1. Introduction
Photodynamic therapy (PDT) is a minimally invasive treatment modality with great promise due to its selective action on specific cell types and its low incidence of adverse effects. PDT involves the administration of a photosensitizer that, upon irradiation with light of a suitable wavelength in the presence of ground-state triplet oxygen, generates reactive oxygen species (ROS), which, although essential for numerous vital cellular functions, induce cytotoxicity when produced in excessive amounts [
1,
2,
3].
To date, the range of photosensitizers approved for clinical use remains limited, with the vast majority being porphyrin derivatives or related analogues [
4]. The identification of new candidates with optimal properties for therapeutic application is therefore crucial. For a molecule to serve effectively as a photosensitizer, several requirements must be met: strong absorption (>10
5 M
−1 cm
−1) within the “phototherapeutic window” (600–850 nm), where reduced tissue scattering allows deeper light penetration [
5]; safety in the absence of light; efficient generation of ROS upon light activation; preferential accumulation in the target tissue; and, ideally, suitability for administration through multiple routes [
6,
7].
Squaraine dyes, a distinctive class of non-porphyrinic organic compounds, consisting of 1,3-disubstituted derivatives of 3,4-dihydroxycyclobut-3-ene-1,2-dione (commonly known as squaric acid) first reported by Treibs and Jacob in 1965 [
8], are characterized by a highly planar, extensively conjugated system centered on a four-membered squaric ring, symmetrically flanked by electron-donating groups. This unique electronic structure endows squaraines with noteworthy optical and electronic properties, making them attractive for a wide range of technological and biomedical applications [
9]. Reported uses include fluorescent probes [
10,
11,
12,
13], active materials for organic light-emitting diodes [
14,
15], sensitizers for organic solar cells [
16,
17,
18,
19], ligands for G-quadruplex structures [
20,
21], and promising candidates as photosensitizers in PDT [
22,
23,
24,
25].
Interestingly, regarding their potential use in PDT, several cyanines and squaraines have been synthesized in recent years and have shown promise as photosensitizers for cancer treatment due to their favorable photophysical and photochemical properties that align well with the requirements of PDT [
26,
27,
28]. Nevertheless, much remains to be understood about this class of compounds, particularly in establishing clear structure–activity relationships [
29]. Moreover, depending on the structural modifications introduced into their scaffold, squaraines may display additional advantageous features, including high photochemical stability and moderate fluorescence quantum yields, along with enhanced fluorescence intensity upon non-covalent binding to specific ligands [
10,
30,
31,
32,
33]. These attributes render squaraine dyes valuable fluorescent probes for protein detection, as their fluorescence can be selectively activated in the presence of target proteins.
The most abundant protein in the human circulatory system is human serum albumin (HSA), which has been extensively studied due to its high bioavailability, low cost, remarkable stability, and ability to transport a wide range of ligands to specific sites [
12]. This protein plays a crucial role in maintaining metabolic processes, including the regulation of plasma oncotic pressure, the reduction in toxin activity, and the control of plasma antioxidant properties [
34,
35]. Its importance stems from its capacity to transport a variety of macromolecules, such as hormones, lipids, metal ions, amino acids, and drugs [
36]. Moreover, HSA is a key biomarker for evaluating a patient’s health status, since fluctuations in its concentration are often associated with diseases such as cardiovascular disorders, liver dysfunction, and nephrotic syndrome [
12,
37]. Consequently, the development of more sensitive methodologies for its detection and quantification is of great relevance in fields such as biochemistry, biotechnology, and immunodiagnostics [
38].
In this work, we report on the synthesis and structural, photophysical, and photochemical characterization of N-propylbenzene indolenine-based squaraines, aiming to explore their potential as fluorescent probes for the detection and quantification of HSA. The in silico and in vitro studies were performed specifically to evaluate their suitability as probes, while in cell experiments were conducted to assess their photobiological properties, particularly regarding their potential as photosensitizers in PDT. Additionally, at the interface of these studies, we investigated the possible role of albumin in mediating the systemic transport of these dyes upon prospective administration.
2. Results
2.1. Chemistry
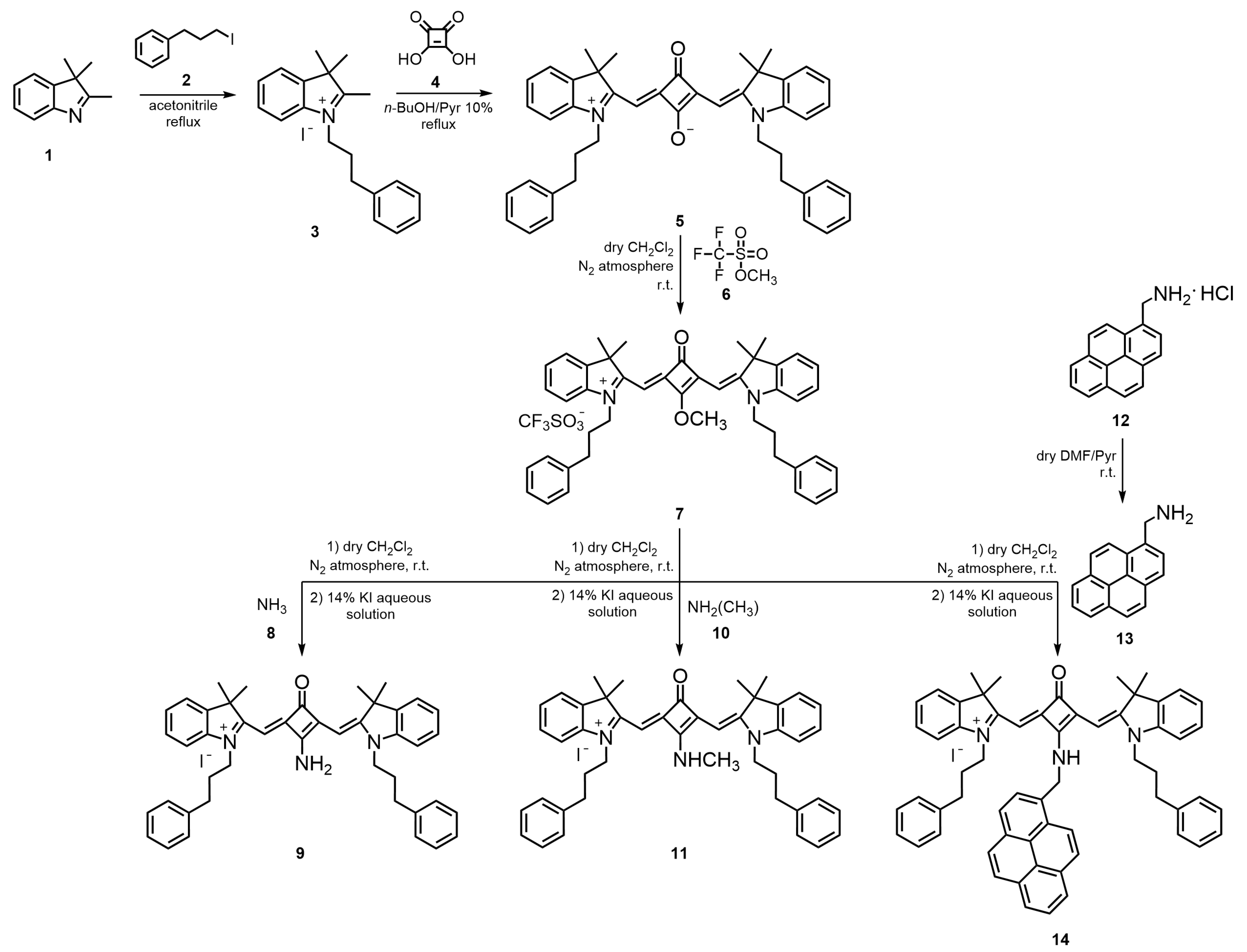
The
N-propylbenzene chain indolenine-based squaraine dyes
5,
7,
9,
11, and
14 were synthesized through a multistep approach, as outlined in
Figure 1. To the best of our knowledge, both the complete structural characterization, together with their photophysical and photochemical properties, are reported here for the first time.
The synthesis began with the N-alkylation of 2,3,3-trimethylindolenine (1) using an excess of 3-iodo-1-phenylpropane (2) in refluxing acetonitrile for six days, affording the corresponding quaternary ammonium salt 3 in 79% yield. Subsequent condensation of salt 3 with 0.5 equivalents of squaric acid (4) in a mixture of n-butanol/pyridine (10%) under reflux for 7 h gave the unsubstituted zwitterionic squaraine dye 5 in 85% yield. From this intermediate, the O-methylated derivative 7 was prepared by the reaction of dye 5 with three molar equivalents of methyl trifluoromethanesulfonate (6) in dry dichloromethane, under nitrogen at room temperature, for 3 h. This derivative served as the key precursor for the preparation of amino-substituted squaraine dyes. The displacement of the methoxy group in O-methylated dye 7 with amines 8, 10, and 13 furnished the corresponding N-propylbenzene aminosquaraines 9, 11, and 14, respectively, in moderate yields (22–43%). For the synthesis of dye 14, the amine 13 was first obtained by neutralization of its hydrochloride salt precursor 12 with pyridine in dry dimethylformamide (DMF) at room temperature, providing the free base suitable for nucleophilic substitution. Finally, all aminosquaraine products were subjected to counterion exchange by treatment with a 14% aqueous potassium iodide solution, yielding the iodide salts.
