Determination of Spectral Characteristics and Moisture Distribution in Wheat Grains After Sorption, Thermal, and Natural Drying
Abstract
1. Introduction
2. Results
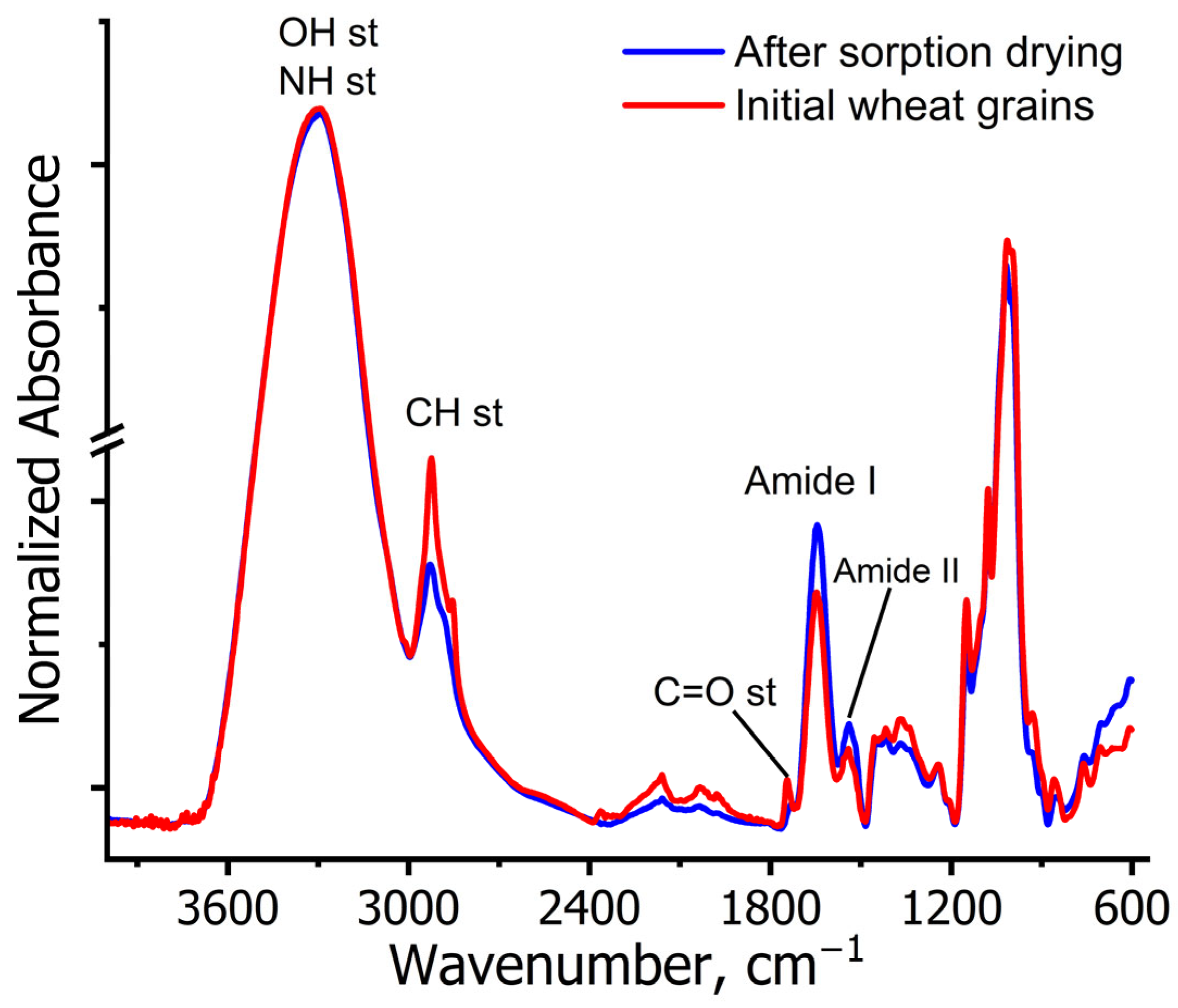
2.1. Infrared Spectroscopy
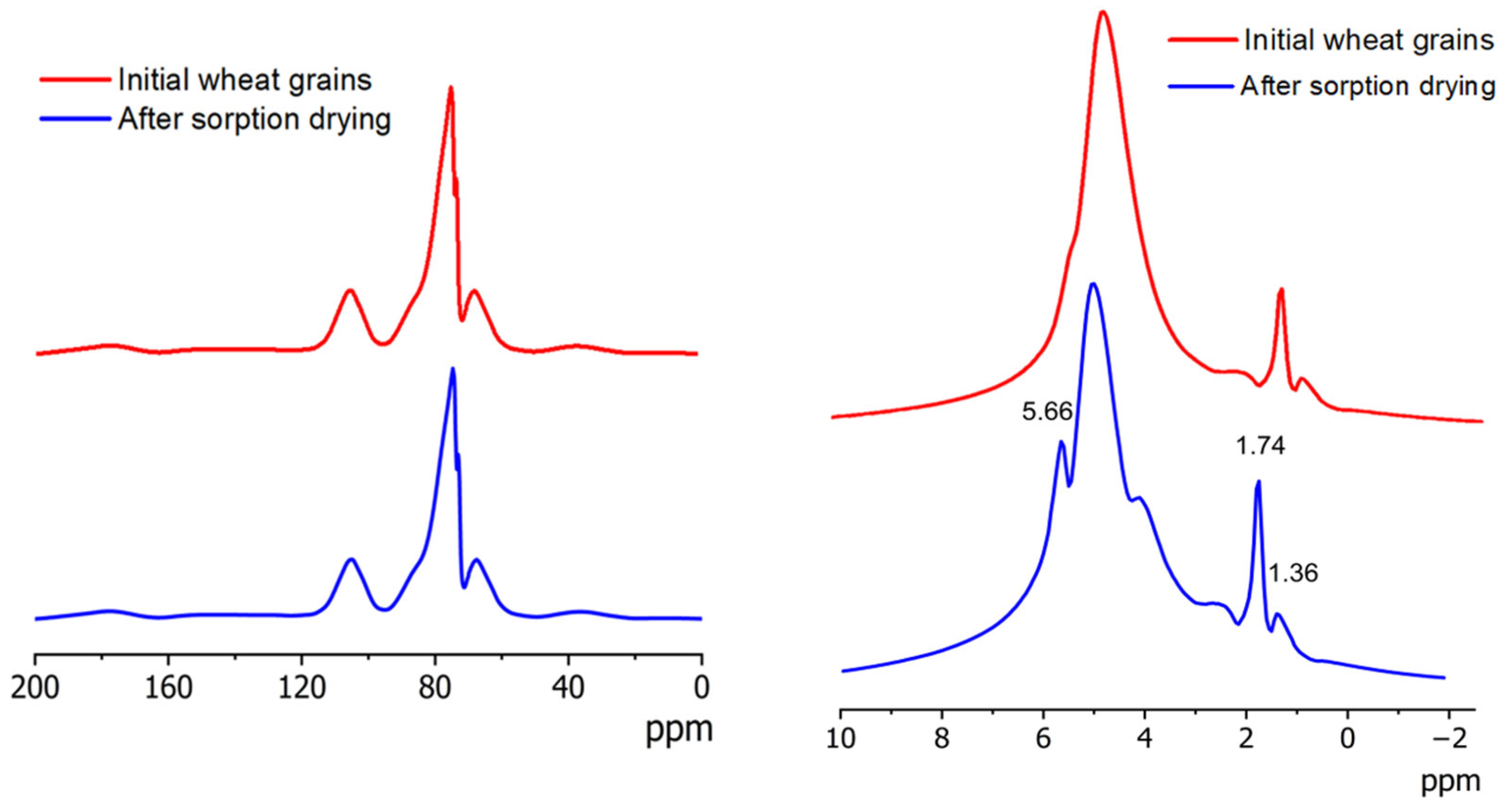
