Impact of Isoquinoline Alkaloids on the Intestinal Barrier in a Colonic Model of Campylobacter jejuni Infection
Abstract
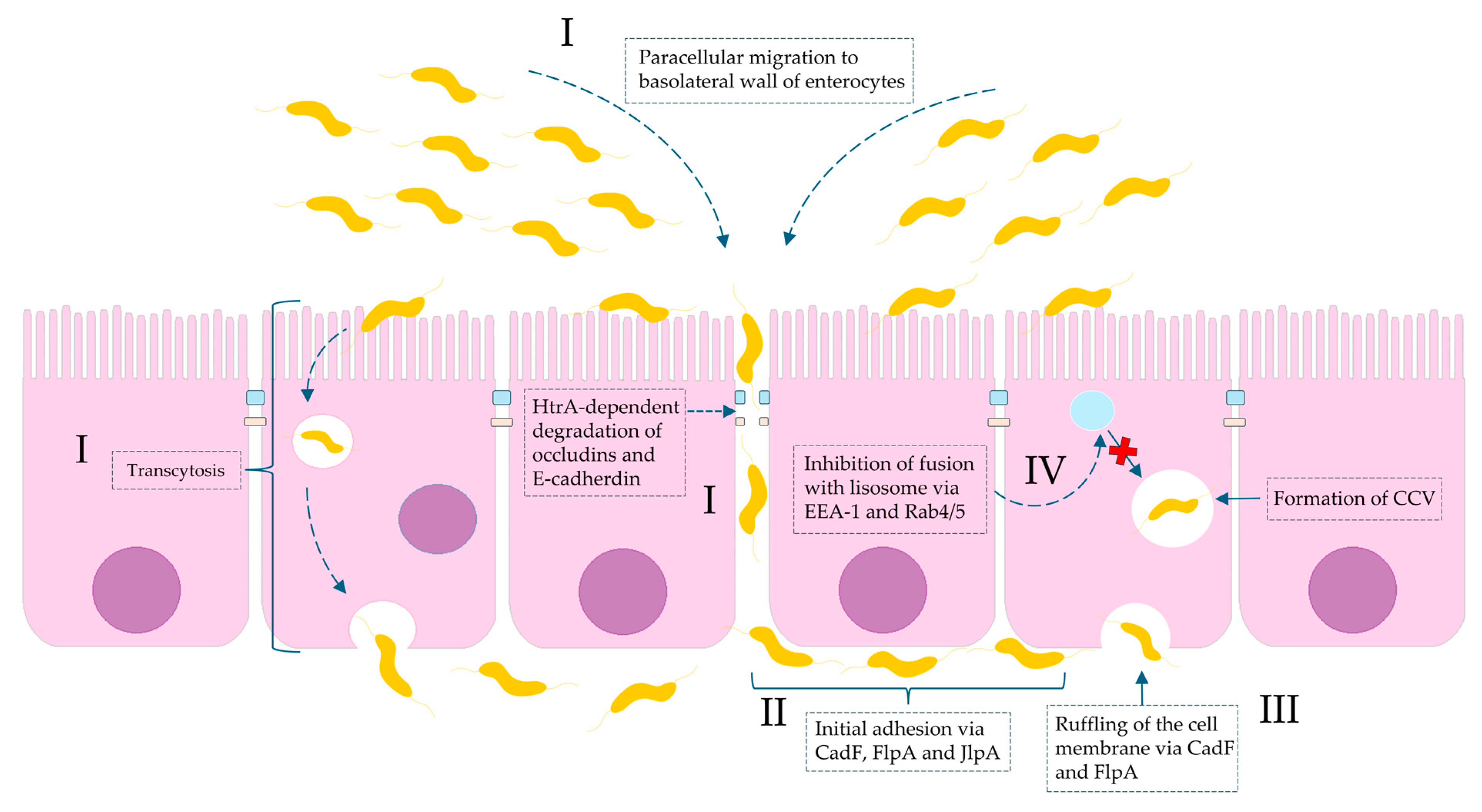
1. Introduction
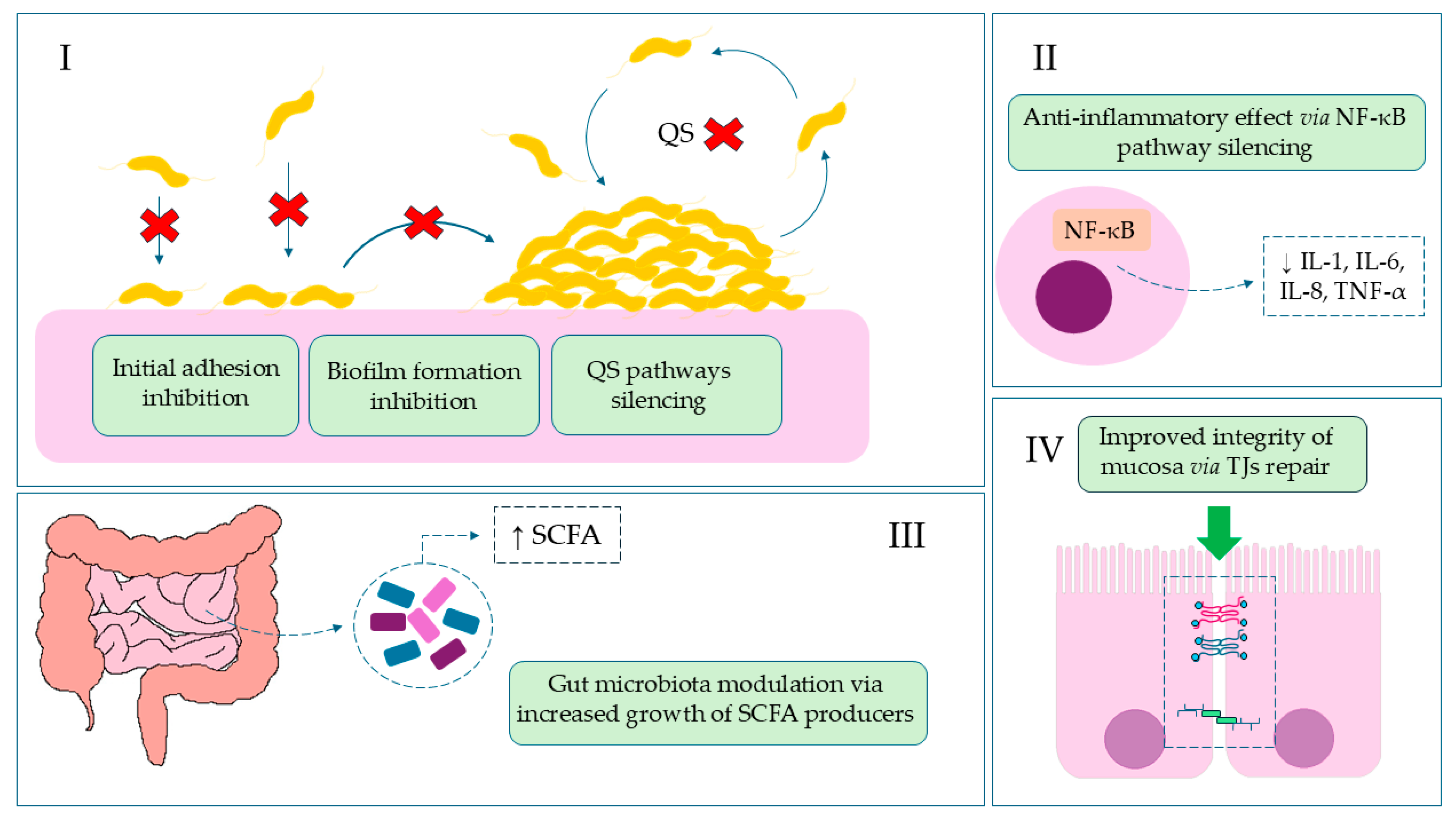
2. Results
3. Discussion
4. Materials and Methods
4.1. Compounds Tested and Culture Media
4.1.1. The Tested Compounds Were Purchased from Chemland (Zgierz, Poland)
4.1.2. LC-MS Analysis of Metabolomics Samples
4.1.3. Bacteriological Media
4.1.4. Cell Line Media
4.2. Antimicrobial Experiments
4.2.1. Revival of the Strain
4.2.2. Experimental Evaluation of the Culture Conditions
4.2.3. Activity Against Planktonic Cells
4.2.4. Activity Against the Biofilm
4.3. Intestinal-Supporting Experiments
4.3.1. Cell Line Tested
4.3.2. Experimental Evaluation of the Culture Conditions
4.3.3. Cytotoxicity Assay
4.3.4. Permeability Assay
4.3.5. Morphostructural State Analysis
4.3.6. Re-Localization of Occludin
4.3.7. Secretory Profile Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foodborne Diseases Estimates. Available online: https://www.who.int/data/gho/data/themes/who-estimates-of-the-global-burden-of-foodborne-diseases (accessed on 26 October 2025).
- Ao, T.T.; Feasey, N.A.; Gordon, M.A.; Keddy, K.H.; Angulo, F.J.; Crump, J.A. Global Burden of Invasive Nontyphoidal Salmonella Disease, 2010. Emerg. Infect. Dis. 2015, 21, 941–949. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Parisi, A.; Sarkar, K.; Blacker, B.F.; Reiner, R.C.; Hay, S.I.; Nixon, M.R.; Dolecek, C.; James, S.L.; Mokdad, A.H.; et al. The Global Burden of Non-Typhoidal Salmonella Invasive Disease: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive Non-Typhoidal Salmonella Disease: An Emerging and Neglected Tropical Disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Erkmen, O.; Bozoglu, T.F. (Eds.) Foodborne Invasive Infections. Food Microbiology Principles into Practice. Volume 1: Microorganisms Related to Foods, Foodborne Diseases and Food Spoilage; Wiley: Hoboken, NJ, USA, 2016; pp. 138–170. [Google Scholar]
- Fabricant, D.S.; Farnsworth, N.R. The Value of Plants Used in Traditional Medicine for Drug Discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [PubMed]
- Fletcher, S.M.; Stark, D.; Ellis, J. Prevalence of Gastrointestinal Pathogens in Sub-Saharan Africa: Systematic Review and Meta-Analysis. J. Public Health Afr. 2011, 2, 127–137. [Google Scholar] [CrossRef]
- Burd, E.M.; Hinrichs, B.H. Gastrointestinal Infections. In Molecular Pathology in Clinical Practice; Leonard, D., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Duda-Madej, A.; Viscardi, S.; Stecko, J.; Szymańska, N.; Topola, E.; Pacyga, K.; Szandruk-Bender, M. Can Nature Overcome Invasive Gastrointestinal Infections? Int. J. Mol. Sci. 2025, 26, 5795. [Google Scholar] [CrossRef]
- Ternhag, A.; Törner, A.; Svensson, Å.; Ekdahl, K.; Giesecke, J. Short- and Long-Term Effects of Bacterial Gastrointestinal Infections. Emerg. Infect. Dis. 2008, 14, 143–148. [Google Scholar] [CrossRef]
- Fischer, G.; Hashmi, M.; Paterek, E. Campylobacter Infection. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2005. [Google Scholar]
- Lopes, G.V.; Ramires, T.; Kleinubing, N.R.; Scheik, L.K.; Fiorentini, Â.M.; Padilha da Silva, W. Virulence Factors of Foodborne Pathogen Campylobacter Jejuni. Microb. Pathog. 2021, 161, 105265. [Google Scholar] [CrossRef]
- Haddad, N.; Marce, C.; Magras, C.; Cappelier, J.M. An Overview of Methods Used to Clarify Pathogenesis Mechanisms of Campylobacter Jejuni. J. Food Prot. 2010, 73, 786–802. [Google Scholar] [CrossRef]
