Targeting Modifiable Risks: Molecular Mechanisms and Population Burden of Lifestyle Factors on Male Genitourinary Health
Abstract
1. Introduction
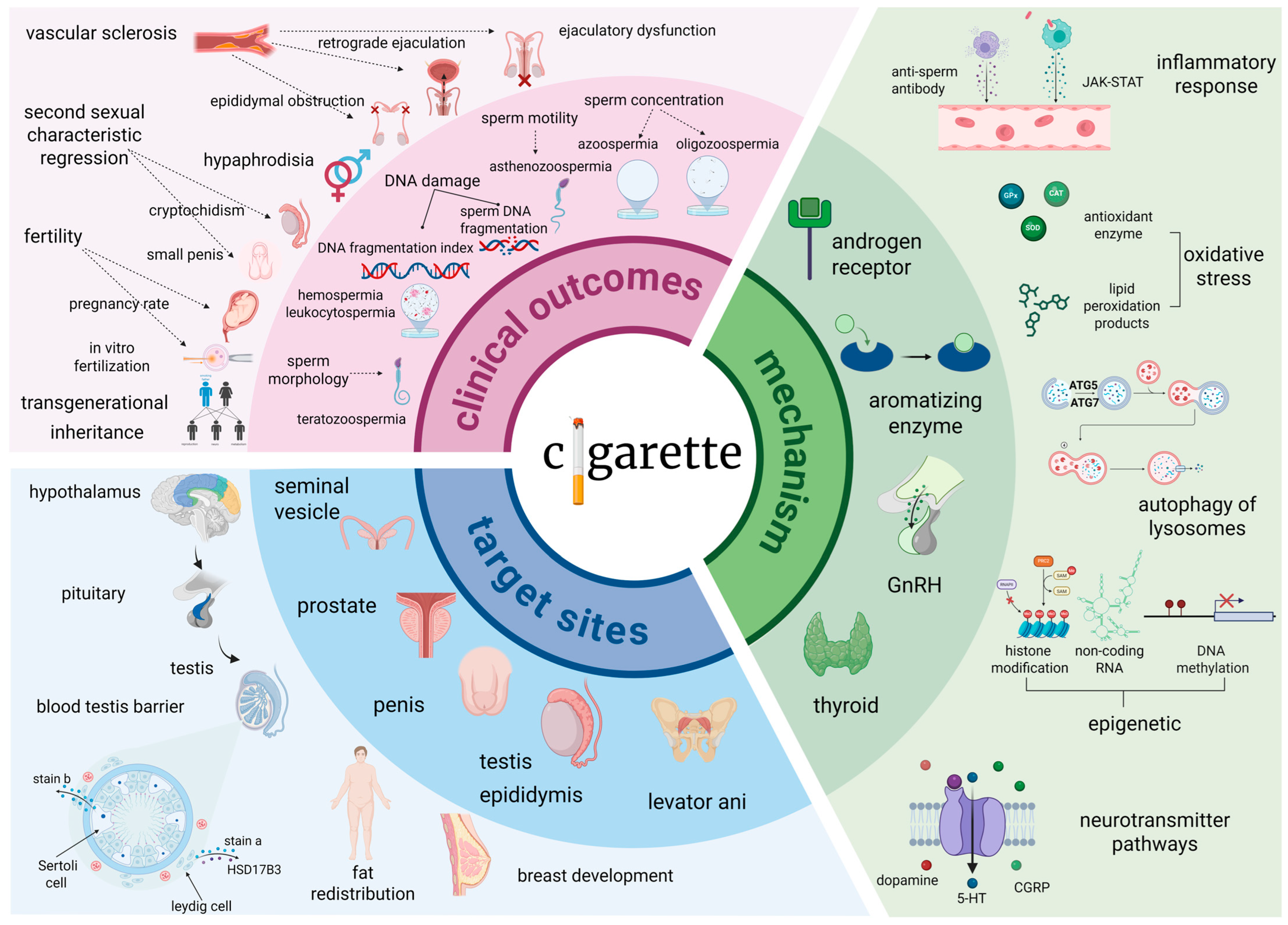
2. Cigarette Smoking: Dose-Dependent Urogenital Toxicity and Spermatogenic Impairment
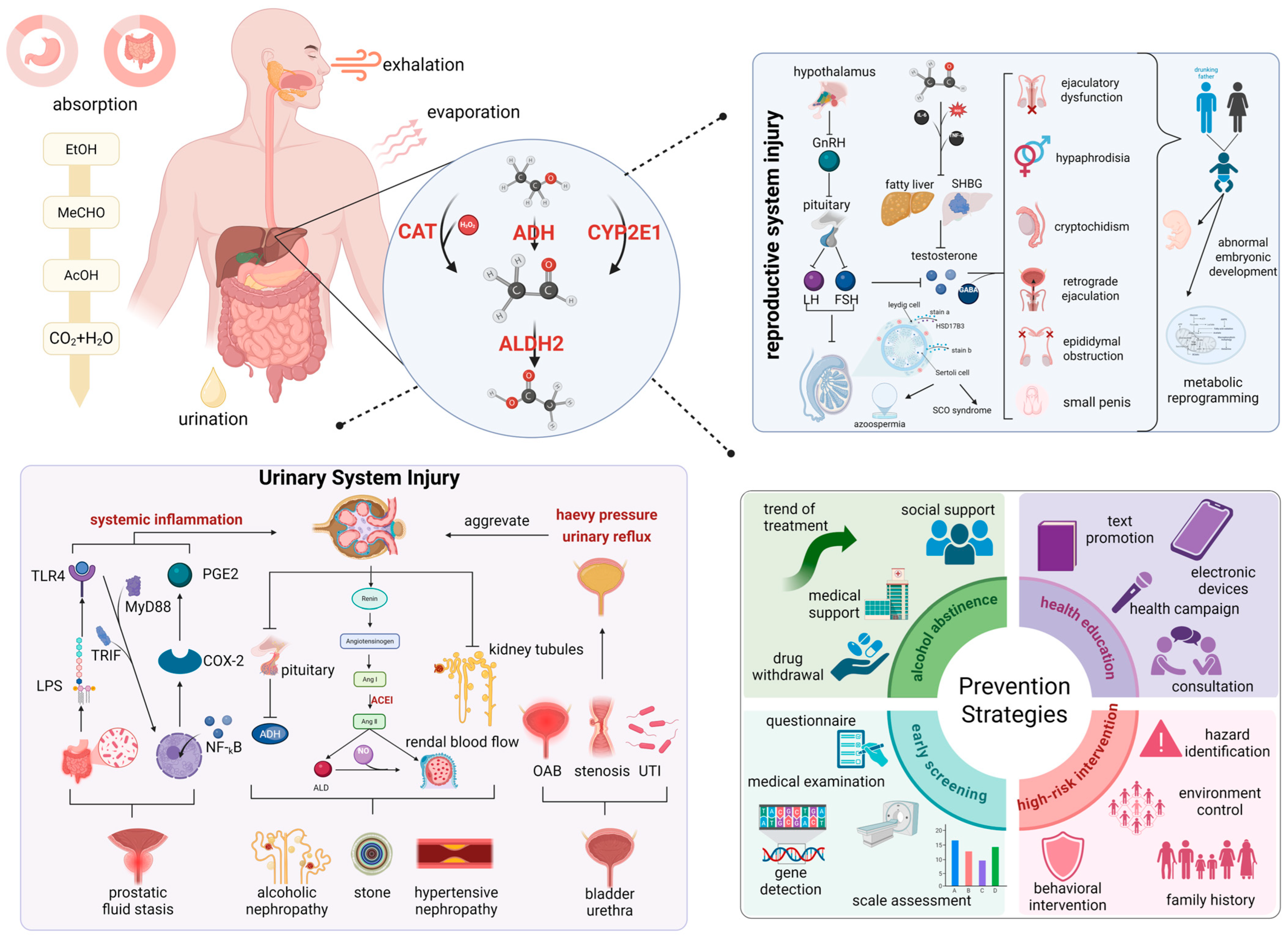
3. Ethanol Exposure: Organ-Specific Pathophysiology and Genetic Polymorphism-Mediated Effects
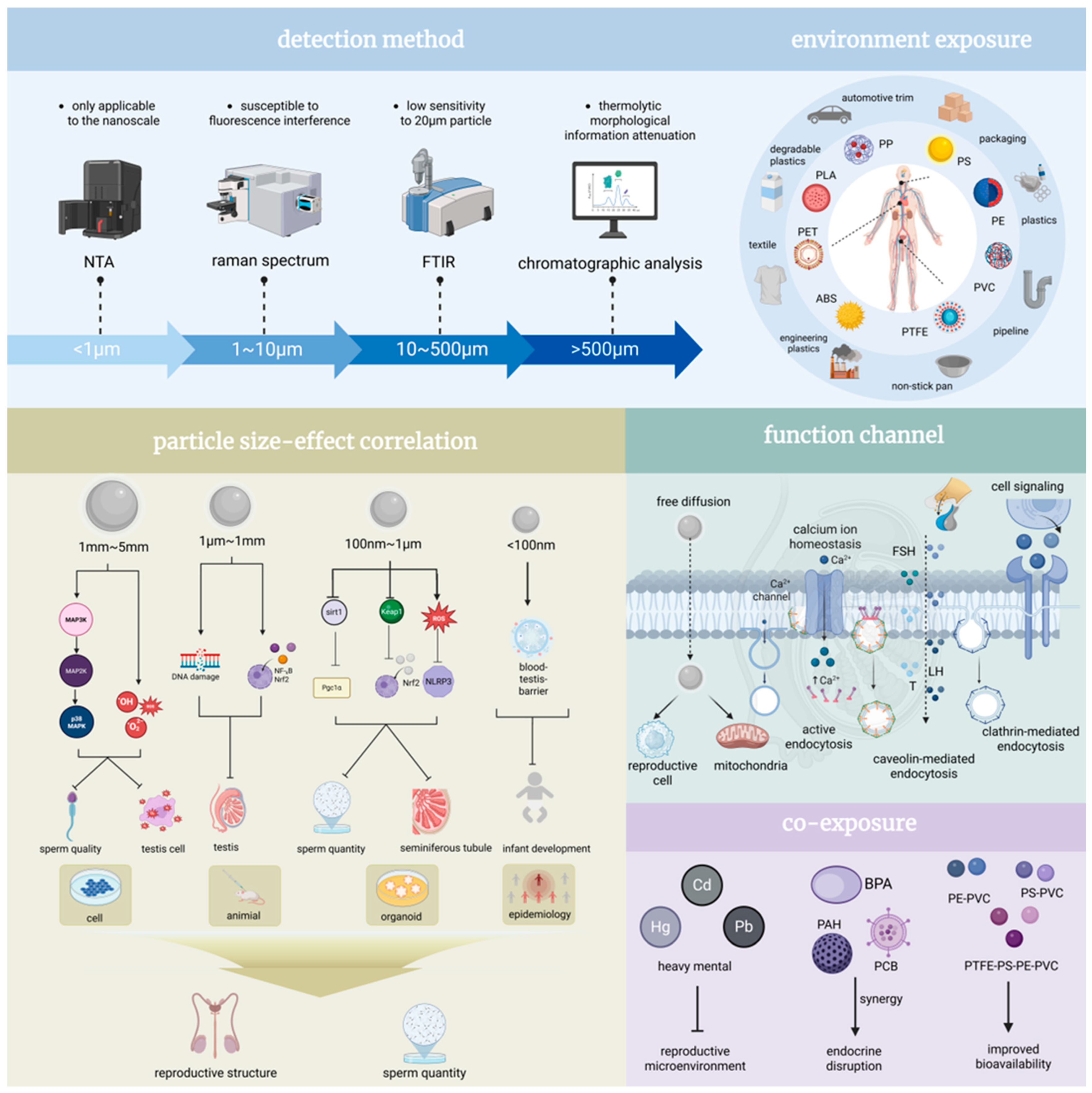
4. Microplastics and Male Health: Multifaceted Mechanisms, Particle Size-Dependent Effects, and Transgenerational Implications
5. Health Risks Associated with Sleep Disorders in Men: Epidemiological Evidence, Disruption Mechanisms and Clinical Implications
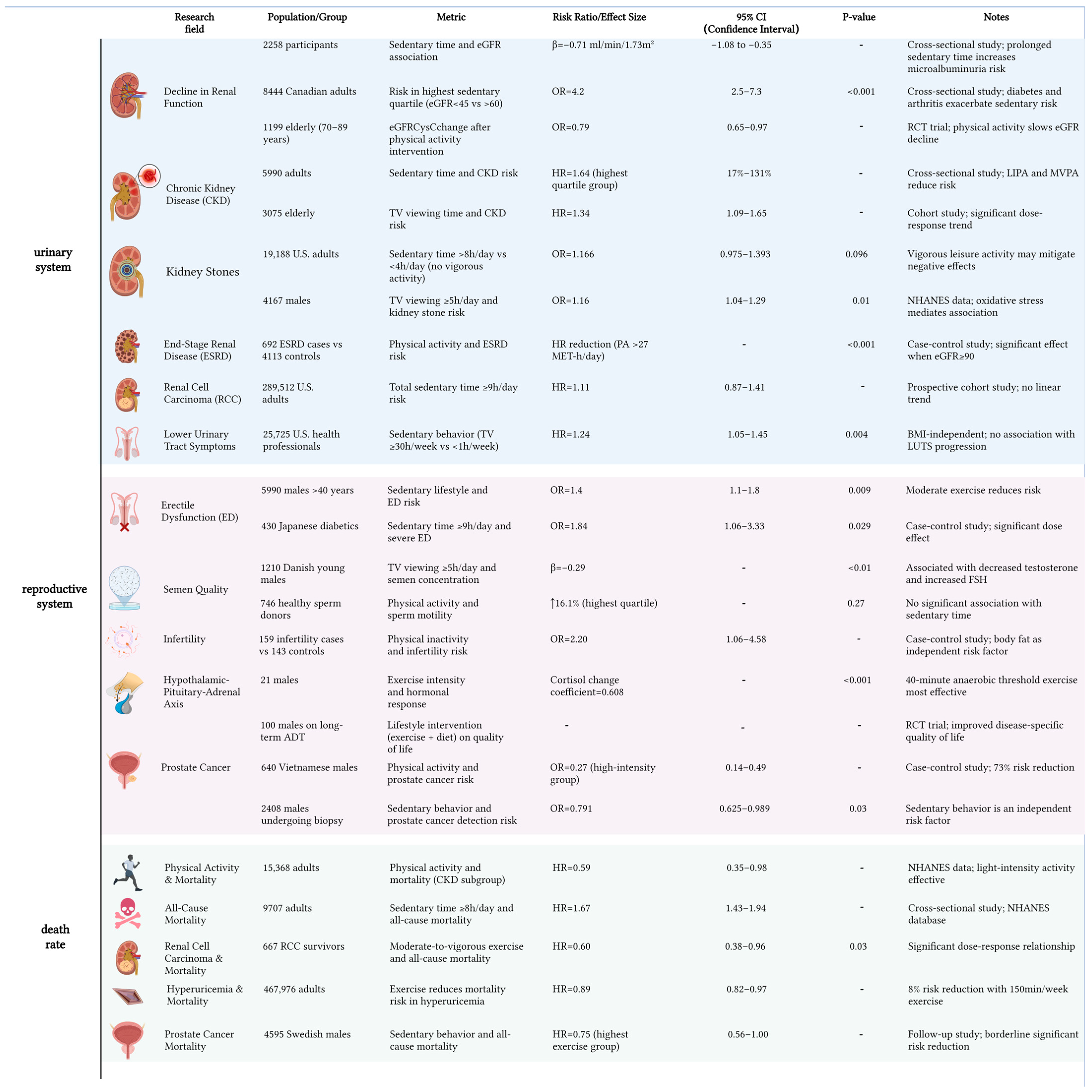
6. Profiles of Adverse Outcomes in Men Under Sedentary: Burden of Genitourinary Diseases and Threat to Life Expectancy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-HT | 5-Hydroxytryptamine |
| AASM | American Academy of Sleep Medicine |
| ACEI | Angiotensin-Converting Enzyme Inhibitor |
| ADH | Alcohol Dehydrogenase |
| ADT | Androgen Deprivation Therapy |
| ALDH2 | Aldehyde Dehydrogenase 2 |
| AMPK | AMP-activated Protein Kinase |
| AOR | Adjusted Odds Ratio |
| ATG5 | Autophagy-related gene 5 |
| ATG7 | Autophagy-related gene 7 |
| BMI | Body Mass Index |
| BPA | Bisphenol A |
| CAT | Catalase |
| CGRP | Calcitonin Gene-Related Peptide |
| CKD | Chronic Kidney Disease |
| COX-2 | Cyclooxygenase-2 |
| CYP2E1 | Cytochrome P450 2E1 |
| DEHP | Di(2-ethylhexyl) Phthalate |
| DNA | Deoxyribonucleic Acid |
| ED | Erectile Dysfunction |
| EDCs | Endocrine-Disrupting Chemicals |
| EEG | Electroencephalogram |
| eGFR | Estimated Glomerular Filtration Rate |
| ESRD | End-stage Renal Disease |
| FSH | Follicle-Stimulating Hormone |
| fPSA | Free Prostate-Specific Antigen |
| GABA | Gamma-Aminobutyric Acid |
| GnRH | Gonadotropin-Releasing Hormone |
| HO-1 | Heme Oxygenase-1 |
| HR | Hazard Ratio |
| HSD17B3 | Hydroxysteroid 17-beta Dehydrogenase 3 |
| IIEF | International Index of Erectile Function |
| JAK-STAT | Janus Kinase-Signal Transducer and Activator of Transcription |
| LH | Luteinizing Hormone |
| LIPA | Lysosomal Acid Lipase |
| LPL | Lipoprotein Lipase |
| LPS | Lipopolysaccharide |
| LUTS | Lower Urinary Tract Symptoms |
| MCLR | Microcystin-LR |
| MPs | Microplastics |
| MVPA | Moderate to Vigorous Physical Activity |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHANES | National Health and Nutrition Examination Survey |
| NMDA | N-Methyl-D-aspartic acid |
| NQO1 | Nitrosylquinoline Oxidase 1 |
| Nrf1 | Nuclear Factor Erythroid 2-Related Factor 1 |
| NPs | Nanoplastics |
| OAB | Overactive Bladder |
| OR | Odds Ratio |
| OSA | Obstructive Sleep Apnea |
| PCA | Protocatechuic Acid |
| PE | Polyethylene |
| PGE2 | Prostaglandin E2 |
| PGC-1α | Peroxisome Proliferator-activated receptor Gamma Coactivator 1-alpha |
| PP | Polypropylene |
| PRC2 | Polycomb Repressive Complex 2 |
| PS | Polystyrene |
| PSQI | Pittsburgh Sleep Quality Index |
| PVC | Polyvinyl Chloride |
| RCC | Renal Cell Carcinoma |
| RCT | Randomized Controlled Trial |
| REM | Rapid Eye Movement |
| RNAPⅡ | RNA Polymerase II |
| RR | Relative Risk |
| SCO | Sertoli Cell-Only Syndrome |
| SHBG | Sex Hormone-Binding Globulin |
| SMD | Standardized Mean Difference |
| SOD2 | Superoxide Dismutase 2 |
| TLR4 | Toll-like receptor 4 |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| UACR | Urine Albumin-to-Creatinine Ratio |
| UTI | Urinary Tract Infection |
| SAM | S-Adenosyl methionine |
| WHO | World Health Organization |
References
- White, A.; Connell, R.; Griffith, D.M.; Baker, P. Defining “Men’s Health”. Int. J. Men’s Soc. Community Health 2023, 6, e1–e9. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yin, C.; Shi, Y.; Du, C.; Pan, X. Global trends in semen quality of young men: A systematic review and regression analysis. J. Assist. Reprod. Genet. 2023, 40, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.W. Chips off the Old Block: How a Father’s Preconception Exposures Might Affect the Health of His Children. Environ. Health Perspect. 2018, 126, 022001. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, Y.; Zhang, X. Impact of Social Capital on Health Behaviors of Middle-Aged and Older Adults in China-An Analysis Based on CHARLS2020 Data. Healthcare 2024, 12, 1154. [Google Scholar] [CrossRef]
- The Lancet. Icd-11. Lancet 2019, 393, 2275. [Google Scholar] [CrossRef]
