P.P.; Mannaerts, G.P. Sphinganine 1-phosphate metabolism in cultured skin fibroblasts: Evidence for the existence of a sphingosine phosphatase. Biochem. J. 1994, 299 Pt 3, 597–601. [Google Scholar] [CrossRef]
- Van Veldhoven, P.P.; Gijsbers, S.; Mannaerts, G.P.; Vermeesch, J.R.; Brys, V. Human sphingosine-1-phosphate lyase: cDNA cloning, functional expression studies and mapping to chromosome 10q22(1). Biochim. Biophys. Acta 2000, 1487, 128–134. [Google Scholar] [CrossRef]
- Okazaki, T.; Bielawska, A.; Domae, N.; Bell, R.M.; Hannun, Y.A. Characteristics and partial purification of a novel cytosolic, magnesium-independent, neutral sphingomyelinase activated in the early signal transduction of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J. Biol. Chem. 1994, 269, 4070–4077. [Google Scholar] [CrossRef] [PubMed]
- Levade, T.; Jaffrézou, J.P. Signalling sphingomyelinases: Which, where, how and why? Biochim. Biophys. Acta 1999, 1438, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Goni, F.M.; Alonso, A. Sphingomyelinases: Enzymology and membrane activity. FEBS Lett. 2002, 531, 38–46. [Google Scholar] [CrossRef]
- Huitema, K.; van den Dikkenberg, J.; Brouwers, J.F.; Holthuis, J.C. Identification of a family of animal sphingomyelin synthases. Embo J. 2004, 23, 33–44. [Google Scholar] [CrossRef]
- Slife, C.W.; Wang, E.; Hunter, R.; Wang, S.; Burgess, C.; Liotta, D.C.; Merrill, A.H., Jr. Free sphingosine formation from endogenous substrates by a liver plasma membrane system with a divalent cation dependence and a neutral pH optimum. J. Biol. Chem. 1989, 264, 10371–10377. [Google Scholar] [CrossRef]
- Tani, M.; Iida, H.; Ito, M. O-glycosylation of mucin-like domain retains the neutral ceramidase on the plasma membranes as a type II integral membrane protein. J. Biol. Chem. 2003, 278, 10523–10530. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Tani, M.; Nakagawa, T.; Okino, N.; Ito, M. Subcellular localization of human neutral ceramidase expressed in HEK293 cells. Biochem. Biophys. Res. Commun. 2005, 331, 37–42. [Google Scholar] [CrossRef]
- Pyne, N.J.; Pyne, S. Recent advances in the role of sphingosine 1-phosphate in cancer. FEBS Lett. 2020, 594, 3583–3601. [Google Scholar] [CrossRef]
- Grassi, S.; Mauri, L.; Prioni, S.; Cabitta, L.; Sonnino, S.; Prinetti, A.; Giussani, P. Sphingosine 1-Phosphate Receptors and Metabolic Enzymes as Druggable Targets for Brain Diseases. Front. Pharmacol. 2019, 10, 807. [Google Scholar] [CrossRef]
- Schengrund, C.L.; Rosenberg, A. Intracellular location and properties of bovine brain sialidase. J. Biol. Chem. 1970, 245, 6196–6200. [Google Scholar] [CrossRef]
- Tettamanti, G.; Preti, A.; Lombardo, A.; Suman, T.; Zambotti, V. Membrane-bound neuraminidase in the brain of different animals: Behaviour of the enzyme on endogenous sialo derivatives and rationale for its assay. J. Neurochem. 1975, 25, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Sagawa, J.; Konno, K.; Tsuiki, S. Immunological discrimination of intralysosomal, cytosolic, and two membrane sialidases present in rat tissues. J. Biochem. 1990, 107, 794–798. [Google Scholar] [CrossRef]
- Papini, N.; Anastasia, L.; Tringali, C.; Croci, G.; Bresciani, R.; Yamaguchi, K.; Miyagi, T.; Preti, A.; Prinetti, A.; Prioni, S.; et al. The plasma membrane-associated sialidase MmNEU3 modifies the ganglioside pattern of adjacent cells supporting its involvement in cell-to-cell interactions. J. Biol. Chem. 2004, 279, 16989–16995. [Google Scholar] [CrossRef]
- Preti, A.; Fiorilli, A.; Lombardo, A.; Caimi, L.; Tettamanti, G. Occurrence of sialyltransferase activity in the synaptosomal membranes prepared from calf brain cortex. J. Neurochem. 1980, 35, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Valaperta, R.; Chigorno, V.; Basso, L.; Prinetti, A.; Bresciani, R.; Preti, A.; Miyagi, T.; Sonnino, S. Plasma membrane production of ceramide from ganglioside GM3 in human fibroblasts. FASEB J. 2006, 20, 1227–1229. [Google Scholar] [CrossRef]
- Ho, Q.W.C.; Zheng, X.; Ali, Y. Ceramide Acyl Chain Length and Its Relevance to Intracellular Lipid Regulation. Int. J. Mol. Sci. 2022, 23, 9697. [Google Scholar] [CrossRef]
- Zelnik, I.D.; Rozman, B.; Rosenfeld-Gur, E.; Ben-Dor, S.; Futerman, A.H. A Stroll Down the CerS Lane. Adv. Exp. Med. Biol. 2019, 1159, 49–63. [Google Scholar]
- Pinto, S.N.; Silva, L.C.; Futerman, A.H.; Prieto, M. Effect of ceramide structure on membrane biophysical properties: The role of acyl chain length and unsaturation. Biochim. Biophys. Acta 2011, 1808, 2753–2760. [Google Scholar] [CrossRef]
- Grösch, S.; Schiffmann, S.; Geisslinger, G. Chain length-specific properties of ceramides. Prog. Lipid Res. 2012, 51, 50–62. [Google Scholar] [CrossRef]