On the infrared (IR) spectra, weak bands characteristic of aromatic C–H bonds appeared in the region between 3102 and 3024 cm−1, and slightly stronger bands related to the vibrations of aliphatic C–H bonds between 2970 and 2860 cm−1. In the case of aminosquaraine dyes 9, 11, and 14, weak vibrations of the NH bonds appeared between 3256 and 3088 cm−1. It would be expected that a characteristic band of C=O in the four-membered central ring would be observed at approximately 1700 cm−1. However, this band only appeared in the spectrum of squaraine dye 7, which is justified by the fact that there is resonance between the nitrogen atom and oxygen atom of the carbonyl group of the other dyes. Around 1600 cm−1, strong bands of C=C bonds appeared in all squaraines.
Regarding the most relevant signals (
Figures S1–S15), in the
1H NMR spectrum of squaraine
9, a singlet of two protons at 9.07 ppm was observed, assigned to the protons of the NH
2 group, which disappeared upon D
2O exchange. For dye
11, a single-proton broad singlet at 9.06 ppm corresponded to the proton of the methylamino group, while a three-proton singlet at 3.27 ppm was attributed to the methyl substituent, further supported in the
13C NMR spectrum by a new signal at 30.46 ppm. For squaraine
14, in the
13C NMR spectrum, a distinct signal at 43.61 ppm corresponded to the methylene carbon of the NHCH
2 group, which was further evidenced in the
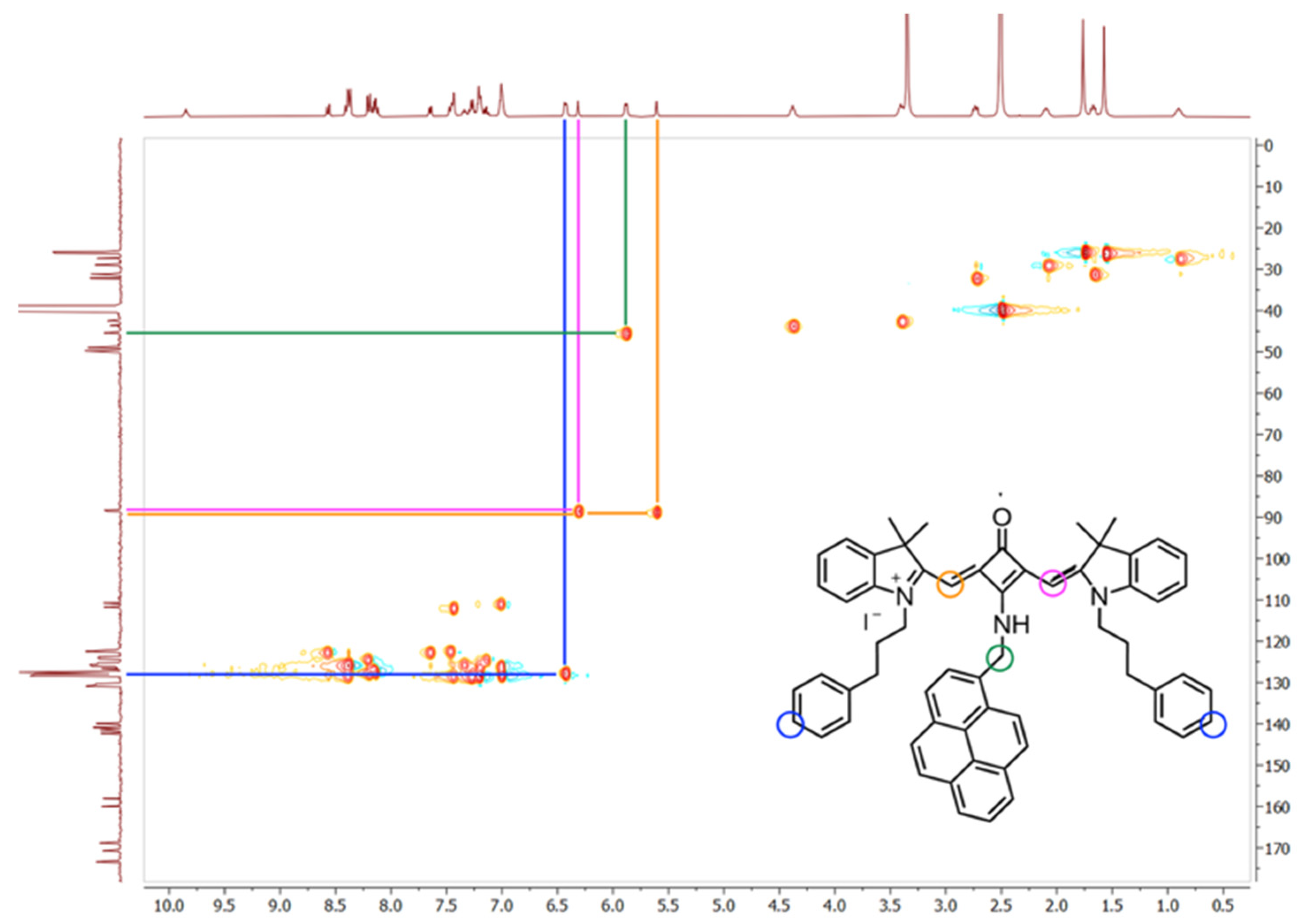
1H NMR spectrum by additional resonances characteristic of the 1-aminomethylpyrene moiety: the N
HCH
2–pyrene proton appeared as a single proton triplet at 9.85 ppm, disappearing upon D
2O exchange, while the methylene protons resonated as a two protons doublet at 5.88 ppm (green correspondence;
Figure 2), collapsing to a singlet after D
2O addition. A multiplet at 6.44–6.42 ppm was assigned to two aromatic protons of the benzene units of
N-propylbenzene chains, confirmed by heteronuclear single quantum coherence spectroscopy (blue correspondence).
Squaraine dyes
5,
7, and
9 were shown to be symmetrical, so their spectra displayed only half of the squaraine-core proton and carbon signals. In contrast, as expected, squaraine dye
11 lost symmetry upon the introduction of a methylamino substituent into the squaric ring, whereas the asymmetry of dye
14 likely arose from the partial double-bond character between the central ring carbon and the nitrogen atom. This effect, highlighted, for instance, by the signals corresponding to the methine protons and carbons marked in orange and pink (
Figure 2), restricts the rotation of the C–NH groups of these molecules.
2.2. Photochemical and Photophysical Characterization
Determining the spectral region in which these molecules absorb, as well as the intensity of that absorption, is highly relevant for their photobiological applications. Ideally, photosensitizers intended for PDT should absorb within the so-called “phototherapeutic window” (600–850 nm), where most endogenous biomolecules do not absorb energy. Furthermore, from the perspective of their use as fluorescent probes, it is important to understand their absorption profile to predict the corresponding emission behavior.
Accordingly, photophysical studies were carried out in three organic solvents (chloroform, methanol, and dimethyl sulfoxide, in order of increasing polarity;
Table 1). The dyes showed absorption within the ideal spectral range (λ
abs = 631–663 nm), accompanied by high molar absorption coefficients (ε = 1.74–4.28 × 10
5 M
−1·cm
−1). The absorption behavior observed in the three solvents cannot be explained solely based on solvent polarity, as the trend did not follow a classical solvatochromic pattern. In methanol, the hypsochromic shift can be attributed to hydrogen bonding between the solvent and the dye, which preferentially stabilizes the ground state over the excited state. In contrast, dimethyl sulfoxide, a polar aprotic solvent, efficiently stabilizes charge-separated excited states through dipole–dipole interactions, leading to a bathochromic shift. Chloroform, being weakly polar and lacking hydrogen-bond donor ability, provides only moderate stabilization of either state, resulting in absorption maxima that lie between those recorded in methanol and dimethyl sulfoxide. Overall, these results highlight the importance of dye–solvent interactions, particularly hydrogen bonding and dipolar stabilization, rather than polarity alone, in dictating the photophysical properties of the dyes.
In terms of fluorescence performance, the squaraine dyes exhibited solvent-dependent behavior. Among them, the unsubstituted squaraine dye 5 showed the highest fluorescence quantum yield, reaching ΦF = 1.00 in dimethyl sulfoxide, which clearly surpassed the substituted derivatives. The introduction of amine groups did not enhance this property; in fact, methylamino- and aminomethylpyrene-bearing dyes 11 and 14 displayed markedly lower quantum yields, with values of 0.04 and 0.05 in dimethyl sulfoxide, respectively. Dye 9, also substituted with an amine, reached only ΦF = 0.12 in dimethyl sulfoxide. Regardless of solvent, all aminosquaraines maintained only modest fluorescence efficiency, with yields further decreasing in chloroform and methanol (ΦF = 0.02–0.08).
Despite these limitations, the dyes consistently exhibited moderate Stokes shifts (ΔS = 9–15 nm), a feature that supports their potential applicability as fluorescent probes, where spectral separation between the absorption and emission bands is desirable.
Squaraine dyes, like other extended π-conjugated scaffold chromophores, exhibit a pronounced tendency to aggregate in aqueous media due to their high planarity and hydrophobic character. In these environments, π–π stacking interactions promote the formation of hypsochromic absorption bands, called H-aggregates, or J-aggregates, that originate bathochromic bands, depending on the relative orientation of the dye molecules.