- Tesarik, J. Lifestyle and Environmental Factors Affecting Male Fertility, Individual Predisposition, Prevention, and Intervention. Int. J. Mol. Sci. 2025, 26, 2797. [Google Scholar] [CrossRef]
- The Lancet. Tobacco control: Far from the finish line. Lancet 2021, 398, 1939. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Wang, L.; Jiang, Y.; Huang, Z.; Zhao, Z.; Zhang, X.; Li, Y.; Liu, S.; Li, C.; et al. Trends in smoking prevalence in urban and rural China, 2007 to 2018: Findings from 5 consecutive nationally representative cross-sectional surveys. PLoS Med. 2022, 19, e1004064. [Google Scholar] [CrossRef]
- Shang, B.; Yao, Y.; Yin, H.; Xie, Y.; Yang, S.; You, X.; Liu, H.; Wang, M.; Ma, J. In utero, childhood, and adolescence tobacco smoke exposure, physical activity, and chronic kidney disease incidence in adulthood: Evidence from a large prospective cohort study. BMC Med. 2024, 22, 528. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.R.; Jerry-Fluker, J.; Atkinson, M.A.; Furth, S.L.; Warady, B.A.; Ng, D.K. Correction to: The association of alcohol, cigarette, e-cigarette, and marijuana use with disease severity in adolescents and young adults with pediatric chronic kidney disease. Pediatr. Nephrol. 2021, 36, 2493–2497. [Google Scholar] [CrossRef]
- Ito, K.; Maeda, T.; Tada, K.; Takahashi, K.; Yasuno, T.; Masutani, K.; Mukoubara, S.; Arima, H.; Nakashima, H. The role of cigarette smoking on new-onset of chronic kidney disease in a Japanese population without prior chronic kidney disease: Iki epidemiological study of atherosclerosis and chronic kidney disease (ISSA-CKD). Clin. Exp. Nephrol. 2020, 24, 919–926. [Google Scholar] [CrossRef]
- Matsumoto, A.; Nagasawa, Y.; Yamamoto, R.; Shinzawa, M.; Yamazaki, H.; Shojima, K.; Shinmura, K.; Isaka, Y.; Iseki, K.; Yamagata, K.; et al. Cigarette smoking and progression of kidney dysfunction: A longitudinal cohort study. Clin. Exp. Nephrol. 2024, 28, 793–802. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Zhang, S.; Chen, X.; Sheng, Y.; Pang, J. Association of cigarette smoking habits with the risk of prostate cancer: A systematic review and meta-analysis. BMC Public Health 2023, 23, 1150. [Google Scholar] [CrossRef]
- Al-Fayez, S.; El-Metwally, A. Cigarette smoking and prostate cancer: A systematic review and meta-analysis of prospective cohort studies. Tob. Induc. Dis. 2023, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Mendoza, E.; Vazquez-Salas, R.A.; Barrientos-Gutierrez, T.; Reynales-Shigematsu, L.M.; Labra-Salgado, I.R.; Manzanilla-Garcia, H.A.; Torres-Sanchez, L.E. Smoking and prostate cancer: A life course analysis. BMC Cancer 2018, 18, 160. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Liang, C. Cigarette smoking and risk of bladder cancer: A dose-response meta-analysis. Int. Urol. Nephrol. 2022, 54, 1169–1185. [Google Scholar] [CrossRef]
- Masaoka, H.; Matsuo, K.; Oze, I.; Kimura, T.; Tamakoshi, A.; Sugawara, Y.; Tsuji, I.; Sawada, N.; Tsugane, S.; Ito, H.; et al. Cigarette Smoking, Smoking Cessation, and Bladder Cancer Risk: A Pooled Analysis of 10 Cohort Studies in Japan. J. Epidemiol. 2023, 33, 582–588. [Google Scholar] [CrossRef]
- Jee, Y.; Jung, K.J.; Back, J.H.; Lee, S.M.; Lee, S.H. Trajectory of smoking and early bladder cancer risk among Korean young adult men. Cancer Causes Control 2020, 31, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef]
- Bao, H.Q.; Sun, L.; Yang, X.N.; Ding, J.; Ma, S.Y.; Yang, L.; Xu, X.O. Impact of cigarette smoking on sperm quality and seminal plasma ROS in preconception males. Zhonghua Nan Ke Xue 2019, 25, 41–45. [Google Scholar]
- Asare-Anane, H.; Bannison, S.B.; Ofori, E.K.; Ateko, R.O.; Bawah, A.T.; Amanquah, S.D.; Oppong, S.Y.; Gandau, B.B.; Ziem, J.B. Tobacco smoking is associated with decreased semen quality. Reprod. Health 2016, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhang, Z.; Wang, H.; Luo, L.; Xu, B. Associations between tobacco inhalation and semen parameters in men with primary and secondary infertility: A cross-sectional study. Front. Endocrinol. 2024, 15, 1396793. [Google Scholar] [CrossRef] [PubMed]
- Kimblad, A.; Ollvik, G.; Lindh, C.H.; Axelsson, J. Decreased sperm counts in Swedish users of oral tobacco. Andrology 2022, 10, 1181–1188. [Google Scholar] [CrossRef]
- Kulaksiz, D.; Toprak, T.; Tokat, E.; Yilmaz, M.; Ramazanoglu, M.A.; Garayev, A.; Sulukaya, M.; Degirmentepe, R.B.; Allahverdiyev, E.; Gul, M.; et al. Sperm concentration and semen volume increase after smoking cessation in infertile men. Int. J. Impot. Res. 2022, 34, 614–619. [Google Scholar] [CrossRef]
