Novel SNP Combination for Predictive Osteoporotic Diagnosis
Abstract
1. Introduction
- COL1A1 (encoding the α1-chain of type I collagen),
- CYP19A1 (encoding aromatase, responsible for converting testosterone to estradiol),
2. Results
2.1. Identification of Osteoporosis-Associated SNPs in GPCR Genes
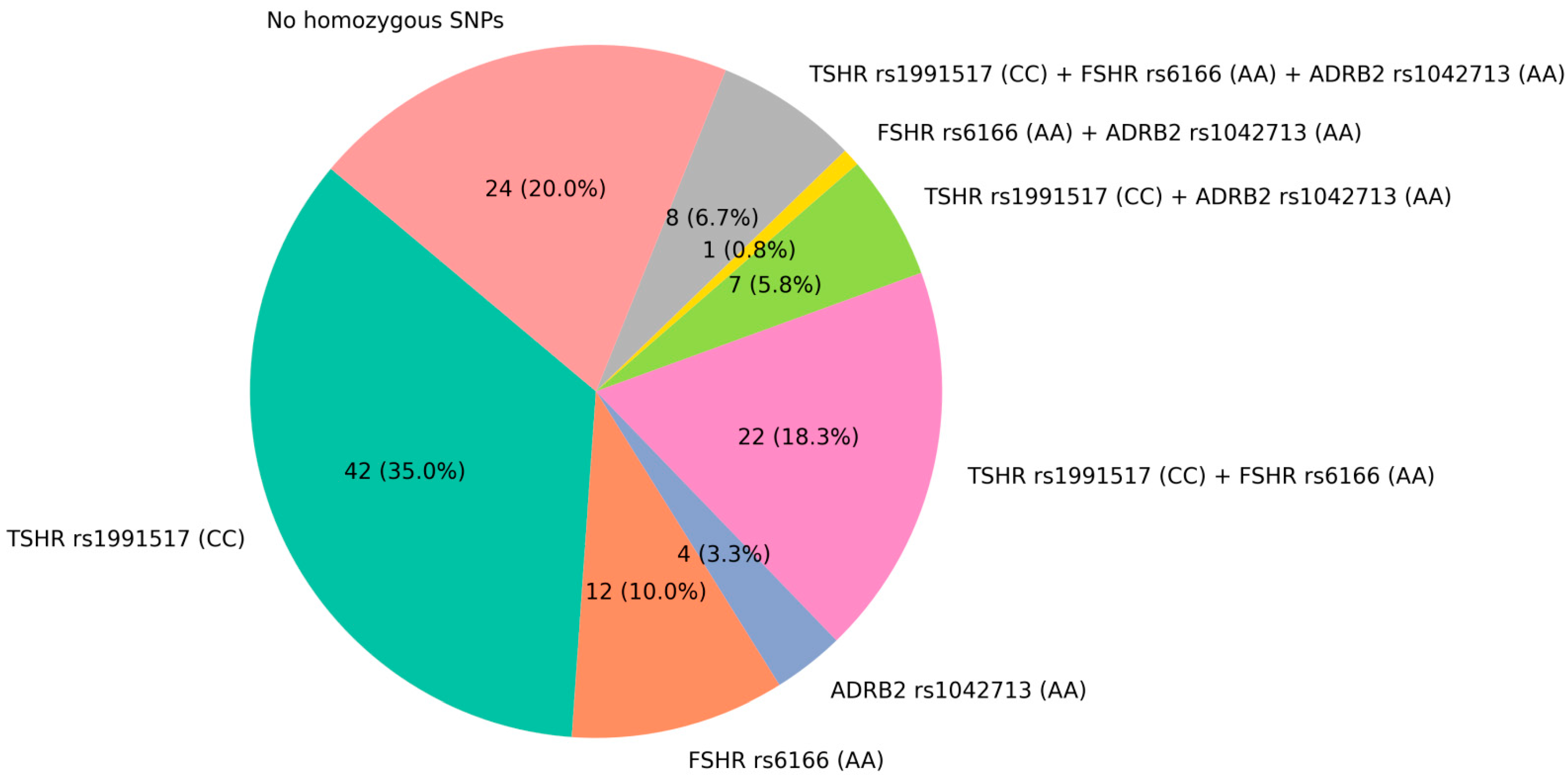
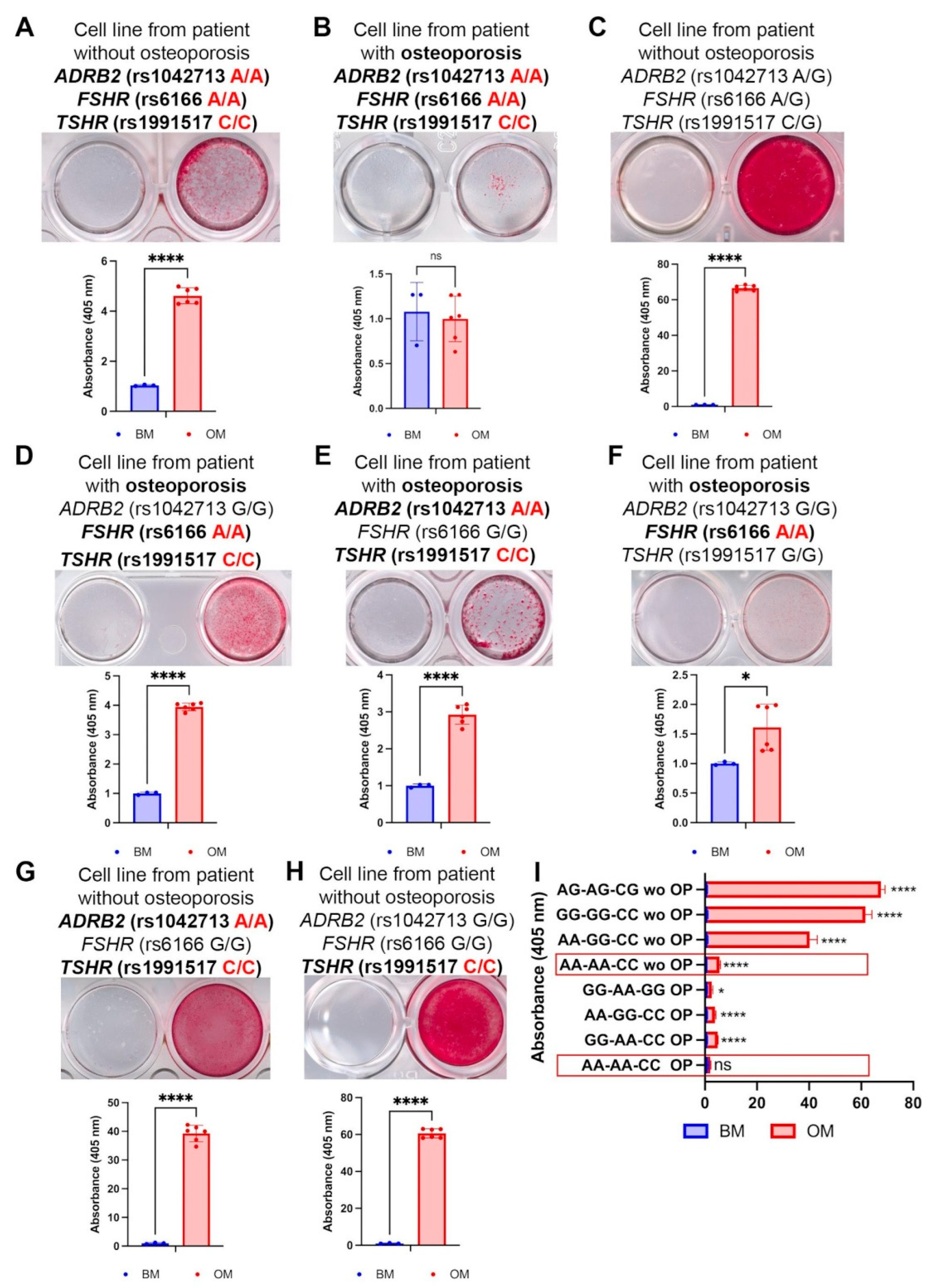
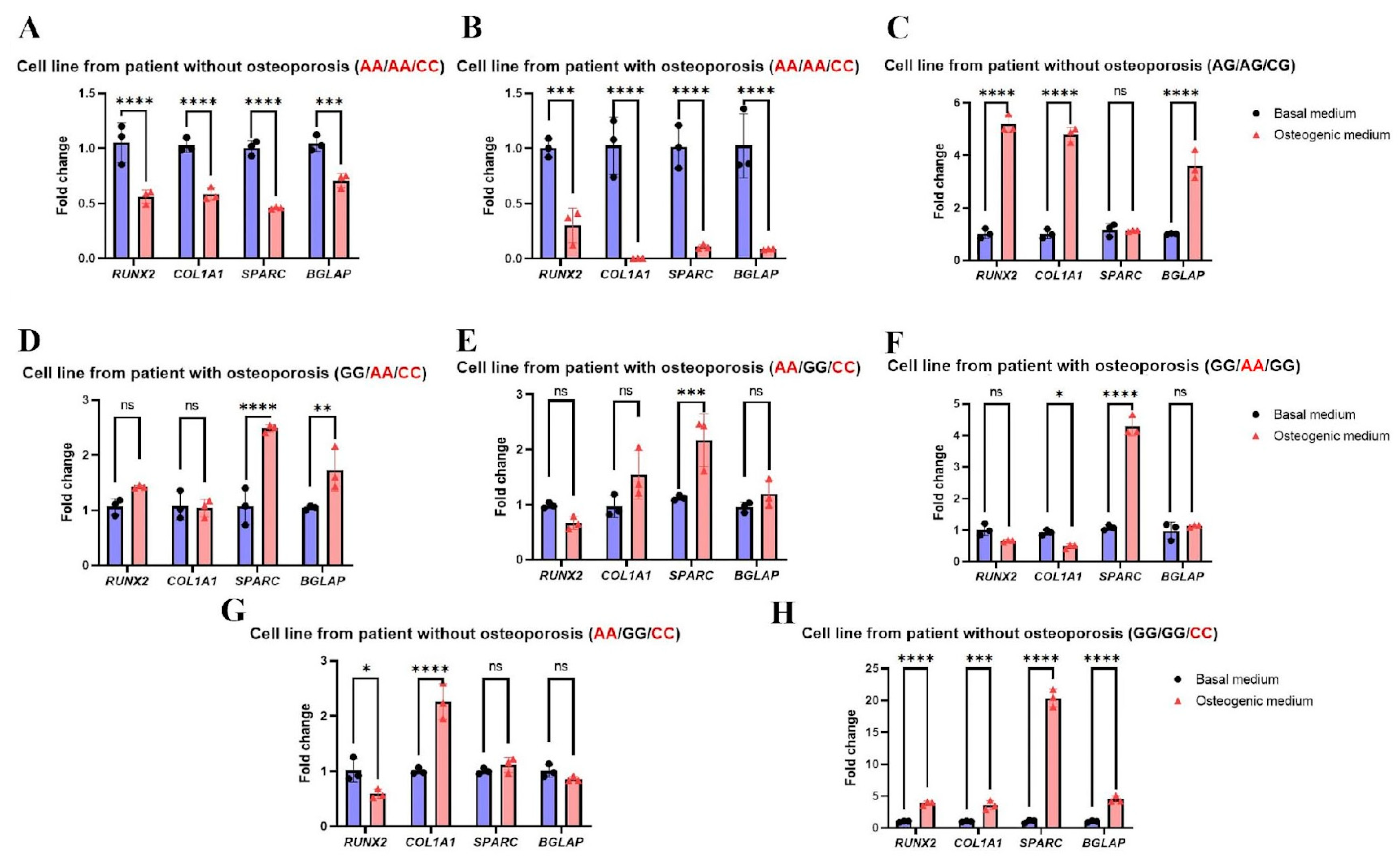
2.2. The Combination of Triple-Homozygous FSHR (rs6166 AA), TSHR (rs1991517 CC), and ADRB2 (rs1042713 AA) Alleles Is Frequent in Patients with Osteoporosis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Whole-Genome Sequencing Protocol
4.3. Read Alignment and Variant Calling
4.4. Genomic DNA Isolation, Sanger Sequencing, and Detection of SNPs Using Real-Time PCR
4.5. Cell Isolation and Cultivation
4.6. Osteogenic Differentiation and Calcium Deposit Staining with Alizarin Red
4.7. Quantitative Polymerase Chain Reaction (qPCR) Analysis of Osteogenic Differentiation Markers’ Gene Expressions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Jeremiah, M.P.; Unwin, B.K.; Greenawald, M.H.; Casiano, V.E. Diagnosis and Management of Osteoporosis. Am. Fam. Physician 2015, 92, 261–268. [Google Scholar]
- Lesnyak, O.M.; Baranova, I.A.; Belova, K.Y.; Gladkova, E.N.; Evstigneeva, L.P.; Ershova, O.B.; Karonova, T.L.; Kochish, A.Y.; Nikitinskaya, O.A.; Skripnikova, I.A.; et al. Osteoporosis in Russian Federation: Epidemiology, socio-medical and economical aspects (review). Traumatol. Orthop. Russ. 2018, 24, 155–168. [Google Scholar] [CrossRef]
- Lesnyak, O.; Svedbom, A.; Belova, K.; Dobrovolskaya, O.; Ershova, O.; Golubev, G.; Grebenshikov, V.; Ivanov, S.; Kochish, A.; Menshikova, L.; et al. Quality of life after fragility fracture in the Russian Federation: Results from the Russian arm of the International Cost and Utility Related to Osteoporotic Fractures Study (ICUROS). Arch. Osteoporos. 2020, 15, 37. [Google Scholar] [CrossRef]
- Stewart, T.L.; Ralston, S.H. Role of genetic factors in the pathogenesis of osteoporosis. J. Endocrinol. 2000, 166, 235–245. [Google Scholar] [CrossRef]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; ACauley, J.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef]
- Johnson, J.; Wang, M.Y. Genome search reveals gene variants associated with low bone mineral density and fractures in osteoporosis. Neurosurgery 2008, 63, N12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, X.; Bai, W.; Zheng, H. Twelve years of GWAS discoveries for osteoporosis and related traits: Advances, challenges and applications. Bone Res. 2021, 9, 23. [Google Scholar] [CrossRef]
- Arden, N.K.; Baker, J.; Hogg, C.; Baan, K.; Spector, T.D. The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: A study of postmenopausal twins. J. Bone Miner. Res. 1996, 11, 530–534. [Google Scholar] [CrossRef]
- Yuan, J.; Tickner, J.; Mullin, B.H.; Zhao, J.; Zeng, Z.; Morahan, G.; Xu, J. Advanced genetic approaches in discovery and characterization of genes involved with osteoporosis in mouse and human. Front. Genet. 2019, 10, 288. [Google Scholar] [CrossRef]
