Endothelial Dysfunction and Hemostatic System Activation in Relation to Shift Workers, Social Jetlag, and Chronotype in Female Nurses
Abstract
1. Introduction
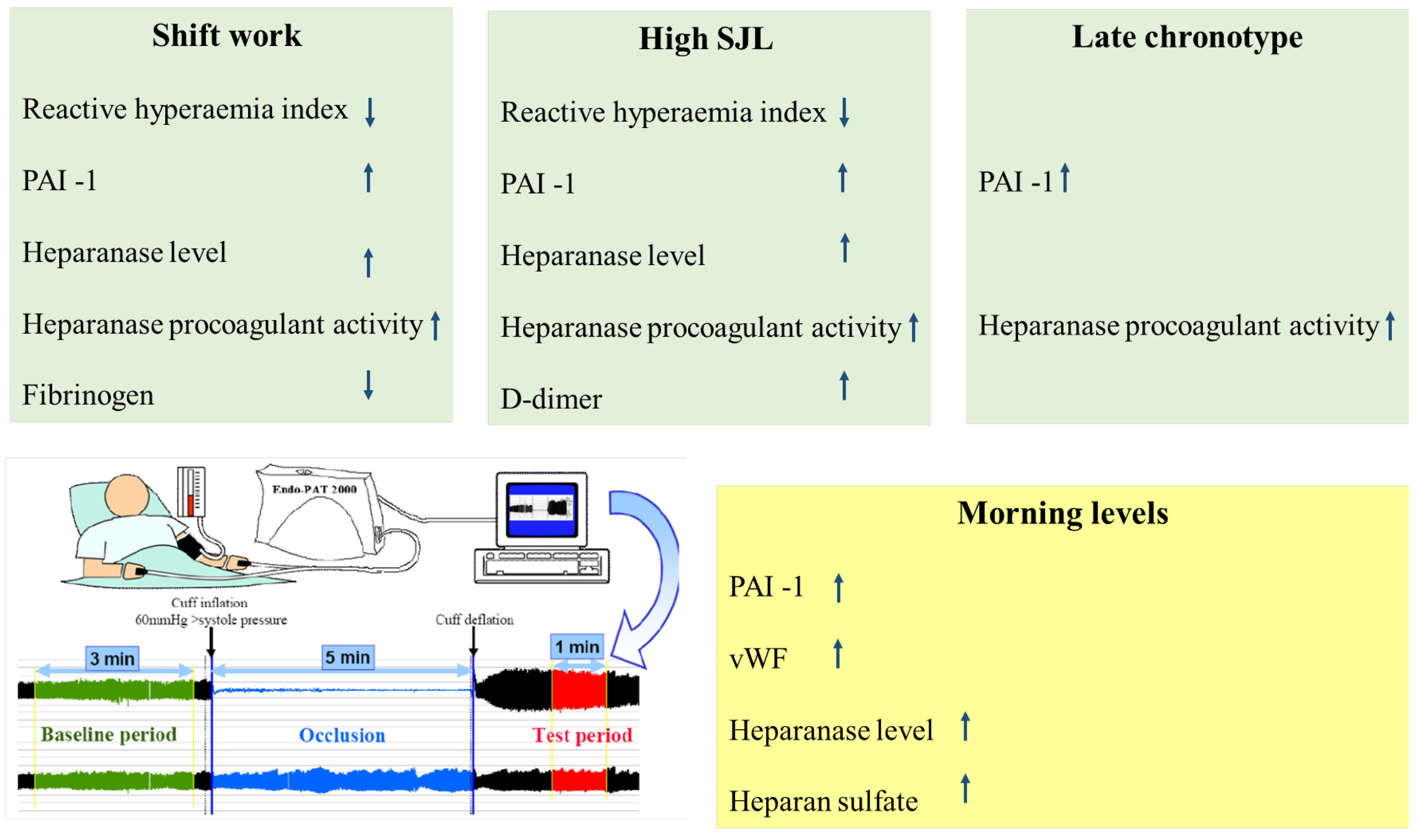
2. Results
3. Discussion
4. Material and Methods
4.1. Study Group
4.2. Reagents and Antibodies
4.3. Endothelial Function Assessment
4.4. Blood Coagulation Parameters
4.5. Heparanase Procoagulant Activity Assay
4.6. Heparanase ELISA
4.7. Heparan Sulfate Chromogenic Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Skogstad, M.; Mamen, A.; Lunde, L.K.; Ulvestad, B.; Matre, D.; Aass, H.C.D.; Ovstebo, R.; Nielsen, P.; Samuelsen, K.N.; Skare, O.; et al. Shift Work Including Night Work and Long Working Hours in Industrial Plants Increases the Risk of Atherosclerosis. Int. J. Environ. Res. Public Health 2019, 16, 521. [Google Scholar] [CrossRef] [PubMed]
- Vetter, C.; Devore, E.E.; Wegrzyn, L.R.; Massa, J.; Speizer, F.E.; Kawachi, I.; Rosner, B.; Stampfer, M.J.; Schernhammer, E.S. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 2016, 315, 1726–1734. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Q.; Fang, B.; Su, Y.; Lu, W.; Liu, M.; Li, X.; Liu, J.; He, L. Shift work is associated with an increased risk of type 2 diabetes and elevated RBP4 level: Cross sectional analysis from the OHSPIW cohort study. BMC Public Health 2023, 23, 1139. [Google Scholar] [CrossRef]
- Manfredini, R.; Portaluppi, F. Night shift and impaired endothelial function: Circadian out-of-synch may play a role. Int. J. Cardiol. 2012, 154, 94–95. [Google Scholar] [CrossRef]
- Charles, L.E.; Zhao, S.; Fekedulegn, D.; Violanti, J.M.; Andrew, M.E.; Burchfiel, C.M. Shiftwork and decline in endothelial function among police officers. Am. J. Ind. Med. 2016, 59, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Pilz, L.K.; Zerbini, G.; Winnebeck, E.C. Chronotype and Social Jetlag: A (Self-) Critical Review. Biology 2019, 8, 54. [Google Scholar] [CrossRef]
- Makarem, N.; Paul, J.; Giardina, E.V.; Liao, M.; Aggarwal, B. Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiol. Int. 2020, 37, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Merikanto, I.; Lahti, T.; Puolijoki, H.; Vanhala, M.; Peltonen, M.; Laatikainen, T.; Vartiainen, E.; Salomaa, V.; Kronholm, E.; Partonen, T. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol. Int. 2013, 30, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Gamboa Madeira, S.; Reis, C.; Paiva, T.; Moreira, C.S.; Nogueira, P.; Roenneberg, T. Social jetlag, a novel predictor for high cardiovascular risk in blue-collar workers following permanent atypical work schedules. J. Sleep Res. 2021, 30, e13380. [Google Scholar] [CrossRef]