- Guha, P.; Bandyopadhyaya, G.; Polumuri, S.K.; Chumsri, S.; Gade, P.; Kalvakolanu, D.V.; Ahmed, H. Nicotine promotes apoptosis resistance of breast cancer cells and enrichment of side population cells with cancer stem cell-like properties via a signaling cascade involving galectin-3, alpha9 nicotinic acetylcholine receptor and STAT3. Breast Cancer Res. Treat. 2014, 145, 5–22. [Google Scholar] [CrossRef]
- Ranjit, S.; Midde, N.M.; Sinha, N.; Patters, B.J.; Rahman, M.A.; Cory, T.J.; Rao, P.S.; Kumar, S. Effect of Polyaryl Hydrocarbons on Cytotoxicity in Monocytic Cells: Potential Role of Cytochromes P450 and Oxidative Stress Pathways. PLoS ONE 2016, 11, e0163827. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Yang, X.; Gong, J.; Lv, J.; Yuan, X.; Shi, M.; Fu, C.; Tan, B.; Fan, Z.; Chen, L.; et al. Patterns of alteration in boar semen quality from 9 to 37 months old and improvement by protocatechuic acid. J. Anim. Sci. Biotechnol. 2024, 15, 78. [Google Scholar] [CrossRef]
- Peacock, A.; Leung, J.; Larney, S.; Colledge, S.; Hickman, M.; Rehm, J.; Giovino, G.A.; West, R.; Hall, W.; Griffiths, P.; et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018, 113, 1905–1926. [Google Scholar] [CrossRef]
- Finelli, R.; Mottola, F.; Agarwal, A. Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 328. [Google Scholar] [CrossRef]
- Yuan, H.C.; Yu, Q.T.; Bai, H.; Xu, H.Z.; Gu, P.; Chen, L.Y. Alcohol intake and the risk of chronic kidney disease: Results from a systematic review and dose-response meta-analysis. Eur. J. Clin. Nutr. 2021, 75, 1555–1567. [Google Scholar] [CrossRef]
- Yang, S.; Tan, W.; Wei, B.; Gu, C.; Li, S.; Wang, S. Association between alcohol and urolithiasis: A mendelian randomization study. Urolithiasis 2023, 51, 103. [Google Scholar] [CrossRef]
- Vartolomei, M.D.; Iwata, T.; Roth, B.; Kimura, S.; Mathieu, R.; Ferro, M.; Shariat, S.F.; Seitz, C. Impact of alcohol consumption on the risk of developing bladder cancer: A systematic review and meta-analysis. World J. Urol. 2019, 37, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, W. Relationship between alcohol use and overactive bladder disease: A cross-sectional study of the NHANES 2005–2016. Front. Public Health 2024, 12, 1418117. [Google Scholar] [CrossRef]
- Hong, S.; Khil, H.; Lee, D.H.; Keum, N.; Giovannucci, E.L. Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis. Nutrients 2020, 12, 2188. [Google Scholar] [CrossRef]
- Michael, J.; Howard, L.E.; Markt, S.C.; De Hoedt, A.; Bailey, C.; Mucci, L.A.; Freedland, S.J.; Allott, E.H. Early-Life Alcohol Intake and High-Grade Prostate Cancer: Results from an Equal-Access, Racially Diverse Biopsy Cohort. Cancer Prev. Res. 2018, 11, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Zuo, L.D.; Fang, F.; Martin, K.; Zheng, Y.; Zhang, H.P.; Li, H.G.; Zhu, C.H.; Xiong, C.L.; Guan, H.T. Association of Alcohol Consumption with Markers of Prostate Health and Reproductive Hormone Profiles: A Multi-Center Study of 4,535 Men in China. PLoS ONE 2015, 10, e0142780. [Google Scholar] [CrossRef]
- Weng, X.; Tan, W.; Wei, B.; Yang, S.; Gu, C.; Wang, S. Interaction between drinking and dietary inflammatory index affects prostate specific antigen: A cross-sectional study. BMC Geriatr. 2023, 23, 537. [Google Scholar] [CrossRef] [PubMed]
- Gianfrilli, D.; Ferlin, A.; Isidori, A.M.; Garolla, A.; Maggi, M.; Pivonello, R.; Santi, D.; Sansone, A.; Balercia, G.; Granata, A.R.M.; et al. Risk behaviours and alcohol in adolescence are negatively associated with testicular volume: Results from the Amico-Andrologo survey. Andrology 2019, 7, 769–777. [Google Scholar] [CrossRef]
- Nguyen-Thanh, T.; Hoang-Thi, A.P.; Anh Thu, D.T. Investigating the association between alcohol intake and male reproductive function: A current meta-analysis. Heliyon 2023, 9, e15723. [Google Scholar] [CrossRef]
- Ricci, E.; Noli, S.; Ferrari, S.; La Vecchia, I.; Cipriani, S.; De Cosmi, V.; Somigliana, E.; Parazzini, F. Alcohol intake and semen variables: Cross-sectional analysis of a prospective cohort study of men referring to an Italian Fertility Clinic. Andrology 2018, 6, 690–696. [Google Scholar] [CrossRef]
- Bai, S.; Wan, Y.; Zong, L.; Li, W.; Xu, X.; Zhao, Y.; Hu, X.; Zuo, Y.; Xu, B.; Tong, X.; et al. Association of Alcohol Intake and Semen Parameters in Men With Primary and Secondary Infertility: A Cross-Sectional Study. Front. Physiol. 2020, 11, 566625. [Google Scholar] [CrossRef]
- Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Pederzoli, F.; Cazzaniga, W.; Chierigo, F.; Deho, F.; Montanari, E.; Montorsi, F.; Salonia, A. Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J. Androl. 2019, 21, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.R.; Bhambhvani, H.P.; Basran, S.S.; Salazar, B.P.; Rios, L.C.; Li, S.J.; Chen, C.H.; Mochly-Rosen, D.; Eisenberg, M.L. ALDH2 Expression, Alcohol Intake and Semen Parameters among East Asian Men. J. Urol. 2022, 208, 406–413. [Google Scholar] [CrossRef]
- Mai, H.; Ke, J.; Zheng, Z.; Luo, J.; Li, M.; Qu, Y.; Jiang, F.; Cai, S.; Zuo, L. Association of diet and lifestyle factors with semen quality in male partners of Chinese couples preparing for pregnancy. Reprod. Health 2023, 20, 173. [Google Scholar] [CrossRef]
- Karmon, A.E.; Toth, T.L.; Chiu, Y.H.; Gaskins, A.J.; Tanrikut, C.; Wright, D.L.; Hauser, R.; Chavarro, J.E.; Earth Study, T. Male caffeine and alcohol intake in relation to semen parameters and in vitro fertilization outcomes among fertility patients. Andrology 2017, 5, 354–361. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Fontana, V.A.; Galotto, C.; Cambiasso, M.Y.; Sobarzo, C.M.A.; Calvo, L.; Calvo, J.C.; Cebral, E. Murine sperm capacitation, oocyte penetration and decondensation following moderate alcohol intake. Reproduction 2018, 155, 529–541. [Google Scholar] [CrossRef]
- Borges, E., Jr.; Braga, D.; Provenza, R.R.; Figueira, R.C.S.; Iaconelli, A., Jr.; Setti, A.S. Paternal lifestyle factors in relation to semen quality and in vitro reproductive outcomes. Andrologia 2018, 50, e13090. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Al Beitawi, S.; Cipriani, S.; Candiani, M.; Chiaffarino, F.; Vigano, P.; Noli, S.; Parazzini, F. Semen quality and alcohol intake: A systematic review and meta-analysis. Reprod. Biomed. Online 2017, 34, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chan, C.P.S.; Waters, T.; Chi, L.; Chan, D.Y.L.; Li, T.C. Lifestyle and demographic factors associated with human semen quality and sperm function. Syst. Biol. Reprod. Med. 2018, 64, 358–367. [Google Scholar] [CrossRef]
- Hu, R.; Yang, X.; Wang, L.; Su, D.; He, Z.; Li, J.; Gong, J.; Zhang, W.; Ma, S.; Shi, M.; et al. Gut microbiota dysbiosis and oxidative damage in high-fat diet-induced impairment of spermatogenesis: Role of protocatechuic acid intervention. Food Front. 2024, 5, 2566–2578. [Google Scholar] [CrossRef]
- Duca, Y.; Aversa, A.; Condorelli, R.A.; Calogero, A.E.; La Vignera, S. Substance Abuse and Male Hypogonadism. J. Clin. Med. 2019, 8, 732. [Google Scholar] [CrossRef] [PubMed]
- Aliabad, M.K.; Nassiri, M.; Kor, K. Microplastics in the surface seawaters of Chabahar Bay, Gulf of Oman (Makran Coasts). Mar. Pollut. Bull. 2019, 143, 125–133. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, Y.; Xu, H. Trojan horse in the intestine: A review on the biotoxicity of microplastics combined environmental contaminants. J. Hazard. Mater. 2022, 439, 129652. [Google Scholar] [CrossRef]
- Cortes-Arriagada, D.; Ortega, D.E.; Miranda-Rojas, S. Mechanistic insights into the adsorption of endocrine disruptors onto polystyrene microplastics in water. Environ. Pollut. 2023, 319, 121017. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Ijaz, M.U.; Rafi, Z.; Hamza, A.; Sayed, A.A.; Albadrani, G.M.; Al-Ghadi, M.Q.; Abdel-Daim, M.M. Mitigative potential of kaempferide against polyethylene microplastics induced testicular damage by activating Nrf-2/Keap-1 pathway. Ecotoxicol. Environ. Saf. 2024, 269, 115746. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.I.; An, Y.J. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J. Hazard. Mater. 2021, 402, 124034. [Google Scholar] [CrossRef]
- Li, T.; Bian, B.; Ji, R.; Zhu, X.; Wo, X.; Song, Q.; Li, Z.; Wang, F.; Jia, Y. Polyethylene Terephthalate Microplastic Exposure Induced Reproductive Toxicity Through Oxidative Stress and p38 Signaling Pathway Activation in Male Mice. Toxics 2024, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Ge, S.J.; Su, Q.L.; Chen, J.J.; Wu, J.; Kang, K. Effects of Polyvinyl Chloride Microplastics on the Reproductive System, Intestinal Structure, and Microflora in Male and Female Mice. Vet. Sci. 2024, 11, 488. [Google Scholar] [CrossRef]
- Zheng, Y.; Nowack, B. Meta-analysis of Bioaccumulation Data for Nondissolvable Engineered Nanomaterials in Freshwater Aquatic Organisms. Environ. Toxicol. Chem. 2022, 41, 1202–1214. [Google Scholar] [CrossRef]
- Hu, M.; Palic, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef]
- Ji, Z.; Huang, Y.; Feng, Y.; Johansen, A.; Xue, J.; Tremblay, L.A.; Li, Z. Effects of pristine microplastics and nanoplastics on soil invertebrates: A systematic review and meta-analysis of available data. Sci. Total Environ. 2021, 788, 147784. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yan, M.; Pan, C.; Liu, Z.; Sha, X.; Jiang, C.; Li, L.; Pan, M.; Li, D.; Han, X.; et al. Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part. Fibre Toxicol. 2022, 19, 13. [Google Scholar] [CrossRef]