- Cools, I.; Uyttendaele, M.; Cerpentier, J.; D’Haese, E.; Nelis, H.J.; Debevere, J. Persistence of Campylobacter jejuni on Surfaces in a Processing Environment and on Cutting Boards. Lett. Appl. Microbiol. 2005, 40, 418–423. [Google Scholar] [CrossRef]
- Same, R.G.; Tamma, P.D. Campylobacter Infections in Children. Pediatr. Rev. 2018, 39, 533–541. [Google Scholar] [CrossRef]
- Sundermann, E.M.; Nauta, M.; Swart, A. A Ready-to-Use Dose-Response Model of Campylobacter jejuni Implemented in the FSKX-Standard. Food Model. J. 2021, 2, e63309. [Google Scholar] [CrossRef]
- Schnee, A.E.; Petri, W.A., Jr. Campylobacter jejuni and Associated Immune Mechanisms: Short-Term Effects and Long-Term Implications for Infants in Low Income Countries. Curr. Opin. Infect. Dis. 2017, 30, 322–328. [Google Scholar] [CrossRef]
- Kalischuk, L.D.; Buret, A.G. A Role for Campylobacter Jejuni-Induced Enteritis in Inflammatory Bowel Disease? Am. J. Physiol.—Gastrointest. Liver Physiol. 2010, 298, G1–G9. [Google Scholar] [CrossRef]
- Schiaffino, F.; Colston, J.M.; Paredes Olortegui, M.; Peñataro Yori, P.; Mourkas, E.; Pascoe, B.; Lima, A.A.M.; Mason, C.J.; Ahmed, T.; Kang, G.; et al. The Epidemiology and Impact of Persistent Campylobacter Infections on Childhood Growth among Children 0–24 Months of Age in Resource-Limited Settings. eClinicalMedicine 2024, 76, 102841. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Backert, S.; Alter, T.; Bereswill, S. Molecular Targets in Campylobacter Infections. Biomolecules 2023, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Esan, O.B.; Pearce, M.; van Hecke, O.; Roberts, N.; Collins, D.R.J.; Violato, M.; McCarthy, N.; Perera, R.; Fanshawe, T.R. Factors Associated with Sequelae of Campylobacter and Non-Typhoidal Salmonella Infections: A Systematic Review. EBioMedicine 2017, 15, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Omarova, S.; Awad, K.; Moos, V.; Püning, C.; Gölz, G.; Schulzke, J.D.; Bücker, R. Intestinal Barrier in Post-Campylobacter jejuni Irritable Bowel Syndrome. Biomolecules 2023, 13, 449. [Google Scholar] [CrossRef]
- Gradel, K.O.; Nielsen, H.L.; Schønheyder, H.C.; Ejlertsen, T.; Kristensen, B.; Nielsen, H. Increased Short- and Long-Term Risk of Inflammatory Bowel Disease After Salmonella or Campylobacter Gastroenteritis. Gastroenterology 2009, 137, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Triggers of Guillain–Barré Syndrome: Campylobacter jejuni Predominates. Int. J. Mol. Sci. 2022, 23, 14222. [Google Scholar] [CrossRef]
- Kalra, V.; Chaudhry, R.; Dua, T.; Dhawan, B.; Sahu, J.K.; Mridula, B. Association of Campylobacter jejuni Infection with Childhood Guillain-Barré Syndrome: A Case-Control Study. J. Child Neurol. 2009, 24, 664–668. [Google Scholar] [CrossRef]
- He, Z.; Yu, J.; Gong, J.; Wu, J.; Zong, X.; Luo, Z.; He, X.; Cheng, W.M.; Liu, Y.; Liu, C.; et al. Campylobacter Jejuni-Derived Cytolethal Distending Toxin Promotes Colorectal Cancer Metastasis. Cell Host Microbe 2024, 32, 2080–2091.e6. [Google Scholar] [CrossRef] [PubMed]
- Gervaz, P.A.; De Campos, Á.; Caeiro, A. Campylobacter jejuni Causes Colorectal Cancer. World J. Color. Surg. 2022, 11, 4–7. [Google Scholar] [CrossRef]
- Huang, G.; Boesze-Battaglia, K.; Walker, L.P.; Zekavat, A.; Schaefer, Z.P.; Blanke, S.R.; Shenker, B.J. The Active Subunit of the Cytolethal Distending Toxin, CdtB, Derived From Both Haemophilus ducreyi and Campylobacter jejuni Exhibits Potent Phosphatidylinositol-3,4,5-Triphosphate Phosphatase Activity. Front. Cell. Infect. Microbiol. 2021, 11, 664221. [Google Scholar] [CrossRef]