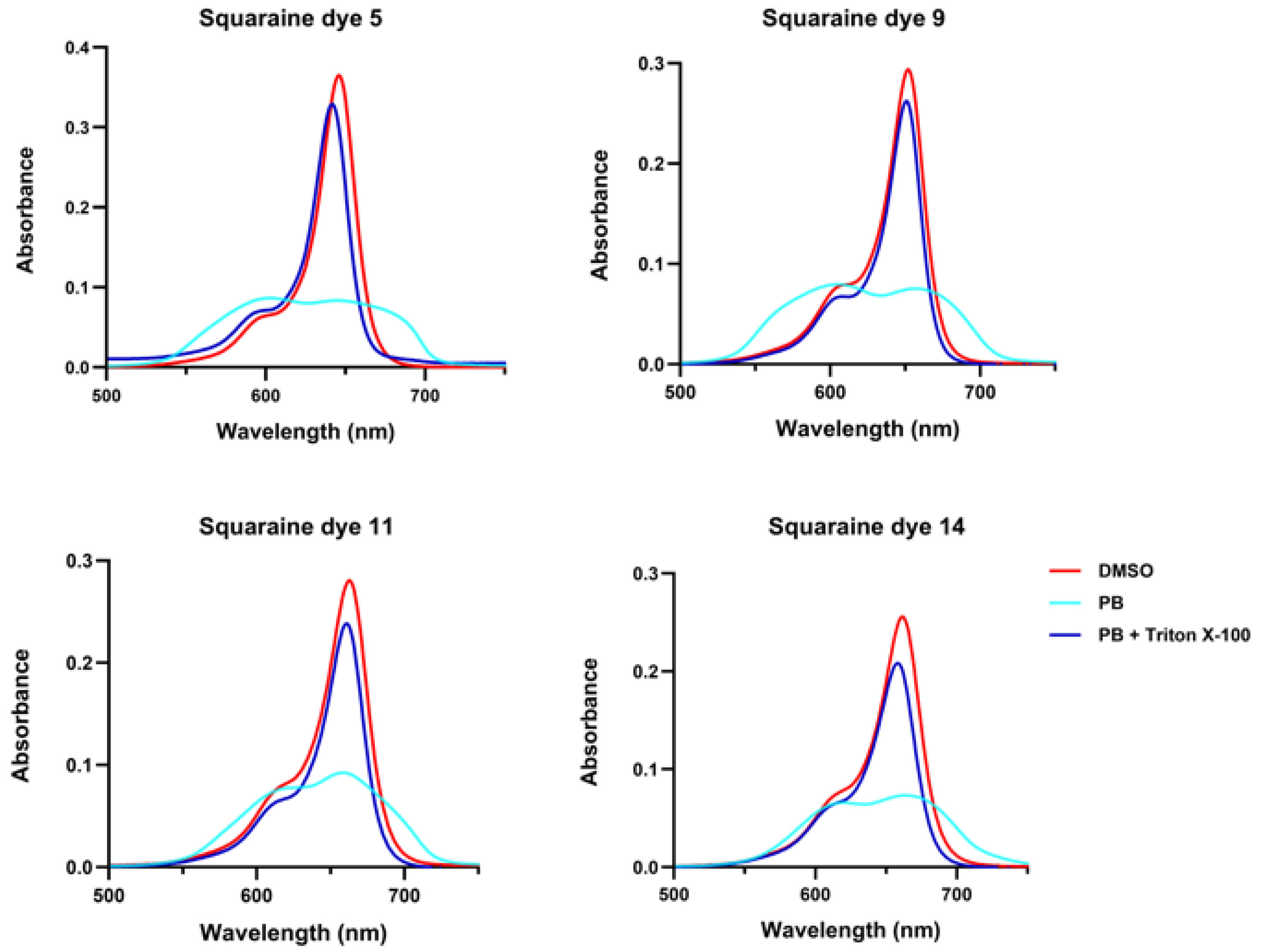
For the synthesized squaraines, it was observed that in phosphate buffer, they tended to aggregate regardless of their molecular structure, which drastically reduced their absorption capacity and led to a pronounced broadening of the absorption band (
Figure 3). This band broadening is a clear indication of aggregate formation. Squaraines
5,
9, and
11 displayed both hypsochromic and bathochromic components compared to their spectra in dimethyl sulfoxide, confirming the coexistence of H- and J-type aggregates. In contrast, the pyrene-containing dye
14 appeared to predominantly form J-aggregates, as suggested by the stronger red-shifted features observed visually. Upon the addition of Triton X-100 detergent, the aggregates were disrupted, and the absorption spectra of all dyes closely resembled those recorded in dimethyl sulfoxide, demonstrating that the compounds dissociated into their monomeric forms under these conditions.
The ability of the squaraine dyes to generate singlet oxygen was assessed using the 1,3-diphenylisobenzofuran (DPBF) assay. In this method, squaraine solutions were incubated with DPBF and irradiated for different time intervals, followed by the monitoring of absorbance at 410 nm. A progressive decrease in DPBF absorbance was observed upon irradiation for all synthesized compounds, confirming their ability to produce this reactive oxygen species. However, it should be emphasized that the reference squaraine dye induced a sharp reduction in DPBF absorbance within a short irradiation period, with the decay being limited only by its intrinsic photoinstability.
Among the new derivatives, the non-substituted dye
5 and the amino-substituted analogue
9 displayed comparatively higher singlet oxygen generation (
Figure 4). Nevertheless, when benchmarked against the reference (Φ
Δ (Ref) = 10%), all dyes synthesized in this work exhibited severely low quantum yields (Φ
Δ < 1%), highlighting their poor efficiency in photosensitization. On the other hand, all
N-propylbenzene indolenine-based squaraines demonstrated good photostability, maintaining their structural integrity under irradiation.
2.3. Squaraine-Albumin Bonding Studies
To assess the interaction between the synthesized squaraine dyes and human serum albumin (HSA), two complementary approaches were employed. First, an in silico strategy was carried out using molecular docking to predict the interactions between the squaraines and the entire protein, with a specific grid defined for Sudlow’s site I and Sudlow’s site II, the main ligand-binding pockets that also accommodate several clinically relevant drugs. The second approach was performed in vitro through fluorometric measurements, in which the protein concentration was kept constant while the concentration of the potential probe was gradually increased, allowing for the collection of various binding and fluorescence-related parameters.
From the perspective of their use as fluorescent probes, an ideal outcome would be a strong and rapid interaction with albumin, as this would facilitate their detection and quantification. Conversely, for potential PDT applications, excessively strong binding could be detrimental, as it would limit the availability of free dye to interact with cellular targets and potentially reduce therapeutic efficacy, since the photosensitizer could remain sequestered in the bloodstream for prolonged periods.
In silico, regardless of the amine introduced into the central ring, all squaraine dyes exhibited lower binding energies (BE) to albumin compared with warfarin and ibuprofen, supporting the prediction of strong interactions with this protein (
Table 2). Warfarin and ibuprofen were chosen as reference ligands since they are well-established standard probes for Sudlow’s sites I and II, respectively, on HSA [
39]. Notably, the aminomethylpyrene-bearing squaraine
14 showed the strongest binding, with calculated BE ranging from −15.57 to −12.59 kcal/mol. In contrast, the introduction of amino and methylamino substituents (squaraines
9 and
11, respectively), which produced comparable predictions, proved to be the least favorable modification, with binding energies between −12.64 and −9.75 kcal/mol, irrespective of the protein site considered. Interestingly, despite presenting higher binding energies than dye
14, the unsubstituted squaraine
5 stood out when compared with the other amino-substituted derivatives, particularly at Sudlow’s site I, where a binding energy of −14.25 kcal/mol was predicted.