- Parish, C.R.; Freeman, C.; Hulett, M.D. Heparanase: A key enzyme involved in cell invasion. Biochim. Biophys. Acta 2001, 1471, M99–M108. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Friedmann, Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J. Clin. Investig. 2001, 108, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Nadir, Y.; Brenner, B.; Zetser, A.; Ilan, N.; Shafat, I.; Zcharia, E.; Goldshmidt, O.; Vlodavsky, I. Heparanase induces tissue factor expression in vascular endothelial and cancer cells. J. Thromb. Haemost. 2006, 4, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Nadir, Y.; Brenner, B.; Gingis-Velitski, S.; Levy-Adam, F.; Ilan, N.; Zcharia, E.; Nadir, E.; Vlodavsky, I. Heparanase induces tissue factor pathway inhibitor expression and extracellular accumulation in endothelial and tumor cells. Thromb. Haemost. 2008, 99, 133–141. [Google Scholar] [CrossRef]
- Nadir, Y.; Brenner, B.; Fux, L.; Shafat, I.; Attias, J.; Vlodavsky, I. Heparanase enhances the generation of activated factor X in the presence of tissue factor and activated factor VII. Haematologica 2010, 95, 1927–1934. [Google Scholar] [CrossRef]
- Axelman, E.; Henig, I.; Crispel, Y.; Attias, J.; Li, J.P.; Brenner, B.; Vlodavsky, I.; Nadir, Y. Novel peptides that inhibit heparanase activation of the coagulation system. Thromb. Haemost. 2014, 112, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Nadir, Y. Decreasing Tumor Growth and Angiogenesis by Inhibition of Coagulation. Semin. Thromb. Hemost. 2019, 45, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.B.; Gibson, W.J.; Kolachalama, V.B.; Golomb, M.; Indolfi, L.; Spruell, C.; Zcharia, E.; Vlodavsky, I.; Edelman, E.R. Heparanase regulates thrombosis in vascular injury and stent-induced flow disturbance. J. Am. Coll. Cardiol. 2012, 59, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Nadir, Y.; Saharov, G.; Hoffman, R.; Keren-Politansky, A.; Tzoran, I.; Brenner, B.; Shochat, T. Heparanase procoagulant activity, factor Xa, and plasminogen activator inhibitor 1 are increased in shift work female nurses. Ann. Hematol. 2015, 94, 1213–1219. [Google Scholar] [CrossRef]
- Turek, S.; Rudan, I.; Smolej-Narancic, N.; Szirovicza, L.; Cubrilo-Turek, M.; Zerjavic-Hrabak, V.; Rak-Kaic, A.; Vrhovski-Hebrang, D.; Prebeg, Z.; Ljubicic, M.; et al. A large cross-sectional study of health attitudes, knowledge, behaviour and risks in the post-war Croatian population (the First Croatian Health Project). Coll. Antropol. 2001, 25, 77–96. [Google Scholar]
- Yamamoto, K.; Takeshita, K.; Kojima, T.; Takamatsu, J.; Saito, H. Aging and plasminogen activator inhibitor-1 (PAI-1) regulation: Implication in the pathogenesis of thrombotic disorders in the elderly. Cardiovasc. Res. 2005, 66, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Tofler, G.H.; Massaro, J.; O’Donnell, C.J.; Wilson, P.W.F.; Vasan, R.S.; Sutherland, P.A.; Meigs, J.B.; Levy, D.; D’Agostino, R.B. Plasminogen activator inhibitor and the risk of cardiovascular disease: The Framingham Heart Study. Thromb. Res. 2016, 140, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Matan, M.; Axelman, E.; Brenner, B.; Nadir, Y. Heparanase procoagulant activity is elevated in women using oral contraceptives. Hum. Reprod. 2013, 28, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Nadir, Y.; Kenig, Y.; Drugan, A.; Shafat, I.; Brenner, B. An assay to evaluate heparanase procoagulant activity. Thromb. Res. 2011, 128, e3–e8. [Google Scholar] [CrossRef]
