Time-Lapse Imaging in IVF: Bridging the Gap Between Promises and Clinical Realities
Abstract
1. Background
2. Technological Foundations of Time-Lapse Imaging
2.1. Time-Lapse Incubators
2.2. Continuous Monitoring vs. Static Evaluation
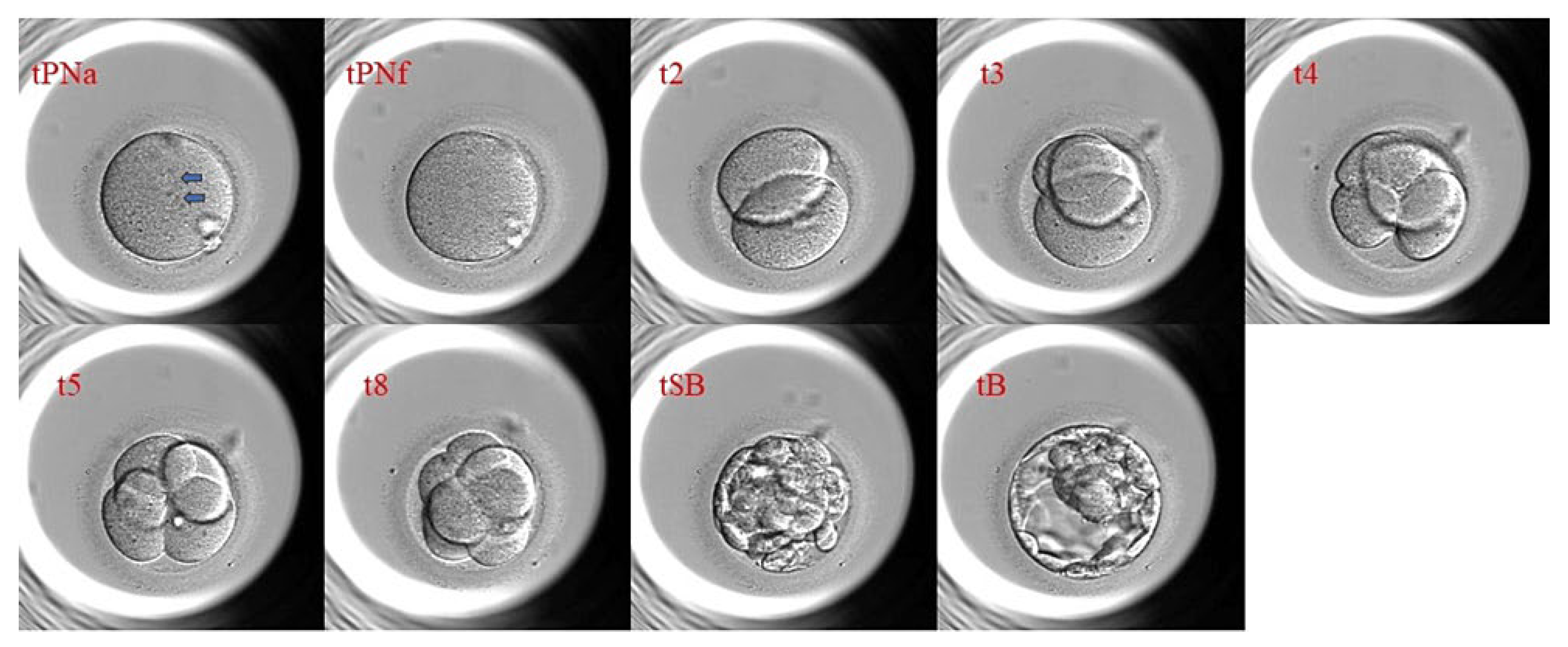
2.3. Morphokinetics: Definition, Parameters, and Recording
2.4. Summary
3. Promises of Time-Lapse Technology
3.1. Improved Embryo Selection Accuracy
3.2. Reduced Embryologist Subjectivity
3.3. Increased Implantation and Live Birth Rates
3.4. Improved Patient Communication and Transparency
3.5. Overview of Industry Claims and Early Study Findings
3.6. Summary
4. Realities and Challenges in Clinical Practice
4.1. Mixed or Inconclusive Evidence in Large Trials and Meta-Analyses
4.2. Limitations in Algorithm Generalizability and Validation
4.3. Outcome Variability Across Populations, Labs, and IVF Protocols
4.4. High Cost of Equipment and Training
4.5. Ethical Concerns and Responsible Patient Communication
4.6. Summary
5. Morphokinetic Parameters and Predictive Algorithms
5.1. Commercial vs. Clinic-Specific Algorithms
5.2. Role of AI and Machine Learning in Interpreting Time-Lapse Data
5.3. Summary
6. Time-Lapse Technology in Specific Contexts
6.1. Advanced Maternal Age (AMA)
6.2. Recurrent Implantation Failure (RIF)
6.3. Comparisons of Outcomes with Traditional Morphology Evaluation
6.4. Summary
7. Future Directions and Recommendations
7.1. Personalized Embryo Selection Protocols
7.2. Integration with Non-Invasive Biomarkers
7.3. Data Standardization Across Centers
7.4. Multicenter Collaboration for Model Training
7.5. Synthesis and Strategic Roadmap
8. Conclusions
8.1. Re-Emphasizing Critical Adoption over Blind Enthusiasm
8.2. Final Perspective
8.3. Clinical Practice Points
- Targeted Application, Not Universal Adoption: The routine use of TLI for all IVF patients is not justified by current evidence. However, its value is most pronounced in specific clinical scenarios:
- ○
- PGT-A cycles: TLI can act as a pre-screening tool to prioritize embryos for biopsy. This has the potential to reduce laboratory workload and costs.
- ○
- RIF and AMA: In these populations, TLI has the ability to detect dynamic anomalies such as abnormal cleavages. This may improve embryo selection precision over static morphology alone.