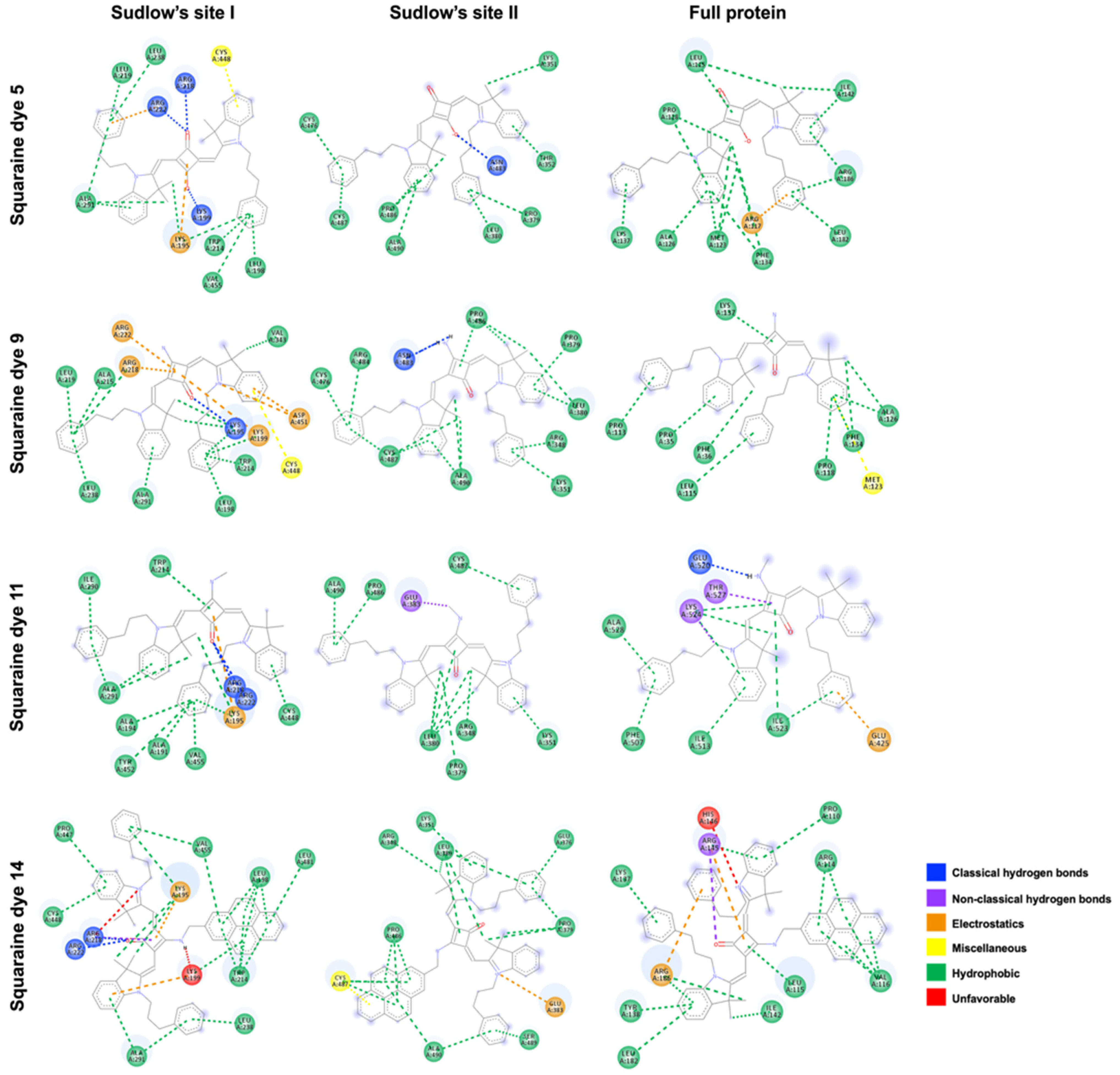
Concerning the nature of the interactions illustrated in
Figure 5, hydrophobic contacts were generally predominant, regardless of the binding site. Interestingly, however, all dyes were predicted to establish hydrogen bonds within Sudlow’s site I, which explains the considerably lower binding energies observed at this site. Specifically, the carbonyl groups of the unsubstituted dye
5 and the methylamino and aminomethylpyrene derivatives
11 and
14 formed hydrogen bonds with residues ARG218 and ARG222, while the negatively charged oxygen atom of dye
5 interacted with LYS199. In addition to potential electrostatic interactions, noteworthy was the predicted engagement of the central ring’s conjugated double bond with residues LYS195 and LYS199. Moreover, π–sulfur interactions were expected between the conjugated aromatic rings of the heterocyclic moieties of squaraine dyes
5 and
9 and residue CYS448. Finally, within the same binding site, an unfavorable interaction was predicted between the amine hydrogen of dye
14 and LYS99 residue. Interestingly, this residue was also expected to engage in electrostatic interactions with the aromatic ring of one of the heterocyclic units, as well as in hydrophobic contacts with the pyrene moiety.
At Sudlow’s site II, classical hydrogen bonds were predicted between residue ASN483 and both the negatively charged oxygen atom of the unsubstituted dye 5 and the amino hydrogens of dye 9. For dyes 11 and 14, additional key interactions were anticipated, including non-conventional hydrogen bonds between the methyl group of the methylamino substituent and GLU383, an electrostatic interaction between the positively charged nitrogen of the heterocyclic unit and GLU383, and a π–sulfur interaction between the pyrene moiety and CYS487 residue, respectively.
At the third binding site, only the dye functionalized with a methylamino group was predicted to establish hydrogen bonding between its labile hydrogen atom and GLU520. Non-conventional hydrogen bonds were also expected between THR527 and the squaric ring double-bond carbons, and between LYS524 and the methylene group directly attached to the non-charged heterocyclic nitrogen of dye 11. In addition, a hydrogen bond was predicted between the carbonyl group of the pyrene-containing dye and ARG145. Except for dye 9, all dyes were expected to engage in electrostatic interactions, particularly involving the phenyl groups of the N-propylbenzyl substituents with residues ARG117, GLU425, and ARG186 for dyes 5, 11, and 14, respectively. Notably, dye 9 was the only compound predicted to establish a π–sulfur interaction, involving the aromatic ring of its heterocyclic unit and residue MET123. Finally, an unfavorable interaction was predicted, arising from charge repulsion between residue HIS146 and the positively charged nitrogen atom of one of the heterocycles.
Experimentally, all evaluated squaraine dyes exhibited very weak fluorescence in phosphate buffer alone (
Figure 6). This behavior is primarily attributed to their strong tendency to aggregate in aqueous media, where π–π stacking and hydrophobic interactions typically lead to efficient fluorescence quenching and a reduction in molar absorptivity compared to organic solvents (
Table 1 and
Table 3). For the higher-wavelength methylamino and aminomethylpyrene dyes
11 and
14, this quenching effect was so pronounced that their maximum emission wavelength could not be determined, with relative fluorescence quantum yields approaching zero. In contrast, dyes
5 and
9 were weakly fluorescent, displaying favorable Stokes shifts of 91 and 37 nm, respectively, a feature that could enhance their suitability as fluorescent probes by reducing self-absorption.
Upon binding to HSA, the most critical observation was an enhancement in fluorescence ability, confirming that the protein environment effectively monomerizes the aggregated dyes and restricts internal rotations, thereby suppressing non-radiative decay pathways (
Figure 6). Aminosquaraines
9,
11, and
14 showed a “turn-on” effect, with relative fluorescence quantum yields increasing from approximately 0.00 to 0.07, 0.04, and 0.01, respectively (
Table 3). Unsubstituted Squaraine dye
5 showed the largest relative increase, with the quantum yield increasing about tenfold to 0.20 in the bound state. Furthermore, the binding led to significant spectral changes: dye
5 underwent a substantial 47 nm blue shift in maximum emission wavelength and a 50% reduction in Stokes shift, consistent with the dye transitioning from an aggregated state to a restricted, less polar microenvironment within the protein.
Fluorescence intensity was measured at 0 min, 3 h, and 24 h to determine the incubation time required for maximum interaction between each dye and HSA. Overall, all squaraines demonstrated binding to the protein, as evidenced by the increase in fluorescence intensity with higher protein concentrations. For amino- and methylamino-bearing dyes 9 and 11, maximum interaction occurred immediately upon addition to the protein solution, indicating that no incubation time is required and that these compounds bind to albumin almost instantaneously. In contrast, unsubstituted and aminomethylpyrene-containing dyes 5 and 14, respectively, reached their maximum fluorescence only after 24 h of incubation at room temperature, protected from light.
The strong interaction between the synthesized squaraine dyes and the albumin observed in the fluorescence studies was quantitatively analyzed to assess their potential as analytical probes (
Table 4). Upon complexation, all dyes exhibited a significant increase in fluorescence intensity, quantified by the ratio of the maximum intensity in the presence of protein (F) to the initial intensity (F
0). Methylamino squaraine
11 displayed the largest relative enhancement, with an F/F
0 ratio of about 361, a value consistent with its near-zero initial fluorescence being efficiently “turned on” by the protein binding. Dye
9 also showed remarkable performance, achieving the highest absolute fluorescence intensity (F = 917.53 a.u.) and an exceptional F/F
0 ratio of near 99. The analytical performance confirmed the high sensitivity of these dyes, with amino-bearing dye
9 standing out as the most sensitive probe (S = 3.18 × 10
5 nM
−1), closely followed by the unsubstituted one
5 (2.09 × 10
5 nM
−1), values that translate into favorable detection abilities. The detection limits ranged from 266 nM for dye
5 to 420 nM for dye
11, while the quantification limits spanned from 886 nM to 1401 nM (values corresponding to squaraines
5 and
11, respectively). Finally, the calculated binding constants confirmed a strong dye–protein affinity consistent with the observed spectral changes, with values ranging from 10
5 to 10
6 M
−1 and squaraine
9 showing the highest affinity at 6.23 × 10
5 M
−1. Collectively, the quantitative parameters, including the high F/F
0 ratios, remarkable sensitivity, and strong binding affinities, highlight these squaraine compounds as highly effective turn-on fluorescent probes for the selective detection and quantification of this protein in an aqueous solution.