- Bassi, R.; Brambilla, S.; Tringali, C.; Giussani, P. Extracellular Sphingosine-1-Phosphate Downstream of EGFR Increases Human Glioblastoma Cell Survival. Int. J. Mol. Sci. 2021, 22, 6824. [Google Scholar] [CrossRef] [PubMed]
- Giussani, P.; Bassi, R.; Anelli, V.; Brioschi, L.; De Zen, F.; Riccitelli, E.; Caroli, M.; Campanella, R.; Gaini, S.M.; Viani, P.; et al. Glucosylceramide synthase protects glioblastoma cells against autophagic and apoptotic death induced by temozolomide and Paclitaxel. Cancer Investig. 2012, 30, 27–37. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191, Correction in Nat. Rev. Mol. Cell Biol. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.T.; Li, T.; Sudalagunta, P.; Nasr, M.; Nyman, K.J.; Alugubelli, R.R.; Meads, M.; Frieling, J.; Nerlakanti, N.; Tauro, M.; et al. Acid ceramidase controls proteasome inhibitor resistance and is a novel therapeutic target for the treatment of relapsed/refractory multiple myeloma. Haematologica 2025, 110, 1351–1367. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Warabi, E.; Mann, G.E. Mechanisms underlying Nrf2 nuclear translocation by non-lethal levels of hydrogen peroxide: p38 MAPK-dependent neutral sphingomyelinase2 membrane trafficking and ceramide/PKCζ/CK2 signaling. Free Radic. Biol. Med. 2022, 191, 191–202. [Google Scholar] [CrossRef]
- Khamrui, E.; Banerjee, S.; Mukherjee, D.D.; Biswas, K. Emerging role of MAPK signaling in glycosphingolipid-associated tumorigenesis. Glycoconj. J. 2024, 41, 343–360. [Google Scholar] [CrossRef]
- Bilal, F.; Soueid, J.; Saab, S.; Makhoul, N.; Hamze, Z.; El-Bazzal, L.; Makoukji, J.; Boustany, R.M. Downregulation of AKT-mediated p27(Kip1) phosphorylation with shift to sphingomyelin synthesis in CLN3 disease. IBRO Neurosci. Rep. 2025, 19, 223–234. [Google Scholar] [CrossRef]
- Czubowicz, K.; Jęśko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer’s Disease and Other Neurodegenerative Disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef] [PubMed]
- Jęśko, H.; Stępień, A.; Lukiw, W.J.; Strosznajder, R.P. The Cross-Talk Between Sphingolipids and Insulin-Like Growth Factor Signaling: Significance for Aging and Neurodegeneration. Mol. Neurobiol. 2019, 56, 3501–3521. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, C.; Martinez-Martinez, P. Ceramide function in the brain: When a slight tilt is enough. Cell. Mol. Life Sci. 2013, 70, 181–203. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Airola, M.V.; Hannun, Y.A. Sphingolipid metabolism and neutral sphingomyelinases. Handb. Exp. Pharmacol. 2013, 215, 57–76. [Google Scholar]
- Cutler, R.G.; Thompson, K.W.; Camandola, S.; Mack, K.T.; Mattson, M.P. Sphingolipid metabolism regulates development and lifespan in Caenorhabditis elegans. Mech. Ageing Dev. 2014, 143–144, 9–18. [Google Scholar] [CrossRef]
- Obeid, L.M.; Hannun, Y.A. Ceramide, stress, and a “LAG” in aging. Sci. Aging Knowl. Environ. 2003, 2003, Pe27. [Google Scholar] [CrossRef]
- Huang, X.; Withers, B.R.; Dickson, R.C. Sphingolipids and lifespan regulation. Biochim. Biophys. Acta 2014, 1841, 657–664. [Google Scholar] [CrossRef]
- Jiang, J.C.; Kirchman, P.A.; Allen, M.; Jazwinski, S.M. Suppressor analysis points to the subtle role of the LAG1 ceramide synthase gene in determining yeast longevity. Exp. Gerontol. 2004, 39, 999–1009. [Google Scholar] [CrossRef]
- Riboni, L.; Viani, P.; Bassi, R.; Stabilini, A.; Tettamanti, G. Biomodulatory role of ceramide in basic fibroblast growth factor-induced proliferation of cerebellar astrocytes in primary culture. Glia 2000, 32, 137–145. [Google Scholar] [CrossRef]
- Riboni, L.; Viani, P.; Bassi, R.; Giussani, P.; Tettamanti, G. Basic fibroblast growth factor-induced proliferation of primary astrocytes—Evidence for the involvement of sphingomyelin biosynthesis. J. Biol. Chem. 2001, 276, 12797–12804. [Google Scholar] [CrossRef]
- Tettamanti, G.; Prinetti, A.; Bassi, R.; Viani, P.; Giussani, P.; Riboni, L. Sphingoid bioregulators in the differentiation of cells of neural origin. J. Lipid Mediat. Cell Signal 1996, 14, 263–275. [Google Scholar] [CrossRef]
- McInnis, J.J.; Sood, D.; Guo, L.; Dufault, M.R.; Garcia, M.; Passaro, R.; Gao, G.; Zhang, B.; Dodge, J.C. Unravelling neuronal and glial differences in ceramide composition, synthesis, and sensitivity to toxicity. Commun. Biol. 2024, 7, 1597. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Selvakumar, S.; Chandrashekar, M. Lipids modulates Tau and amyloid-β proteins in Alzheimer’s disease. Adv. Protein Chem. Struct. Biol. 2025, 146, 137–159. [Google Scholar] [PubMed]
- Yuyama, K.; Mitsutake, S.; Igarashi, Y. Pathological roles of ceramide and its metabolites in metabolic syndrome and Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1841, 793–798. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Huang, Y.; Li, B.; Gong, C.X.; Schuchman, E.H. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol. Aging 2010, 31, 398–408. [Google Scholar] [CrossRef]