- Sule, G.; Campeau, P.M.; Zhang, V.W.; Nagamani, S.C.S.; Dawson, B.C.; Grover, M.; Bacino, C.A.; Sutton, V.R.; Brunetti-Pierri, N.; Lu, J.T.; et al. Next-generation sequencing for disorders of low and high bone mineral density. Osteoporos. Int. 2013, 24, 2253–2259. [Google Scholar] [CrossRef]
- Wu, Q.; Jung, J. Genome-wide polygenic risk score for major osteoporotic fractures in postmenopausal women using associated single nucleotide polymorphisms. J. Transl. Med. 2023, 21, 127. [Google Scholar] [CrossRef]
- Du, X.-P.; Zheng, M.-L.; Yang, X.-C.; Zheng, M.-L. High blood pressure is associated with increased risk of future fracture, but not vice versa. Sci. Rep. 2024, 14, 8005. [Google Scholar] [CrossRef]
- Tomasiuk, J.M.; Nowakowska-Płaza, A.; Wisłowska, M.; Głuszko, P. Osteoporosis and diabetes—Possible links and diagnostic difficulties. Reumatologia 2023, 61, 294–304. [Google Scholar] [CrossRef]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Hypertension is a risk factor for fractures. Calcif. Tissue Int. 2009, 84, 103–111. [Google Scholar] [CrossRef]
- Rejnmark, L.; Vestergaard, P.; Mosekilde, L. Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: A nationwide case-control study. J. Hypertens. 2006, 24, 581–589. [Google Scholar] [CrossRef]
- Kim, K.M.; Hwang, E.J.; Lee, S.; Yoon, J.-H. The impact of Renin-Angiotensin System Inhibitors on bone fracture risk: A nationwide nested case-control study. BMC Musculoskelet. Disord. 2024, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, E.; Aida, K.; Chen, J.; Hayashi, J.I.; Isobe, K.; Tawata, M.; Onaya, T. A patient with type 2 diabetes mellitus associated with mutations in calcium sensing receptor gene and mitochondrial DNA. Biochem. Biophys. Res. Commun. 2000, 278, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Keinan, D.; Yang, S.; Cohen, R.E.; Yuan, X.; Liu, T.; Li, Y.-P. Role of regulator of G protein signaling proteins in bone. Front. Biosci. (Landmark Ed) 2014, 19, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Sopova, J.; Krasnova, O.; Vasilieva, G.; Zhuk, A.; Lesnyak, O.; Karelkin, V.; Neganova, I. SNPs in GPCR Genes and Impaired Osteogenic Potency in Osteoporotic Patient Lines-Based Study. Int. J. Mol. Sci. 2024, 25, 13594. [Google Scholar] [CrossRef]
- Krasnova, O.; Sopova, J.; Kovaleva, A.; Semenova, P.; Zhuk, A.; Smirnova, D.; Perepletchikova, D.; Bystrova, O.; Martynova, M.; Karelkin, V.; et al. Unraveling the Mechanism of Impaired Osteogenic Differentiation in Osteoporosis: Insights from ADRB2 Gene Polymorphism. Cells 2024, 13, 2110. [Google Scholar] [CrossRef]
- Luo, J.; Sun, P.; Siwko, S.; Liu, M.; Xiao, J. The role of GPCRs in bone diseases and dysfunctions. Bone Res. 2019, 7, 19. [Google Scholar] [CrossRef]
- Domnina, A.P.; Krasnova, O.A.; Kulakova, K.A.; Sopova, Y.V.; Karelkin, V.V.; Lesnyak, O.M.; Neganova, I.E. Role of G protein-associated membrane receptors in the pathogenesis of osteoporosis. Transl. Med. 2022, 9, 41–61. (In Russian) [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Diepenhorst, N.; Rueda, P.; Cook, A.E.; Pastoureau, P.; Sabatini, M.; Langmead, C.J. G protein-coupled receptors as anabolic drug targets in osteoporosis. Pharmacol. Ther. 2018, 184, 1–12. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Liu, X.; Huang, W.; Zhu, C.; Xu, Y.; Yang, H.; Bai, J.; Geng, D. Targeting strategies for bone diseases: Signaling pathways and clinical studies. Signal Transduct. Target. Ther. 2023, 8, 202. [Google Scholar] [CrossRef]