- Lee, S.; Kang, K.K.; Sung, S.E.; Choi, J.H.; Sung, M.; Seong, K.Y.; Lee, S.; Yang, S.Y.; Seo, M.S.; Kim, K. Toxicity Study and Quantitative Evaluation of Polyethylene Microplastics in ICR Mice. Polymers 2022, 14, 402. [Google Scholar] [CrossRef]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef]
- Park, E.J.; Han, J.S.; Park, E.J.; Seong, E.; Lee, G.H.; Kim, D.W.; Son, H.Y.; Han, H.Y.; Lee, B.S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Shahzadi, S.; Samad, A.; Ehsan, N.; Ahmed, H.; Tahir, A.; Rehman, H.; Anwar, H. Dose-Dependent Effect of Polystyrene Microplastics on the Testicular Tissues of the Male Sprague Dawley Rats. Dose Response 2021, 19, 15593258211019882. [Google Scholar] [CrossRef]
- Fu, G.; Wu, Q.; Dai, J.; Lu, S.; Zhou, T.; Yang, Z.; Shi, Y. piRNA array analysis provide insight into the mechanism of DEHP-induced testicular toxicology in pubertal male rats. Ecotoxicol. Environ. Saf. 2024, 287, 117282. [Google Scholar] [CrossRef]
- Han, B.; Hua, L.; Yu, S.; Ge, W.; Huang, C.; Tian, Y.; Li, C.; Yan, J.; Qiao, T.; Guo, J.; et al. Revealing the core suppression effects of various Di (2-ethylhexyl) phthalate exposure on early meiosis progression in postnatal male mice via single-cell RNA sequencing. Ecotoxicol. Environ. Saf. 2025, 291, 117866. [Google Scholar] [CrossRef]
- Kitaoka, M.; Hirai, S.; Terayama, H.; Naito, M.; Qu, N.; Hatayama, N.; Miyaso, H.; Matsuno, Y.; Komiyama, M.; Itoh, M.; et al. Effects on the local immunity in the testis by exposure to di-(2-ethylhexyl) phthalate (DEHP) in mice. J. Reprod. Dev. 2013, 59, 485–490. [Google Scholar] [CrossRef]
- Bolling, A.K.; Ovrevik, J.; Samuelsen, J.T.; Holme, J.A.; Rakkestad, K.E.; Mathisen, G.H.; Paulsen, R.E.; Korsnes, M.S.; Becher, R. Mono-2-ethylhexylphthalate (MEHP) induces TNF-alpha release and macrophage differentiation through different signalling pathways in RAW264.7 cells. Toxicol. Lett. 2012, 209, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Jin, Z.; Zhao, Q.; Weng, J.; Zhang, Z.; Yang, Y.; Jiang, H. Multi-omics revealed activation of TNF-alpha induced apoptosis signaling pathway in testis of DEHP treated prepubertal male rat. Reprod. Toxicol. 2025, 132, 108758. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lin, R.; Wu, F.; Sun, Q.; Jia, L. Decreased Capacity for Sperm Production Induced by Perinatal Bisphenol A Exposure Is Associated with an Increased Inflammatory Response in the Offspring of C57BL/6 Male Mice. Int. J. Environ. Res. Public Health 2018, 15, 2158. [Google Scholar] [CrossRef]
- Shi, M.; Lin, Z.; Ye, L.; Chen, X.; Zhang, W.; Zhang, Z.; Luo, F.; Liu, Y.; Shi, M. Estrogen receptor-regulated SOCS3 modulation via JAK2/STAT3 pathway is involved in BPF-induced M1 polarization of macrophages. Toxicology 2020, 433–434, 152404. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, S.; Tan, L.; Gao, X.; Fan, W.; Ding, C.; Li, M.; Tang, Z.; Shi, X.; Luo, Y.; et al. Testicular toxicity of bisphenol compounds: Homeostasis disruption of cholesterol/testosterone via PPARalpha activation. Sci. Total Environ. 2022, 836, 155628. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dong, Y.; Tian, E.; Xie, L.; Wang, G.; Li, X.; Chen, X.; Chen, Y.; Lv, Y.; Ni, C.; et al. 4-Bromodiphenyl ether delays pubertal Leydig cell development in rats. Chemosphere 2018, 211, 986–997. [Google Scholar] [CrossRef]
- Wu, D.; Huang, C.J.; Jiao, X.F.; Ding, Z.M.; Zhang, S.X.; Miao, Y.L.; Huo, L.J. Bisphenol AF compromises blood-testis barrier integrity and sperm quality in mice. Chemosphere 2019, 237, 124410. [Google Scholar] [CrossRef]
- Hassine, M.B.H.; Venditti, M.; Rhouma, M.B.; Minucci, S.; Messaoudi, I. Combined effect of polystyrene microplastics and cadmium on rat blood-testis barrier integrity and sperm quality. Environ. Sci. Pollut. Res. Int. 2023, 30, 56700–56712. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Qiao, B.; Zhong, G.; Li, T.; Su, Q.; Wu, S.; Tang, Z.; Hu, L. Arsenic and polystyrene-nano plastics co-exposure induced testicular toxicity: Triggers oxidative stress and promotes apoptosis and inflammation in mice. Environ. Toxicol. 2024, 39, 264–276. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Huang, Y.; Ren, H.; Zhang, Y. Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (Mus musculus). J. Hazard. Mater. 2021, 406, 124644. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, W.; Jiang, X.; Yu, Z.; Xia, Y.; Cheng, S.; Mao, L.; Luo, S.; Tang, S.; Xu, S.; et al. Polystyrene nanoparticles enhance the adverse effects of di-(2-ethylhexyl) phthalate on male reproductive system in mice. Ecotoxicol. Environ. Saf. 2022, 245, 114104. [Google Scholar] [CrossRef]
