Anti-Cytokine Drugs in the Treatment of Canine Atopic Dermatitis
Abstract
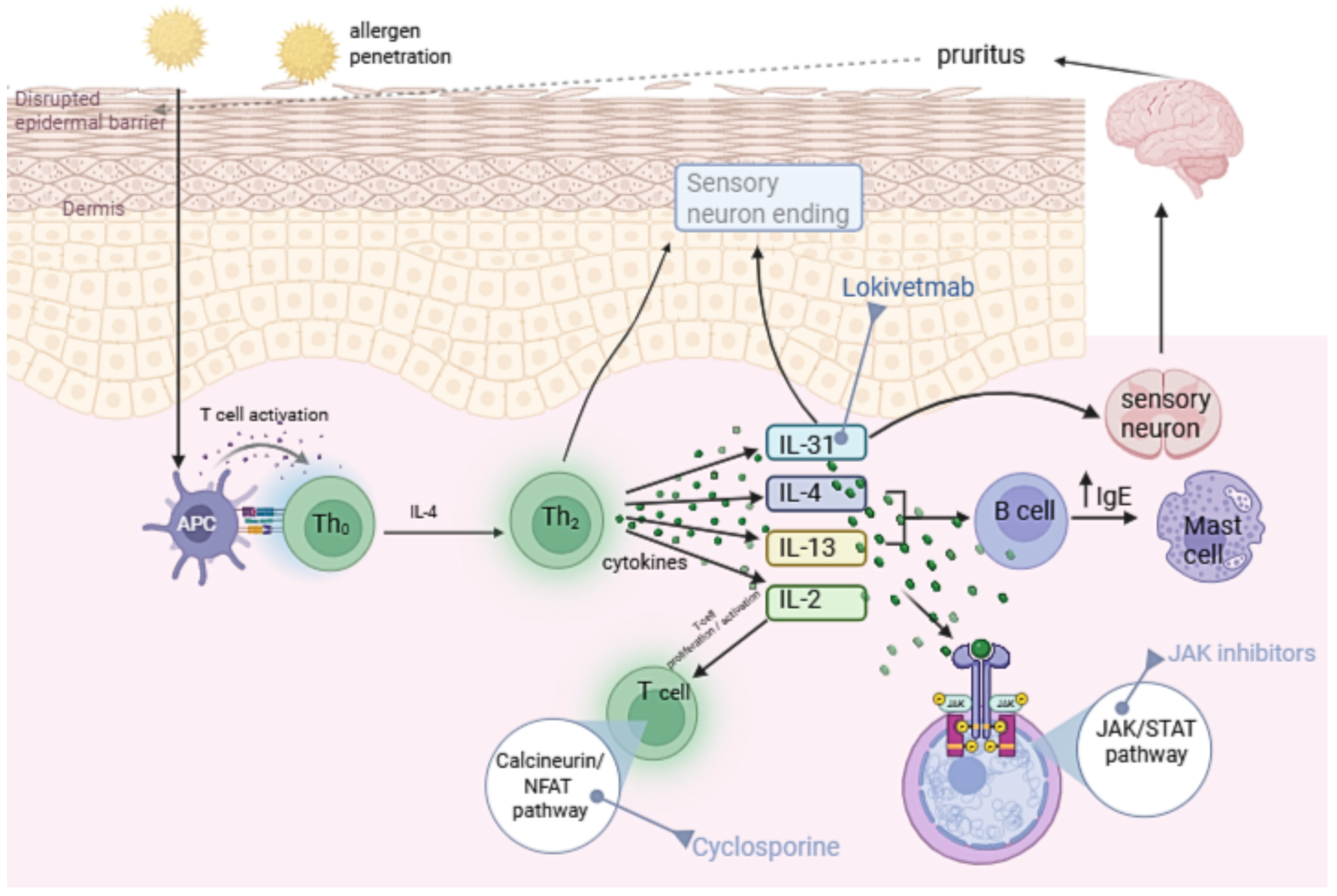
1. Introduction
1.1. Immunopathogenesis of Canine Atopic Dermatitis
1.2. IL-31 and Other Cytokines in cAD
2. Characteristics of Anti-Cytokine Drugs Used in cAD
2.1. Drugs That Directly Neutralize Cytokines
2.2. Drugs Indirectly Affecting Cytokine Signaling—JAK Inhibitors
2.2.1. Oclacitinib
2.2.2. Ilunocitinib
2.2.3. Atinvicitinib
2.3. Drugs Indirectly Affecting Cytokine Signaling—Immunosuppressants Inhibiting Cytokine Synthesis
2.3.1. Ciclosporin
2.3.2. Tacrolimus
3. Therapeutic Guidelines for Anti-Cytokine Drugs in cAD Treatment
4. Discussion—Limitations and Perspectives
4.1. Breed-Related and Individual Variability
4.2. Pharmacokinetic Variability and Influencing Factors
4.3. Cytokine Heterogeneity, Allergen Dependence, and Personalized Therapy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernandes, B.; Alves, S.; Schmidt, V.; Bizarro, A.; Pinto, M.; Pereira, H.; Marto, J.; Lourenço, A. Primary Prevention of Canine Atopic Dermatitis: Breaking the Cycle—A Narrative Review. Vet. Sci. 2023, 10, 659. [Google Scholar] [CrossRef]
- Eisenschenk, M.C.; Hensel, P.; Saridomichelakis, M.N.; Tamamoto-Mochizuki, C.; Pucheu-Haston, C.M.; Santoro, D. Introduction to the ICADA 2023 Canine Atopic Dermatitis Pathogenesis Review Articles and Updated Definition. Vet. Dermatol. 2024, 35, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y.M. The Immunology of Atopic Dermatitis and Its Reversibility with Broad-Spectrum and Targeted Therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef]
- Outerbridge, C.; Jordan, T. Current Knowledge on Canine Atopic Dermatitis—Pathogenesis and Treatment. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Hensel, P.; Santoro, D.; Favrot, C.; Hill, P.; Griffin, C. Canine Atopic Dermatitis: Detailed Guidelines for Diagnosis and Allergen Identification. BMC Vet. Res. 2015, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, Y.; Dong, C.; Clark, D.; Kaur, G. Canine Atopic Dermatitis: Prevalence, Impact, and Management Strategies. Vet. Med. 2024, 15, 15–29. [Google Scholar] [CrossRef]
- Hensel, P.; Saridomichelakis, M.; Eisenschenk, M.; Tamamoto-Mochizuki, C.; Pucheu-Haston, C.; Santoro, D.; For the International Committee on Allergic Diseases of Animals (ICADA). Update on the Role of Genetic Factors, Environmental Factors and Allergens in Canine Atopic Dermatitis. Vet. Dermatol. 2024, 35, 15–24. [Google Scholar] [CrossRef]