- Malaplate-Armand, C.; Florent-Bechard, S.; Youssef, I.; Koziel, V.; Sponne, I.; Kriem, B.; Leininger-Muller, B.; Olivier, J.L.; Oster, T.; Pillot, T. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiol. Dis. 2006, 23, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.G.; Kelly, J.; Storie, K.; Pedersen, W.A.; Tammara, A.; Hatanpaa, K.; Troncoso, J.C.; Mattson, M.P. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2070–2075. [Google Scholar] [CrossRef]
- Katsel, P.; Li, C.; Haroutunian, V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: A shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease? Neurochem. Res. 2007, 32, 845–856. [Google Scholar] [CrossRef]
- Chua, X.Y.; Torta, F.; Chong, J.R.; Venketasubramanian, N.; Hilal, S.; Wenk, M.R.; Chen, C.P.; Arumugam, T.V.; Herr, D.R.; Lai, M.K.P. Lipidomics profiling reveals distinct patterns of plasma sphingolipid alterations in Alzheimer’s disease and vascular dementia. Alzheimers Res. Ther. 2023, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef] [PubMed]
- Furuya, S.; Ono, K.; Hirabayashi, Y. Sphingolipid biosynthesis is necessary for dendrite growth and survival of cerebellar Purkinje cells in culture. J. Neurochem. 1995, 65, 1551–1561. [Google Scholar] [CrossRef]
- Harel, R.; Futerman, A.H. Inhibition of sphingolipid synthesis affects axonal outgrowth in cultured hippocampal neurons. J. Biol. Chem. 1993, 268, 14476–14481. [Google Scholar] [CrossRef]
- Marasas, W.F.; Kellerman, T.S.; Gelderblom, W.C.; Coetzer, J.A.; Thiel, P.G.; van der Lugt, J.J. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J. Vet. Res. 1988, 55, 197–203. [Google Scholar]
- Ross, P.F.; Ledet, A.E.; Owens, D.L.; Rice, L.G.; Nelson, H.A.; Osweiler, G.D.; Wilson, T.M. Experimental equine leukoencephalomalacia, toxic hepatosis, and encephalopathy caused by corn naturally contaminated with fumonisins. J. Vet. Diagn. Investig. 1993, 5, 69–74. [Google Scholar] [CrossRef]
- Suarez, L.; Felkner, M.; Brender, J.D.; Canfield, M.; Zhu, H.; Hendricks, K.A. Neural tube defects on the Texas-Mexico border: What we’ve learned in the 20 years since the Brownsville cluster. Birth Defects Res. A Clin. Mol. Teratol. 2012, 94, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Maetzler, W.; Haughey, N.J.; Bandaru, V.V.; Savica, R.; Deuschle, C.; Gasser, T.; Hauser, A.K.; Graber-Sultan, S.; Schleicher, E.; et al. Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson’s disease and associated with cognitive impairment: A pilot study. PLoS ONE 2013, 8, e73094. [Google Scholar] [CrossRef]
- Abbott, S.K.; Li, H.; Muñoz, S.S.; Knoch, B.; Batterham, M.; Murphy, K.E.; Halliday, G.M.; Garner, B. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov. Disord. 2014, 29, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Riboni, L.; Campanella, R.; Bassi, R.; Villani, R.; Gaini, S.M.; Martinelli-Boneschi, F.; Viani, P.; Tettamanti, G. Ceramide levels are inversely associated with malignant progression of human glial tumors. Glia 2002, 39, 105–113. [Google Scholar] [CrossRef]
- Bassi, R.; Cas, M.D.; Tringali, C.; Compostella, F.; Paroni, R.; Giussani, P. Ceramide Is Involved in Temozolomide Resistance in Human Glioblastoma U87MG Overexpressing EGFR. Int. J. Mol. Sci. 2023, 24, 15394. [Google Scholar] [CrossRef]
- Mohamud Yusuf, A.; Hagemann, N.; Hermann, D.M. The Acid Sphingomyelinase/Ceramide System as Target for Ischemic Stroke Therapies. Neurosignals 2019, 27, 32–43. [Google Scholar]
- Yu, J.; Novgorodov, S.A.; Chudakova, D.; Zhu, H.; Bielawska, A.; Bielawski, J.; Obeid, L.M.; Kindy, M.S.; Gudz, T.I. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J. Biol. Chem. 2007, 282, 25940–25949. [Google Scholar] [CrossRef] [PubMed]
- Novgorodov, S.A.; Gudz, T.I. Ceramide and mitochondria in ischemic brain injury. Int. J. Biochem. Mol. Biol. 2011, 2, 347–361. [Google Scholar]
- Imgrund, S.; Hartmann, D.; Farwanah, H.; Eckhardt, M.; Sandhoff, R.; Degen, J.; Gieselmann, V.; Sandhoff, K.; Willecke, K. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 2009, 284, 33549–33560. [Google Scholar] [CrossRef]
- Tzou, F.Y.; Hornemann, T.; Yeh, J.Y.; Huang, S.Y. The pathophysiological role of dihydroceramide desaturase in the nervous system. Prog. Lipid Res. 2023, 91, 101236. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Ormseth, C.; Chiang, J.; Cipolla, M.; Arakaki, X.; Harrington, M.G. Sphingolipid metabolism correlates with cerebrospinal fluid Beta amyloid levels in Alzheimer’s disease. PLoS ONE 2015, 10, e0125597. [Google Scholar] [CrossRef]
- Huynh, K.; Lim, W.L.F.; Giles, C.; Jayawardana, K.S.; Salim, A.; Mellett, N.A.; Smith, A.A.T.; Olshansky, G.; Drew, B.G.; Chatterjee, P.; et al. Concordant peripheral lipidome signatures in two large clinical studies of Alzheimer’s disease. Nat. Commun. 2020, 11, 5698. [Google Scholar] [CrossRef] [PubMed]