- No Universal Superiority in Live Birth Rates: Existing evidence from large trials shows that TLI does not significantly improve live birth rates compared to conventional incubation and morphological assessment.
- Cost–Benefit Considerations are Paramount: TLI systems have high upfront and operational costs. Hence, a cost–benefit analysis is essential. This is because the marginal gains in selection accuracy rarely justify the expense for average-prognosis patients.
- Algorithmic Insights are Advisory, Not Deterministic: The predictive algorithms used in TLI are tools to aid embryologist judgment; they are not there to replace it. Clinicians must maintain oversight and communicate the probabilistic nature of these predictions to patients. This is necessary to manage expectations appropriately.
- Standardization is a Prerequisite for Validity: The clinical utility of TLI is highly dependent on the core aspects of standardized laboratory protocols and validated, context-specific algorithms. The current widespread variability in practice limits the generalizability of findings and tool performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AMA | Advanced Maternal Age |
| ART | Assisted Reproductive Technology |
| CNN | Convolutional Neural Network (AI architecture for image analysis) |
| Eeva® | Early Embryo Viability Assessment (commercial TLI system) |
| ESHRE | European Society of Human Reproduction and Embryology |
| ICSI | Intracytoplasmic Sperm Injection |
| IGF2 | Insulin-like Growth Factor 2 (biomarker) |
| IVF | In Vitro Fertilization |
| LBR | Live Birth Rate |
| ML | Machine Learning |
| PGT-A | Preimplantation Genetic Testing for Aneuploidies |
| RCT | Randomized Controlled Trial |
| RIF | Recurrent Implantation Failure |
| SCM | Spent Culture Media (source of non-invasive biomarkers) |
| sHLA-G | Soluble Human Leukocyte Antigen-G (implantation biomarker) |
| t2 | Time to 2-cell stage (hours post-insemination) |
| t3 | Time to 3-cell stage |
| t4 | Time to 4-cell stage |
| t5 | Time to 5-cell stage |
| tB | Time to Blastocyst formation |
| tB–t2 | Duration between 2-cell stage and blastocyst formation (morphokinetic marker) |
| TLI | Time-Lapse Imaging |
| TILT | Time-Lapse Imaging Systems for Embryo Incubation and Selection Trial |
| ICCP | Intercellular Contact Points |
| tPB2 | Timing of second Polar Body extrusion |
| tPNa | Timing of pronuclei Appearance |
| tPNf | Timing of pronuclei Fading |
| tM | Time of Morula |
| tSB | Time of Starting Blastulation |
| tEB | Time of Expanding Blastocyst |
| tHB | Time of Hatching Blastocyst |
References
- Abdullah, K.A.L.; Atazhanova, T.; Chavez-Badiola, A.; Shivhare, S.B. Automation in ART: Paving the way for the future of infertility treatment. Reprod. Sci. 2023, 30, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Ahlström, A.; Lundin, K.; Cimadomo, D.; Coticchio, G.; Selleskog, U.; Westlander, G.; Winerdal, J.; Stenfelt, C.; Callender, S.; Nyberg, C.; et al. No major differences in perinatal and maternal outcomes between uninterrupted embryo culture in time-lapse system and conventional embryo culture. Hum. Reprod. 2023, 38, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Alammari, K. Time-Lapse Embryo Morphokinetics and Clinical Outcomes for Patients Seeking IVF in a Private Hospital in Riyadh. Master’s Thesis, Alfaisal University, Riyadh, Saudi Arabia, 2022. [Google Scholar]
- AlSaad, R.; Abusarhan, L.; Odeh, N.; Abd-Alrazaq, A.; Choucair, F.; Zegour, R.; Ahmed, A.; Aziz, S.; Sheikh, J. Deep learning applications for human embryo assessment using time-lapse imaging: Scoping review. Front. Reprod. Health 2025, 7, 1549642. [Google Scholar] [CrossRef]
- Armstrong, S. IVF Add-Ons: The Quantitative and Qualitative Evidence Behind Their Use. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2023. [Google Scholar]
- Armstrong, S.; Pacey, A.; Farquhar, C.; Lensen, S. O-227 Has time-lapse technology finally proven its clinical benefit? Hum. Reprod. 2022, 37 (Suppl. S1), deac106-005. [Google Scholar] [CrossRef]
- Armstrong, S.; Vail, A.; Mastenbroek, S.; Jordan, V.; Farquhar, C. Time-lapse in the IVF-lab: How should we assess potential benefit? Hum. Reprod. 2015, 30, 3–8. [Google Scholar] [CrossRef]
- Avila Perez, C.; Parnell, L.; Florensa Bargallo, M.; Herreros Cuesta, J.; Larreategui Laiseca, Z.; Prados Dodd, N.; Ruiz Perez, M.; Wells, D. P-731 The accuracy of truly non-invasive PGT using spent culture media is insufficient to justify routine clinical use. Hum. Reprod. 2023, 38 (Suppl. S1), dead093-1050. [Google Scholar] [CrossRef]
- Barrie, A.; McDowell, G.; Troup, S. An investigation into the effect of potential confounding patient and treatment parameters on human embryo morphokinetics. Fertil. Steril. 2021, 115, 1014–1022. [Google Scholar] [CrossRef]
- Barrie, A.; Smith, R.; Campbell, A.; Fishel, S. Optimisation of the timing of fertilisation assessment for oocytes cultured in standard incubation: Lessons learnt from time-lapse imaging of 78,348 embryos. Hum. Reprod. 2021, 36, 2840–2847. [Google Scholar] [CrossRef]