- Pucheu-Haston, C.M.; Bizikova, P.; Marsella, R.; Santoro, D.; Nuttall, T.; Eisenschenk, M.N.C. Review: Lymphocytes, Cytokines, Chemokines and the T-helper 1–T-helper 2 Balance in Canine Atopic Dermatitis. Vet. Dermatol. 2015, 26, 124-e32. [Google Scholar] [CrossRef]
- Schlotter, Y.M.; Rutten, V.P.M.G.; Riemers, F.M.; Knol, E.F.; Willemse, T. Lesional Skin in Atopic Dogs Shows a Mixed Type-1 and Type-2 Immune Responsiveness. Vet. Immunol. Immunopathol. 2011, 143, 20–26. [Google Scholar] [CrossRef]
- Marsella, R.; Sousa, C.; Gonzales, A.; Fadok, V. Current Understanding of Pathophysiologic Mechanisms of Canine Atopic Dermatitis. J. Am. Vet. Med. Assoc. 2012, 241, 194–207. [Google Scholar] [CrossRef]
- Majewska, A.; Dembele, K.; Dziendzikowska, K.; Prostek, A.; Gajewska, M. Cytokine and Lymphocyte Profiles in Dogs with Atopic Dermatitis after Allergen-Specific Immunotherapy. Vaccines 2022, 10, 1037. [Google Scholar] [CrossRef]
- Hill, P.B.; Olivry, T. The ACVD Task Force on Canine Atopic Dermatitis (V): Biology and Role of Inflammatory Cells in Cutaneous Allergic Reactions. Vet. Immunol. Immunopathol. 2001, 81, 187–198. [Google Scholar] [CrossRef]
- Tamamoto-Mochizuki, C.; Santoro, D.; Saridomikelakis, M.N.; Eisenschenk, M.N.C.; Hensel, P.; Pucheu-Haston, C.; For the International Committee on Allergic Diseases of Animals (ICADA). Update on the Role of Cytokines and Chemokines in Canine Atopic Dermatitis. Vet. Dermatol. 2024, 35, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2017, 10, a028415. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.; Leon, R.; Starr, H.; Kim, S.; Fogle, J.; Banovic, F. Establishment of an Intradermal Canine IL-31-Induced Pruritus Model to Evaluate Therapeutic Candidates in Atopic Dermatitis. Vet. Sci. 2023, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.J.; Humphrey, W.R.; Messamore, J.E.; Fleck, T.J.; Fici, G.J.; Shelly, J.A.; Teel, J.F.; Bammert, G.F.; Dunham, S.A.; Fuller, T.E.; et al. Interleukin-31: Its Role in Canine Pruritus and Naturally Occurring Canine Atopic Dermatitis. Vet. Dermatol. 2013, 24, 48-e12. [Google Scholar] [CrossRef]
- Asahina, R.; Nishida, H.; Kamishina, H.; Maeda, S. Expression of IL-33 in Chronic Lesional Skin of Canine Atopic Dermatitis. Vet. Dermatol. 2018, 29, 246-e91. [Google Scholar] [CrossRef]
- Borek, F.; Nagashima, S.; Roldán Villalobos, W.; Gmyterco, V.; Sell, T.; Farias, M.; Bechara, G. Immunoexpression of IL-33 in the Different Clinical Aspects of Canine Atopic Dermatitis. Vet. Immunol. Immunopathol. 2024, 273, 110786. [Google Scholar] [CrossRef]