- Karsai, G.; Kraft, F.; Haag, N.; Korenke, G.C.; Hänisch, B.; Othman, A.; Suriyanarayanan, S.; Steiner, R.; Knopp, C.; Mull, M.; et al. DEGS1-associated aberrant sphingolipid metabolism impairs nervous system function in humans. J. Clin. Investig. 2019, 129, 1229–1239. [Google Scholar] [CrossRef]
- Pant, D.C.; Dorboz, I.; Schluter, A.; Fourcade, S.; Launay, N.; Joya, J.; Aguilera-Albesa, S.; Yoldi, M.E.; Casasnovas, C.; Willis, M.J.; et al. Loss of the sphingolipid desaturase DEGS1 causes hypomyelinating leukodystrophy. J. Clin. Investig. 2019, 129, 1240–1256. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, V.; Straussberg, R.; Xu, R.; Mileva, I.; Yogev, Y.; Khoury, R.; Konen, O.; Barhum, Y.; Zvulunov, A.; Mao, C.; et al. DEGS1 variant causes neurological disorder. Eur. J. Hum. Genet. 2019, 27, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, N.B.; Michael, L.F.; Meikle, P.J.; Peralta, J.M.; Mosior, M.; McAhren, S.; Bui, H.H.; Bellinger, M.A.; Giles, C.; Kumar, S.; et al. Rare DEGS1 variant significantly alters de novo ceramide synthesis pathway. J. Lipid Res. 2019, 60, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Casasampere, M.; Ordóñez, Y.F.; Casas, J.; Fabrias, G. Dihydroceramide desaturase inhibitors induce autophagy via dihydroceramide-dependent and independent mechanisms. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 264–275. [Google Scholar] [CrossRef]
- Hla, T.; Brinkmann, V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 2011, 76, S3–S8. [Google Scholar] [CrossRef]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Olivera, A.; Spiegel, S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 1993, 365, 557–560. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Choi, J.W.; Chun, J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta 2013, 1831, 20–32. [Google Scholar] [CrossRef]
- Wu, Y.P.; Mizugishi, K.; Bektas, M.; Sandhoff, R.; Proia, R.L. Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum. Mol. Genet. 2008, 17, 2257–2264. [Google Scholar] [CrossRef]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef]
- Chun, J.; Hla, T.; Lynch, K.R.; Spiegel, S.; Moolenaar, W.H. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2010, 62, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Payne, S.G.; Milstien, S.; Spiegel, S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim. Biophys. Acta 2002, 1585, 193–201. [Google Scholar] [CrossRef]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, F.N.; Hernandez-Corbacho, M.; Trayssac, M.; Stith, J.L.; Bonica, J.; Jean, B.; Pulkoski-Gross, M.J.; Carroll, B.L.; Salama, M.F.; Hannun, Y.A.; et al. Bioactive sphingolipids: Advancements and contributions from the laboratory of Dr. Lina M. Obeid. Cell Signal 2021, 79, 109875. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Larrauri, A.; Larrea, A.; Martín, C.; Gomez-Muñoz, A. The critical roles of bioactive sphingolipids in inflammation. J. Biol. Chem. 2025, 301, 110475. [Google Scholar] [CrossRef]
- Mohamud Yusuf, A.; Zhang, X.; Gulbins, E.; Peng, Y.; Hagemann, N.; Hermann, D.M. Signaling roles of sphingolipids in the ischemic brain and their potential utility as therapeutic targets. Neurobiol. Dis. 2024, 201, 106682. [Google Scholar] [CrossRef]
- Lee, M.J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999, 99, 301–312. [Google Scholar] [CrossRef]
- Lee, O.H.; Kim, Y.M.; Lee, Y.M.; Moon, E.J.; Lee, D.J.; Kim, J.H.; Kim, K.W.; Kwon, Y.G. Sphingosine 1-phosphate induces angiogenesis: Its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 1999, 264, 743–750. [Google Scholar] [CrossRef]
- Chun, J.; Hartung, H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Anelli, V.; Giussani, P.; Tettamanti, G.; Viani, P.; Riboni, L. Sphingosine-1-phosphate is released by cerebellar astrocytes in response to bFGF and induces astrocyte proliferation through G(i)-protein-coupled receptors. Glia 2006, 53, 621–630. [Google Scholar] [CrossRef]
- Giussani, P.; Ferraretto, A.; Gravaghi, C.; Bassi, R.; Tettamanti, G.; Riboni, L.; Viani, P. Sphingosine-1-phosphate and calcium signaling in cerebellar astrocytes and differentiated granule cells. Neurochem. Res. 2007, 32, 27–37. [Google Scholar] [CrossRef]
- An, S.; Zheng, Y.; Bleu, T. Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. J. Biol. Chem. 2000, 275, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Stepanovska, B.; Huwiler, A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol. Res. 2020, 154, 104170. [Google Scholar] [CrossRef] [PubMed]
- Motyl, J.A.; Strosznajder, J.B.; Wencel, A.; Strosznajder, R.P. Recent Insights into the Interplay of Alpha-Synuclein and Sphingolipid Signaling in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 6277. [Google Scholar] [CrossRef]