- Bartolacci, A.; Dal Canto, M.; Guglielmo, M.C.; Mura, L.; Brigante, C.; Renzini, M.M.; Buratini, J. Early embryo morphokinetics is a better predictor of post-ICSI live birth than embryo morphology: Speed is more important than beauty at the cleavage stage. Zygote 2021, 29, 495–502. [Google Scholar] [CrossRef]
- Berman, A.; Anteby, R.; Efros, O.; Klang, E.; Soffer, S. Deep learning for embryo evaluation using time-lapse: A systematic review of diagnostic test accuracy. Am. J. Obstet. Gynecol. 2023, 229, 490–501. [Google Scholar] [CrossRef]
- Berntsen, J.; Rimestad, J.; Lassen, J.T.; Tran, D.; Kragh, M.F. Robust and generalizable embryo selection based on artificial intelligence and time-lapse image sequences. PLoS ONE 2022, 17, e0262661. [Google Scholar] [CrossRef]
- Bhide, P.; Chan, D.Y.; Lanz, D.; Alqawasmeh, O.; Barry, E.; Baxter, D.; Carreras, F.; Choudhyry, Y.; Cheong, Y.; Pui Wah Chung, J.; et al. Clinical effectiveness and safety of time-lapse imaging systems for embryo incubation and selection in in-vitro fertilisation treatment (TILT): A multicentre, three-parallel-group, double-blind, randomised controlled trial. Lancet 2024, 404, 256–265. [Google Scholar] [CrossRef]
- Bhide, P.; Maheshwari, A.; Cutting, R.; Seenan, S.; Patel, A.; Khan, K.; Homburg, R. Time lapse imaging: Is it time to incorporate this technology into routine clinical practice? Hum. Fertil. 2017, 20, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Boueilh, T.; Reignier, A.; Barrière, P.; Freour, T. Time-lapse imaging systems in IVF laboratories: A French national survey. J. Assist. Reprod. Genet. 2018, 35, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Blais, I.; Koifman, M.; Feferkorn, I.; Dirnfeld, M.; Lahav-Baratz, S. Improving embryo selection by the development of a laboratory-adapted time-lapse model. F&S Sci. 2021, 2, 176–197. [Google Scholar]
- Boucret, L.; Tramon, L.; Saulnier, P.; Ferré-L’Hôtellier, V.; Bouet, P.E.; May-Panloup, P. Change in the strategy of embryo selection with time-lapse system implementation—Impact on clinical pregnancy rates. J. Clin. Med. 2021, 10, 4111. [Google Scholar] [CrossRef]
- Campbell, A. 11 Embryo Selection. In Manual of Embryo Selection in Human Assisted Reproduction; CRC Press: Boca Raton, FL, USA, 2023; p. 113. [Google Scholar]
- Campbell, A.; Yosef, D.B.; Escrivá, M.M.; Freour, T. Evaluating time-lapse imaging systems for embryo incubation and selection in ART: A critical review of a randomized controlled trial. Reprod. Biomed. Online 2025, 50, 104937. [Google Scholar] [CrossRef]
- Cannarella, R.; Rando, O.J.; Condorelli, R.A.; Chamayou, S.; Romano, S.; Guglielmino, A.; Yin, Q.; Hans, T.; Mancuso, F.; Arato, I.; et al. Sperm-carried IGF2: Towards the discovery of a spark contributing to embryo growth and development. Mol. Hum. Reprod. 2024, 30, gaae034. [Google Scholar] [CrossRef]
- Casciani, V.; Galliano, D.; Franasiak, J.M.; Mariani, G.; Meseguer, M. Are we approaching automated assisted reproductive technology? Embryo culture, metabolomics, and cryopreservation. F&S Rev. 2021, 2, 251–264. [Google Scholar] [CrossRef]
- Chen, P.; Li, T.; Jia, L.; Fang, C.; Liang, X. Should all embryos be cultured to blastocyst for advanced maternal age women with low ovarian reserve: A single center retrospective study. Gynecol. Endocrinol. 2018, 34, 761–765. [Google Scholar] [CrossRef]
- Chera-Aree, P.; Thanaboonyawat, I.; Thokha, B.; Laokirkkiat, P. Comparison of pregnancy outcomes using a time-lapse monitoring system for embryo incubation versus a conventional incubator in in vitro fertilization: An age-stratification analysis. Clin. Exp. Reprod. Med. 2021, 48, 174–181. [Google Scholar] [CrossRef]
- Cohen, J.; Silvestri, G.; Paredes, O.; Martin-Alcala, H.E.; Chavez-Badiola, A.; Alikani, M.; Palmer, G. Artificial intelligence in assisted reproductive technology: Separating the dream from reality. Reprod. Biomed. Online 2025, 50, 104855. [Google Scholar] [CrossRef]
- Coticchio, G.; Bartolacci, A.; Cimadomo, V.; Trio, S.; Innocenti, F.; Borini, A.; Vaiarelli, A.; Rienzi, L.; Ahlstrom, A.; Cimadomo, D. Time will tell: Time-lapse technology and artificial intelligence to set time cut-offs indicating embryo incompetence. Hum. Reprod. 2024, 39, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Nogueira, D.; Dissler, N.; Maskani Filali, M.; Delestro Matos, F.; Chansel-Debordeaux, L.; Ferrer-Buitrago, M.; Ferrer, E.; Antequera, V.; Ruiz-Jorro, M.; et al. A hybrid artificial intelligence model leverages multi-centric clinical data to improve fetal heart rate pregnancy prediction across time-lapse systems. Hum. Reprod. 2023, 38, 596–608. [Google Scholar] [CrossRef] [PubMed]
- ESHRE Working Group on Recurrent Implantation Failure; Cimadomo, D.; de Los Santos, M.J.; Griesinger, G.; Lainas, G.; Le Clef, N.; McLernon, D.; Montjean, D.; Toth, B.; Vermeulen, N.; et al. ESHRE good practice recommendations on recurrent implantation failure. Hum. Reprod. Open 2023, 2023, hoad023. [Google Scholar]
- Fadon, P.; Gallegos, E.; Jalota, S.; Muriel, L.; Diaz-Garcia, C. Time-lapse systems: A comprehensive analysis on effectiveness. Semin. Reprod. Med. 2021, 39, e12–e18. [Google Scholar] [CrossRef]