- Peled, E.; Rovitsky, A.; Axelman, E.; Norman, D.; Brenner, B.; Nadir, Y. Increased heparanase level and procoagulant activity in orthopedic surgery patients receiving prophylactic dose of enoxaparin. Thromb. Res. 2012, 130, 129–134. [Google Scholar] [CrossRef]
- Peled, E.; Melamed, E.; Portal, T.B.; Axelman, E.; Norman, D.; Brenner, B.; Nadir, Y. Heparanase procoagulant activity as a predictor of wound necrosis following diabetic foot amputation. Thromb. Res. 2016, 139, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Nadir, Y.; Sarig, G.; Axelman, E.; Meir, A.; Wollner, M.; Shafat, I.; Hoffman, R.; Brenner, B.; Vlodavsky, I.; Haim, N. Heparanase procoagulant activity is elevated and predicts survival in non-small cell lung cancer patients. Thromb. Res. 2014, 134, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Atik, A.; Yu, H.; Li, T.; Zhang, B.; Li, D.; Cai, N.; Yu, Y.; Chen, J.; Li, G.; et al. Serum heparanase concentration and heparanase activity in patients with retinal vein occlusion. Acta Ophthalmol. 2017, 95, e62–e66. [Google Scholar] [CrossRef][Green Version]
- Bayam, E.; Kalcik, M.; Gurbuz, A.S.; Yesin, M.; Guner, A.; Gunduz, S.; Gursoy, M.O.; Karakoyun, S.; Cersit, S.; Kilicgedik, A.; et al. The relationship between heparanase levels, thrombus burden and thromboembolism in patients receiving unfractionated heparin treatment for prosthetic valve thrombosis. Thromb. Res. 2018, 171, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I.; Friedmann, Y.; Elkin, M.; Aingorn, H.; Atzmon, R.; Ishai-Michaeli, R.; Bitan, M.; Pappo, O.; Peretz, T.; Michal, I.; et al. Mammalian heparanase: Gene cloning, expression and function in tumor progression and metastasis. Nat. Med. 1999, 5, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Digre, A.; Singh, K.; Abrink, M.; Reijmers, R.M.; Sandler, S.; Vlodavsky, I.; Li, J.P. Overexpression of heparanase enhances T lymphocyte activities and intensifies the inflammatory response in a model of murine rheumatoid arthritis. Sci. Rep. 2017, 7, 46229. [Google Scholar] [CrossRef] [PubMed]
- Meirovitz, A.; Goldberg, R.; Binder, A.; Rubinstein, A.M.; Hermano, E.; Elkin, M. Heparanase in inflammation and inflammation-associated cancer. FEBS J. 2013, 280, 2307–2319. [Google Scholar] [CrossRef]