- Giussani, P.; Prinetti, A.; Tringali, C. The role of Sphingolipids in myelination and myelin stability and their involvement in childhood and adult demyelinating disorders. J. Neurochem. 2021, 156, 403–414. [Google Scholar] [CrossRef]
- Grassi, S.; Giussani, P.; Prioni, S.; Button, D.; Cao, J.; Hakimi, I.; Sarmiere, P.; Srinivas, M.; Cabitta, L.; Sonnino, S.; et al. Human Remyelination Promoting Antibody Stimulates Astrocytes Proliferation Through Modulation of the Sphingolipid Rheostat in Primary Rat Mixed Glial Cultures. Neurochem. Res. 2019, 44, 1460–1474. [Google Scholar] [CrossRef]
- Al-Hashmi, G.K.H.; Al-Asmi, A.; Islam, M.M.; Al-Zakwani, I.; Butt, M.; Al-Qassabi, A.; Al-Abri, H.; Gujjar, A.R. Effectiveness and Safety Profile of Fingolimod in Treating Omani Patients with Multiple Sclerosis: A single tertiary centre experience. Sultan Qaboos Univ. Med. J. 2025, 25, 225–232. [Google Scholar] [CrossRef]
- Shang, Y.; Huang, Y.; Meng, Q.; Yu, Z.; Wen, Z.; Yu, F. Fingolimod as a potent anti-Staphylococcus aureus: pH-dependent cell envelope damage and eradication of biofilms/persisters. BMC Microbiol. 2025, 25, 299. [Google Scholar] [CrossRef]
- Dayani, L.; Haddadi, F.; Aliomrani, M.; Taheri, A. Preparation and In vitro/In vivo Evaluation of Fingolimod hydrochloride Loaded Polymeric Mixed Nano-Micelles for Treatment of Multiple Sclerosis. J. Neuroimmune Pharmacol. 2025, 20, 41. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Loberto, N.; Schiumarini, D.; Samarani, M.; Mancini, G.; Tamanini, A.; Lippi, G.; Dechecchi, M.C.; Bassi, R.; Giussani, P.; et al. Sphingolipids role in the regulation of inflammatory response: From leukocyte biology to bacterial infection. J. Leukoc. Biol. 2018, 103, 445–456. [Google Scholar] [CrossRef]
- Maktabi, B.; Shehjar, F.; Senger, Z.; Kountz, L.; Hasan, S.; Maaieh, K.; Hoersten, K.; Duric, J.; Shah, Z.A. Sphingosine-1-Phosphate Modulation in Neurological Disorders: Insights from MS and Stroke. Brain Sci. 2025, 15, 436. [Google Scholar] [CrossRef]
- Murayama, R.; Cai, Y.; Nakamura, H.; Hashimoto, K. Demyelination in psychiatric and neurological disorders: Mechanisms, clinical impact, and novel therapeutic strategies. Neurosci. Biobehav. Rev. 2025, 174, 106209. [Google Scholar] [CrossRef]
- Abulaban, A.A.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Shokr, M.M.; Alexiou, A.; Papadakis, M.; Batiha, G.E. The janus face of astrocytes in multiple sclerosis: Balancing protection and pathology. Brain Res. Bull. 2025, 226, 111356. [Google Scholar] [CrossRef]
- Yuan, X.; Klein, D.; Maier, A.M.; Martini, R. Therapeutic sphingosine-1-phosphate receptor modulation by repurposing fingolimod (FTY720) leads to mitigated neuropathy and improved clinical outcome in a mouse model for Charcot-Marie-Tooth 1X disease. Neuromuscul. Disord. 2025, 50, 105345. [Google Scholar] [CrossRef]
- Takasugi, N.; Sasaki, T.; Ebinuma, I.; Osawa, S.; Isshiki, H.; Takeo, K.; Tomita, T.; Iwatsubo, T. FTY720/fingolimod, a sphingosine analogue, reduces amyloid-beta production in neurons. PLoS ONE 2013, 8, e64050. [Google Scholar] [CrossRef] [PubMed]
- Asle-Rousta, M.; Kolahdooz, Z.; Oryan, S.; Ahmadiani, A.; Dargahi, L. FTY720 (fingolimod) attenuates beta-amyloid peptide (Abeta42)-induced impairment of spatial learning and memory in rats. J. Mol. Neurosci. 2013, 50, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Jiang, L.; Zhao, J.; Wang, H.; Hu, M.; Chen, L.; Chen, Y. Bioactive lipids and their metabolism: New therapeutic opportunities for Parkinson’s disease. Eur. J. Neurosci. 2022, 55, 846–872. [Google Scholar] [CrossRef] [PubMed]
- Pyszko, J.A.; Strosznajder, J.B. The key role of sphingosine kinases in the molecular mechanism of neuronal cell survival and death in an experimental model of Parkinson’s disease. Folia Neuropathol. 2014, 52, 260–269. [Google Scholar] [CrossRef]
- Sivasubramanian, M.; Kanagaraj, N.; Dheen, S.T.; Tay, S.S. Sphingosine kinase 2 and sphingosine-1-phosphate promotes mitochondrial function in dopaminergic neurons of mouse model of Parkinson’s disease and in MPP+ -treated MN9D cells in vitro. Neuroscience 2015, 290, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Pyszko, J.; Strosznajder, J.B. Sphingosine kinase 1 and sphingosine-1-phosphate in oxidative stress evoked by 1-methyl-4-phenylpyridinium (MPP+) in human dopaminergic neuronal cells. Mol. Neurobiol. 2014, 50, 38–48. [Google Scholar] [CrossRef]
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 2010, 10, 489–503. [Google Scholar] [CrossRef]
- Pyne, N.J.; Tonelli, F.; Lim, K.G.; Long, J.S.; Edwards, J.; Pyne, S. Sphingosine 1-phosphate signalling in cancer. Biochem. Soc. Trans. 2012, 40, 94–100. [Google Scholar] [CrossRef]
- Yester, J.W.; Tizazu, E.; Harikumar, K.B.; Kordula, T. Extracellular and intracellular sphingosine-1-phosphate in cancer. Cancer Metastasis Rev. 2011, 30, 577–597. [Google Scholar] [CrossRef]