- Fernandes, I.; Sankar, P.; Figueiredo, D. Artificial Intelligence (AI) Application in In Vitro Fertilization (IVF) Imaging Techniques. In AI-Powered Systems for Healthcare Diagnostics and Treatment; IGI Global: Hershey, PA, USA, 2025; pp. 141–178. [Google Scholar]
- Futo, M.; Široki, T.; Koska, S.; Čorak, N.; Tušar, A.; Domazet-Lošo, M.; Domazet-Lošo, T. A novel time-lapse imaging method for studying developing bacterial biofilms. Sci. Rep. 2022, 12, 21120. [Google Scholar] [CrossRef]
- Gardner, D.K. IVF–through the looking glass. Reprod. Biomed. Online 2025, 50, 104835. [Google Scholar] [CrossRef]
- Garg, A.; Valera, M.Á.; Meseguer, M. Evaluation of embryo quality: Time-lapse imaging to assess embryo morphokinesis. In Textbook of Assisted Reproductive Techniques, 6th ed.; Racowsky, C., Schlegel, P.N., Fauser, B.C.J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 182–201. [Google Scholar]
- Geampana, A.; Perrotta, M. Predicting success in the embryology lab: The use of algorithmic technologies in knowledge production. Sci. Technol. Human. Values 2023, 48, 212–233. [Google Scholar] [CrossRef]
- Ghelardi, C. Non-invasive biomarkers of embryo quality: How they can contribute to the success of Assisted Reproduction Techniques. Reprod. Fertil. 2022, 3, C46–C51. [Google Scholar]
- Giménez, C.; Conversa, L.; Murria, L.; Meseguer, M. Time-lapse imaging: Morphokinetic analysis of in vitro fertilization outcomes. Fertil. Steril. 2023, 120, 218–227. [Google Scholar] [CrossRef]
- Kaser, J.; Racowsky, C. Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: A systematic review. Hum. Reprod. Update 2014, 20, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Hew, Y.; Kutuk, D.; Duzcu, T.; Ergun, Y.; Basar, M. Artificial Intelligence in IVF Laboratories: Elevating Outcomes Through Precision and Efficiency. Biology 2024, 13, 988. [Google Scholar] [CrossRef] [PubMed]
- Hop, V.D.; Cuong, A.M.; Anh, P.T.; Huong, N.T.; Hoang, L.; Hanh, N.V. Clinical validation of the early embryo viability assessment system: Analysis for the blastocyst morphology and pregnancy outcomes. Asian Pac. J. Reprod. 2024, 13, 219–227. [Google Scholar] [CrossRef]
- Huang, B.; Tan, W.; Li, Z.; Jin, L. An artificial intelligence model (euploid prediction algorithm) can predict embryo ploidy status based on time-lapse data. Reprod. Biol. Endocrinol. 2021, 19, 185. [Google Scholar] [CrossRef]
- Hunter, H.R. Investigating Human Embryo Implantation–Developing Clinical Applications from In Vitro Models. Ph.D. Thesis, Manchester Metropolitan University, Manchester, UK, 2024. [Google Scholar]
- Iaconelli, C.; Braga, D.; Setti, A.; Guilherme, P.; Iaconelli, A., Jr.; Borges Junior, E. P-182 The impact of ethnic differences on embryo morphokinetics and clinical outcomes: The importance of racial admixture. Hum. Reprod. 2022, 37 (Suppl. S1), deac107-175. [Google Scholar] [CrossRef]
- Iannone, A.; Carfì, A.; Mastrogiovanni, F.; Zaccaria, R.; Manna, C. On the role of artificial intelligence in analysing oocytes during in vitro fertilisation procedures. Artif. Intell. Med. 2024, 157, 102997. [Google Scholar] [CrossRef]
- Illingworth, P.J.; Venetis, C.; Gardner, D.K.; Nelson, S.M.; Berntsen, J.; Larman, M.G.; Agresta, F.; Ahitan, S.; Ahlstrom, A.; Cattrall, F.; et al. Deep learning versus manual morphology-based embryo selection in IVF: A randomized, double-blind noninferiority trial. Nat. Med. 2024, 30, 3114–3120. [Google Scholar] [CrossRef]
- Jeong, T. Deep Learning-based Video Object Detection for Single-and Multi-Cell Analysis and Evaluation in Time-Lapse Imaging. Ph.D. Thesis, Hallym University, Chuncheon, Republic of Korea, 2025. [Google Scholar]
- Jiang, Y.; Wang, L.; Wang, S.; Shen, H.; Wang, B.; Zheng, J.; Yang, J.; Ma, B.; Zhang, X. The effect of embryo selection using time-lapse monitoring on IVF/ICSI outcomes: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2023, 49, 2792–2803. [Google Scholar] [CrossRef]
- Johansen, M.N.; Parner, E.T.; Kragh, M.F.; Kato, K.; Ueno, S.; Palm, S.; Kernbach, M.; Balaban, B.; Keles, I.; Gabrielsen, A.; et al. Comparing performance between clinics of an embryo evaluation algorithm based on time-lapse images and machine learning. J. Assist. Reprod. Genet. 2023, 40, 2129–2137. [Google Scholar] [CrossRef]
- Kieslinger, D.C.; Vergouw, C.G.; Ramos, L.; Arends, B.; Curfs, M.H.J.M.; Slappendel, E.; Kostelijk, E.H.; Pieters, M.H.E.C.; Consten, D.; Verhoeven, M.O.; et al. Clinical outcomes of uninterrupted embryo culture with or without time-lapse-based embryo selection versus interrupted standard culture (SelecTIMO): A three-armed, multicentre, double-blind, randomised controlled trial. Lancet 2023, 401, 1438–1446. [Google Scholar] [CrossRef]