- Velazquez-Kronen, R.; MacDonald, L.A.; Akinyemiju, T.F.; Cushman, M.; Howard, V.J. Shiftwork, long working hours and markers of inflammation in a national US population-based sample of employed black and white men and women aged >/=45 years. Occup. Environ. Med. 2023, 80, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Fukuda, Y.; Yokoo, T.; Yamaoka, K. Interleukin-6 Level among Shift and Night Workers in Japan: Cross-Sectional Analysis of the J-HOPE Study. J. Atheroscler. Thromb. 2018, 25, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Matre, D.; Christensen, J.O.; Mork, P.J.; Ferreira, P.; Sand, T.; Nilsen, K.B. Shift work, inflammation and musculoskeletal pain-The HUNT Study. Occup. Med. 2021, 71, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Lingas, E.C. A Narrative Review of the Carcinogenic Effect of Night Shift and the Potential Protective Role of Melatonin. Cureus 2023, 15, e43326. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jang, S.J. Social jetlag and depression in female rotating-shift nurses: A secondary analysis. Perspect. Psychiatr. Care 2022, 58, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Petruso, F.; Giff, A.E.; Milano, B.A.; De Rossi, M.M.; Saccaro, L.F. Inflammation and emotion regulation: A narrative review of evidence and mechanisms in emotion dysregulation disorders. Neuronal Signal. 2023, 7, NS20220077. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, F.; Davies, G.J.; Hackett, D.R.; Khan, M.I.; De Bart, A.C.; Aber, V.R.; Maseri, A.; Kluft, C. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am. J. Cardiol. 1988, 62, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Angleton, P.; Chandler, W.L.; Schmer, G. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1). Circulation 1989, 79, 101–106. [Google Scholar] [CrossRef]
- Bridges, A.B.; McLaren, M.; Scott, N.A.; Pringle, T.H.; McNeill, G.P.; Belch, J.J. Circadian variation of tissue plasminogen activator and its inhibitor, von Willebrand factor antigen, and prostacyclin stimulating factor in men with ischaemic heart disease. Br. Heart J. 1993, 69, 121–124. [Google Scholar] [CrossRef][Green Version]
- Klimes-Dougan, B.; Hastings, P.D.; Granger, D.A.; Usher, B.A.; Zahn-Waxler, C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev. Psychopathol. 2001, 13, 695–719. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.A.; Shirtcliff, E.A.; Zahn-Waxler, C.; Usher, B.; Klimes-Dougan, B.; Hastings, P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: Individual differences and developmental effects. Dev. Psychopathol. 2003, 15, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Induruwa, I.; Moroi, M.; Bonna, A.; Malcor, J.D.; Howes, J.M.; Warburton, E.A.; Farndale, R.W.; Jung, S.M. Platelet collagen receptor Glycoprotein VI-dimer recognizes fibrinogen and fibrin through their D-domains, contributing to platelet adhesion and activation during thrombus formation. J. Thromb. Haemost. 2018, 16, 389–404. [Google Scholar] [CrossRef]
- Yeung, C.C.; Svensson, R.B.; Yurchenko, K.; Malmgaard-Clausen, N.M.; Tryggedsson, I.; Lendal, M.; Jokipii-Utzon, A.; Olesen, J.L.; Lu, Y.; Kadler, K.E.; et al. Disruption of day-to-night changes in circadian gene expression with chronic tendinopathy. J. Physiol. 2024, 602, 6509–6524. [Google Scholar] [CrossRef] [PubMed]