- Sakamoto, M.; Asahina, R.; Kamishina, H.; Maeda, S. Transcription of Thymic Stromal Lymphopoietin via Toll-like Receptor 2 in Canine Keratinocytes: A Possible Association of Staphylococcus Spp. in the Deterioration of Allergic Inflammation in Canine Atopic Dermatitis. Vet. Dermatol. 2016, 27, 184-e46. [Google Scholar] [CrossRef]
- Klukowska-Rötzler, J.; Chervet, L.; Müller, E.J.; Roosje, P.; Marti, E.; Janda, J. Expression of Thymic Stromal Lymphopoietin in Canine Atopic Dermatitis. Vet. Dermatol. 2013, 24, 54-e14. [Google Scholar] [CrossRef]
- Olivry, T.; Mayhew, D.; Paps, J.S.; Linder, K.E.; Peredo, C.; Rajpal, D.; Hofland, H.; Cote-Sierra, J. Early Activation of Th2/Th22 Inflammatory and Pruritogenic Pathways in Acute Canine Atopic Dermatitis Skin Lesions. J. Investig. Dermatol. 2016, 136, 1961–1969. [Google Scholar] [CrossRef]
- Jebbawi, F.; Olomski, F.; Inversini, V.; Keller, G.; Rhiner, T.; Waldern, N.; Lam, J.; Pantelyushin, S.; Canonica, F.; Birkmann, K.; et al. Anti-IL-5 Vaccination Dampens Allergen-Specific IgE Levels and Modulates IL-4 and IL-5 Th2 Cytokines in Skin Allergy of Mice and Horses. Allergy, 2025; Early View. [Google Scholar] [CrossRef]
- Nuttall, T.; Marsella, R.; Rosenbaum, M.; Gonzales, A.; Fadok, V. Update on Pathogenesis, Diagnosis, and Treatment of Atopic Dermatitis in Dogs. J. Am. Vet. Med. Assoc. 2019, 254, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Mazrier, H.; Vogelnest, L.J.; Taylor, R.M.; Williamson, P. Altered Plasma Cytokines in Dogs with Atopic Dermatitis. Vet. Dermatol. 2022, 33, 131-e38. [Google Scholar] [CrossRef]
- Shiomitsu, S.; Gillen, J.; Frasca, S.; Santoro, D. Evaluation of the Cutaneous Expression of IL-17, IL-22, IL-31, and Their Receptors in Canine Atopic Dermatitis. Res. Vet. Sci. 2021, 136, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Gow, D.J.; Jackson, H.; Forsythe, P.; Nuttall, T.; Gow, A.G.; Mellanby, R.J.; Hume, D.A. Measurement of Serum Interleukin 34 (IL-34) and Correlation with Severity and Pruritus Scores in Client-Owned Dogs with Atopic Dermatitis. Vet. Dermatol. 2020, 31, 359-e94. [Google Scholar] [CrossRef] [PubMed]
- Moyaert, H.; Van Brussel, L.; Borowski, S.; Escalada, M.; Mahabir, S.P.; Walters, R.R.; Stegemann, M.R. A Blinded, Randomized Clinical Trial Evaluating the Efficacy and Safety of Lokivetmab Compared to Ciclosporin in Client-Owned Dogs with Atopic Dermatitis. Vet. Dermatol. 2017, 28, 593-e145. [Google Scholar] [CrossRef]
- Gober, M.; Hillier, A.; Vasquez, M.; Amodie, D.; Mellencamp, M. Use of Cytopoint in the Allergic Dog. Front. Vet. Sci. 2022, 9, 909776. [Google Scholar] [CrossRef]