- Lecuit, M.; Abachin, E.; Martin, A.; Poyart, C.; Pochart, P.; Suarez, F.; Bengoufa, D.; Feuillard, J.; Lavergne, A.; Gordon, J.I.; et al. Immunoproliferative Small Intestinal Disease Associated with Campylobacter jejuni. N. Engl. J. Med. 2004, 350, 239–248. [Google Scholar] [CrossRef]
- Rossi, D.A.; Melo, R.T.; Mendonça, E.P.; Monteiro, G.P. Biofilms of Salmonella and Campylobacter in the Poultry Industry. Poult. Sci. 2017, 15, 93–114. [Google Scholar] [CrossRef]
- Whelan, M.V.X.; Simpson, J.C.; Cróinín, T.Ó. A Novel High-Content Screening Approach for the Elucidation of C. jejuni Biofilm Composition and Integrity. BMC Microbiol. 2021, 21, 2. [Google Scholar] [CrossRef]
- Kreling, V.; Falcone, F.H.; Kehrenberg, C.; Hensel, A. Campylobacter Sp.: Pathogenicity Factors and Prevention Methods—New Molecular Targets for Innovative Antivirulence Drugs? Appl. Microbiol. Biotechnol. 2020, 104, 10409–10436. [Google Scholar] [CrossRef] [PubMed]
- Tram, G.; Day, C.J.; Korolik, V. Bridging the Gap: A Role for Campylobacter jejuni Biofilms. Microorganisms 2020, 8, 452. [Google Scholar] [CrossRef]
- Kemper, L.; Hensel, A. Campylobacter jejuni: Targeting Host Cells, Adhesion, Invasion, and Survival. Appl. Microbiol. Biotechnol. 2023, 107, 2725–2754. [Google Scholar] [CrossRef]
- Sharafutdinov, I.; Tegtmeyer, N.; Müsken, M.; Backert, S. Campylobacter jejuni Serine Protease HtrA Induces Paracellular Transmigration of Microbiota across Polarized Intestinal Epithelial Cells. Biomolecules 2022, 12, 4–17. [Google Scholar] [CrossRef]
- Sharafutdinov, I.; Tegtmeyer, N.; Rohde, M.; Olofsson, A.; Rehman, Z.U.; Arnqvist, A.; Backert, S. Campylobacter jejuni Surface-Bound Protease HtrA, but Not the Secreted Protease nor Protease in Shed Membrane Vesicles, Disrupts Epithelial Cell-to-Cell Junctions. Cells 2024, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Sharafutdinov, I.; Linz, B.; Tegtmeyer, N.; Backert, S. Therapeutic and Protective Approaches to Combat Campylobacter jejuni Infections. Front. Pharmacol. 2025, 16, 1572616. [Google Scholar] [CrossRef] [PubMed]
- Čerňáková, M.; Košťálová, D. Antimicrobial Activity of Berberine—A Constituent of Mahonia Aquifolium. Folia Microbiol. 2002, 47, 375–378. [Google Scholar] [CrossRef]
- Obiang-Obounou, B.W.; Kang, O.H.; Choi, J.G.; Keum, J.H.; Kim, S.B.; Mun, S.H.; Shin, D.W.; Kim, K.W.; Park, C.B.; Kim, Y.G.; et al. The Mechanism of Action of Sanguinarine against Methicillin-Resistant Staphylococcus Aureus. J. Toxicol. Sci. 2011, 36, 277–283. [Google Scholar] [CrossRef]
- Assaf, A.M.; Haddadin, R.N.; Aldouri, N.A.; Alabbassi, R.; Mashallah, S.; Mohammad, M.; Bustanji, Y. Anti-Cancer, Anti-Inflammatory and Anti-Microbial Activities of Plant Extracts Used against Hematological Tumors in Traditional Medicine of Jordan. J. Ethnopharmacol. 2013, 145, 728–736. [Google Scholar] [CrossRef]
- Li, X.L.; Sun, Y.P.; Wang, M.; Wang, Z.B.; Kuang, H.X. Alkaloids in Chelidonium majus L.: A Review of Its Phytochemistry, Pharmacology and Toxicology. Front. Pharmacol. 2024, 15, 1440979. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Inhibition of Biofilm Formation and Quorum Sensing Mediated Phenotypes by Berberine in Pseudomonas Aeruginosa and Salmonella Typhimurium. RSC Adv. 2018, 8, 36133–36141. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, W.; Liu, M.; Zhang, J.; Yang, M.; Wang, T.; Qian, W. In Vitro Anti-Biofilm Efficacy of Sanguinarine against Carbapenem-Resistant Serratia Marcescens. Biofouling 2021, 37, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G. Antibiofilm and Antimicrobial-Enhancing Activity of Chelidonium Majus and Corydalis Cheilanthifolia Extracts. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef]