- Lu, Y.; Ren, X.; Wang, Y.; Li, T.; Li, F.; Wang, S.; Xu, C.; Wu, G.; Li, H.; Li, G.; et al. Mutational and structural characteristics of four novel heterozygous C-propeptide mutations in the proα1(I) collagen gene in Chinese osteogenesis imperfecta patients. Clin. Endocrinol. 2014, 80, 524–531. [Google Scholar] [CrossRef]
- Braga, V.; Mottes, M.; Mirandola, S.; Lisi, V.; Malerba, G.; Sartori, L.; Bianchi, G.; Gatti, D.; Rossini, M.; Bianchini, D.; et al. Association of CTR and COLIA1 alleles with BMD values in peri- and postmenopausal women. Calcif. Tissue Int. 2000, 67, 361–366. [Google Scholar] [CrossRef]
- Hong, X.; Hsu, Y.-H.; Terwedow, H.; Arguelles, L.M.; Tang, G.; Liu, X.; Zhang, S.; Xu, X.; Xu, X. CYP19A1 polymorphisms are associated with bone mineral density in Chinese men. Hum. Genet. 2007, 121, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kaur, M.; Khetarpal, P. CYP19 gene rs2414096 variant and differential genetic risk of polycystic ovary syndrome: A systematic review and meta-analysis. Gynecol. Endocrinol. 2021, 37, 126–131. [Google Scholar] [CrossRef]
- Mondockova, V.; Adamkovicova, M.; Lukacova, M.; Grosskopf, B.; Babosova, R.; Galbavy, D.; Martiniakova, M.; Omelka, R. The estrogen receptor 1 gene affects bone mineral density and osteoporosis treatment efficiency in Slovak postmenopausal women. BMC Med Genet. 2018, 19, 174. [Google Scholar] [CrossRef]
- Ji, Y.-F.; Jiang, X.; Li, W.; Ge, X. Impact of interleukin-6 gene polymorphisms and its interaction with obesity on osteoporosis risk in Chinese postmenopausal women. Environ. Heal. Prev. Med. 2019, 24, 48. [Google Scholar] [CrossRef]
- Kruk, M.; Ralston, S.H.; Albagha, O.M.E. LRP5 Polymorphisms and response to risedronate treatment in osteoporotic men. Calcif. Tissue Int. 2009, 84, 171–179. [Google Scholar] [CrossRef]
- Zhu, D.-L.; Chen, X.-F.; Hu, W.-X.; Dong, S.-S.; Lu, B.-J.; Rong, Y.; Chen, Y.-X.; Chen, H.; Thynn, H.N.; Wang, N.-N.; et al. Multiple Functional Variants at 13q14 Risk Locus for Osteoporosis Regulate RANKL Expression Through Long-Range Super-Enhancer. J. Bone Miner. Res. 2018, 33, 1335–1346. [Google Scholar] [CrossRef]
- Roshandel, D.; Holliday, K.L.; Pye, S.R.; Boonen, S.; Borghs, H.; Vanderschueren, D.; Huhtaniemi, I.T.; E Adams, J.; A Ward, K.; Bartfai, G.; et al. Genetic variation in the RANKL/RANK/OPG signaling pathway is associated with bone turnover and bone mineral density in men. J. Bone Miner. Res. 2010, 25, 1830–1838. [Google Scholar] [CrossRef]
- Yureneva, S.V.; E Donnikov, A.; Bordakova, E.V.; Yakushevskaya, O.V.; Smetnik, A.A.; Trofimov, D.Y. Clinical and prog-nostic significance of molecular genetic factors in postmenopausal osteoporosis. Osteoporos. Bone Dis. 2015, 18, 3–6. (In Russian) [Google Scholar] [CrossRef]
- Lorentzon, M.; Lorentzon, R.; Lerner, U.; Nordstrom, P. Calcium sensing receptor gene polymorphism, circulating calcium concentrations and bone mineral density in healthy adolescent girls. Eur. J. Endocrinol. 2001, 144, 257–261. [Google Scholar] [CrossRef]