- Ugwoke, C.K.; Sink, Z.; Pusnik, L.; Umek, N. Tendon circadian clock and diurnal regulation of collagen homeostasis as novel aetiological and therapeutic binoculars in chronic tendinopathy. J. Physiol. 2024, 602, 6527–6529. [Google Scholar] [CrossRef]
- Bao, A.M.; Liu, R.Y.; van Someren, E.J.; Hofman, M.A.; Cao, Y.X.; Zhou, J.N. Diurnal rhythm of free estradiol during the menstrual cycle. Eur. J. Endocrinol. 2003, 148, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Santos, C.V.; Vieira Neto, L.; Gadelha, M.R. Adverse effects of glucocorticoids: Coagulopathy. Eur. J. Endocrinol. 2015, 173, M11–M21. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Minnetti, M.; Sbardella, E.; Graziadio, C.; Grossman, A.B. Mechanisms in endocrinology: The spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur. J. Endocrinol. 2015, 173, R101–R113. [Google Scholar] [CrossRef]
- Treger, S.; Ackerman, S.; Kaplan, V.; Ghanem, S.; Nadir, Y. Progestin type affects the increase of heparanase level and procoagulant activity mediated by the estrogen receptor. Hum. Reprod. 2021, 36, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ding, J.; Rao, G.; Shen, J.; Prinz, R.A.; Rana, N.; Dmowski, W.P. Estradiol induces heparanase-1 expression and heparan sulphate proteoglycan degradation in human endometrium. Hum. Reprod. 2007, 22, 927–937. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakao, T.; Yasumoto, A.; Tokuoka, S.; Kita, Y.; Kawahara, T.; Daimon, M.; Yatomi, Y. The impact of night-shift work on platelet function in healthy medical staff. J. Occup. Health 2018, 60, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Juda, M.; Vetter, C.; Roenneberg, T. The Munich ChronoType Questionnaire for Shift-Workers (MCTQShift). J. Biol. Rhythms 2013, 28, 130–140. [Google Scholar] [CrossRef]
- Juda, M.; Vetter, C.; Roenneberg, T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J. Biol. Rhythm 2013, 28, 141–151. [Google Scholar] [CrossRef]
- Hayden, J.; O’Donnell, G.; deLaunois, I.; O’Gorman, C. Endothelial Peripheral Arterial Tonometry (Endo-PAT 2000) use in paediatric patients: A systematic review. BMJ Open 2023, 13, e062098. [Google Scholar] [CrossRef]
- Hansen, A.S.; Butt, J.H.; Holm-Yildiz, S.; Karlsson, W.; Kruuse, C. Validation of Repeated Endothelial Function Measurements Using EndoPAT in Stroke. Front. Neurol. 2017, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Rajai, N.; Toya, T.; Sara, J.D.; Rajotia, A.; Lopez-Jimenez, F.; Lerman, L.O.; Lerman, A. Prognostic value of peripheral endothelial function on major adverse cardiovascular events above traditional risk factors. Eur. J. Prev. Cardiol. 2023, 30, 1781–1788. [Google Scholar] [CrossRef]
- Shafat, I.; Zcharia, E.; Nisman, B.; Nadir, Y.; Nakhoul, F.; Vlodavsky, I.; Ilan, N. An ELISA method for the detection and quantification of human heparanase. Biochem. Biophys. Res. Commun. 2006, 341, 958–963. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hardak, E.; Peled, E.; Crispel, Y.; Ghanem, S.; Attias, J.; Asayag, K.; Kogan, I.; Nadir, Y. Heparan sulfate chains contribute to the anticoagulant milieu in malignant pleural effusion. Thorax 2020, 75, 143–152. [Google Scholar] [CrossRef]