- Qian, W.; Huang, J.; Zhang, J.; Li, X.; Kong, Y.; Wang, T.; Li, Y. Antimicrobial and Antibiofilm Activities and Mechanism of Action of Chelerythrine Against Carbapenem-Resistant Serratia Marcescens In Vitro. Microb. Drug Resist. 2021, 27, 1105–1116. [Google Scholar] [CrossRef]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The Anti-Inflammatory Potential of Berberine in Vitro and in Vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef]
- Niu, X.; Fan, T.; Li, W.; Xing, W.; Huang, H. The Anti-Inflammatory Effects of Sanguinarine and Its Modulation of Inflammatory Mediators from Peritoneal Macrophages. Eur. J. Pharmacol. 2012, 689, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Hao, D.J.; Fan, T.; Huang, H.M.; Yao, H.; Niu, X.F. Protective Effect of Chelerythrine against Ethanol-Induced Gastric Ulcer in Mice. Chem. Biol. Interact. 2014, 208, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, P.L.; Kêdzia, B.; Marcin, O.; Kujawski, R.; Bogacz, A.; Bartkowiak-Wieczorek, J.; Bialas, W.; Gryszczyñska, A.; Buchwald, W.; Szulc, M.; et al. Evaluation of Anti-Inflammatory and Analgesic Activities of Extracts from Herb of Chelidonium majus L. Cent. Eur. J. Immunol. 2015, 40, 400–410. [Google Scholar] [CrossRef]
- Xu, F.; Liu, M.; Liao, Y.; Zhou, Y.; Zhang, P.; Zeng, Y.; Liu, Z. Improvement of Anticancer Effect of Berberine by Salt Formation Modifications. Phytomedicine 2022, 104, 154314. [Google Scholar] [CrossRef] [PubMed]
- Achkar, I.W.; Mraiche, F.; Mohammad, R.M.; Uddin, S. Anticancer Potential of Sanguinarine for Various Human Malignancies. Future Med. Chem. 2017, 9, 933–950. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Y.; Zhang, G.; Wu, Y.; Zhong, W.; Chu, C.; Qian, Y.; Zhu, G. Chelerythrine Inhibits Human Hepatocellular Carcinoma Metastasis in Vitro. Biol. Pharm. Bull. 2018, 41, 36–46. [Google Scholar] [CrossRef]
- Deljanin, M.; Nikolic, M.; Baskic, D.; Todorovic, D.; Djurdjevic, P.; Zaric, M.; Stankovic, M.; Todorovic, M.; Avramovic, D.; Popovic, S. Chelidonium Majus Crude Extract Inhibits Migration and Induces Cell Cycle Arrest and Apoptosis in Tumor Cell Lines. J. Ethnopharmacol. 2016, 190, 362–371. [Google Scholar] [CrossRef]
- Gu, L.; Li, N.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine Ameliorates Intestinal Epithelial Tight-Junction Damage and down-Regulates Myosin Light Chain Kinase Pathways in a Mouse Model of Endotoxinemia. J. Infect. Dis. 2011, 203, 1602–1612. [Google Scholar] [CrossRef]
- Li, N.; Gu, L.; Qu, L.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine Attenuates Pro-Inflammatory Cytokine-Induced Tight Junction Disruption in an in Vitro Model of Intestinal Epithelial Cells. Eur. J. Pharm. Sci. 2010, 40, 1–8. [Google Scholar] [CrossRef]
- Peng, J.; Li, H.; Olaolu, O.A.; Ibrahim, S.; Ibrahim, S.; Wang, S. Natural Products: A Dependable Source of Therapeutic Alternatives for Inflammatory Bowel Disease through Regulation of Tight Junctions. Molecules 2023, 28, 6293. [Google Scholar] [CrossRef]