- Di Nisio, A.; Rocca, M.S.; Ghezzi, M.; Ponce, M.D.R.; Taglianetti, S.; Plebani, M.; Ferlin, A.; Foresta, C. Calcium-sensing receptor polymorphisms increase the risk of osteoporosis in ageing males. Endocrine 2017, 61, 349–352. [Google Scholar] [CrossRef]
- Liang, X.; Wu, C.; Zhao, H.; Liu, L.; Du, Y.; Li, P.; Wen, Y.; Zhao, Y.; Ding, M.; Cheng, B.; et al. Assessing the genetic correlations between early growth parameters and bone mineral density: A polygenic risk score analysis. Bone 2018, 116, 301–306. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.; Ku, S.-Y.; Choi, Y.M.; Kim, J.H.; Kim, J.G. Association between polymorphisms in leptin, leptin receptor, and β-adrenergic receptor genes and bone mineral density in postmenopausal Korean women. Menopause 2014, 21, 67–73. [Google Scholar] [CrossRef]
- Woo, J.H.; Kim, H.; Kim, J.H.; Kim, J.G. Cannabinoid receptor gene polymorphisms and bone mineral density in Korean postmenopausal women. Menopause 2015, 22, 512–519. [Google Scholar] [CrossRef]
- Yamada, Y.; Ando, F.; Shimokata, H. Association of candidate gene polymorphisms with bone mineral density in community-dwelling Japanese women and men. Int. J. Mol. Med. 2007, 19, 791–801. [Google Scholar] [CrossRef]
- Karsak, M.; Cohen-Solal, M.; Freudenberg, J.; Ostertag, A.; Morieux, C.; Kornak, U.; Essig, J.; Erxlebe, E.; Bab, I.; Kubisch, C.; et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum. Mol. Genet. 2005, 14, 3389–3396. [Google Scholar] [CrossRef]
- Chiang, T.-I.; Lane, H.-Y.; Lin, C.-H. D2 dopamine receptor gene (DRD2) Taq1A (rs1800497) affects bone density. Sci. Rep. 2020, 10, 13236. [Google Scholar] [CrossRef]
- Yamada, Y.; Ando, F.; Niino, N.; Shimokata, H. Association of a polymorphism of the dopamine receptor D4 gene with bone mineral density in Japanese men. J. Hum. Genet. 2003, 48, 629–633. [Google Scholar] [CrossRef][Green Version]
- Garg, G.; Kumar, J.; McGuigan, F.E.; Ridderstråle, M.; Gerdhem, P.; Luthman, H.; Åkesson, K. Variation in the MC4R gene is associated with bone phenotypes in elderly Swedish women. PLOS ONE 2014, 9, e88565. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Yeo, G.S.; Keogh, J.M.; Aminian, S.; Jebb, S.A.; Butler, G.; Cheetham, T.; O’rAhilly, S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Investig. 2000, 106, 271–279. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Wu, Y.; Lu, T.; Yuan, M.; Cui, Y.; Zhou, Y.; Yang, G.; Hong, Y. Association of osteoporosis with genetic variants of circadian genes in Chinese geriatrics. Osteoporos. Int. 2016, 27, 1485–1492. [Google Scholar] [CrossRef]
- Dennison, E.M.; Syddall, H.E.; Jameson, K.A.; Sayer, A.A.; Gaunt, T.R.; Rodriguez, S.; Day, I.N.; Cooper, C.; Lips, M.A. A study of relationships between single nucleotide polymorphisms from the growth hormone-insulin-like growth factor axis and bone mass: The Hertfordshire cohort study. J. Rheumatol. 2009, 36, 1520–1526. [Google Scholar] [CrossRef]