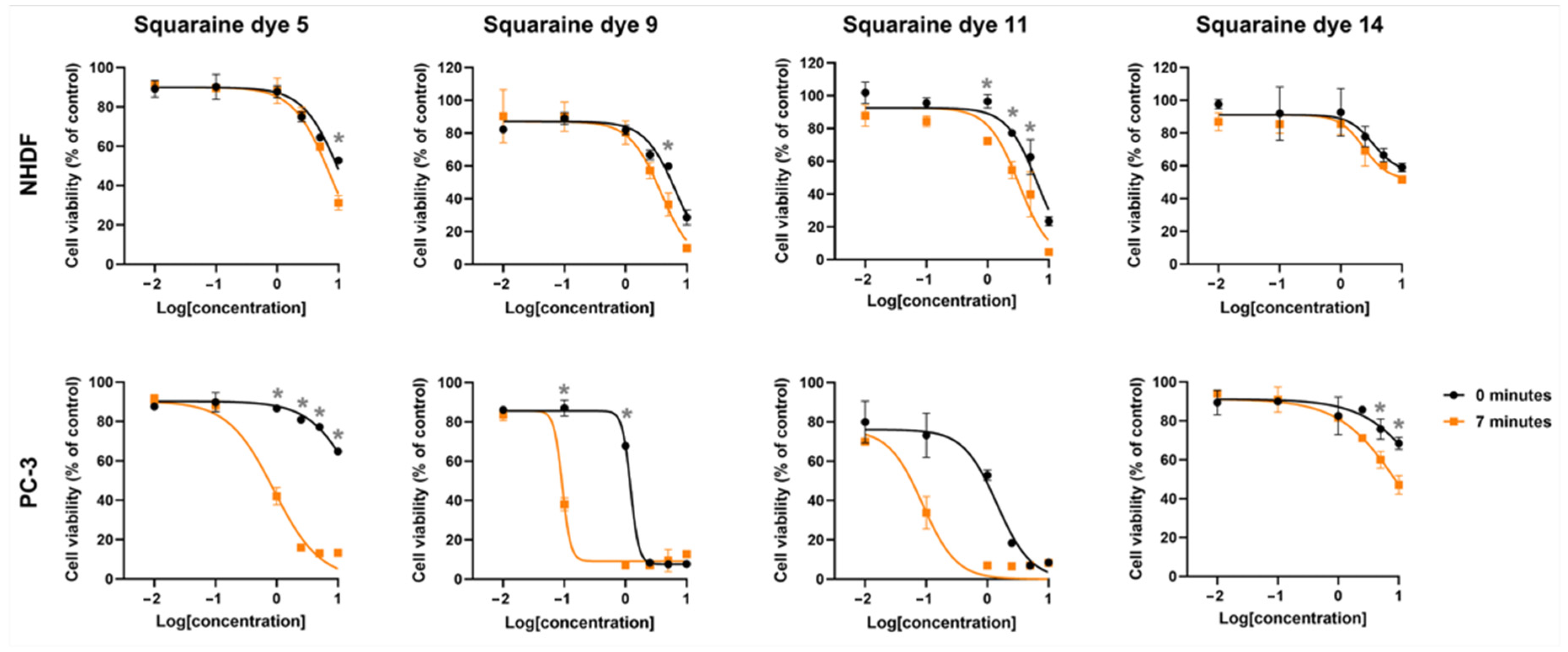
2.4. In Vitro Photodynamic Effects
For potential applicability as photosensitizers in PDT, these candidates are expected to display cytotoxic effects only after photoactivation, namely, and preferably restricted to target tissue cells, without inducing damage to healthy counterparts. To evaluate this, prostate adenocarcinoma PC-3 cells and non-tumoral NHDF fibroblasts were incubated with increasing concentrations of the squaraine dyes for 24 h. Subsequently, the cells were irradiated using an LED system centered at 663 nm, a wavelength close to the absorption maximum of the monomeric dye forms. Twenty-four hours post-irradiation, cellular metabolic activity was assessed by the MTT assay.
Photodynamically, all dyes demonstrated light responsiveness to varying degrees, as evidenced by reduced cell viability following irradiation. The unsubstituted squaraine dye
5 showed marked differences in photocytotoxic activity between the two cell lines: while no reduction in viability greater than 30% was observed in normal fibroblasts up to the highest tested concentration of 2.5 μM, a much more pronounced effect was detected in PC-3 cells, where approximately 60% reduction in viability occurred at just 1.0 μM (
Figure 7). This effect is reflected in the calculated photodynamic ratio, which indicates that this dye is at least 11.1-fold more cytotoxic upon irradiation compared to its inherent dark cytotoxicity (
Table 5). Moreover, the tumor selectivity ratio revealed that dye
5 was about 8.2-fold more toxic toward tumor cells than toward normal fibroblasts under light treatment.
The introduction of different amine substituents into the four-membered central ring resulted in markedly distinct biological outcomes. Functionalization with amino and methylamino groups (squaraines 9 and 11, respectively) rendered the dyes highly promising, showing 13- and 16-fold increases in cytotoxicity upon irradiation, as well as 40.8- and 36-fold higher photocytotoxicity toward PC-3 cells compared to normal fibroblasts. In contrast, functionalization of the squaric ring with an aminomethylpyrene group (dye 14) did not prove to be as advantageous. Despite the slight differences in cell viability reduction observed for this latter dye across both cell lines, no significant effects were detected when compared to the other dyes evaluated. This was reflected in IC50 values exceeding 10 μM, the highest concentration tested.
3. Discussion
This study aimed to investigate how subtle structural modifications in N-propylbenzene indolenine-based squaraine dyes affect their interaction with HSA. Understanding these effects provides valuable insights not only into their potential use as fluorescent probes for the detection and quantification of this protein, but also into their possible transport and biodistribution mechanisms in the body when envisioned as drug candidates for PDT. Furthermore, the work included an evaluation of their in vitro photodynamic activity against prostate cancer cells.
Photophysically, the synthesized squaraine dyes exhibited several key properties required for applications in both areas of interest: unlike porphyrins, the main class of clinically approved photosensitizers that absorb at shorter wavelengths [
40,
41], they displayed maximum absorption within a spectral region where most biomolecules do not absorb efficiently, high molar absorptivity coefficients indicative of strong light-harvesting capability, and emission maxima moderately shifted from their absorption bands, depending on the solvent. Squaraines’ fluorescence quantum yields were also moderate and solvent-dependent, while they demonstrated remarkable photostability. The latter was indeed expected, as indolenine-based dyes are generally known for their excellent light stability [
42,
43], in contrast to other heterocyclic derivatives such as benzoselenazole and benzothiazole [
44,
45]. It is therefore understood that regarding this property, the heterocyclic core from which these dyes are derived plays a decisive role in ensuring such robustness.
The synthesized dyes were found to exhibit a marked tendency to aggregate in aqueous media, a common feature among squaraine compounds due to their highly planar and π-conjugated structures that promote strong intermolecular interactions. Such aggregation is generally disadvantageous for their intended applications, as it often results in fluorescence quenching through non-radiative deactivation of excited states [
46,
47] and may also reduce the generation of reactive oxygen species by limiting the availability of excited singlet and triplet states [
48]. Nevertheless, for these particular structures, the impact of aggregation on their photobiological performance may not be critical, since high molar absorption coefficients were still obtained at the absorption maximum of the monomeric form even in buffer solution. Interestingly, and quite distinctly, methylamino squaraine dye
11 showed the lowest tendency to aggregate, displaying a well-defined absorption band corresponding to its monomeric form, in contrast to all other derivatives.
Despite the promising in silico interaction profile predicted for dye 14, bearing the aminomethylpyrene group, this high potential was not fully corroborated by the in vitro results. Instead, methylamino-functionalized dye 11 exhibited markedly higher binding constants, indicating a stronger actual interaction with the protein than computationally anticipated. This discrepancy highlights the influence of factors beyond static docking predictions, such as solvation effects, dye aggregation, and the conformational flexibility of HSA, on the real binding behavior in solution. The pronounced interaction of this dye with the protein was further evidenced by an approximately 360-fold fluorescence enhancement, far exceeding that of the other dyes tested, none of which surpassed a 100-fold increase relative to their baseline emission. From the perspective of their potential as fluorescent probes, the unsubstituted dye 5 proved most effective in vitro, enabling the detection and quantification of albumin at considerably lower concentrations than its amino-substituted squaraine analogues, which displayed higher detection and quantification limits. However, a notable limitation was the slow kinetics of binding, as the interaction required at least one hour of incubation to reach equilibrium, a drawback that may restrict its suitability for diagnostic, biochemical, or biotechnological applications demanding rapid response times.
Far below the expectations, only low to moderate fluorescence quantum yields were determined after protein addition, significantly lower than those observed for other analogous dyes recently investigated by some of us. For comparison, squaraine derivatives functionalized on the central ring with dimethylbarbituric, dimethylthiobarbituric, and diphenylthiobarbituric acids, as reported by Gomes et al. [
13,
49], exhibited remarkable increases in fluorescence quantum yield, from about 1% up to 57% in the presence of albumin. These compounds also displayed binding constants one order of magnitude higher than those of the dyes reported herein (10
6 M
−1), except for dye
11, whose binding affinity was comparable to that of the barbituric derivatives. In comparison with the
N-hexyl chain indolenine- and benz[
e]indole-based derivatives reported by Sousa et al. [
50], although the squaraines presented herein exhibited greater fluorescence enhancement in the presence of protein relative to its absence, the previously reported analogues displayed properties more consistent with the requirements for probe applications. Indeed, those compounds showed considerably higher fluorescence quantum yields, greater sensitivity, and overall, lower detection and quantification limits.