- Cao, M.; Wang, P.; Sun, C.; He, W.; Wang, F. Amelioration of IFN-γ and TNF-α-Induced Intestinal Epithelial Barrier Dysfunction by Berberine via Suppression of MLCK-MLC Phosphorylation Signaling Pathway. PLoS ONE 2013, 8, e61944. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5154, Sanguinarine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/sanguinarine (accessed on 24 October 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2703, Chelerythrine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/chelerythrine (accessed on 24 October 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2353, Berberine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/berberine (accessed on 24 October 2025).
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Neurol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Hacker, K. The Burden of Chronic Disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar] [CrossRef]
- Watson, K.; Wiltz, J.; Nhim, K.; Kaufmann, R.; Thomas, C.; Greenlund, K. Trends in Multiple Chronic Conditions Among US Adults, By Life Stage, Behavioral Risk Factor Surveillance System, 2013–2023. Prev. Chronic. Dis. 2025, 22, E15. [Google Scholar] [CrossRef]
- Benavidez, G.; Zahnd, W.; Hung, P.; Eberth, J. Chronic Disease Prevalence in the US: Sociodemographic and Geographic Variations by Zip Code Tabulation Area. Prev. Chronic. Dis. 2024, 21, E14. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.H.S. Integration of Herbal Medicine into Modern Medical Practices: Issues and Prospects. Integr. Cancer Ther. 2002, 1, 287–293. [Google Scholar] [CrossRef]
- Thamizhoviya, G. Mini Review Global Integration of Traditional and Modern Medicine: Policy Developments, Regulatory Frameworks, and Clinical Integration Model. Future Integr. Med. 2025, 4, 180–190. [Google Scholar] [CrossRef]
- Li, Z.; Geng, Y.-N.; Jiang, J.-D.; Kong, W.-J. Antioxidant and Anti-Inflammatory Activities of Berberine in the Treatment of Diabetes Mellitus. Evid. Based Complement. Alternat. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef]
- Cheng, Z.; Kang, C.; Che, S.; Su, J.; Sun, Q.; Ge, T.; Guo, Y.; Lv, J.; Sun, Z.; Yang, W.; et al. Berberine: A Promising Treatment for Neurodegenerative Diseases. Front. Pharmacol. 2022, 13, 845591. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Gilani, A.U.H.; Abdollahi, M.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Berberine and Neurodegeneration: A Review of Literature. Pharmacol. Rep. 2015, 67, 970–979. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Viscardi, S.; Szewczyk, W.; Topola, E. Natural Alkaloids in Cancer Therapy: Berberine, Sanguinarine and Chelerythrine against Colorectal and Gastric Cancer. Int. J. Mol. Sci. 2024, 25, 8375. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Lipska, P.; Viscardi, S.; Bazan, H.; Sobieraj, J. Targeting Skin Neoplasms: A Review of Berberine’s Anticancer Properties. Cells 2025, 14, 1041. [Google Scholar] [CrossRef]
- Pacyga, K.; Pacyga, P.; Topola, E.; Viscardi, S.; Duda-Madej, A. Bioactive Compounds from Plant Origin as Natural Antimicrobial Agents for the Treatment of Wound Infections. Int. J. Mol. Sci. 2024, 25, 2100. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, K.; Hong, Y.; Gong, Y.; Ni, J.; Yang, N.; Ding, W. A New Perspective on the Antimicrobial Mechanism of Berberine Hydrochloride Against Staphylococcus Aureus Revealed by Untargeted Metabolomic Studies. Front. Microbiol. 2022, 13, 917414. [Google Scholar] [CrossRef]
- Fukamachi, H.; Matsumoto, C.; Omiya, Y.; Arimoto, T.; Morisaki, H.; Kataoka, H.; Kadena, M.; Funatsu, T.; Fukutake, M.; Kase, Y.; et al. Effects of Hangeshashinto on Growth of Oral Microorganisms. Evid.-Based Complement. Altern. Med. 2015, 2015, 512947. [Google Scholar] [CrossRef]