- Michels, G.M.; Walsh, K.F.; Kryda, K.A.; Mahabir, S.P.; Walters, R.R.; Hoevers, J.D.; Martinon, O.M. A Blinded, Randomized, Placebo-Controlled Trial of the Safety of Lokivetmab (ZTS-00103289), a Caninized Anti-Canine IL-31 Monoclonal Antibody in Client-Owned Dogs with Atopic Dermatitis. Vet. Dermatol. 2016, 27, 505-e136. [Google Scholar] [CrossRef]
- Bağci, I.S.; Ruzicka, T. IL-31: A New Key Player in Dermatology and Beyond. J. Allergy Clin. Immunol. 2018, 141, 858–866. [Google Scholar] [CrossRef]
- Krautmann, M.; Walters, R.R.; King, V.L.; Esch, K.; Mahabir, S.P.; Gonzales, A.; Dominowski, P.J.; Sly, L.; Mwangi, D.; Foss, D.L.; et al. Laboratory Safety Evaluation of Lokivetmab, a Canine Anti-Interleukin-31 Monoclonal Antibody, in Dogs. Vet. Immunol. Immunopathol. 2023, 258, 110574. [Google Scholar] [CrossRef] [PubMed]
- Kasper, B.; Zablotski, Y.; Mueller, R.S. Long-term Use of Lokivetmab in Dogs with Atopic Dermatitis. Vet. Dermatol. 2024, 35, 683–693. [Google Scholar] [CrossRef]
- Souza, C.P.; Rosychuk, R.A.W.; Contreras, E.T.; Schissler, J.R.; Simpson, A.C. A Retrospective Analysis of the Use of Lokivetmab in the Management of Allergic Pruritus in a Referral Population of 135 Dogs in the Western USA. Vet. Dermatol. 2018, 29, 489-e164. [Google Scholar] [CrossRef]
- Bruet, V.; Mosca, M.; Briand, A.; Bourdeau, P.; Pin, D.; Cochet-Faivre, N.; Cadiergues, M.-C. Clinical Guidelines for the Use of Antipruritic Drugs in the Control of the Most Frequent Pruritic Skin Diseases in Dogs. Vet. Sci. 2022, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Van Brussel, L.; Moyaert, H.; Escalada, M.; Mahabir, S.P.; Stegemann, M.R. A Masked, Randomised Clinical Trial Evaluating the Efficacy and Safety of Lokivetmab Compared to Saline Control in Client-Owned Dogs with Allergic Dermatitis. Vet. Dermatol. 2021, 32, 477-e131. [Google Scholar] [CrossRef]
- Cosgrove, S.; Wren, J.; Cleaver, D.; Martin, D.; Walsh, K.; Harfst, J.; Follis, S.; King, V.; Boucher, J.; Stegemann, M. Efficacy and Safety of Oclacitinib for the Control of Pruritus and Associated Skin Lesions in Dogs with Canine Allergic Dermatitis. Vet. Dermatol. 2013, 24, 479-e114. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.J.; Bowman, J.W.; Fici, G.J.; Zhang, M.; Mann, D.W.; Mitton-Fry, M. Oclacitinib (APOQUEL®) Is a Novel Janus Kinase Inhibitor with Activity against Cytokines Involved in Allergy. J. Vet. Pharmacol. Ther. 2014, 37, 317–324. [Google Scholar] [CrossRef]