| Day Workers n = 49 | Shift Workers n = 51 | p-Value | |
|---|---|---|---|
| Age (years) | 44.0 ± 4.5 | 35.3 ± 7.5 | 0.001 |
| Total nurse experience (years) | 19.9 ± 6.4 | 10.3 ± 6.7 | 0.001 |
| Experience in current work (years) | 7.5 ± 5.9 | 8.3 ± 6.4 | 0.52 |
| Scope of the job | 0.024 | ||
| 100% | 41 (84%) | 30 (59%) | |
| 88% | 3 (6%) | 8 (16%) | |
| 75% | 5 (10%) | 13 (25%) | |
| Birth control pills use (n) | 6 (12%) | 5 (10%) | 0.76 |
| BMI | 24.2 ± 3.6 | 23.7 ± 3.5 | 0.49 |
| Smoking (n) | 8 (16%) | 5 (10%) | 0.38 |
| Family history of heart disease at young age (n) | 10 (20%) | 8 (16%) | 0.54 |
| Last menstruation date before the first blood test | 0.64 | ||
| no menstruation | 8 (16%) | 4 (8%) | |
| 1–10 days | 13 (27%) | 15 (29%) | |
| 11–20 days | 9 (18%) | 10 (20%) | |
| 21–35 days | 19 (39%) | 22 (43%) | |
| Hours of sitting during working day | 0.001 | ||
| 0–1 | 4 (8%) | 22 (43%) | |
| 1–3 | 30 (61%) | 25 (49%) | |
| 3–6 | 15 (31%) | 4 (8%) |
| Day Workers n = 49 | Shift Workers n = 51 | p Value | |
|---|---|---|---|
| Morning reactive hyperaemia index | 0.67 ± 0.26 | 0.58 ± 0.27 | 0.083 |
| Evening reactive hyperaemia index | 0.71 ± 0.21 | 0.62 ± 0.3 | 0.001 |
| Morning plasmin activator inhibitor-1 (ng/mL) | 13 ± 4.83 | 15.9 ± 9.1 | 0.053 |
| Evening plasmin activator inhibitor-1 | 8.3 ± 3.48 | 11.1 ± 6.48 | 0.009 |
| Morning von Willebrand factor (%) | 136 ± 42 | 126 ± 43 | 0.28 |
| Evening von Willebrand factor | 127 ± 41 | 117 ± 44 | 0.25 |
| Morning heparanase levels (pg/mL) | 2653 ± 1247 | 3258 ± 1516 | 0.03 |
| Evening heparanase levels | 2543 ± 1122 | 3121 ± 1513 | 0.03 |
| Morning heparanase procoagulant activity (ng/mL) | 414 ± 279 | 585 ± 513 | 0.043 |
| Evening heparanase procoagulant activity | 341 ± 259 | 555 ± 626 | 0.029 |
| Morning D-Dimer (ng/mL) | 294 ± 152 | 337 ± 195 | 0.22 |
| Evening D-Dimer | 312 ± 235 | 369 ± 241 | 0.23 |
| Morning fibrinogen (mg/dL) | 252 ± 47 | 227 ± 38 | 0.005 |
| Evening fibrinogen | 247 ± 46 | 228 ± 41 | 0.033 |
| Morning heparan sulfate levels (µg/mL) | 27 ± 25 | 34 ± 32 | 0.3 |
| Evening heparan sulfate levels | 18 ± 16 | 26 ± 24 | 0.28 |
| Low SJL < 1.2 h n = 50 | High SJL ≥ 1.2 h n = 50 | p-Value | |
|---|---|---|---|
| Age (years) | 40.9 ± 6.6 | 35.4 ± 8.3 | 0.099 |
| Total nursing experience (years) | 16.4 ± 7.5 | 13.7 ± 8.6 | 0.098 |
| Experience in current work (years) | 7.6 ± 5.8 | 8.1 ± 6.5 | 0.68 |
| Scope of the job | 0.62 | ||
| 100% | 36 (74%) | 35 (68%) | |
| 88% | 6 (12%) | 5 (10%) | |
| 75% | 7 (14%) | 11 (22%) | |
| Birth control pills use (n) | 3 (6.1%) | 8 (15.7%) | 0.20 |
| BMI | 23.7 ± 3.3 | 24.2 ± 3.8 | 0.48 |
| Smoking (n) | 8 (16.3%) | 5 (9.8%) | 0.38 |
| Family history of heart disease at young age (n) | 9 (18.4%) | 9 (17.6%) | 0.93 |