- Van Brocklyn, J.; Letterle, C.; Snyder, P.; Prior, T. Sphingosine-1-phosphate stimulates human glioma cell proliferation through Gi-coupled receptors: Role of ERK MAP kinase and phosphatidylinositol 3-kinase beta. Cancer Lett. 2002, 181, 195–204. [Google Scholar] [CrossRef]
- Van Brocklyn, J.R.; Young, N.; Roof, R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett. 2003, 199, 53–60. [Google Scholar] [CrossRef]
- Mahajan-Thakur, S.; Bien-Moller, S.; Marx, S.; Schroeder, H.; Rauch, B.H. Sphingosine 1-phosphate (S1P) signaling in glioblastoma multiforme-A systematic review. Int. J. Mol. Sci. 2017, 18, 2448. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Sun, S.; Yu, S.; Zhang, M.; Zou, F. Inhibition of sphingosine kinase 1 suppresses proliferation of glioma cells under hypoxia by attenuating activity of extracellular signal-regulated kinase. Cell Prolif. 2012, 45, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Edsall, L.C.; Cuvillier, O.; Twitty, S.; Spiegel, S.; Milstien, S. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12. J. Neurochem. 2001, 76, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Kapitonov, D.; Allegood, J.C.; Mitchell, C.; Hait, N.C.; Almenara, J.A.; Adams, J.K.; Zipkin, R.E.; Dent, P.; Kordula, T.; Milstien, S.; et al. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009, 69, 6915–6923. [Google Scholar] [CrossRef]
- Van Brocklyn, J.R.; Jackson, C.A.; Pearl, D.K.; Kotur, M.S.; Snyder, P.J.; Prior, T.W. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: Roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J. Neuropathol. Exp. Neurol. 2005, 64, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.G.; Vanetti, C.; Samarani, M.; Aureli, M.; Bassi, R.; Sonnino, S.; Giussani, P. Crosstalk between sphingosine-1-phosphate and EGFR signalling pathways enhances human glioblastoma cell invasiveness. FEBS Lett. 2018, 592, 949–961. [Google Scholar] [CrossRef]
- Sonnino, S.; Ghidoni, R.; Marchesini, S.; Tettamanti, G. Cytosolic gangliosides: Occurrence in calf brain as ganglioside--protein complexes. J. Neurochem. 1979, 33, 117–121. [Google Scholar] [CrossRef]
- Sonnino, S.; Prinetti, A.; Mauri, L.; Chigorno, V.; Tettamanti, G. Dynamic and structural properties of sphingolipids as driving forces for the formation of membrane domains. Chem. Rev. 2006, 106, 2111–2125. [Google Scholar] [CrossRef]
- Chigorno, V.; Negroni, E.; Nicolini, M.; Sonnino, S. Activity of 3-ketosphinganine synthase during differentiation and aging of neuronal cells in culture. J. Lipid Res. 1997, 38, 1163–1169. [Google Scholar] [CrossRef]
- Sandhoff, R.; Sandhoff, K. Emerging concepts of ganglioside metabolism. FEBS Lett. 2018, 592, 3835–3864. [Google Scholar] [CrossRef] [PubMed]
- Aureli, M.; Mauri, L.; Carsana, E.V.; Dobi, D.; Breviario, S.; Lunghi, G.; Sonnino, S. Gangliosides and Cell Surface Ganglioside Metabolic Enzymes in the Nervous System. Adv. Neurobiol. 2023, 29, 305–332. [Google Scholar] [PubMed]
- Miyagi, T.; Yamaguchi, K. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology 2012, 22, 880–896. [Google Scholar] [CrossRef]
- Chowdhury, S.; Wu, G.; Lu, Z.H.; Kumar, R.; Ledeen, R. Age-Related Decline in Gangliosides GM1 and GD1a in Non-CNS Tissues of Normal Mice: Implications for Peripheral Symptoms of Parkinson’s Disease. Biomedicines 2023, 11, 209. [Google Scholar] [CrossRef]
- Sonnino, S.; Chigorno, V. Ganglioside molecular species containing C18- and C20-sphingosine in mammalian nervous tissues and neuronal cell cultures. Biochim. Biophys. Acta 2000, 1469, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Aureli, M.; Mauri, L.; Ciampa, M.G.; Prinetti, A.; Toffano, G.; Secchieri, C.; Sonnino, S. GM1 Ganglioside: Past Studies and Future Potential. Mol. Neurobiol. 2016, 53, 1824–1842. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, T.; Hamano, T.; Yano, S.; Koga, H.; Yamamoto, H.; Furukawa, K.; Ledeen, R.W. Stable transfection of GM1 synthase gene into GM1-deficient NG108-15 cells, CR-72 cells, rescues the responsiveness of Trk-neurotrophin receptor to its ligand, NGF. Neurochem. Res. 2002, 27, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, T.; Tokuda, A.; Miyadai, T.; Hamaguchi, M.; Fujiki, N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. USA 1995, 92, 5087–5091. [Google Scholar] [CrossRef]
- Duchemin, A.M.; Ren, Q.; Neff, N.H.; Hadjiconstantinou, M. GM1-induced activation of phosphatidylinositol 3-kinase: Involvement of Trk receptors. J. Neurochem. 2008, 104, 1466–1477. [Google Scholar] [CrossRef]
- Duchemin, A.M.; Ren, Q.; Mo, L.; Neff, N.H.; Hadjiconstantinou, M. GM1 ganglioside induces phosphorylation and activation of Trk and Erk in brain. J. Neurochem. 2002, 81, 696–707. [Google Scholar] [CrossRef]