- Marsella, R.; Doerr, K.; Gonzales, A.; Rosenkrantz, W.; Schissler, J.; White, A. Oclacitinib 10 Years Later: Lessons Learned and Directions for the Future. J. Am. Vet. Med. Assoc. 2023, 261, S36–S47. [Google Scholar] [CrossRef]
- Gadeyne, C.; Little, P.; King, V.; Edwards, N.; Davis, K.; Stegemann, M. Efficacy of Oclacitinib (Apoquel®) Compared with Prednisolone for the Control of Pruritus and Clinical Signs Associated with Allergic Dermatitis in Client-Owned Dogs in Australia. Vet. Dermatol. 2014, 25, 512-e86. [Google Scholar] [CrossRef]
- Collard, W.T.; Hummel, B.D.; Fielder, A.F.; King, V.L.; Boucher, J.F.; Mullins, M.A.; Malpas, P.B.; Stegemann, M.R. The Pharmacokinetics of Oclacitinib Maleate, a Janus Kinase Inhibitor, in the Dog. J. Vet. Pharmacol. Ther. 2014, 37, 279–285. [Google Scholar] [CrossRef]
- Cosgrove, S.B.; Cleaver, D.M.; King, V.L.; Gilmer, A.R.; Daniels, A.E.; Wren, J.A.; Stegemann, M.R. Long-Term Compassionate Use of Oclacitinib in Dogs with Atopic and Allergic Skin Disease: Safety, Efficacy and Quality of Life. Vet. Dermatol. 2015, 26, 171-e35. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Apoquel (Oclacitinib)—Summary of Product Characteristics; European Medicines Agency: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Carrasco, I.; Ferrer, L.; Puigdemont, A. Efficacy of Oclacitinib for the Control of Feline Atopic Skin Syndrome: Correlating Plasma Concentrations with Clinical Response. J. Feline Med. Surg. 2021, 24, 787–793. [Google Scholar] [CrossRef]
- Noli, C.; Matricoti, I.; Schievano, C. A Double-blinded, Randomized, Methylprednisolone-controlled Study on the Efficacy of Oclacitinib in the Management of Pruritus in Cats with Nonflea Nonfood-induced Hypersensitivity Dermatitis. Vet. Dermatol. 2019, 30, 110-e30. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.L.; Campos, D.R.; Machado, M.A.; Alves, M.S.R.; de Souza, M.S.G.; da Veiga, C.C.P.; Merlo, A.; Scott, F.B.; Fernandes, J.I. A Blinded, Randomized, Placebo-Controlled Trial of the Safety of Oclacitinib in Cats. BMC Vet. Res. 2019, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.; Boegel, A.; Despa, S.; Trout, C.; King, S. Comparative Efficacy and Safety of Ilunocitinib and Oclacitinib for the Control of Pruritus and Associated Skin Lesions in Dogs with Atopic Dermatitis. Vet. Dermatol. 2025, 36, 165–176. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration, Center for Veterinary Medicine (FDA CVM). Freedom of Information (FOI) Summary: NADA 141-585, ZenreliaTM (Ilunocitinib Tablets); U.S. Food and Drug Administration, Center for Veterinary Medicine: Laurel, MD, USA, 2024.