- Kirkegaard, K.; Agerholm, I.E.; Ingerslev, H.J. Time-lapse monitoring as a tool for clinical embryo assessment. Hum. Reprod. 2015, 30, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Kozyra, O.; Medvediev, M.; Tinelli, A. Predictions of live birth in IVF programs of patients with recurrent implantation failure. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 303, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R. Use of time-lapse monitoring in medically assisted reproduction treatments: A mini-review. Zygote 2021, 29, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.I.; Su, Y.R.; Chen, C.H.; Chang, T.A.; Kuo, E.E.S.; Zheng, W.L.; Hsieh, W.; Huang, C.; Lee, M.; Liu, M. End-to-end deep learning for recognition of ploidy status using time-lapse videos. J. Assist. Reprod. Genet. 2021, 38, 1655–1663. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, G.; Huang, G.; Hou, W.; Cai, B.; Zhou, S.; Xu, Y. The safety of time-lapse incubation system for embryo culture in assisted reproductive laboratory: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2022, 20, 89. [Google Scholar]
- Magata, F. Time-lapse monitoring technologies for the selection of bovine in vitro fertilized embryos with high implantation potential. J. Reprod. Dev. 2023, 69, 57–64. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Owen, S.J.; French, E.; Thompson, K.; Balen, A.H. Impact of time-lapse imaging incubators with single-step culture medium on cumulative live birth rate in IVF cycles: A retrospective cohort study. Fertil. Steril. 2022, 117, e15. [Google Scholar]
- Meijering, E.; Smal, I.; Dzyubachyk, O.; Olivo-Marin, J.C. Time-lapse imaging. In Microscope Image Processing; Academic Press: Cambridge, MA, USA, 2008; pp. 401–440. ISBN 978-0-12-372578-3. [Google Scholar]
- Meng, Q.; Xu, Y.; Zheng, A.; Li, H.; Ding, J.; Xu, Y.; Pu, Y.; Wang, W.; Wu, H. Noninvasive embryo evaluation and selection by time-lapse monitoring vs. conventional morphologic assessment in women undergoing in vitro fertilization/intracytoplasmic sperm injection: A single-center randomized controlled study. Fertil. Steril. 2022, 117, 1203–1212. [Google Scholar] [CrossRef]
- Meseguer, F.; Piluso, C.; Meseguer, M. Time-lapse imaging. In Handbook of Current and Novel Protocols for the Treatment of Infertility; Carrell, D.T., Schlegel, P.N., Racowsky, C., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 405–417. [Google Scholar]
- Meseguer, M.; Herrero, J.; Tejera, A.; Hilligsøe, K.M.; Ramsing, N.B.; Remohí, J. The use of morphokinetics in embryo selection: Where are we now? Reprod. Biomed. Online 2019, 38, 708–719. [Google Scholar]
- Minasi, M.G.; Greco, P.; Varricchio, M.T.; Barillari, P.; Greco, E. The clinical use of time-lapse in human-assisted reproduction. Ther. Adv. Reprod. Health 2020, 14, 2633494120976921. [Google Scholar] [CrossRef]
- Perrotta, M. Biomedical Innovation in Fertility Care: Evidence Challenges, Commercialization, and the Market for Hope; Bristol University Press: Bristol, UK, 2024. [Google Scholar]
- Montgomery, K.; Montgomery, S.; Campbell, A.; Nash, D.M. A comparison of the morphokinetic profiles of embryos developed from vitrified versus fresh oocytes. Reprod. Biomed. Online 2023, 47, 51–60. [Google Scholar] [CrossRef]
- Pandit, S.; Sharma, R. Noninvasive assessment of human oocytes and embryos in assisted reproduction: Review on present practices and future trends. Med. J. Armed Forces India 2022, 78, 7–16. [Google Scholar] [CrossRef]
- Perrotta, M.; Zampino, L.; Geampana, A.; Bhide, P. The provision of information on time-lapse imaging: A systematic analysis of UK fertility clinics websites. medRxiv 2023. [Google Scholar] [CrossRef]
- Picou, A.; Wirka, K.A.; Catherino, A.B.; Hayward, B.; VerMilyea, M.D. Introducing time-lapse for flexible embryo assessment in in vitro fertilization: Implications for grading confidence and workflow efficiency. F&S Rep. 2025, 6, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Borot, L.; Lorenzon, A.R.; Lopes, A.L.R.D.C.; Sakkas, D.; Lledó, B.; Morales, R.; Ortiz, J.A.; Polyzos, N.P.; Parriego, M.; et al. Implicit bias in diagnosing mosaicism amongst preimplantation genetic testing providers: Results from a multicenter study of 36 395 blastocysts. Hum. Reprod. 2024, 39, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Milewski, R.; Ajduk, A. Time-lapse imaging of cleavage divisions in embryo quality assessment. Reproduction 2017, 154, R37–R53. [Google Scholar] [CrossRef] [PubMed]
- Racowsky, C.; Martins, W.P. Effectiveness and safety of time-lapse imaging for embryo culture and selection: It is still too early for any conclusions? Fertil. Steril. 2017, 108, 450–452. [Google Scholar] [CrossRef][Green Version]
- Racowsky, C.; Kovacs, P.; Martins, W.P. A critical appraisal of time-lapse imaging for embryo selection: Where are we and where do we need to go? J. Assist. Reprod. Genet. 2015, 32, 1025–1030. [Google Scholar] [CrossRef]
- Rajendran, S.; Brendel, M.; Barnes, J.; Zhan, Q.; Malmsten, J.E.; Zisimopoulos, P.; Sigaras, A.; Ofori-Atta, K.; Meseguer, M.; Miller, K.A.; et al. Automatic ploidy prediction and quality assessment of human blastocysts using time-lapse imaging. Nat. Commun. 2024, 15, 7756. [Google Scholar] [CrossRef]