- Grari, O.; Ezrari, S.; El Yandouzi, I.; Benaissa, E.; Ben Lahlou, Y.; Lahmer, M.; Saddari, A.; Elouennass, M.; Maleb, A. A Comprehensive Review on Biofilm-Associated Infections: Mechanisms, Diagnostic Challenges, and Innovative Therapeutic Strategies. Microbe 2025, 8, 100436. [Google Scholar] [CrossRef]
- Krzyżek, P. What Is a Biofilm? Lessons Learned from Interactions with Immune Cells. Int. J. Mol. Sci. 2024, 25, 11684. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Feng, J.; Zhang, J.; Lu, X. Campylobacter Biofilms. Microbiol. Res. 2022, 264, 127149. [Google Scholar] [CrossRef] [PubMed]
- Elgamoudi, B.A.; Korolik, V. Campylobacter Biofilms: Potential of Natural Compounds to Disrupt Campylobacter jejuni Transmission. Int. J. Mol. Sci. 2021, 22, 12159. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Viscardi, S.; Bazan, H.; Sobieraj, J. Exploring the Role of Berberine as a Molecular Disruptor in Antimicrobial Strategies. Pharmaceuticals 2025, 18, 947. [Google Scholar] [CrossRef]
- Veronese, P.; Dodi, I. Campylobacter jejuni/Coli Infection: Is It Still a Concern? Microorganisms 2024, 12, 2669. [Google Scholar] [CrossRef]
- Mills, D.C.; Gundogdu, O.; Elmi, A.; Bajaj-Elliott, M.; Taylor, P.W.; Wren, B.W.; Dorrell, N. Increase in Campylobacter jejuni Invasion of Intestinal Epithelial Cells under Low-Oxygen Coculture Conditions That Reflect the in Vivo Environment. Infect. Immun. 2012, 80, 1690–1698. [Google Scholar] [CrossRef]
- Watson, R.O.; Galán, J.E. Campylobacter jejuni Survives within Epithelial Cells by Avoiding Delivery to Lysosomes. PLoS Pathog. 2008, 4, e14. [Google Scholar] [CrossRef]
- Boehm, M.; Haenel, I.; Hoy, B.; Brøndsted, L.; Smith, T.G.; Hoover, T.; Wessler, S.; Tegtmeyer, N. Extracellular Secretion of Protease HtrA from Campylobacter jejuni Is Highly Efficient and Independent of Its Protease Activity and Flagellum. Eur. J. Microbiol. Immunol. 2013, 3, 163–173. [Google Scholar] [CrossRef]
- Boehm, M.; Lind, J.; Backert, S.; Tegtmeyer, N. Campylobacter jejuni Serine Protease HtrA Plays an Important Role in Heat Tolerance, Oxygen Resistance, Host Cell Adhesion, Invasion, and Transmigration. Eur. J. Microbiol. Immunol. 2015, 5, 68–80. [Google Scholar] [CrossRef]
- Lobo de Sá, F.D.; Heimesaat, M.M.; Bereswill, S.; Nattramilarasu, P.K.; Schulzke, J.D.; Bücker, R. Resveratrol Prevents Campylobacter jejuni—Induced Leaky Gut by Restoring Occludin and Claudin-5 in the Paracellular Leak Pathway. Front. Pharmacol. 2021, 12, 640572. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, H.; Pinjari, J.; Honrao, P.; Praband, S.; Somani, R. The Impact of Permeability Enhancers on Assessment for Monolayer of Colon Adenocarcinoma Cell Line (Caco-2) Used in in Vitro Permeability Assay. J. Drug Deliv. Ther. 2013, 3, 20–29. [Google Scholar] [CrossRef]
- Awad, W.A.; Ruhnau, D.; Hess, C.; Hess, M. Campylobacter jejuni Increases the Paracellular Permeability of Broiler Chickens in a Dose-Dependent Manner. Poult. Sci. 2020, 99, 5407–5414. [Google Scholar] [CrossRef]
- Awad, W.A.; Molnár, A.; Aschenbach, J.R.; Ghareeb, K.; Khayal, B.; Hess, C.; Liebhart, D.; Dublecz, K.; Hess, M. Campylobacter Infection in Chickens Modulates the Intestinal Epithelial Barrier Function. Innate Immun. 2015, 21, 151–160. [Google Scholar] [CrossRef]
- Beltinger, J.; del Buono, J.; Skelly, M.M.; Thornley, J.; Spiller, R.C.; Stack, W.A.; Hawkey, C.J. Disruption of Colonic Barrier Function and Induction of Mediator Release by Strains of Campylobacter jejuni That Invade Epithelial Cells. World J. Gastroenterol. 2008, 14, 7345–7352. [Google Scholar] [CrossRef]
- Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 12 May 2025).