- Ma, T.; Liu, X.; Xiong, T.; Li, H.; Zhou, Y.; Liang, J. Polystyrene nanoplastics aggravated dibutyl phthalate-induced blood-testis barrier dysfunction via suppressing autophagy in male mice. Ecotoxicol. Environ. Saf. 2023, 264, 115403. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Q.; Yang, N.; Xu, S. Polystyrene-microplastics and DEHP co-exposure induced DNA damage, cell cycle arrest and necroptosis of ovarian granulosa cells in mice by promoting ROS production. Sci. Total Environ. 2023, 871, 161962. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, Y.; Yao, C.; Yu, S.; Qu, J.; Chen, G.; Wei, H. Combined effects of polystyrene nanoplastics and lipopolysaccharide on testosterone biosynthesis and inflammation in mouse testis. Ecotoxicol. Environ. Saf. 2024, 273, 116180. [Google Scholar] [CrossRef]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Li, S.; Wang, Q.; Yu, H.; Yang, L.; Sun, Y.; Xu, N.; Wang, N.; Lei, Z.; Hou, J.; Jin, Y.; et al. Polystyrene microplastics induce blood-testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 47921–47931. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.; Le Goic, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J. Exposure to polystyrene microplastics impairs gonads of zebrafish (Danio rerio). Chemosphere 2021, 263, 128161. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Z.; Ji, S.; Lo, H.S.; Billah, B.; Sharmin, A.; Han, X.; Lui, W.Y.; Tse, W.K.F.; Fang, J.K.; et al. Size-dependent deleterious effects of nano- and microplastics on sperm motility. Toxicology 2024, 506, 153834. [Google Scholar] [CrossRef]
- Wen, Y.; Cai, J.; Zhang, H.; Li, Y.; Yu, M.; Liu, J.; Han, F. The Potential Mechanisms Involved in the Disruption of Spermatogenesis in Mice byNanoplastics and Microplastics. Biomedicines 2024, 12, 1714. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, C.; Xiong, Y. Polystyrene microplastics induce male reproductive toxicity in mice by activating spermatogonium mitochondrial oxidative stress and apoptosis. Chem. Biol. Interact. 2024, 396, 111043. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Bao, T.T.; Lan, H. Long-term exposure to polystyrene microplastics triggers premature testicular aging. Part Fibre Toxicol. 2023, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, A.; Sisman, A.B.; Gunaydin, S.; Balci, B.P. Sleep Disorders in Patients with Epilepsy. Noro Psikiyatr Ars. 2025, 62, 172–178. [Google Scholar] [PubMed]
- Ford, E.S.; Cunningham, T.J.; Croft, J.B. Trends in Self-Reported Sleep Duration among US Adults from 1985 to 2012. Sleep 2015, 38, 829–832. [Google Scholar] [CrossRef]
- Geng, T.; Li, X.; Ma, H.; Heianza, Y.; Qi, L. Adherence to a Healthy Sleep Pattern and Risk of Chronic Kidney Disease: The UK Biobank Study. Mayo Clin. Proc. 2022, 97, 68–77. [Google Scholar] [CrossRef]
- Bo, Y.; Yeoh, E.K.; Guo, C.; Zhang, Z.; Tam, T.; Chan, T.C.; Chang, L.Y.; Lao, X.Q. Sleep and the Risk of Chronic Kidney Disease: A Cohort Study. J. Clin. Sleep. Med. 2019, 15, 393–400. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Z.; Tang, Z.; Wang, R.; Wu, J.; Na, N.; Zhang, J. Insomnia and sleep duration for kidney function: Mendelian randomization study. Ren. Fail. 2024, 46, 2387430. [Google Scholar] [CrossRef]
- Jiang, B.; Tang, D.; Dai, N.; Huang, C.; Liu, Y.; Wang, C.; Peng, J.; Qin, G.; Yu, Y.; Chen, J. Association of Self-Reported Nighttime Sleep Duration with Chronic Kidney Disease: China Health and Retirement Longitudinal Study. Am. J. Nephrol. 2023, 54, 249–257. [Google Scholar] [CrossRef]
- Yan, B.; Yu, J.; Fang, Q.; Qiu, H.; Shen, C.; Wang, J.; Li, J.; Huang, Y.; Dai, L.; Zhi, Y.; et al. Association between kidney stones and poor sleep factors in U.S. adults. Medicine 2024, 103, e38210. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.Q.; Yu, C.Q.; Guo, Y.; Pei, P.; Chen, J.S.; Chen, Z.M.; Lyu, J.; Li, L. Associations between sleep status and risk for kidney stones in Chinese adults: A prospective cohort study. Zhonghua Liu Xing Bing Xue Za Zhi 2022, 43, 1002–1009. [Google Scholar]
- Li, J.; Huang, Z.; Hou, J.; Sawyer, A.M.; Wu, Z.; Cai, J.; Curhan, G.; Wu, S.; Gao, X. Sleep and CKD in Chinese Adults: A Cross-Sectional Study. Clin. J. Am. Soc. Nephrol. 2017, 12, 885–892. [Google Scholar] [CrossRef]