- Rubio, I.; Galán, A.; Larreategui, Z.; Ayerdi, F.; Bellver, J.; Herrero, J.; Meseguer, M. Clinical validation of embryo culture and selection by morphokinetic analysis: A randomized, controlled trial of the EmbryoScope. Fertil. Steril. 2014, 102, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G.C.; Mozes, H.; Ronn, R.; Elder-Geva, T.; Schonberger, O.; Ben-Ami, I.; Srebnik, N. Time-Lapse Incubation for Embryo Culture-Morphokinetics and Environmental Stability May Not Be Enough: Results from a Pilot Randomized Controlled Trial. J. Clin. Med. 2024, 13, 1701. [Google Scholar] [CrossRef] [PubMed]
- Sainte-Rose, R.; Petit, C.; Dijols, L.; Frapsauce, C.; Guerif, F. Extended embryo culture is effective for patients of an advanced maternal age. Sci. Rep. 2021, 11, 13499. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Sato, T.; Nagaya, M.; Saito, C.; Yoshihara, H.; Banno, C.; Matsumoto, Y.; Matsuda, Y.; Yoshikai, K.; Sawada, T.; et al. Evaluation of artificial intelligence using time-lapse images of IVF embryos to predict live birth. Reprod. Biomed. Online 2021, 43, 843–852. [Google Scholar] [CrossRef]
- Sayed, S.; Reigstad, M.M.; Petersen, B.M.; Schwennicke, A.; Hausken, J.W.; Storeng, R. Nucleation status of Day 2 pre-implantation embryos, acquired by time-lapse imaging during IVF, is associated with live birth. PLoS ONE 2022, 17, e0274502. [Google Scholar] [CrossRef]
- Serdarogullari, M.; Ammar, O.F.; Sharma, K.; Kohlhepp, F.; Montjean, D.; Meseguer, M.; Farire-Zamora, J.J. #ESHREjc report: Seeing is believing! How time lapse imaging can improve IVF practice and take it to the future clinic. Hum. Reprod. 2022, 37, 1370–1372. [Google Scholar] [CrossRef]
- Serrano-Novillo, C.; Uroz, L.; Márquez, C. Novel time-lapse parameters correlate with embryo ploidy and suggest an improvement in non-invasive embryo selection. J. Clin. Med. 2023, 12, 2983. [Google Scholar] [CrossRef]
- Sharma, A.; Dorobantiu, A.; Ali, S.; Iliceto, M.; Stensen, M.H.; Delbarre, E.; Riegler, M.A.; Hammer, H.L. Deep learning methods to forecasting human embryo development in time-lapse videos. PLos ONE 2024, 20. [Google Scholar] [CrossRef]
- Taniguchi, R.; Hatakeyama, S.; Ohgi, S.; Yanaihara, A. Effect of Male Cigarette Smoking on In Vitro Fertilization (IVF) Outcomes and Embryo Morphokinetic Parameters. Cureus 2024, 16, e52788. [Google Scholar] [CrossRef]
- Stevens Brentjens, L.B.; Roumen, R.J.; Smits, L.; Derhaag, J.; Romano, A.; van Golde, R.J.; den Hartog, J.E. Pregnancy rate and time to pregnancy after recurrent implantation failure (RIF)—A prospective cohort follow-up study. J. Assist. Reprod. Genet. 2024, 41, 3061–3070. [Google Scholar] [CrossRef]
- Stevens Brentjens, L.; Roumen, R.; Romano, A.; Van Golde, R.; Den Hartog, J. P-479 Prognosis for ongoing pregnancy after recurrent implantation failure (RIF) following IVF/ICSI treatment. Hum. Reprod. 2023, 38 (Suppl. S1), dead093-823. [Google Scholar] [CrossRef]
- Takeda, S.; Watanabe, H.; Kida, Y.; Kondo, F.; Kitasaka, H.; Fukunaga, N.; Asada, Y. P-513 Non-invasive PGT (niPGT) of vitrified blastocysts cultured for 24 hours after thawing. Hum. Reprod. 2024, 39 (Suppl. S1), deae108-853. [Google Scholar] [CrossRef]
- Tartia, A.P.; Wu, C.Q.; Gale, J.; Shmorgun, D.; Léveillé, M.C. Time-lapse KIDScoreD5 for prediction of embryo pregnancy potential in fresh and vitrified-warmed single-embryo transfers. Reprod. Biomed. Online 2022, 45, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tatishcheva, Y.; Slominskaya, N.; Kuzminih, N.; Pryadkina, O.; Gerkulov, D.; Byankina, Y.; Chezhina, M.; Kalugina, A. P-181 Morphokinetic analysis may reveal associations between embryo development to the blastocyst stage and ploidy status. Hum. Reprod. 2022, 37 (Suppl. S1), deac107-174. [Google Scholar] [CrossRef]
- Tesarik, J. Noninvasive Biomarkers of Human Embryo Developmental Potential. Reprod. Biomed. Online 2025, 50, 104836. [Google Scholar] [CrossRef]
- Toschi, M.; Bori, L.; Rocha, J.C.; Hickman, C.; Nogueira, M.F.G.; Ferreira, A.S.; Maffeis, M.C.; Malmstein, J.; Zhan, Q.; Zaninovic, N.; et al. A combination of artificial intelligence with genetic algorithms on static time-lapse images improves consistency in blastocyst assessment, an interpretable tool to automate human embryo evaluation: A retrospective cohort study. Int. J. Fertil. Steril. 2024, 18, 378–385. [Google Scholar]
- van Duijn, L.; Rousian, M.; Kramer, C.S.; van Marion, E.S.; Willemsen, S.P.; Speksnijder, J.P.; Laven, J.S.E.; Steegers-Theunissen, R.P.M.; Baart, E.B. The impact of culture medium on morphokinetics of cleavage stage embryos: An observational study. Reprod. Sci. 2022, 29, 2179–2189. [Google Scholar] [CrossRef]
- Wang, F.; Hao, S.; Park, K.; Ahmady, A.; Zhou, C. Label-free evaluation of mouse embryo quality using time-lapse bright field and optical coherence microscopy. Commun. Biol. 2025, 8, 612. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Zhang, N.; Li, T. Research progress of time-lapse imaging technology and embryonic development potential: A review. Medicine 2023, 102, e35203. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Q.; Huang, W.; Yin, L.; Ma, T. Can time-lapse culture combined with artificial intelligence improve ongoing pregnancy rates in fresh transfer cycles of single cleavage stage embryos? Front. Endocrinol. 2024, 15, 1449035. [Google Scholar] [CrossRef]