From a photodynamic perspective, the dyes presented herein exhibited significant potential, except for the aminomethylpyrene-bearing derivative
14, which showed no appreciable cytotoxicity toward the tumor cell line, regardless of irradiation conditions. Among the remaining compounds, the amino- and methylamino-substituted dyes, squaraines
9 and
11, respectively, stood out, as they not only displayed pronounced phototoxicity but also exhibited a tendency toward selective activity in tumor cells compared with the normal cell line. Although these dyes showed relatively low fluorescence quantum yields, this is consistent with their strong photodynamic activity. After light absorption, excited dyes can either emit fluorescence or undergo intersystem crossing to the triplet state, generating ROS. Efficient intercrossing system competes with fluorescence, so dyes with lower fluorescence typically produce more ROS, explaining the inverse relationship between fluorescence efficiency and photodynamic cytotoxicity [
51,
52]. Although the two cell lines originate from different embryonic tissues, the lower cytotoxicity observed in NHDF suggests a preference for prostate adenocarcinoma cells. Although the two cell lines originate from different embryonic tissues, the markedly lower cytotoxicity observed in NHDF indicates a preferential accumulation and activity of these dyes in PC-3 cells. Such pronounced tumor selectivity underscores the potential safety of these dyes for the skin, an organ frequently exposed to light both during PDT and in daily life post-treatment. The introduction of amino substituents into the squaric ring, while enhancing certain molecular interactions, led to an undesirable increase in dark cytotoxicity. Importantly, this modification did not compromise photodynamic efficacy: upon irradiation, the dyes exhibited cumulative cytotoxic effects, maintaining their therapeutic potential. Conversely, the unsubstituted squaraine
5, although less selective, emerged as particularly attractive for photodynamic applications due to its minimal dark toxicity across both cell lines, offering a favorable safety profile even if its light-activated potency was comparatively lower.
Regarding their photodynamic mechanism of action, further studies are clearly required to elucidate the underlying processes. Although the measured singlet oxygen quantum yields were relatively low, our experience suggests that this reactive oxygen species may still contribute to the biological activity of these photosensitizing candidates [
45,
53]. Since both type I and type II photodynamic reactions may occur, these molecules are particularly attractive from a clinical perspective for treating tumors with hypoxic microenvironments [
54,
55,
56]. Type II mechanisms are oxygen-dependent, leading mainly to the formation of singlet oxygen, whereas type I processes involve electron or hydrogen transfer reactions that generate reactive oxygen species such as superoxide anion, hydroxyl radicals, or hydrogen peroxide, and can therefore proceed even under oxygen-limited conditions. Indeed, the intracellular site and nature of ROS generation are often more critical than the absolute amount produced [
57]. In contrast to porphyrin-based photosensitizers, which mainly rely on singlet oxygen generation [
58,
59], squaraine dyes are predicted to mainly engage in type I photoreactions, potentially enhancing their performance under hypoxic conditions commonly found in tumors [
60,
61,
62]. Therefore, additional investigations are needed to fully understand how these dyes exert their effects upon photodynamic activation. The intrinsically low cytotoxicity and favorable safety profile of these dyes toward healthy cells also highlight their potential for future exploration as photoantibacterial and -antifungal candidates within the broader scope of PDT applications.
Overall, the poor fluorescence performance of the aminomethylpyrene-functionalized dye, both in organic and aqueous environments, together with its weak photodynamic activity, does not support its potential for further applications in this context. Nevertheless, it is worth noting that a wide range of other dye molecules, such as porphyrins [
63], imidazoles [
64], quinolines [
65], and naphthalenes [
66] functionalized with pyrene moieties, have been reported in the literature as effective stabilizers or destabilizers of G-quadruplex structures, encouraging further exploration of this pyrene-functionalized dye for applications in nucleic acid targeting.
A few limitations of this study should be acknowledged. The in vitro assays provide useful preliminary insights but cannot fully capture the complexity of in vivo environments, including biodistribution, metabolism, and protein interactions. The low singlet oxygen quantum yields also indicate the need for further mechanistic studies to clarify type I versus type II pathways. Future work will address these gaps through more detailed in vitro experiments using specific ROS quenchers and scavengers, subcellular localization analysis, evaluation of cell death mechanisms, and assessment of cell cycle effects. Promising results will pave the way for studies in three-dimensional cell cultures and animal models, alongside structural optimization to enhance protein binding and overall phototherapeutic efficacy.
4. Materials and Methods
All reagents used in this work, except those synthesized during the experimental procedure, were commercially obtained and used without further purification. Dichloromethane employed in the organic synthesis reactions was first dried over anhydrous calcium chloride and subsequently refluxed over calcium hydride.
The progress of the synthetic reactions and the chromatographic assessment of the purity of the obtained crystals were monitored by thin-layer chromatography on aluminum plates coated with silica gel (Merck, 60 F254, thickness 0.25 mm; Merck, Darmstadt, Germany). Dichloromethane/methanol mixtures (5% and 2%) were used as mobile phases. After elution, the chromatograms were visualized under UV light at 254 and/or 366 nm. Plates containing compounds corresponding to quaternary ammonium salts were further developed with Dragendorff’s reagent [1:1 (v/v) mixture of 2% bismuth nitrate in 20% aqueous acetic acid and 40% aqueous potassium iodide], which produces an orange coloration.
Melting points (m.p.) were determined with a binocular microscope equipped with a heated stage and digital thermometer (URA Technic, Lisbon, Portugal).
UV–Vis spectra were recorded on a Perkin-Elmer Lambda 25 spectrophotometer (Perkin-Elmer, Waltham, MA, USA) using Hellma Analytics quartz cuvettes (1 cm path length; Hellma Analytics, Müllheim, Germany). Spectral data are reported as maximum absorption wavelength (λmax, nm) and molar absorptivity (ε, M−1·cm−1). The latter was calculated according to the Lambert–Beer law from calibration plots of absorbance at λmax versus known dye concentrations.
Fluorescence spectra were acquired on a Varian Cary Eclipse fluorometer (Agilent Technologies, Santa Clara, CA, USA) using 1 cm path length, four-sided polished Hellma Analytics quartz cuvettes.
IR spectra were recorded on a Shimadzu IRAffinity-1S spectrophotometer (Shimadzu, Kyoto, Japan) using KBr pellets. Spectral data are reported as follows: sample form [KBr]; absorption maxima (ʋmax, cm−1); band intensity [strong (s), medium (m), weak (w)], and, when possible, characteristic functional group assignments.
1H and 13C NMR spectra were obtained on a Bruker Avance III 400 spectrometer (University of Beira Interior; Bruker, Billerica, MA, USA) using DMSO-d6 as the solvent. Chemical shifts (δ, ppm) are reported with reference to solvent signals (1H DMSO-d6 2.50 ppm and 13C DMSO-d6 39.52 ppm), along with the number of protons, multiplicity [singlet (s), broad singlet (br s), doublet (d), broad triplet (br t), triplet (t), quintet (qt), multiplet (m)], coupling constants (J, Hz), and assignments. 13C NMR data were supported by DEPT-135 experiments to distinguish CH3, CH2, and CH signals.
High-resolution mass spectra (HRMS) were obtained using a Bruker solariX XR spectrometer (Bruker, Billerica, MA, USA) at the Mass Spectrometry Services of C.A.C.T.I., University of Vigo, equipped with an electrospray ionization (ESI) and Fourier transform ion cyclotron resonance analyzer. Data are reported as m/z values of the molecular ion, molecular formula, and calculated exact mass.
5. Synthesis of N-Propylbenzene Indolenine-Based Aminosquaraines
5.1. 2,3,3-Trimethyl-1-(3-phenylpropyl)-indol-1-ium iodide (3)
In a 50 mL round bottom flask, 2,3,3-trimethylindolenine (1; 2.00 g; 12.6 mmol) was mixed with the alkylating agent 3-iodo-1-phenylpropane (2; 6.20 g; 25.2 mmol), in acetonitrile (25 mL), under stirring and reflux (90 °C), for 6 days. After this time, the reaction mixture was removed from heating and placed in an ice bath, and diethyl ether was added to promote precipitation. The resulting solid was filtered at reduced pressure and washed several times with diethyl ether, and then recrystallized by dissolving them in the minimum amount of methanol and adding diethyl ether until crystallization began. After vacuum filtration followed by drying under vacuum, cream-colored needle-shaped crystals of compound 3 were obtained, with a yield of 79%. M.p.: 135–137 °C; IR ʋmax (KBr): 3024 (s, ArCH), 2970–2860 (m, CH), 1626 (m, C=C), 1593 (m, ArC=C), 1497 (m), 1479 (s), 1462 (s), 1366 (w), 1126 (w), 1042 (w), 937 (w), 768 (s), 750 (s), 698 (s), 496 (m) cm−1; 1H NMR (DMSO-d6, 400.13 MHz) δ: 7.98–7.93 (1H, m, ArH), 7.85–7.82 (1H, m, ArH), 7.65–7.61 (2H, m, ArH), 7.33–7.27 (4H, m, ArH), 7.24–7.20 (1H, m, ArH), 4.49 (2H, t, J = 7.8, NCH2CH2CH2Ph), 2.81 (2H, t, J = 8.0, NCH2CH2CH2Ph), 2.81 (3H, s, CH3), 2.17 (2H, qt, J = 8.0, NCH2CH2CH2Ph), 1.52 (6H, s, C(CH3)2) ppm. 13C NMR (DMSO-d6, 100.62 MHz) δ: 196.56, 141.83, 141.04, 140.51, 129.37 (ArCH), 128.90 (ArCH), 128.37 (ArCH), 128.23 (ArCH), 126.14 (ArCH), 123.48 (ArCH), 115.32 (ArCH), 54.14 (C(CH3)2), 47.30 (NCH2CH2CH2Ph), 31.77 (NCH2CH2CH2Ph), 28.70 (NCH2CH2CH2Ph), 21.96 (CH3), 13.98 (C(CH3)2) ppm; HRMS m/z: 278.190601 [M–I]+ (C20H24N calc. 278.190326).