- Iwasaki, H.; Emi, M.; Ezura, Y.; Ishida, R.; Kajita, M.; Kodaira, M.; Yoshida, H.; Suzuki, T.; Hosoi, T.; Inoue, S.; et al. Association of a Trp16Ser variation in the gonadotropin releasing hormone signal peptide with bone mineral density, revealed by SNP-dependent PCR typing. Bone 2003, 32, 185–190. [Google Scholar] [CrossRef]
- Chun, E.H.; Kim, H.; Suh, C.S.; Kim, J.H.; Kim, D.Y.; Kim, J.G. Polymorphisms in neuropeptide genes and bone mineral density in Korean postmenopausal women. Menopause 2015, 22, 1256–1263. [Google Scholar] [CrossRef]
- Eraltan, H.; Cacina, C.; Kahraman, O.T.; Kurt, O.; Aydogan, H.Y.; Uyar, M.; Can, A.; Cakmakoğlu, B. MCP-1 and CCR2 Gene Variants and the Risk for Osteoporosis and Osteopenia. Genet. Test. Mol. Biomarkers 2012, 16, 229–233. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, L.-J.; Chen, X.-D.; Papasian, C.J.; Wu, K.-H.; Tan, L.-J.; Wang, Z.-E.; Pei, Y.-F.; Tian, Q.; Deng, H.-W. Bivariate genome-wide association analyses identified genetic pleiotropic effects for bone mineral density and alcohol drinking in Caucasians. J. Bone Miner. Metab. 2016, 35, 649–658. [Google Scholar] [CrossRef]
- Rendina, D.; Gianfrancesco, F.; De Filippo, G.; Merlotti, D.; Esposito, T.; Mingione, A.; Nuti, R.; Strazzullo, P.; Mossetti, G.; Gennari, L. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. Eur. J. Endocrinol. 2010, 163, 165–172. [Google Scholar] [CrossRef]
- Shi, S.-Q.; Li, S.-S.; Zhang, X.-Y.; Wei, Z.; Fu, W.-Z.; He, J.-W.; Hu, Y.-Q.; Li, M.; Zheng, L.-L.; Zhang, Z.-L. LGR4 Gene Polymorphisms Are Associated With Bone and Obesity Phenotypes in Chinese Female Nuclear Families. Front. Endocrinol. 2021, 12, 656077. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, H.; Shen, H.; Ehrlich, M. Prioritization of osteoporosis-associated genome-wide association study (GWAS) single-nucleotide polymorphisms (SNPs) using epigenomics and transcriptomics. JBMR Plus 2021, 5, e10481. [Google Scholar] [CrossRef]
- Styrkarsdottir, U.; Thorleifsson, G.; Sulem, P.; Gudbjartsson, D.F.; Sigurdsson, A.; Jonasdottir, A.; Jonasdottir, A.; Oddsson, A.; Helgason, A.; Magnusson, O.T.; et al. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature 2013, 497, 517–520. [Google Scholar] [CrossRef]
- Wesselius, A.; Bours, M.J.L.; Henriksen, Z.; Syberg, S.; Petersen, S.; Schwarz, P.; Jørgensen, N.R.; van Helden, S.; Dagnelie, P.C. Association of P2Y(2) receptor SNPs with bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Purinergic Signal. 2012, 9, 41–49. [Google Scholar] [CrossRef]
- Ferlin, A.; Pepe, A.; Gianesello, L.; Garolla, A.; Feng, S.; Giannini, S.; Zaccolo, M.; Facciolli, A.; Morello, R.; I Agoulnik, A.; et al. Mutations in the insulin-like factor 3 receptor are associated with osteoporosis. J. Bone Miner. Res. 2008, 23, 683–693. [Google Scholar] [CrossRef]
- Van Der Deure, W.M.; Uitterlinden, A.G.; Hofman, A.; Rivadeneira, F.; Pols, H.A.P.; Peeters, R.P.; Visser, T.J. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: The Rotterdam Study. Clin. Endocrinol. 2008, 68, 175–181. [Google Scholar] [CrossRef]