- Luong, T.M.T.; Le, N.Q.K. Artificial intelligence in time-lapse system: Advances, applications, and future perspectives in reproductive medicine. J. Assist. Reprod. Genet. 2024, 41, 239–252. [Google Scholar] [CrossRef]
- Watanabe, H.; Kida, Y.; Kondo, F.; Takeda, S.; Kitasaka, H.; Fukunaga, N.; Asada, Y. P-136 Non-invasive PGT (niPGT) using spent culture medium: Relationship between time lapse embryo dynamics and accuracy of niPGT analysis. Hum. Reprod. 2024, 39 (Suppl. S1), deae108-510. [Google Scholar] [CrossRef]
- Wong, C.; Chen, A.A.; Behr, B.; Shen, S. Time-lapse microscopy and image analysis in basic and clinical embryo development research. Reprod. Biomed. Online 2013, 26, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Wu, S.; Xu, H.; Ma, Y.; Bao, N.; Gao, M.; Han, X.; Gao, S.; Zhang, S.; Zhao, X.; et al. Non-invasive prediction of human embryonic ploidy using artificial intelligence: A systematic review and meta-analysis. EClinicalMedicine 2024, 77, 102452. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Li, W.; Li, M. Optimal number of high-quality cleavage-stage embryos for extended culture to blastocyst-stage for transfer in women 38 years and older. Gynecol. Endocrinol. 2023, 39, 2181642. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Ezoe, K.; Ueno, S.; Yoshino, O.; Takahashi, T. Use of time-lapse technology on fertilization verification, embryo evaluation, and utilization: A national survey in Japan. AJOG Glob. Rep. 2024, 4, 100397. [Google Scholar] [CrossRef]
- Yanai, A.; Horie, A.; Sakurai, A.; Imakita, S.; Nakamura, M.; Ikeda, A.; Shitanaka, S.; Ohara, T.; Nakakita, B.; Ueda, A.; et al. Innovative AI models for clinical decision-making: Predicting blastocyst formation and quality from time-lapse embryo images up to embryonic day 3. Comput. Biol. Med. 2025, 195, 110637. [Google Scholar] [CrossRef]
- Yang, L.; Leynes, C.; Pawelka, A.; Lorenzo, I.; Chou, A.; Lee, B.; Heaney, J.D. Machine learning in time-lapse imaging to differentiate embryos from young vs old mice. Biol. Reprod. 2024, 110, 1115–1124. [Google Scholar] [CrossRef]
| Feature | EmbryoScope (Vitrolife) | Early Embryo Viability Assessment—Eeva (Merck KGaA) |
|---|---|---|
| Core Technology | Integrated microscope and camera Continuous imaging in a stable incubator. | Automated algorithms for early-stage morphokinetic analysis (first 48 h). |
| Primary Strength | Comprehensive morphokinetic profiling (t2 to tB) Widely adopted and studied Improves workflow standardization. | Aims to simplify and standardize selection. This is potentially beneficial for labs with less embryology expertise. |
| Key Algorithm/Focus | Proprietary algorithms, mainly EmbryoScope+, which uses a wide range of morphokinetic parameters. | Generates a viability score based on early cleavage events. For instance, first cytokinesis. |
| Main Criticism/Limitation | Questionable clinical superiority. This is because it fails to consistently and significantly improve live birth rates over conventional methods [2]. It is also expensive | Limited predictive scope. The reliance on early-stage markers raises questions about accuracy for blastocyst-stage outcomes [31]. |
| Common Challenges |
|
| Feature | Continuous Monitoring (TLI) | Static Evaluation | Key References |
|---|---|---|---|
| Data Capture | Dynamic, uninterrupted morphokinetic tracking | Snapshot assessments at fixed intervals such as Day 3 or Day 5 | [49,51,59] |
| Embryo Stress | Minimal—embryos remain in stable culture conditions | High—repeated removal from incubator | [15,16,17,18,19] |
| Detection of Anomalies | High—Identifies transient events (multinucleation, abnormal cleavages) | Low—misses dynamic anomalies | [7,49] |
| Subjectivity | Low—algorithm-driven analysis | High—depends on the expertise of the embryologist | [51,59] |
| Aneuploidy Correlation | Stronger link via morphokinetic markers (e.g., delayed t2/tB) | Weak correlation with ploidy | [51] |
| Cost and Accessibility | High—expensive equipment and training needed | Low—widely accessible | [5,61] |
| Clinical Utility | Context-dependent—superior in RIF/AMA; mixed in general IVF | Consistent but limited in complex cases | [14,23,50] |
| Promise of TLI | Proposed Mechanism and Supporting Evidence | Limitations and Contradictory Evidence |
|---|---|---|
| Improved Embryo Selection Accuracy | Continuous monitoring captures dynamic morphokinetic parameters such as t2, tB, and cleavage synchronicity amongst others. It also captures transient anomalies that tend to be missed by static assessment such as multinucleation and direct cleavage [49,59,67]. TLI also links certain specific patterns such as delayed t2/tB to aneuploidy and reduced viability [11,75,91]. | The generalizability of algorithms is limited by two key factors: inter-clinic variability in culture conditions and diversity of patient populations [47,51]. Large RCTs like SelecTIMO [48] and TILT [14] have found no significant improvement in overall live birth rates versus conventional methods. |