5.2. 4-[3,3-Dimethyl-1-(3-phenylpropyl)-indol-1-ium-2-ylmethylene]-2-[3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidenemethyl]-3-oxocyclobut-1-en-1-olate (5)
In a 100 mL round bottom flask, the indolenine derivative salt 3 (1.50 g; 3.70 mmol) was mixed with 3,4-dihydroxycyclobut-3-ene-1,2-dione (4; 0.21 g; 1.85 mmol) in 30 mL of a mixture of n-butanol and pyridine (10%), under stirring and reflux (140 °C), for 6 h. The reaction mixture was removed from heating and placed in an ice bath, and diethyl ether was added to the flask to initiate the first precipitation. Then, for recrystallization, the resulting solid was dissolved with methanol and some drops of heated dichloromethane, and after complete dissolution, diethyl ether was added, and the flask was left in an ice bath. Green metallic crystals were obtained of compound 5, after filtration under reduced pressure, with a yield of 85%. M.p.: 234–235 °C. Vis λmax (DMSO): 646 nm; ε = 292,993 M−1·cm−1. IR ʋmax (KBr): 3055 (w, ArCH), 3024 (w, ArCH), 2956–2864 (w, CH), 1597 (s, ArC=C), 1576 (m, ArC=C), 1493 (s), 1450 (s), 1427 (s), 1398 (m), 1354 (m), 1283 (s), 1231 (m), 1219 (m), 1161 (s), 1098 (s), 1076 (s), 1059 (s), 1018 (m), 966 (m), 924 (m), 791 (m), 752 (m), 698 (m), 683 (m), 554 (w) cm−1. 1H NMR (DMSO-d6, 400.13 MHz) δ: 7.53 (2H, d, J = 7.2, ArH), 7.34 (2H, t, J = 7.6, ArH), 7.30–7.28 (4H, m, ArH), 7.25–7.19 (8H, m, ArH), 7.17 (2H, d, J = 7.6, ArH), 5.83 (2H, s, CH=C), 4.14 (4H, br t, NCH2CH2CH2Ph), 2.74 (4H, t, J = 8.0, NCH2CH2CH2Ph), 2.02 (4H, qt, J = 6.4, NCH2CH2CH2Ph), 1.69 (12 H, s, C(CH3)2) ppm. 13C NMR (DMSO-d6, 100.62 MHz) δ: 180.68, 178.70, 169.10, 142.19, 141.44, 140.93, 128.46 (ArCH), 128.17 (ArCH), 127.96 (ArCH), 126.04 (ArCH), 123.70 (ArCH), 122.29 (ArCH), 110.19 (ArCH), 86.15 (CH=C), 48.74 (C(CH3)2), 42.71 (NCH2CH2CH2Ph), 32.29 (NCH2CH2CH2Ph), 28.32 (NCH2CH2CH2Ph), 26.54 (C(CH3)2) ppm. HRMS m/z: 632.3399654 [M]+ (C44H44N2O2 calc. 632.339730).
5.3. 2-{3-[3,3-Dimethyl-1-(3-phenylpropyl)indolin-2-ylidenemethyl]-2-methoxy-4-oxocyclobut-2-en-1-ylidenemethyl}-3,3-dimethyl-1-(3-phenylpropyl)-indol-1-ium trifluoromethanesulfonate (7)
In a 100 mL round bottom flask, compound 5 (0.50 g; 0.79 mmol) was mixed with methyl trifluoromethanesulfonate (6; 0.39 g; 2.37 mmol) under stirring in 30 mL of dry dichloromethane. The reaction took place at room temperature, under a nitrogen atmosphere, for 3 h. Once finished, the reaction mixture was treated, placing it in an extraction ampoule and washed with an ice-cold 5% (w/v) sodium hydrogen carbonate solution (100 mL). Then, distilled water was used to wash the organic phase that was posteriorly dried with anhydrous sodium sulfate. The solvent was removed to dryness using a rotary evaporator, yielding a golden film, which was converted into a paste upon the addition of ether. To precipitate this paste, a mixture of dichloromethane and methanol, followed by petroleum ether, was added. The solid was then washed several times with heated diethyl ether to remove impurities and subsequently recrystallized from a mixture of dichloromethane and diethyl ether. After filtration to reduced pressure, compound 7 was obtained as dark green metallic crystals, with a yield of 75%. M.p.: 151–152 °C. IR ʋmax (KBr): 3057 (w, ArCH), 3024 (w, ArCH), 2968–2859 (w, CH), 1757 (m, C=O), 1647 (m, ArC=C), 1601 (m, ArC=C), 1524 (s), 1499 (s), 1360 (m), 1314 (m, C–O), 1296 (m, C–O), 1148 (m), 1049 (m), 922 (m), 824 (m), 791 (m), 748 (m), 691 (m), 637 (s), 554 (w) cm−1. 1H NMR (DMSO-d6, 400.13 MHz) δ: 7.62 (2H, d, J = 7.2, ArH), 7.49 (2H, t, J = 8.2, ArH), 7.45 (2H, t, J = 7.0, ArH), 7.35–7.29 (6H, m, ArH), 7.24–7.22 (6H, m, ArH), 5.80 (2H, s, CH=C), 4.55 (3H, s, OCH3), 4.25 (4H, t, J = 7.8, NCH2CH2CH2Ph), 2.73 (4H, t, J = 7.8, NCH2CH2CH2Ph), 2.04 (4H, qt, J = 7.6, NCH2CH2CH2Ph), 1.65 (12 H, s, C(CH3)2) ppm. 13C NMR (DMSO-d6, 100.62 MHz) δ: 179.09, 176.91, 173.13, 160.71, 141.92, 141.39, 140.65, 128.43 (ArCH), 128.36 (ArCH), 128.22 (ArCH), 126.09 (ArCH), 125.56 (ArCH), 122.41 (ArCH), 111.85 (ArCH), 86.75 (CH=C), 61.03 (OCH3), 49.62 (C(CH3)2), 43.65 (NCH2CH2CH2Ph), 32.01 (NCH2CH2CH2Ph), 28.41 (NCH2CH2CH2Ph), 25.52 (C(CH3)2) ppm. HRMS m/z: 647.363165 [M–CF3SO3]+ (C45H47N2O2 calc. 647.363205).
5.4. 2-{2-Amino-3-[3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidenemethyl]-4-oxocyclobut-2-en-1-ylidenemethyl}-3,3-dimethyl-1-(3-phenylpropyl)-indol-1-ium Iodide (9)
In a 100 mL round bottom flask, the O-methylated derivative 7 (0.20 g; 0.25 mmol) was mixed with 2M ammonia solution in methanol (8; 0.013 g; 0.75 mmol), in dry dichloromethane (30 mL), under stirring, at room temperature, under a nitrogen atmosphere, for 3 h. The content of the flask was transferred to an extraction ampoule and washed three times with distilled water; the collected organic phase was dried over anhydrous sodium sulfate, filtered, and the solvent removed to dryness using a rotary evaporator. To the resulting residue, 10 mL of methanol and 10 mL of a 14% potassium iodide aqueous solution were added (1.4 g; 8.43 mmol), and the mixture was stirred at room temperature for 30 min. After decanting, the resulting residue, which was shown chromatographically to be a complex mixture of several compounds, was washed with distilled water and crushed with diethyl ether. After decanting, the solid was treated with heated ether and a minimal amount of dichloromethane, then placed in an ice bath until precipitation began. After vacuum filtration followed by drying under vacuum, pure bluish/greenish crystals were obtained, with a yield of 43%. M.p.: 156–157 °C. Vis λmax (DMSO): 652 nm; ε = 232,117 M−1·cm−1. IR ʋmax (KBr): 3256 (w, NH), 3102 (m, ArCH), 3024 (w, ArCH), 2961–2860 (w, CH), 1641 (s, ArC=C), 1535 (s, ArC=C), 1497 (s), 1454 (s), 1366 (s), 1314 (s), 1283 (s), 1159 (s), 1121 (w), 1098 (w), 1063 (s), 964 (m), 924 (w), 839 (w), 793 (m), 746 (m), 687 (w) cm−1. 1H NMR (DMSO-d6, 400.13 MHz) δ: 9.07 (2H, s, NH2, exchange with D2O), 7.59 (2H, d, J = 7.6, ArH), 7.41 (2H, dt, J = 7.6, 1.2, ArH), 7.35 (2H, d, J = 7.6, ArH), 7.31–7.25 (6H, m, ArH), 7.22–7.17 (6H, m, ArH), 6.06 (2H, s, CH=C), 4.28 (4H, t, J = 7.2, NCH2CH2CH2Ph), 2.74 (4H, t, J = 8.2, NCH2CH2CH2Ph), 2.05 (4H, qt, J = 7.7, NCH2CH2CH2Ph), 1.70 (12 H, s, C(CH3)2) ppm. 13C NMR (DMSO-d6, 100.62 MHz) δ: 172.99, 171.94, 170.91, 159.45, 141.84, 141.63, 140.93, 128.38 (ArCH), 128.17 (ArCH), 128.12 (ArCH), 126.01 (ArCH), 124.82 (ArCH), 122.34 (ArCH), 111.15 (ArCH), 87.77 (CH=C), 49.21 (C(CH3)2), 43.27 (NCH2CH2CH2Ph), 32.11 (NCH2CH2CH2Ph), 28.75 (NCH2CH2CH2Ph), 25.99 (C(CH3)2) ppm. HRMS m/z: 632.363460 [M–I]+ (C44H46N3O calc. 632.363540).