- Morris, J.A.; Kemp, J.P.; Youlten, S.E.; Laurent, L.; Logan, J.G.; Chai, R.C.; Vulpescu, N.A.; Forgetta, V.; Kleinman, A.; Mohanty, S.T.; et al. Author Correction: An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2019, 51, 920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kuipers, A.L.; Yerges-Armstrong, L.M.; Nestlerode, C.S.; Jin, Z.; Wheeler, V.W.; Patrick, A.L.; Bunker, C.H.; Zmuda, J.M. Functional and association analysis of frizzled 1 (FZD1) promoter haplotypes with femoral neck geometry. Bone 2010, 46, 1131–1137. [Google Scholar] [CrossRef]
- Masi, L.; Becherini, L.; Colli, E.; Gennari, L.; Mansani, R.; Falchetti, A.; Becorpi, A.M.; Cepollaro, C.; Gonnelli, S.; Tanini, A.; et al. Polymorphisms of the calcitonin receptor gene are associated with bone mineral density in postmenopausal Italian women. Biochem. Biophys. Res. Commun. 1998, 248, 190–195. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, S.-Y.; Kim, G.S.; Hwang, J.-Y.; Kim, Y.-J.; Jeong, B.; Kim, T.-H.; Park, E.K.; Lee, S.H.; Kim, H.-L.; et al. Fracture, bone mineral density, and the effects of calcitonin receptor gene in postmenopausal Koreans. Osteoporos. Int. 2010, 21, 1351–1360. [Google Scholar] [CrossRef]
- The Genetic Factors for Osteoporosis (GEFOS) Consortium. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat. Genet. 2009, 41, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Torekov, S.S.; Harsløf, T.; Rejnmark, L.; Eiken, P.; Jensen, J.B.; Herman, A.P.; Hansen, T.; Pedersen, O.; Holst, J.J.; Langdahl, B.L. A functional amino acid substitution in the glucose-dependent insulinotropic polypeptide receptor (GIPR) gene is associated with lower bone mineral density and increased fracture risk. J. Clin. Endocrinol. Metab. 2014, 99, E729–E733. [Google Scholar] [CrossRef]
- Abdi, S.; Almiman, A.A.; Ansari, M.G.A.; Alnaami, A.M.; Mohammed, A.K.; Aljohani, N.J.; Alenad, A.; Alghamdi, A.; Alokail, M.S.; Al-Daghri, N.M. PTHR1 Genetic Polymorphisms Are Associated with Osteoporosis among Postmenopausal Arab Women. BioMed Res. Int. 2021, 2021, 2993761. [Google Scholar] [CrossRef]
- Roshandel, D.; Thomson, W.; Pye, S.R.; Boonen, S.; Borghs, H.; Vanderschueren, D.; Huhtaniemi, I.T.; Adams, J.E.; Ward, K.A.; Bartfai, G.; et al. Polymorphisms in genes involved in the NF-κB Signalling pathway are associated with bone mineral density, geometry and turnover in men. PLoS ONE 2011, 6, e28031. [Google Scholar] [CrossRef]
- Ye, G.; Huang, Y.; Yin, L.; Wang, J.; Huang, X.; Bin, X. Association between LEPR polymorphism and susceptibility of osteoporosis in Chinese Mulao people. Artif. Cells, Nanomedicine, Biotechnol. 2022, 50, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Abe, E.; Marians, R.C.; Yu, W.; Wu, X.-B.; Ando, T.; Li, Y.; Iqbal, J.; Eldeiry, L.; Rajendren, G.; Blair, H.C.; et al. TSH is a negative regulator of skeletal remodeling. Cell 2003, 115, 151–162. [Google Scholar] [CrossRef]
- Bassett, J.H.; Williams, G.R. Critical role of the hypothalamic-pituitary-thyroid axis in bone. Bone 2008, 43, 418–426. [Google Scholar] [CrossRef]