| Last menstruation date before the first blood test | 0.69 | ||
| No menstruation | 6 (12%) | 6 (12%) | |
| 1–10 days | 15 (31%) | 13 (25.5%) | |
| 11–20 days | 7 (14%) | 12 (23.5%) | |
| 21–35 days | 21 (43%) | 20 (39%) | |
| Hours of sitting during working day | 0.23 | ||
| 0–1 | 9 (18%) | 17 (33%) | |
| 1–3 | 30 (61%) | 25 (49%) | |
| 3–6 | 10 (20%) | 9 (18%) |
| Low SJL < 1.2 h n = 50 | High SJL ≥ 1.2 h n = 50 | p Value | |
|---|---|---|---|
| Morning reactive hyperaemia index | 0.69 ± 0.28 | 0.56 ± 0.23 | 0.014 |
| Evening reactive hyperaemia index | 0.7 ± 0.23 | 0.53 ± 0.28 | 0.002 |
| Morning plasmin activator inhibitor-1 (ng/mL) | 12.3 ± 5.6 | 16.5 ± 8.4 | 0.004 |
| Evening plasmin activator inhibitor-1 | 8.8 ± 5.1 | 10.5 ± 5.4 | 0.12 |
| Morning von Willebrand factor (%) | 124 ± 43 | 136 ± 42 | 0.16 |
| Evening von Willebrand factor | 118 ± 38 | 125 ± 46 | 0.46 |
| Morning heparanase levels (pg/mL) | 2539 ± 995 | 3366 ± 1637 | 0.003 |
| Evening heparanase levels | 2548 ± 1067 | 3115 ± 1553 | 0.037 |
| Morning heparanase procoagulant activity (ng/mL) | 401 ± 358 | 596 ± 459 | 0.021 |
| Evening heparanase procoagulant activity | 442 ± 439 | 458 ± 542 | 0.87 |
| Morning D-Dimer (ng/mL) | 267 ± 141 | 365 ± 194 | 0.006 |
| Evening D-Dimer | 311 ± 245 | 370 ± 230 | 0.22 |
| Morning fibrinogen (mg/dL) | 238 ± 47 | 241 ± 42 | 0.74 |
| Evening fibrinogen | 242 ± 41 | 234 ± 48 | 0.35 |
| Morning heparan sulfate levels (µg/mL) | 31 ± 30 | 30 ± 28 | 0.9 |
| Evening heparan sulfate levels | 25 ± 21 | 24 ± 22 | 0.8 |
| Early Chronotype MSF < 4 AM n = 57 | Late Chronotype MSF ≥ 4 AM n = 43 | p-Value | |
|---|---|---|---|
| Age (years) | 40.86 ± 6.7 | 37.9 ± 8.4 | 0.051 |
| Total nurse experience (years) | 16.3 ± 7.8 | 13.3 ± 8.3 | 0.068 |
| Experience in current work (years) | 7.6 ± 5.5 | 8.3 ± 6.9 | 0.56 |
| Scope of the job | 0.012 | ||
| 100% | 46 (81%) | 25 (58%) | |
| 88% | 2 (3%) | 9 (21%) | |
| 75% | 9 (16%) | 9 (21%) | |
| Birth control pills use (n) | 6 (10.5%) | 5 (11.6%) | 0.86 |
| BMI | 23.8 ± 3.3 | 24.2 ± 3.8 | 0.58 |
| Smoking (n) | 11 (19%) | 2 (4.7%) | 0.037 |
| Family history of heart disease at young age (n) | 12 (21%) | 6 (14%) | 0.36 |
| Last menstruation date before the first blood test | 0.78 | ||
| no menstruation | 8 (14%) | 4 (9%) | |
| 1–10 days | 15 (26%) | 13 (30%) | |
| 11–20 days | 12 (21%) | 7 (16%) | |
| 21–35 days | 22 (39%) | 19 (44%) | |
| Hours of sitting during working day | 0.11 | ||
| 0–1 | 11 (19%) | 15 (35%) | |
| 1–3 | 32 (56%) | 23 (53%) | |
| 3–6 | 14 (25%) | 5 (12%) |
| Early Chronotype MSF < 4 AM n = 51 | Late Chronotype MSF ≥ 4 AM n = 49 | p Value | |
|---|---|---|---|
| Morning reactive hyperaemia index | 0.65 ± 0.28 | 0.59 ± 0.23 | 0.27 |
| Evening reactive hyperaemia index | 0.62 ± 0.23 | 0.61 ± 0.32 | 0.82 |
| Morning plasmin activator inhibitor-1 (ng/mL) | 12.7 ± 5.6 | 16.6 ± 8.9 | 0.009 |
| Evening plasmin activator inhibitor-1 | 9.2 ± 4.2 | 10.4 ± 6.6 | 0.25 |