| Reduced Embryologist Subjectivity | Automated, algorithm-driven analysis standardizes embryo evaluation. This in turn reduces inter-observer variability. For instance, Armstrong et al., 2022 [6] reported a 30% reduction in grading discrepancies. On top of this, AI integration further minimizes human bias [38,44]. | Significant risk of overreliance on yet-to-be-validated algorithms [25,51]. It also requires significant training and expertise to interpret data correctly. This will not completely phase out subjectivity but shift its nature [25]. |
| Increased Implantation and Live Birth Rates (LBR) | Observational studies and early single-center trials reported 15–20% higher implantation rates. This is more particularly in niche populations like RIF [71] and AMA [23]. | The major multicenter TILT RCT Bhide et al., 2024 [14] found no significant difference in LBR (32.1% vs. 31.4%). SelecTIMO RCT Kieslinger et al., 2023 [48] also reported comparable LBRs. Overall, pertinent benefits appear highly context-dependent. |
| Enhanced Patient Communication and Transparency | Visual timelines of embryo development improve patient understanding of embryo quality. It also helps with their grasp of the treatment rationale. On this, studies report higher patient satisfaction and the feeling of being informed among individuals [6,65]. | Presents ethical concerns due to predictive algorithms being communicated as overly definitive. This potentially inflates patient expectations and anxiety if outcomes are either unsuccessful or what they did not expect [51]. |
| Superiority in Specific Populations: AMA | Detects subtle morphokinetic delays (t2 > 28 h, tB > 120 h). These are linked to higher aneuploidy rates in AMA patients. In their report, Chen et al., 2018 [23] note a 12% improvement in implantation rates compared to static methods in AMA. | Chera-Aree et al. (2021) [24] found no significant difference in pregnancy outcomes between TLI and conventional incubation. This was after an age-stratified analysis. Live birth rates have also often remained comparable despite improved implantation [23]. |
| Superiority in Specific Populations: RIF | TLI identifies dynamic dysmorphisms, that is, irregular cleavages, which are the main cause of previous failures. In their study, Rubio et al. (2014) observed a 23% higher pregnancy rate in RIF patients using TLI [71]. Stevens Brentjens et al., 2024 [80] also reported a 27% ongoing pregnancy rate compared to 19% with static methods. | Subgroup analysis of the large TILT trial [14] found no significant benefit for RIF patients. This highlights the fact that the positive outcomes may be protocol-dependent, thus not universal. |
| Synergy with PGT-A | Acts as a pre-screening tool to preselect embryos likely to be euploid based on morphokinetics. This significantly reduces the number of unnecessary biopsies. For instance, Popovic et al., 2024 [66] noted a 32% reduction in biopsy cycles. | While improving euploid identification efficiency, live birth rates often remain comparable to morphology-based selection in PGT-A cycles [8,82]. This undermines and questions TLI’s additive value in all cases [8,82]. |
| Metric | TLI Outcomes | Traditional Morphology Outcomes | Key Studies |
|---|---|---|---|
| Embryo Selection | Prioritizes dynamic markers (t2, tB); reduces subjectivity | Rely on static snapshots (cell number, fragmentation) | [57,71,73] |
| Aneuploidy Detection | 65–75% accuracy via morphokinetics vs. 50–60% with morphology | Limited to indirect markers (fragmentation, asymmetry) | [23,82,94] |
| Live Birth Rates | Context-dependent: +15–20% in RIF/AMA; no difference in general populations | Consistent across broad populations but lower in complex cases | [14,50,80] |
| Cost Efficiency | High upfront costs; justified in PGT-A/RIF cohorts | Lower costs but higher repeat cycles in complex cases | [8,61,82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrugacz, G.; Bołkun, I.; Magoń, T.; Korowaj, I.; Golka, B.; Pluta, T.; Fedak, O.; Cieśla, P.; Zowczak, J.; Skórka, E. Time-Lapse Imaging in IVF: Bridging the Gap Between Promises and Clinical Realities. Int. J. Mol. Sci. 2025, 26, 9609. https://doi.org/10.3390/ijms26199609
Mrugacz G, Bołkun I, Magoń T, Korowaj I, Golka B, Pluta T, Fedak O, Cieśla P, Zowczak J, Skórka E. Time-Lapse Imaging in IVF: Bridging the Gap Between Promises and Clinical Realities. International Journal of Molecular Sciences. 2025; 26(19):9609. https://doi.org/10.3390/ijms26199609
Chicago/Turabian StyleMrugacz, Grzegorz, Igor Bołkun, Tomasz Magoń, Izabela Korowaj, Beata Golka, Tomasz Pluta, Olena Fedak, Paulina Cieśla, Joanna Zowczak, and Ewelina Skórka. 2025. "Time-Lapse Imaging in IVF: Bridging the Gap Between Promises and Clinical Realities" International Journal of Molecular Sciences 26, no. 19: 9609. https://doi.org/10.3390/ijms26199609
APA StyleMrugacz, G., Bołkun, I., Magoń, T., Korowaj, I., Golka, B., Pluta, T., Fedak, O., Cieśla, P., Zowczak, J., & Skórka, E. (2025). Time-Lapse Imaging in IVF: Bridging the Gap Between Promises and Clinical Realities. International Journal of Molecular Sciences, 26(19), 9609. https://doi.org/10.3390/ijms26199609