- Zhong, X.P.; Xia, W.F. Regulation of bone metabolism mediated by β-adrenergic receptor and its clinical application. World J. Clin. Cases 2021, 9, 8967–8973. [Google Scholar] [CrossRef]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Simões, R.; Ferreira, J.; Barbosa, A.P.; Mascarenhas, M.R.; Bicho, M. Beta-2 adrenergic receptor (ADRB2) gene polymorphisms as risk factors for reduced bone mineral density. Rev. Port. Endocrinol. Diabetes E Metab. 2016, 11, 12–16. [Google Scholar] [CrossRef]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.N.; Komm, B.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Sekaran, S.; Dhanasekaran, A.; Warrier, S. Type 1 collagen: Synthesis, structure and key functions in bone mineralization. Differentiation 2024, 136, 100757. [Google Scholar] [CrossRef]
- Musso, G.; Paschetta, E.; Gambino, R.; Cassader, M.; Molinaro, F. Interactions among bone, liver, and adipose tissue predisposing to diabesity and fatty liver. Trends Mol. Med. 2013, 19, 522–535. [Google Scholar] [CrossRef]
- Rosset, E.M.; Bradshaw, A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016, 52–54, 78–87. [Google Scholar] [CrossRef]
- Fairbrother, U.L.; Tankó, L.B.; Walley, A.J.; Christiansen, C.; Froguel, P.; Blakemore, A.I. Leptin receptor genotype at Gln223Arg is associated with body composition, BMD, and vertebral fracture in postmenopausal Danish women. J. Bone Miner. Res. 2007, 22, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, L.; Harrison, D.G. Hypertension and osteoporosis: Common pathophysiological mechanisms. Med. Nov. Technol. Devices 2020, 8, 100047. [Google Scholar] [CrossRef]
- Chen, J.; Liao, Y.; Sheng, Y.; Yao, H.; Li, T.; He, Z.; Ye, W.W.Y.; Yin, M.; Tang, H.; Zhao, Y.; et al. FSH exacerbates bone loss by promoting osteoclast energy metabolism through the CREB-MDH2-NAD+ axis. Metabolism 2025, 165, 156147. [Google Scholar] [CrossRef]
- Boutin, A.; Gershengorn, M.C.; Neumann, S. β-Arrestin 1 in Thyrotropin Receptor Signaling in Bone: Studies in Osteoblast-Like Cells. Front. Endocrinol. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.J.; Landao-Bassonga, E.; Ralston, S.H.; Idris, A.I. Beta2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch. Biochem. Biophys. 2009, 482, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Becker, F.; van El, C.G.; Ibarreta, D.; Zika, E.; Hogarth, S.; Borry, P.; Cambon-Thomsen, A.; Cassiman, J.J.; Evers-Kiebooms, G.; Hodgson, S.; et al. Genetic testing and common disorders in a public health framework: How to assess relevance and possibilities. Background Document to the ESHG recommendations on genetic testing and common disorders. Eur. J. Hum. Genet. EJHG 2011, 19 (Suppl. 1), S6–S44. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Babraham Bioinformatics. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 12 November 2025).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Poplin, R.; Ruano-Rubio, V.; DePristo, M.A.; Fennell, T.J.; Carneiro, M.O.; Van der Auwera, G.A.; Kling, D.E.; Gauthier, L.D.; Levy-Moonshine, A.; Roazen, D.; et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv 2018. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef]
- Bernar, A.; Gebetsberger, J.V.; Bauer, M.; Streif, W.; Schirmer, M. Optimization of the Alizarin Red S Assay by Enhancing Mineralization of Osteoblasts. Int. J. Mol. Sci. 2022, 24, 723. [Google Scholar] [CrossRef] [PubMed]
| Gene | rsID | Type | Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 | Patient #6 |
|---|---|---|---|---|---|---|---|---|
| LEPR | rs1137100 | Missense variant | A/G | A/G | A/G | GG | AA | A/G |