- Zhang, L.; Wu, X.; Yang, R.; Chen, F.; Liao, Y.; Zhu, Z.; Wu, Z.; Sun, X.; Wang, L. Effects of Berberine on the Gastrointestinal Microbiota. Front. Cell. Infect. Microbiol. 2021, 10, 588517. [Google Scholar] [CrossRef]
- Tang, M.; Yuan, D.; Liao, P. Berberine Improves Intestinal Barrier Function and Reduces Inflammation, Immunosuppression, and Oxidative Stress by Regulating the NF-ΚB/MAPK Signaling Pathway in Deoxynivalenol-Challenged Piglets. Environ. Pollut. 2021, 289, 117865. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Wang, X.M.; Jiang, T.; Gong, J.F.; Niu, L.Y.; Li, N. Berberine Prevents Intestinal Mucosal Barrier Damage during Early Phase of Sepsis in Rat through the Toll-like Receptors Signaling Pathway. Korean J. Physiol. Pharmacol. 2015, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Kiani, S.; Rahimi, G.; Boskabady, M.H. Anti-Inflammatory, Antioxidant, and Immunomodulatory Effects of Berberis Vulgaris and Its Constituent Berberine, Experimental and Clinical, a Review. Phyther. Res. 2024, 38, 1882–1902. [Google Scholar] [CrossRef]
- Jael Teresa de Jesús, Q.V.; Gálvez-Ruíz, J.C.; Márquez Ibarra, A.A.; Leyva-Peralta, M.A. Perspectives on Berberine and the Regulation of Gut Microbiota: As an Anti-Inflammatory Agent. Pharmaceuticals 2025, 18, 193. [Google Scholar] [CrossRef]
- Wolf, P.G.; Devendran, S.; Doden, H.L.; Ly, L.K.; Moore, T.; Takei, H.; Nittono, H.; Murai, T.; Kurosawa, T.; Chlipala, G.E.; et al. Berberine Alters Gut Microbial Function through Modulation of Bile Acids. BMC Microbiol. 2021, 21, 24. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.C.; Huang, W.H.; Selwyn, F.P.; Klaassen, C.D. Dose-Response Effect of Berberine on Bile Acid Profile and Gut Microbiota in Mice. BMC Complement. Altern. Med. 2016, 16, 394. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cagliero, C.; Guo, B.; Barton, Y.; Maurel, M.; Payot, S.; Zhang, Q. Bile Salts Modulate Expression of the CmeABC Multidrug Efflux Pump in Campylobacter jejuni. J. Bacteriol. 2005, 187, 7417–7424. [Google Scholar] [CrossRef]
- Gillard, J.; Leclercq, I.A. Biological Tuners to Reshape the Bile Acid Pool for Therapeutic Purposes in Non-Alcoholic Fatty Liver Disease. Clin. Sci. 2023, 137, 65–85. [Google Scholar] [CrossRef]
- Sun, R.; Yang, N.; Kong, B.; Cao, B.; Feng, D.; Yu, X.; Ge, C.; Huang, J.; Shen, J.; Wang, P.; et al. Orally Administered Berberine Modulates Hepatic Lipid Metabolism by Altering Microbial Bile Acid Metabolism and the Intestinal FXR Signaling Pathway. Mol. Pharmacol. 2017, 91, 110–122. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Tan, S.; Chen, Z.; Wu, B.; Wu, X. Therapeutic Effects of Berberine on Liver Fibrosis Are Associated With Lipid Metabolism and Intestinal Flora. Front. Pharmacol. 2022, 13, 814871. [Google Scholar] [CrossRef]
- Guo, Q.; Lu, T.; Zhang, M.; Wang, Q.; Zhao, M.; Wang, T.; Du, M. Protective Effect of Berberine on Acute Gastric Ulcer by Promotion of Tricarboxylic Acid Cycle-Mediated Arachidonic Acid Metabolism. J. Inflamm. Res. 2024, 17, 15–28. [Google Scholar] [CrossRef]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of Berberine and Its Main Metabolites in Conventional and Pseudo Germ-Free Rats Determined by Liquid Chromatography/Ion Trap Mass Spectrometry. Drug Metab. Dispos. 2006, 34, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Allijn, I.E.; Czarny, B.M.S.; Wang, X.; Chong, S.Y.; Weiler, M.; da Silva, A.E.; Metselaar, J.M.; Lam, C.S.P.; Pastorin, G.; de Kleijn, D.P.V.; et al. Liposome Encapsulated Berberine Treatment Attenuates Cardiac Dysfunction after Myocardial Infarction. J. Control. Release 2017, 247, 127–133. [Google Scholar] [CrossRef]