- Lozano-Lorca, M.; Olmedo-Requena, R.; Vega-Galindo, M.V.; Vazquez-Alonso, F.; Jimenez-Pacheco, A.; Salcedo-Bellido, I.; Sanchez, M.J.; Jimenez-Moleon, J.J. Night Shift Work, Chronotype, Sleep Duration, and Prostate Cancer Risk: CAPLIFE Study. Int. J. Environ. Res. Public Health 2020, 17, 6300. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Dong, Q. Association between sleep quality and benign prostate hyperplasia among middle-aged and older men in India. BMC Public Health 2023, 23, 1147. [Google Scholar] [CrossRef] [PubMed]
- Du, C.Q.; Yang, Y.Y.; Chen, J.; Feng, L.; Lin, W.Q. Association Between Sleep Quality and Semen Parameters and Reproductive Hormones: A Cross-Sectional Study in Zhejiang, China. Nat. Sci. Sleep 2020, 12, 11–18. [Google Scholar] [CrossRef]
- Kyrkou, K.; Alevrakis, E.; Baou, K.; Alchanatis, M.; Poulopoulou, C.; Kanopoulos, C.; Vagiakis, E.; Dikeos, D. Impaired Human Sexual and Erectile Function Affecting Semen Quality, in Obstructive Sleep Apnea: A Pilot Study. J. Pers. Med. 2022, 12, 980. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, H.; Zhou, N.; Sun, L.; Bao, H.; Tan, L.; Chen, H.; Ling, X.; Zhang, G.; Huang, L.; et al. Inverse U-shaped Association between Sleep Duration and Semen Quality: Longitudinal Observational Study (MARHCS) in Chongqing, China. Sleep 2016, 39, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gulec, M.; Ozkol, H.; Selvi, Y.; Tuluce, Y.; Aydin, A.; Besiroglu, L.; Ozdemir, P.G. Oxidative stress in patients with primary insomnia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 37, 247–251. [Google Scholar] [CrossRef]
- Alvarez, J.D.; Hansen, A.; Ord, T.; Bebas, P.; Chappell, P.E.; Giebultowicz, J.M.; Williams, C.; Moss, S.; Sehgal, A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J. Biol. Rhythm. 2008, 23, 26–36. [Google Scholar] [CrossRef]
- Hvidt, J.E.M.; Knudsen, U.B.; Zachariae, R.; Ingerslev, H.J.; Philipsen, M.T.; Frederiksen, Y. Associations of bedtime, sleep duration, and sleep quality with semen quality in males seeking fertility treatment: A preliminary study. Basic. Clin. Androl. 2020, 30, 5. [Google Scholar] [CrossRef]
- Liu, P.Y. Light pollution: Time to consider testicular effects. Front. Toxicol. 2024, 6, 1481385. [Google Scholar] [CrossRef]
- Gaml-Sorensen, A.; Frolich, M.K.; Brix, N.; Ernst, A.; Bonde, J.P.E.; Hougaard, K.S.; Tottenborg, S.S.; Clemmensen, P.J.; Toft, G.; Ramlau-Hansen, C.H. Sleep duration and biomarkers of fecundity in young men: A cross-sectional study from a population-based cohort. Andrology 2024, 12, 1125–1136. [Google Scholar] [CrossRef]
- Vigano, P.; Chiaffarino, F.; Bonzi, V.; Salonia, A.; Ricci, E.; Papaleo, E.; Mauri, P.A.; Parazzini, F. Sleep disturbances and semen quality in an Italian cross sectional study. Basic. Clin. Androl. 2017, 27, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, X.; Zhang, Y.; Liu, G.; Wu, X.; Huang, H.; Jiang, H.; Zhang, X. Attenuation Effect of Recovery Sleep for Impaired Reproductive Function in Male Rats by Sleep Deprivation. World J. Men’s Health 2023, 41, 671–679. [Google Scholar] [CrossRef]
- Wilmot, E.G.; Edwardson, C.L.; Achana, F.A.; Davies, M.J.; Gorely, T.; Gray, L.J.; Khunti, K.; Yates, T.; Biddle, S.J. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Erratum in Diabetologia 2012, 55, 2895–2905. [CrossRef]
- Dunstan, D.W.; Howard, B.; Healy, G.N.; Owen, N. Too much sitting—A health hazard. Diabetes Res. Clin. Pract. 2012, 97, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Heydari, H.; Ghiasi, R.; Ghaderpour, S.; Keyhanmanesh, R. The Mechanisms Involved in Obesity-Induced Male Infertility. Curr. Diabetes Rev. 2021, 17, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Glavinovic, T.; Ferguson, T.; Komenda, P.; Rigatto, C.; Duhamel, T.A.; Tangri, N.; Bohm, C. CKD and Sedentary Time: Results from the Canadian Health Measures Survey. Am. J. Kidney Dis. 2018, 72, 529–537. [Google Scholar] [CrossRef]
- Hannan, M.; Ricardo, A.C.; Cai, J.; Franceschini, N.; Kaplan, R.; Marquez, D.X.; Rosas, S.E.; Schneiderman, N.; Sotres-Alvarez, D.; Talavera, G.A.; et al. Sedentary Behavior and Change in Kidney Function: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Kidney360 2021, 2, 245–253. [Google Scholar] [CrossRef]