- Mo, L.; Ren, Q.; Duchemin, A.M.; Neff, N.H.; Hadjiconstantinou, M. GM1 and ERK signaling in the aged brain. Brain Res. 2005, 1054, 125–134. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Biase, E.D.; Maggioni, M.; Lunghi, G.; Fazzari, M.; Pomè, D.Y.; Casellato, R.; Loberto, N.; Mauri, L.; Sonnino, S. GM1 promotes TrkA-mediated neuroblastoma cell differentiation by occupying a plasma membrane domain different from TrkA. J. Neurochem. 2019, 149, 231–241. [Google Scholar] [CrossRef]
- Schengrund, C.L.; Prouty, C. Oligosaccharide portion of GM1 enhances process formation by S20Y neuroblastoma cells. J. Neurochem. 1988, 51, 277–282. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Di Biase, E.; Lunghi, G.; Fazzari, M.; Loberto, N.; Aureli, M.; Mauri, L.; Sonnino, S. Turning the spotlight on the oligosaccharide chain of GM1 ganglioside. Glycoconj. J. 2021, 38, 101–117. [Google Scholar] [CrossRef]
- Fazzari, M.; Audano, M.; Lunghi, G.; Di Biase, E.; Loberto, N.; Mauri, L.; Mitro, N.; Sonnino, S.; Chiricozzi, E. The oligosaccharide portion of ganglioside GM1 regulates mitochondrial function in neuroblastoma cells. Glycoconj. J. 2020, 37, 293–306. [Google Scholar] [CrossRef]
- Di Biase, E.; Lunghi, G.; Fazzari, M.; Maggioni, M.; Pomè, D.Y.; Valsecchi, M.; Samarani, M.; Fato, P.; Ciampa, M.G.; Prioni, S.; et al. Gangliosides in the differentiation process of primary neurons: The specific role of GM1-oligosaccharide. Glycoconj. J. 2020, 37, 329–343. [Google Scholar] [CrossRef]
- Tansey, M.G.; Baloh, R.H.; Milbrandt, J.; Johnson, E.M., Jr. GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron 2000, 25, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Decressac, M.; Kadkhodaei, B.; Mattsson, B.; Laguna, A.; Perlmann, T.; Björklund, A. α-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci. Transl. Med. 2012, 4, 163ra156, Erratum in Sci. Transl. Med. 2016, 8, 335er334.. [Google Scholar] [CrossRef] [PubMed]
- Bartels, T.; Kim, N.C.; Luth, E.S.; Selkoe, D.J. N-alpha-acetylation of α-synuclein increases its helical folding propensity, GM1 binding specificity and resistance to aggregation. PLoS ONE 2014, 9, e103727. [Google Scholar] [CrossRef]
- Martinez, Z.; Zhu, M.; Han, S.; Fink, A.L. GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry 2007, 46, 1868–1877. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.H.; Kulkarni, N.; Ledeen, R.W. Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans. J. Neurosci. Res. 2012, 90, 1997–2008. [Google Scholar] [CrossRef]
- Hadaczek, P.; Wu, G.; Sharma, N.; Ciesielska, A.; Bankiewicz, K.; Davidow, A.L.; Lu, Z.H.; Forsayeth, J.; Ledeen, R.W. GDNF signaling implemented by GM1 ganglioside; failure in Parkinson’s disease and GM1-deficient murine model. Exp. Neurol. 2015, 263, 177–189. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.H.; Wang, J.; Wang, Y.; Xie, X.; Meyenhofer, M.F.; Ledeen, R.W. Enhanced susceptibility to kainate-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraose gangliosides: Protection with LIGA 20, a membrane-permeant analog of GM1. J. Neurosci. 2005, 25, 11014–11022. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lu, Z.H.; Xie, X.; Li, L.; Ledeen, R.W. Mutant NG108-15 cells (NG-CR72) deficient in GM1 synthase respond aberrantly to axonogenic stimuli and are vulnerable to calcium-induced apoptosis: They are rescued with LIGA-20. J. Neurochem. 2001, 76, 690–702. [Google Scholar] [CrossRef]
- Wu, G.; Xie, X.; Lu, Z.H.; Ledeen, R.W. Cerebellar neurons lacking complex gangliosides degenerate in the presence of depolarizing levels of potassium. Proc. Natl. Acad. Sci. USA 2001, 98, 307–312. [Google Scholar] [CrossRef]
- Newburn, E.N.; Duchemin, A.M.; Neff, N.H.; Hadjiconstantinou, M. GM1 ganglioside enhances Ret signaling in striatum. J. Neurochem. 2014, 130, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Snead, D.; Eliezer, D. Intrinsically disordered proteins in synaptic vesicle trafficking and release. J. Biol. Chem. 2019, 294, 3325–3342. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef]
- Bartels, T.; Choi, J.G.; Selkoe, D.J. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2011, 477, 107–110. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Mauri, L.; Lunghi, G.; Di Biase, E.; Fazzari, M.; Maggioni, M.; Valsecchi, M.; Prioni, S.; Loberto, N.; Pome, D.Y.; et al. Parkinson’s disease recovery by GM1 oligosaccharide treatment in the B4galnt1(+/−) mouse model. Sci. Rep. 2019, 9, 19330. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Aras, R.; Williams, C.K.; Koprich, J.B.; Brotchie, J.M.; Singh, V. GM1 Ganglioside Modifies alpha-Synuclein Toxicity and is Neuroprotective in a Rat alpha-Synuclein Model of Parkinson’s Disease. Sci. Rep. 2019, 9, 8362. [Google Scholar] [CrossRef]