- Forster, S.; Trout, C.M.; Despa, S.; Boegel, A.; Berger, D.; King, S. Efficacy and Field Safety of Ilunocitinib for the Control of Atopic Dermatitis in Client-Owned Dogs: A Multicentre, Double-Masked, Randomised, Placebo-Controlled Clinical Trial. Vet. Dermatol. 2025, 36, 647–659. [Google Scholar] [CrossRef]
- Boerngen, K.; Patel, Y.; Pittorino, M.; Toutain, C. Pharmacokinetics of Ilunocitinib, a New Janus Kinase Inhibitor, in Dogs. J. Vet. Pharmacol. Ther. 2025; Early View. [Google Scholar] [CrossRef]
- Kuntz, E.A.; Gabor, L.; Toutain, C.E. Safety of Ilunocitinib Tablets (Zenrelia™) after Once Daily Oral Administration in Dogs. BMC Vet. Res. 2025, 21, 144. [Google Scholar] [CrossRef]
- Fent, G.M.; Despa, S.; Gabor, L.; Earll, M.; McCandless, E.E.; O’Kelley, S.; Patch, J.R.; Snyder, J.; King, S. Response to Primary Canine Core Vaccination in 10-Month-Old Seronegative Dogs Treated with Three Times the Recommended Therapeutic Dose of Ilunocitinib Tablets (Zenrelia™). BMC Vet. Res. 2025, 21, 461. [Google Scholar] [CrossRef]
- Fent, G.M.; Jacela, J.; Plazola-Ortiz, R.; Olps, J.; McCandless, E.E.; Toutain, C.E.; O’Kelley, S.; King, S. Immunologic Response to First Booster Vaccination in Dogs Treated with Zenrelia™ (Ilunocitinib Tablets) at up to Three Times the Recommended Therapeutic Dose Compared to Untreated Controls. BMC Vet. Res. 2025, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Nederveld, S.; Krautmann, M.; Mitchell, J. Safety of the Selective JAK1 Inhibitor Oclacitinib in Dogs. J. Vet. Pharmacol. Ther. 2025, 48, 135–145. [Google Scholar] [CrossRef]
- Choy, E. Clinical Significance of Janus Kinase Inhibitor Selectivity. Rheumatol. Oxf. Engl. 2019, 58, 953–962, Erratum in Rheumatol. Oxf. Engl. 2019, 58, 1122. https://doi.org/10.1093/rheumatology/kez002. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Numelvi (Atinvicitinib)—Summary of Product Characteristics; European Medicines Agency: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Parmentier, J.M.; Voss, J.; Graff, C.; Schwartz, A.; Argiriadi, M.; Friedman, M.; Camp, H.S.; Padley, R.J.; George, J.S.; Hyland, D.; et al. In Vitro and in Vivo Characterization of the JAK1 Selectivity of Upadacitinib (ABT-494). BMC Rheumatol. 2018, 2, 23. [Google Scholar] [CrossRef]
- Olivry, T.; Steffan, J.; Fisch, R.; Prélaud, P.; Fontaine, J.; Carlotti, D.; Guaguère, E. Randomised Controlled Trial of the Efficacy of Ciclosporin in the Treatment of Atopic Dermatitis in Dogs. J. Am. Vet. Med. Assoc. 2002, 221, 370–377. [Google Scholar] [CrossRef]
- Matsuda, S.; Koyasu, S. Mechanisms of Action of Cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef]