- Bharakhada, N.; Yates, T.; Davies, M.J.; Wilmot, E.G.; Edwardson, C.; Henson, J.; Webb, D.; Khunti, K. Association of sitting time and physical activity with CKD: A cross-sectional study in family practices. Am. J. Kidney Dis. 2012, 60, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Park, Y.S.; Kim, H.; Hurh, K.; Park, E.C.; Jang, S.Y. Association between sedentary behavior and chronic kidney disease in Korean adults. BMC Public Health 2023, 23, 306. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Di, X.; Liu, M.; Wei, J.; Li, T.; Liao, B. Association between daily sitting time and kidney stones based on the National Health and Nutrition Examination Survey (NHANES) 2007–2016: A cross-sectional study. Int. J. Surg. 2024, 110, 4624–4632. [Google Scholar] [CrossRef]
- Mondul, A.M.; Giovannucci, E.; Platz, E.A. A Prospective Study of Physical Activity, Sedentary Behavior, and Incidence and Progression of Lower Urinary Tract Symptoms. J. Gen. Intern. Med. 2020, 35, 2281–2288. [Google Scholar] [CrossRef]
- Foucaut, A.M.; Faure, C.; Julia, C.; Czernichow, S.; Levy, R.; Dupont, C.; ALIFERT collaborative group. Sedentary behavior, physical inactivity and body composition in relation to idiopathic infertility among men and women. PLoS ONE 2019, 14, e0210770. [Google Scholar] [CrossRef]
- Priskorn, L.; Jensen, T.K.; Bang, A.K.; Nordkap, L.; Joensen, U.N.; Lassen, T.H.; Olesen, I.A.; Swan, S.H.; Skakkebaek, N.E.; Jorgensen, N. Is Sedentary Lifestyle Associated with Testicular Function? A Cross-Sectional Study of 1,210 Men. Am. J. Epidemiol. 2016, 184, 284–294. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Afeiche, M.C.; Hauser, R.; Williams, P.L.; Gillman, M.W.; Tanrikut, C.; Petrozza, J.C.; Chavarro, J.E. Paternal physical and sedentary activities in relation to semen quality and reproductive outcomes among couples from a fertility center. Hum. Reprod. 2014, 29, 2575–2582. [Google Scholar] [CrossRef]
- Sun, B.; Messerlian, C.; Sun, Z.H.; Duan, P.; Chen, H.G.; Chen, Y.J.; Wang, P.; Wang, L.; Meng, T.Q.; Wang, Q.; et al. Physical activity and sedentary time in relation to semen quality in healthy men screened as potential sperm donors. Hum. Reprod. 2019, 34, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Lalinde-Acevedo, P.C.; Mayorga-Torres, B.J.M.; Agarwal, A.; du Plessis, S.S.; Ahmad, G.; Cadavid, A.P.; Cardona Maya, W.D. Physically Active Men Show Better Semen Parameters than Their Sedentary Counterparts. Int. J. Fertil. Steril. 2017, 11, 156–165. [Google Scholar]
- Beddhu, S.; Baird, B.C.; Zitterkoph, J.; Neilson, J.; Greene, T. Physical activity and mortality in chronic kidney disease (NHANES III). Clin. J. Am. Soc. Nephrol. 2009, 4, 1901–1906. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Guo, Z.; Dong, Y.; Yu, B. Prolonged sitting time and all-cause mortality: The mediating and predictive role of kidney function markers. Ren. Fail. 2025, 47, 2486568. [Google Scholar] [CrossRef]
- Schmid, D.; Matthews, C.E.; Leitzmann, M.F. Physical activity and sedentary behavior in relation to mortality among renal cell cancer survivors. PLoS ONE 2018, 13, e0198995. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Wen, C.P.; Wu, S.B.; Lan, J.L.; Tsai, M.K.; Tai, Y.P.; Lee, J.H.; Hsu, C.C.; Tsao, C.K.; Wai, J.P.; et al. Attenuating the mortality risk of high serum uric acid: The role of physical activity underused. Ann. Rheum. Dis. 2015, 74, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Bonn, S.E.; Holmberg, E.; Hugosson, J.; Balter, K. Is leisure time sitting associated with mortality rates among men diagnosed with localized prostate cancer? Eur. J. Cancer Prev. 2020, 29, 134–140. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Lan, M.; Yang, J.; Xia, Y.; Han, L.; Zhang, L.; Fang, Y. Targeting Modifiable Risks: Molecular Mechanisms and Population Burden of Lifestyle Factors on Male Genitourinary Health. Int. J. Mol. Sci. 2025, 26, 9698. https://doi.org/10.3390/ijms26199698
Yang X, Lan M, Yang J, Xia Y, Han L, Zhang L, Fang Y. Targeting Modifiable Risks: Molecular Mechanisms and Population Burden of Lifestyle Factors on Male Genitourinary Health. International Journal of Molecular Sciences. 2025; 26(19):9698. https://doi.org/10.3390/ijms26199698
Chicago/Turabian StyleYang, Xingcheng, Meiping Lan, Jiawen Yang, Yuyi Xia, Linxiang Han, Ling Zhang, and Yu Fang. 2025. "Targeting Modifiable Risks: Molecular Mechanisms and Population Burden of Lifestyle Factors on Male Genitourinary Health" International Journal of Molecular Sciences 26, no. 19: 9698. https://doi.org/10.3390/ijms26199698
APA StyleYang, X., Lan, M., Yang, J., Xia, Y., Han, L., Zhang, L., & Fang, Y. (2025). Targeting Modifiable Risks: Molecular Mechanisms and Population Burden of Lifestyle Factors on Male Genitourinary Health. International Journal of Molecular Sciences, 26(19), 9698. https://doi.org/10.3390/ijms26199698