- Fazzari, M.; Di Biase, E.; Zaccagnini, L.; Henriques, A.; Callizot, N.; Ciampa, M.G.; Mauri, L.; Carsana, E.V.; Loberto, N.; Aureli, M.; et al. GM1 oligosaccharide efficacy against α-synuclein aggregation and toxicity in vitro. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159350. [Google Scholar] [CrossRef] [PubMed]
- Fortin, D.L.; Nemani, V.M.; Voglmaier, S.M.; Anthony, M.D.; Ryan, T.A.; Edwards, R.H. Neural activity controls the synaptic accumulation of alpha-synuclein. J. Neurosci. 2005, 25, 10913–10921. [Google Scholar] [CrossRef]
- Fortin, D.L.; Troyer, M.D.; Nakamura, K.; Kubo, S.; Anthony, M.D.; Edwards, R.H. Lipid rafts mediate the synaptic localization of alpha-synuclein. J. Neurosci. 2004, 24, 6715–6723. [Google Scholar] [CrossRef]
- Ottico, E.; Prinetti, A.; Prioni, S.; Giannotta, C.; Basso, L.; Chigorno, V.; Sonnino, S. Dynamics of membrane lipid domains in neuronal cells differentiated in culture. J. Lipid Res. 2003, 44, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Chigorno, V.; Valsecchi, M.; Acquotti, D.; Sonnino, S.; Tettamanti, G. Formation of a cytosolic ganglioside-protein complex following administration of photoreactive ganglioside GM1 to human fibroblasts in culture. FEBS Lett. 1990, 263, 329–331. [Google Scholar] [CrossRef]
- Del Tredici, K.; Rüb, U.; De Vos, R.A.; Bohl, J.R.; Braak, H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 2002, 61, 413–426. [Google Scholar] [CrossRef]
- Ledeen, R.; Wu, G. Gangliosides of the Nervous System. Methods Mol. Biol. 2018, 1804, 19–55. [Google Scholar]
- Ledeen, R.W.; Wu, G. Gangliosides, α-Synuclein, and Parkinson’s Disease. Prog. Mol. Biol. Transl. Sci. 2018, 156, 435–454. [Google Scholar] [PubMed]
- Schneider, J.S. Altered expression of genes involved in ganglioside biosynthesis in substantia nigra neurons in Parkinson’s disease. PLoS ONE 2018, 13, e0199189. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Lunghi, G.; Di Biase, E.; Fazzari, M.; Sonnino, S.; Mauri, L. GM1 Ganglioside Is a Key Factor in Maintaining the Mammalian Neuronal Functions Avoiding Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 868. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lu, Z.H.; Seo, J.H.; Alselehdar, S.K.; DeFrees, S.; Ledeen, R.W. Mice deficient in GM1 manifest both motor and non-motor symptoms of Parkinson’s disease; successful treatment with synthetic GM1 ganglioside. Exp. Neurol. 2020, 329, 113284. [Google Scholar] [CrossRef]
- Di Biase, E.; Lunghi, G.; Maggioni, M.; Fazzari, M.; Pomè, D.Y.; Loberto, N.; Ciampa, M.G.; Fato, P.; Mauri, L.; Sevin, E.; et al. GM1 Oligosaccharide Crosses the Human Blood-Brain Barrier In Vitro by a Paracellular Route. Int. J. Mol. Sci. 2020, 21, 2858. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Aureli, M.; Mauri, L.; Di Biase, E.; Lunghi, G.; Fazzari, M.; Valsecchi, M.; Carsana, E.V.; Loberto, N.; Prinetti, A.; et al. Glycosphingolipids. Adv. Exp. Med. Biol. 2021, 1325, 61–102. [Google Scholar]
- Davies, L.; Fassbender, K.; Walter, S. Sphingolipids in neuroinflammation. Handb. Exp. Pharmacol. 2013, 421–430. [Google Scholar] [CrossRef]
- Hawkins-Salsbury, J.A.; Parameswar, A.R.; Jiang, X.; Schlesinger, P.H.; Bongarzone, E.; Ory, D.S.; Demchenko, A.V.; Sands, M.S. Psychosine, the cytotoxic sphingolipid that accumulates in globoid cell leukodystrophy, alters membrane architecture. J. Lipid Res. 2013, 54, 3303–3311. [Google Scholar] [CrossRef] [PubMed]
- Mizugishi, K.; Yamashita, T.; Olivera, A.; Miller, G.F.; Spiegel, S.; Proia, R.L. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell Biol. 2005, 25, 11113–11121. [Google Scholar] [CrossRef] [PubMed]
- Penno, A.; Reilly, M.M.; Houlden, H.; Laurá, M.; Rentsch, K.; Niederkofler, V.; Stoeckli, E.T.; Nicholson, G.; Eichler, F.; Brown, R.H., Jr.; et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 2010, 285, 11178–11187. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giussani, P.; Mauri, L.; Sonnino, S. The Involvement of Ceramide, Sphingosine-1-Phosphate and Ganglioside GM1 in Regulating Some Nervous System Functions. Int. J. Mol. Sci. 2025, 26, 11118. https://doi.org/10.3390/ijms262211118
Giussani P, Mauri L, Sonnino S. The Involvement of Ceramide, Sphingosine-1-Phosphate and Ganglioside GM1 in Regulating Some Nervous System Functions. International Journal of Molecular Sciences. 2025; 26(22):11118. https://doi.org/10.3390/ijms262211118
Chicago/Turabian StyleGiussani, Paola, Laura Mauri, and Sandro Sonnino. 2025. "The Involvement of Ceramide, Sphingosine-1-Phosphate and Ganglioside GM1 in Regulating Some Nervous System Functions" International Journal of Molecular Sciences 26, no. 22: 11118. https://doi.org/10.3390/ijms262211118
APA StyleGiussani, P., Mauri, L., & Sonnino, S. (2025). The Involvement of Ceramide, Sphingosine-1-Phosphate and Ganglioside GM1 in Regulating Some Nervous System Functions. International Journal of Molecular Sciences, 26(22), 11118. https://doi.org/10.3390/ijms262211118