- Guo, H.H.; Feng, C.L.; Zhang, W.X.; Luo, Z.G.; Zhang, H.J.; Zhang, T.T.; Ma, C.; Zhan, Y.; Li, R.; Wu, S.; et al. Liver-Target Nanotechnology Facilitates Berberine to Ameliorate Cardio-Metabolic Diseases. Nat. Commun. 2019, 10, 1981. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; De Feo, V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int. J. Mol. Sci. 2020, 21, 2217. [Google Scholar] [CrossRef]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef]
- Luo, G.; Lin, X.; Li, Z.; Xiao, M.; Li, X.; Zhang, D.; Xiang, H. Structure-Guided Modification of Isoxazole-Type FXR Agonists: Identification of a Potent and Orally Bioavailable FXR Modulator. Eur. J. Med. Chem. 2021, 209, 112910. [Google Scholar] [CrossRef]
- Andima, M.; Boese, A.; Paul, P.; Koch, M.; Loretz, B.; Lehr, C.-M. Targeting Intracellular Bacteria with Dual Drug-Loaded Lactoferrin Nanoparticles. ACS Infect. Dis. 2024, 10, 1696–1710. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/Body-on-a-Chip Based on Microfluidic Technology for Drug Discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Siramshetty, V.B.; Drwal, M.N.; Preissner, R. Computational Methods for Prediction of in Vitro Effects of New Chemical Structures. J. Cheminform. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Ganot, N.; Meker, S.; Reytman, L.; Tzubery, A.; Tshuva, E.Y. Anticancer Metal Complexes: Synthesis and Cytotoxicity Evaluation by the MTT Assay. J. Vis. Exp. 2013, 1–6, 50767. [Google Scholar] [CrossRef]
- Pires, C.L.; Praça, C.; Martins, P.A.T.; Batista de Carvalho, A.L.M.; Ferreira, L.; Marques, M.P.M.; Moreno, M.J. Re-Use of Caco-2 Monolayers in Permeability Assays—Validation Regarding Cell Monolayer Integrity. Pharmaceutics 2021, 13, 1563. [Google Scholar] [CrossRef]

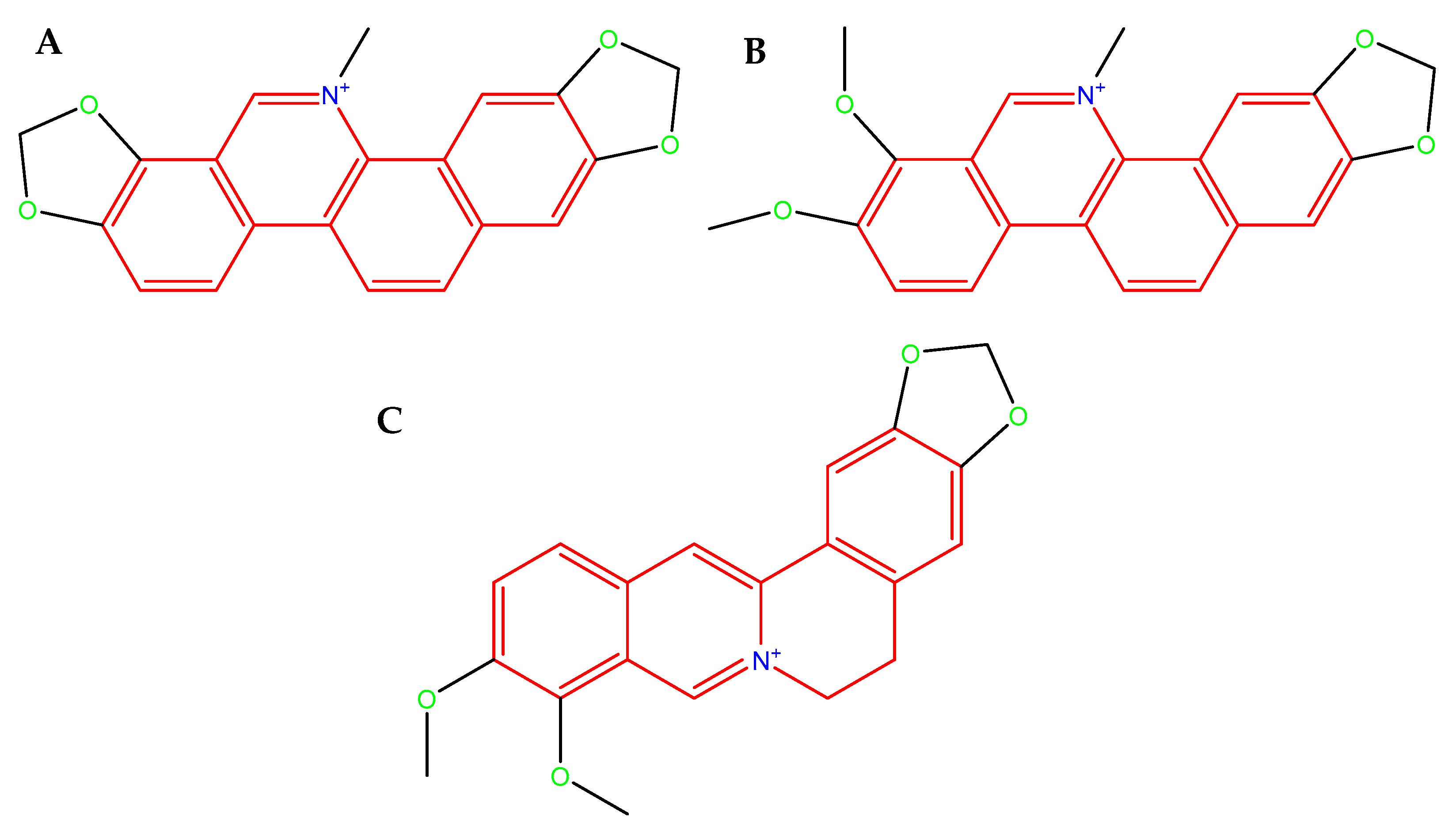






| Condition | MIC [µg/mL] | ||
|---|---|---|---|
| CHE | BBR | SAN | |
| BHI | 32 | 32 | 8 |
| BHI5 | 32 | 64 | 16 |
| ACF10 | 32 | 64 | 16 |
| selected MIC | 32 | 64 | 16 |
| Conditions | Size of Aggregates [%] | |||
|---|---|---|---|---|
| Small | Medium | Big | Large | |
| E/Amix | 40.8 | 46.5 | 9.0 | 3.7 |
| E/Amix + BBR | 87.2 | 12.8 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda-Madej, A.; Gagat, P.; Wiśniewski, J.; Viscardi, S.; Krzyżek, P. Impact of Isoquinoline Alkaloids on the Intestinal Barrier in a Colonic Model of Campylobacter jejuni Infection. Int. J. Mol. Sci. 2025, 26, 10634. https://doi.org/10.3390/ijms262110634
Duda-Madej A, Gagat P, Wiśniewski J, Viscardi S, Krzyżek P. Impact of Isoquinoline Alkaloids on the Intestinal Barrier in a Colonic Model of Campylobacter jejuni Infection. International Journal of Molecular Sciences. 2025; 26(21):10634. https://doi.org/10.3390/ijms262110634
Chicago/Turabian StyleDuda-Madej, Anna, Przemysław Gagat, Jerzy Wiśniewski, Szymon Viscardi, and Paweł Krzyżek. 2025. "Impact of Isoquinoline Alkaloids on the Intestinal Barrier in a Colonic Model of Campylobacter jejuni Infection" International Journal of Molecular Sciences 26, no. 21: 10634. https://doi.org/10.3390/ijms262110634
APA StyleDuda-Madej, A., Gagat, P., Wiśniewski, J., Viscardi, S., & Krzyżek, P. (2025). Impact of Isoquinoline Alkaloids on the Intestinal Barrier in a Colonic Model of Campylobacter jejuni Infection. International Journal of Molecular Sciences, 26(21), 10634. https://doi.org/10.3390/ijms262110634