| Morning von Willebrand factor (%) | 126 ± 42 | 136 ± 43 | 0.28 |
| Evening von Willebrand factor | 118 ± 37 | 126 ± 48 | 0.33 |
| Morning heparanase levels (pg/mL) | 2828 ± 1327 | 3137 ± 1525 | 0.28 |
| Evening heparanase levels | 2718 ± 1303 | 2996 ± 1432 | 0.31 |
| Morning heparanase procoagulant activity (ng/mL) | 436 ± 366 | 586 ± 478 | 0.07 |
| Evening heparanase procoagulant activity | 336 ± 263 | 601 ± 661 | 0.007 |
| Morning D-Dimer (ng/mL) | 311 ± 142 | 321 ± 214 | 0.78 |
| Evening D-Dimer | 338 ± 238 | 344 ± 242 | 0.91 |
| Morning fibrinogen (mg/dL) | 245 ± 42 | 235 ± 47 | 0.38 |
| Evening fibrinogen | 243 ± 40 | 232 ± 49 | 0.22 |
| Morning heparan sulfate levels (µg/mL) | 31 ± 30 | 30 ± 29 | 0.9 |
| Evening heparan sulfate levels | 25 ± 23 | 20 ± 19 | 0.5 |
| Morning n = 100 | Evening n = 100 | p Value | |
|---|---|---|---|
| Reactive hyperaemia index | 0.62 ± 0.26 | 0.61 ± 0.27 | 0.8 |
| Plasmin activator inhibitor-1 (ng/mL) | 14.4 ± 7.4 | 9.7 ± 5.3 | 0.0001 |
| von Willebrand factor (%) | 131 ± 43 | 121 ± 42 | 0.005 |
| Heparanase levels (pg/mL) | 2961 ± 1416 | 2838 ± 1360 | 0.019 |
| Heparanase procoagulant activity (ng/mL) | 500 ± 422 | 450 ± 492 | 0.26 |
| D-Dimer (ng/mL) | 316 ± 176 | 341 ± 238 | 0.16 |
| Fibrinogen (mg/dL) | 239 ± 44 | 238 ± 45 | 0.6 |
| Heparan sulfate levels (µg/mL) | 31 ± 28 | 23 ± 19 | 0.005 |
| Pearson’s Correlation | p Value | |
|---|---|---|
| Shift/day workers/chronotype * | 0.427 | 0.0001 |
| Shift/day workers/SJL * | 0.37 | 0.0001 |
| Chronotype */SJL * | 0.374 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saharov, G.; Salti, B.; Bareya, M.; Keren-Politansky, A.; Fodi, M.; Shochat, T.; Nadir, Y. Endothelial Dysfunction and Hemostatic System Activation in Relation to Shift Workers, Social Jetlag, and Chronotype in Female Nurses. Int. J. Mol. Sci. 2025, 26, 482. https://doi.org/10.3390/ijms26020482
Saharov G, Salti B, Bareya M, Keren-Politansky A, Fodi M, Shochat T, Nadir Y. Endothelial Dysfunction and Hemostatic System Activation in Relation to Shift Workers, Social Jetlag, and Chronotype in Female Nurses. International Journal of Molecular Sciences. 2025; 26(2):482. https://doi.org/10.3390/ijms26020482
Chicago/Turabian StyleSaharov, Gleb, Barbara Salti, Maram Bareya, Anat Keren-Politansky, Muhammed Fodi, Tamar Shochat, and Yona Nadir. 2025. "Endothelial Dysfunction and Hemostatic System Activation in Relation to Shift Workers, Social Jetlag, and Chronotype in Female Nurses" International Journal of Molecular Sciences 26, no. 2: 482. https://doi.org/10.3390/ijms26020482
APA StyleSaharov, G., Salti, B., Bareya, M., Keren-Politansky, A., Fodi, M., Shochat, T., & Nadir, Y. (2025). Endothelial Dysfunction and Hemostatic System Activation in Relation to Shift Workers, Social Jetlag, and Chronotype in Female Nurses. International Journal of Molecular Sciences, 26(2), 482. https://doi.org/10.3390/ijms26020482