| FSHR | rs6166 | AA | AA | GG | GG | GG | AA | |
| CASR | rs1801725 | G/T | GG | GG | G/T | G/T | G/T | |
| ADRB2 | rs1042713 | G/A | GG | GG | G/A | AA | AA | |
| CALCR | rs1801197 | TT | CC | TT | TT | TT | T/C | |
| GNRH1 | rs6185 | CC | CC | GG | CC | C/G | CC | |
| P2RY2 | rs2511241 | TT | C/T | TT | TT | TT | TT | |
| DRD2 | rs1800497 | CC | C/T | CC | C/T | C/T | TT | |
| TSHR | rs1991517 | CC | G/C | CC | G/C | CC | CC | |
| GIPR | rs1800437 | G/C | GG | GG | GG | GG | G/C | |
| NPY2R | rs2880415 | Synonymous variant | TT | TT | C/T | C/T | C/T | TT |
| NPY2R | rs6857715 | Intron variant | TT | C/T | C/T | C/T | TT | TT |
| OPRM1 | rs4870268 | TT | T/C | T/C | CC | CC | T/C | |
| OPRM1 | rs9479769 | TT | T/C | T/C | CC | CC | T/C | |
| OPRM1 | rs1998221 | TT | T/C | T/C | CC | CC | T/C | |
| CALCR | rs2051748 | A/G | AA | AA | A/G | AA | A/G | |
| CALCR | rs2051748 | A/G | AA | AA | A/G | AA | A/G | |
| LGR4 | rs7936621 | G/A | G/A | GG | G/A | GG | G/A | |
| MTNR1B | rs3781638 | TT | TT | G/T | TT | G/T | TT | |
| ADGRD1 | rs1880842 | GG | GG | GG | GG | GG | GG | |
| LGR4 | rs10835153 | Intergenic variant | TT | TT | A/T | TT | TT | TT |
| MC4R | rs17782313 | TT | TT | T/C | TT | TT | TT | |
| FZD1 | rs2232157 | 5′prime UTR variant | GG | T/G | T/G | T/G | T/G | TT |
| FZD1 | rs2232158 | GG | T/G | T/G | T/G | T/G | TT | |
| CALCR | rs1042138 | 3′prime UTR variant | GG | G/A | GG | GG | GG | GG |
| Gene | Primers |
|---|---|
| RUNX2 | Forward GAG TGG ACG AGG CAA GAG T Reverse GGG TTC CCG AGG TCC ATC TA |
| COL1A1 | Forward GAC CTA AAG GTG CTG CTG GAG Reverse CTT GTT CAC CTC TCT CGC CA |
| SPARC | Forward GGC CTG GAT CTT CTT TCT C Reverse CCC ACA GAT ACC TCA GTC A |
| BGLAP | Forward GGC AGC GAG GTA GTG AAG AG Reverse CTG GAG AGG AGC AGA ACT GG |
| GAPDH | Forward TGC ACC ACC AAC TGC TTA GC Reverse GGC ATG GAC TGT GGT CAT GAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sopova, J.V.; Krasnova, O.A.; Semenova, P.I.; Kryukova, J.D.; Vasileva, G.V.; Zhuk, A.S.; Lesnyak, O.M.; Karelkin, V.V.; Neganova, I.E. Novel SNP Combination for Predictive Osteoporotic Diagnosis. Int. J. Mol. Sci. 2025, 26, 11117. https://doi.org/10.3390/ijms262211117
Sopova JV, Krasnova OA, Semenova PI, Kryukova JD, Vasileva GV, Zhuk AS, Lesnyak OM, Karelkin VV, Neganova IE. Novel SNP Combination for Predictive Osteoporotic Diagnosis. International Journal of Molecular Sciences. 2025; 26(22):11117. https://doi.org/10.3390/ijms262211117
Chicago/Turabian StyleSopova, Julia V., Olga A. Krasnova, Polina I. Semenova, Julia D. Kryukova, Giomar V. Vasileva, Anna S. Zhuk, Olga M. Lesnyak, Vitaliy V. Karelkin, and Irina E. Neganova. 2025. "Novel SNP Combination for Predictive Osteoporotic Diagnosis" International Journal of Molecular Sciences 26, no. 22: 11117. https://doi.org/10.3390/ijms262211117
APA StyleSopova, J. V., Krasnova, O. A., Semenova, P. I., Kryukova, J. D., Vasileva, G. V., Zhuk, A. S., Lesnyak, O. M., Karelkin, V. V., & Neganova, I. E. (2025). Novel SNP Combination for Predictive Osteoporotic Diagnosis. International Journal of Molecular Sciences, 26(22), 11117. https://doi.org/10.3390/ijms262211117