5.5. 2-{3-[3,3-Dimethyl-1-(3-phenylpropyl)indolin-2-ylidenemethyl]-2-(methylamino)-4-oxocyclobut-2-en-1-ylidenemethyl}-3,3-dimethyl-1-(3-phenylpropyl)-indol-1-ium Iodide (11)
In a 100 mL round bottom flask, the O-methylated derivative 7 (0.20 g; 0.25 mmol) was mixed with a 2 M solution of methylamine (10; 0.023 g; 0.75 mmol) in methanol, in dry dichloromethane (30 mL), at room temperature under stirring and nitrogen atmosphere, for 2 h. The mixture was washed twice with distilled water, recovering the organic phase and drying it with anhydrous sodium sulfate. After gravimetric filtration, the solvent was removed to dryness on the rotary evaporator, and the forming thick reddish paste was dissolved in 10 mL of methanol, and then 10 mL of ice-cold 14% potassium iodide solution (1.4 g; 8.43 mmol) was slowly added. The mixture was stirred for 2 h at room temperature. At the end of this time, the reaction mixture was transferred to an extraction ampoule, diluted in dichloromethane, and washed three times with distilled water. The organic phase was separated from the aqueous one, dried with anhydrous sodium sulfate, and the solvent was removed under reduced pressure. Successive recrystallizations were carried out with different solvents (acetonitrile/diethyl ether, methanol/petroleum ether/diethyl ether), and the last one with a mixture of dichloromethane and diethyl ether guaranteed the formation of pure bordeaux crystals with a 22% yield. M.p. 145–148 °C. Vis λmax (DMSO): 663 nm; ε = 212,228 M−1·cm−1. IR ʋmax (KBr): 3146 (w, NH), 3102 (w, ArCH), 3049 (w, ArCH), 2959–2859 (w, CH), 1632 (s, ArC=C), 1611 (m), 1568 (s), 1493 (s), 1479 (s), 1456 (s), 1398 (m), 1356 (m), 1285 (s); 1204 (w), 1159 (m), 1107 (m); 1080 (m); 1020 (m), 961 (m), 922 (m), 793 (m), 748 (m); 698 (m), 677 (m), 617 (w), 552 (w), 494 (w); 446 (w) cm−1; 1H NMR (DMSO-d6, 400.13 MHz) δ: 9.06 (1H, br s, NH, exchange with D2O), 7.61–7.55 (2H, m, ArH), 7.44–7.40 (4H, m, ArH), 7.32–7.26 (6H, m, ArH), 7.23–7.19 (6H, m, ArH), 6.00 (1H, s, CH=C), 5.95 (1H, s, CH=C), 4.30 (2H, t, J = 7.2, NCH2CH2CH2Ph), 4.20 (2H, t, J = 7.6, NCH2CH2CH2Ph), 3.28 (3H, s, NHCH3, observable after D2O exchange), 2.72 (4H, br t, J = 7.4, NCH2CH2CH2Ph), 2.04–1.98 (4H, m, NCH2CH2CH2Ph), 1.69 (12 H, s, C(CH3)2) ppm. 13C NMR (DMSO-d6, 100.62 MHz) δ: 172.77, 172.73, 170.95, 168.50, 159.60, 158.67, 142.08, 141.69, 141.49, 140.88, 140.78, 128.42 (ArCH), 128.27 (ArCH), 128.14 (ArCH), 126.09 (ArCH), 125.23 (ArCH), 124.54 (ArCH), 122.42 (ArCH), 122.30 (ArCH), 111.51 (ArCH), 111.00 (ArCH), 88.62 (CH=C), 87.73 (CH=C), 49.52 (C(CH3)2), 49.10 (C(CH3)2), 43.47 (NCH2CH2CH2Ph), 43.34 (NCH2CH2CH2Ph), 32.25 (NCH2CH2CH2Ph), 32.08 (NCH2CH2CH2Ph), 30.46 (NHCH3), 28.86 (NCH2CH2CH2Ph), 28.37 (NCH2CH2CH2Ph), 26.18 (C(CH3)2), 25.76 (C(CH3)2) ppm. HRMS m/z: 646.378971 [M–I]+ (C45H48N3O calc. 646.379190).
5.6. Synthesis of 2-({3-[3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidenemethyl]-4-oxo-2-[(pyren-1-ylmethyl)amino]cyclobut-2-en-1-ylidene}methyl)-3,3-dimethyl-1-(3-phenylpropyl)-indol-1-ium Iodide (14)
In a 100 mL round bottom flask, pyrenemethylamine hydrochloride (0.20 g; 0.75 mmol), 0.061 mL of dry pyridine (0.059 g; 0.75 mmol), and 6 mL of dry dimethylformamide were mixed at room temperature, under stirring and a nitrogen atmosphere. After complete dissolution, a solution of the O-methylated dye 5 (0.20 g; 0.25 mmol) in dry dichloromethane (25 mL) was slowly added and allowed to react for 3 h. The mixture was transferred to a 100 mL extraction ampoule, and dichloromethane was added to dilute, and 3 washes with distilled water were performed. The organic phase was recovered, dried with anhydrous sodium sulfate, filtered gravimetrically, and the solvent removed to dryness on a rotary evaporator. After the addition of 10 mL of methanol and 10 mL of a 14% potassium iodide aqueous solution (1.4 g; 8.43 mmol), the mixture was allowed to react, under stirring, for 30 min. A paste was obtained, which was dissolved with dichloromethane and washed with distilled water 3 times in an extraction ampoule. After separation of the organic phase, dried with anhydrous sodium sulfate, gravimetrically filtered, and the solvent removed in the rotary evaporator, the obtained residue was washed several times with a 1:1 mixture of ice-cold ether and methanol, and proceeding with recrystallizations with these same solvents, it was possible to obtain pure green crystals with a 37% yield. M.p.: 231–232 °C. Vis λmax (DMSO): 661 nm; ε = 196,583 M−1·cm−1. IR ʋmax (KBr): 3088 (w, NH), 3036 (w, ArCH), 2957 (w, CH), 2922 (w, CH), 1630 (m, ArC=C), 1611 (w), 1560 (m), 1491 (s), 1479 (s), 1458 (s), 1356 (w), 1281 (s), 1240 (m), 1150 (s), 1117 (m), 1096 (m), 1057 (w), 1020 (w), 968 (w), 924 (w), 843 (w), 795 (w), 746 (m), 683 (w), 552 (w) cm−1. 1H NMR (DMSO-d6, 400.13 MHz) δ: 9.84 (1H, br t, J = 5.6, NHCH2, exchange with D2O), 8.57 (1H, d, J = 9.2, ArH), 8.41–8.36 (4H, m, ArH), 8.21–8.12 (4H, m, ArH), 7.65 (1H, d, J = 7.6, ArH), 7.48–7.43 (3H, m, ArH), 7.34 (1H, t, J = 7.6), 7.30–7.24 (2H, m, ArH), 7.23–7.19 (4H, m, ArH), 7.14 (1H, t, J = 6.8), 7.02–6.99 (4H, m, ArH), 6.44–6.42 (2H, m, ArH), 6.32 (1H, s, CH=C), 5.88 (2H, d, J = 6.0, NHCH2Pyrene, collapse to s with D2O), 5.61 (1H, s, CH=C), 4.37 (2H, t, J = 6.8, NCH2CH2CH2Ph), 3.40 (2H, d, J = 6.8, NCH2CH2CH2Ph), 2.74 (2H, t, J = 8.2, NCH2CH2CH2Ph), 2.10 (2H, qt, J = 7.6, NCH2CH2CH2Ph), 1.76 (6H, s, C(CH3)2), 1.67 (2H, t, J = 8.4, NCH2CH2CH2Ph), 1.57 (6H, s, C(CH3)2), 0.90 (2H, qt, J = 7.2, NCH2CH2CH2Ph) ppm. 13C NMR (DMSO-d6, 100.62 MHz) δ: 173.42, 173.28, 170.73, 168.85, 160.03, 158.06, 142.27, 141.60, 141.46, 141.38, 140.86, 139.93, 130.91, 130.54, 130.34, 128.41 (ArCH), 128.30 (ArCH), 128.14 (ArCH), 128.00 (ArCH), 127.50 (ArCH), 127.33 (ArCH), 126.60 (ArCH), 125.77 (ArCH), 125.72 (ArCH), 125.61 (ArCH), 125.46 (ArCH), 125.26 (ArCH), 124.38 (ArCH), 124.29 (ArCH), 124.18 (ArCH), 123.81 (ArCH), 122.47 (ArCH), 122.16 (ArCH), 111.74 (ArCH), 110.76 (ArCH), 88.57 (CH=C), 88.29 (CH=C), 49.75 (C(CH3)2), 48.97 (C(CH3)2), 45.41 (NHCH2Pyrene), 43.61 (NCH2CH2CH2Ph), 42.48 (NCH2CH2CH2Ph), 32.11 (NCH2CH2CH2Ph), 31.13 (NCH2CH2CH2Ph), 28.94 (NCH2CH2CH2Ph), 27.35 (NCH2CH2CH2Ph), 26.11 (C(CH3)2), 25.77 (C(CH3)2) ppm. HRMS m/z: 846.441816 [M–I]+ (C61H56N3O calc. 846.441790).