- Kobayashi, T.; Momoi, Y.; Iwasaki, T. Cyclosporine A Inhibits the mRNA Expressions of IL-2, IL-4 and IFN-γ, but Not TNF-α, in Canine Mononuclear Cells. J. Vet. Med. Sci. 2007, 69, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; DeBoer, D.J.; Favrot, C.; Jackson, H.A.; Mueller, R.S.; Nuttall, T.; Prélaud, P.; For the International Committee on Allergic Diseases of Animals. Treatment of Canine Atopic Dermatitis: 2015 Updated Guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet. Res. 2015, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Archer, T.M.; Boothe, D.M.; Langston, V.C.; Fellman, C.L.; Lunsford, K.V.; Mackin, A.J. Oral Cyclosporine Treatment in Dogs: A Review of the Literature. J. Vet. Intern. Med. 2014, 28, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Panteri, A.; Strehlau, G.; Helbig, R.; Prost, C.; Doucette, K. Repeated Oral Dose Tolerance in Dogs Treated Concomitantly with Ciclosporin and Oclacitinib for Three Weeks. Vet. Dermatol. 2016, 27, 22-e7. [Google Scholar] [CrossRef]
- Little, P.; King, V.; Davis, K.; Cosgrove, S.; Stegemann, M. A Blinded, Randomized Clinical Trial Comparing the Efficacy and Safety of Oclacitinib and Ciclosporin for the Control of Atopic Dermatitis in Client-Owned Dogs. Vet. Dermatol. 2014, 26, 23-e8. [Google Scholar] [CrossRef]
- Marsella, R.; Nicklin, C.; Saglio, S.; Lopez, J. Investigation on the Clinical Efficacy and Safety of 0.1% Tacrolimus Ointment (Protopic®) in Canine Atopic Dermatitis: A Randomized, Double-Blinded, Placebo-Controlled, Cross-over Study. Vet. Dermatol. 2004, 15, 294–303. [Google Scholar] [CrossRef]
- Bensignor, E.; Olivry, T. Treatment of Localized Lesions of Canine Atopic Dermatitis with Tacrolimus Ointment: A Blinded Randomized Controlled Trial. Vet. Dermatol. 2005, 16, 52–60. [Google Scholar] [CrossRef]
- Marsella, R.; Ahrens, K.; Wilkes, R.; Trujillo, A.; Dorr, M. Comparison of Various Treatment Options for Canine Atopic Dermatitis: A Blinded, Randomized, Controlled Study in a Colony of Research Atopic Beagle Dogs. Vet. Dermatol. 2020, 31, 284-e69. [Google Scholar] [CrossRef]
- Cheung, B. In Dogs with Atopic Skin Disease, Is Lokivetmab More Effective than Oclacitinib in Reducing the Score of a Recognised Scoring System? Vet. Evid. 2022, 7. [Google Scholar] [CrossRef]
- Haugh, I.; Watson, I.; Menter MD, A. Successful Treatment of Atopic Dermatitis with the JAK1 Inhibitor Oclacitinib. Bayl. Univ. Med. Cent. Proc. 2018, 31, 524–525. [Google Scholar] [CrossRef]
- DeBoer, D. The Future of Immunotherapy for Canine Atopic Dermatitis: A Review. Vet. Dermatol. 2017, 28, 25-e6. [Google Scholar] [CrossRef]
- Bizikova, P.; Santoro, D.; Marsella, R.; Nuttall, T.; Eisenschenk, M.N.C.; Pucheu-Haston, C.M. Review: Clinical and Histological Features of Canine Atopic Dermatitis. Vet. Dermatol. 2015, 26, 79-e24. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Tseng, C.M.; Roskos, L.K. Elimination Mechanisms of Therapeutic Monoclonal Antibodies. Drug Discov. Today 2006, 11, 81–88. [Google Scholar] [CrossRef]
- Olivry, T.; Bizikova, P. A Systematic Review of Randomized Controlled Trials for Prevention or Treatment of Atopic Dermatitis in Dogs: 2008–2018 update. Vet. Dermatol. 2013, 24, 97-e26. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From Its Discovery to Clinical Applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Merbl, Y.; Lopez Baltazar, J.M.; Byron, M.; Chamberlin, T.; Brown, A.; Stuebing, E.; Eves, W.; Tatarniuk, D.; Brodie, D.; Copland, G.; et al. Tocilizumab Binds to Canine IL-6 Receptor and Elicits In Vitro Inhibitory Biological Response. Front. Vet. Sci. 2025, 12, 1645414. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Elazab, S.T.; Huang, J.; Hsu, W.H. Current Review of Monoclonal Antibody Therapeutics in Small Animal Medicine. Animals 2025, 15, 472. [Google Scholar] [CrossRef] [PubMed]

| Drug | Target/Mechanism | Formulation/Route | Typical Dose & Schedule | Main Advantages | Limitations/Adverse Effects |
|---|---|---|---|---|---|
| Lokivetmab | Neutralizes IL-31 directly (mAb) | Injectable (SC) | 2 mg/kg q4w (monthly) | Rapid onset, long dosing interval, minimal side effects | Weight limit (>3 kg), GI signs, rare ADAs |
| Oclacitinib | Selective JAK1 inhibitor (IL-31, IL-4, IL-13) | Oral tablets | 0.4–0.6 mg/kg BID × 14 d → QD | Very fast pruritus relief, flexible dosing, convenient | Contraindicated <12 mo, infection/neoplasia risk, daily dosing |
| Ilnocitinib | JAK1/JAK2/TYK2 inhibitor (broader) | Oral tablets | Once daily | Strong itch and lesion control, simple q24h dosing | Broader immunosuppression, infection risk |
| Atinivicytinib | Next-gen JAK inhibitor (JAK1/3, TYK2) | Oral tablets | Once daily (investigational) | Potent, broad cytokine modulation, promising efficacy | Limited clinical data; possible immunosuppression, infection risk |
| Ciclosporin | Calcineurin inhibitor → ↓ IL-2, IL-4, IFN-γ synthesis | Oral solution; Oral capsules | ≈5 mg/kg QD | Effective long-term control, steroid-sparing | Slow onset, GI side effects, contraindications |
| Tacrolimus | Calcineurin inhibitor (topical) | Topical ointment | Local application (0.1% oint.) | Useful for localized lesions, avoids systemic exposure | Limited to focal lesions, local irritation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wichtowska, A.; Olejnik, M. Anti-Cytokine Drugs in the Treatment of Canine Atopic Dermatitis. Int. J. Mol. Sci. 2025, 26, 10990. https://doi.org/10.3390/ijms262210990
Wichtowska A, Olejnik M. Anti-Cytokine Drugs in the Treatment of Canine Atopic Dermatitis. International Journal of Molecular Sciences. 2025; 26(22):10990. https://doi.org/10.3390/ijms262210990
Chicago/Turabian StyleWichtowska, Agnieszka, and Małgorzata Olejnik. 2025. "Anti-Cytokine Drugs in the Treatment of Canine Atopic Dermatitis" International Journal of Molecular Sciences 26, no. 22: 10990. https://doi.org/10.3390/ijms262210990
APA StyleWichtowska, A., & Olejnik, M. (2025). Anti-Cytokine Drugs in the Treatment of Canine Atopic Dermatitis. International Journal of Molecular Sciences, 26(22), 10990. https://doi.org/10.3390/ijms262210990