2.2. MAS NMR Spectroscopy
2.3. Photoluminescence Spectroscopy
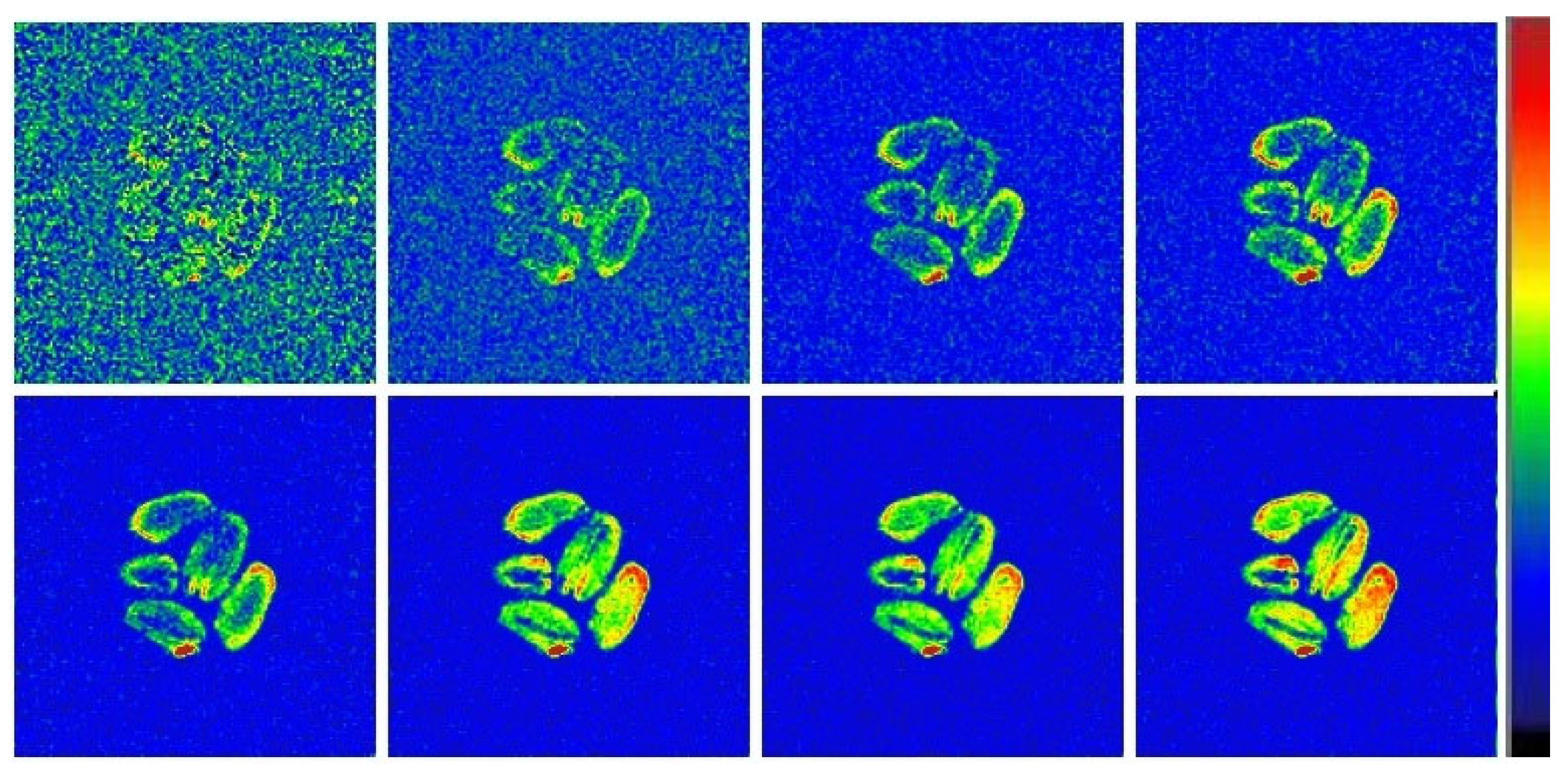
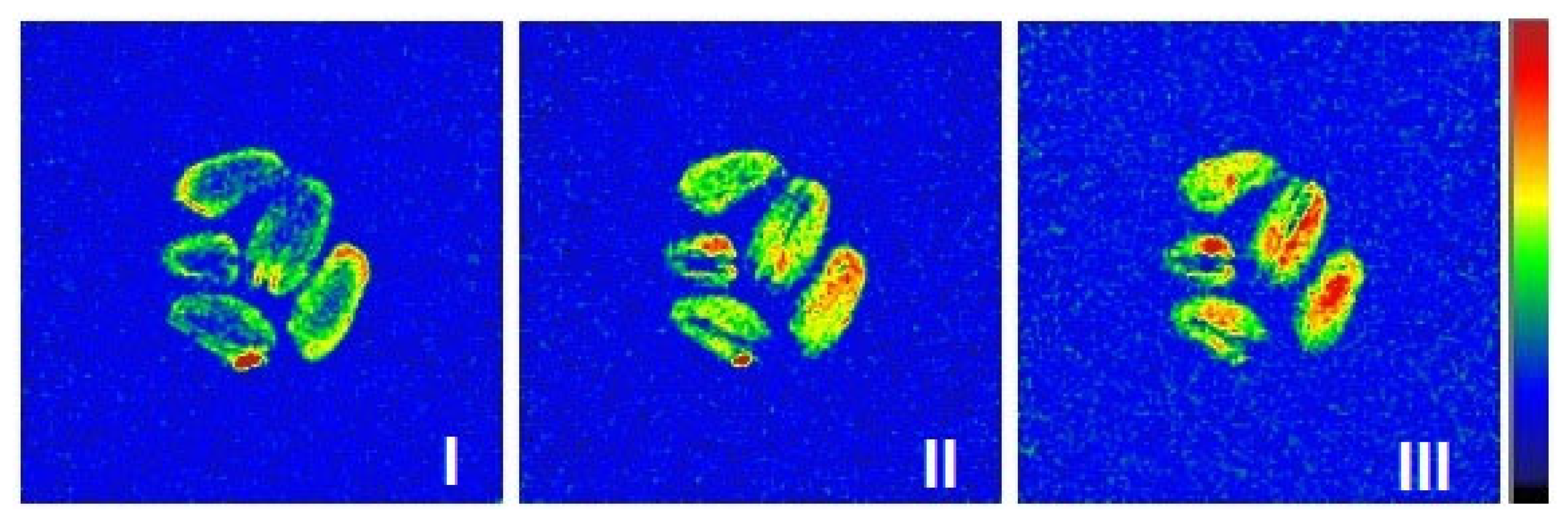
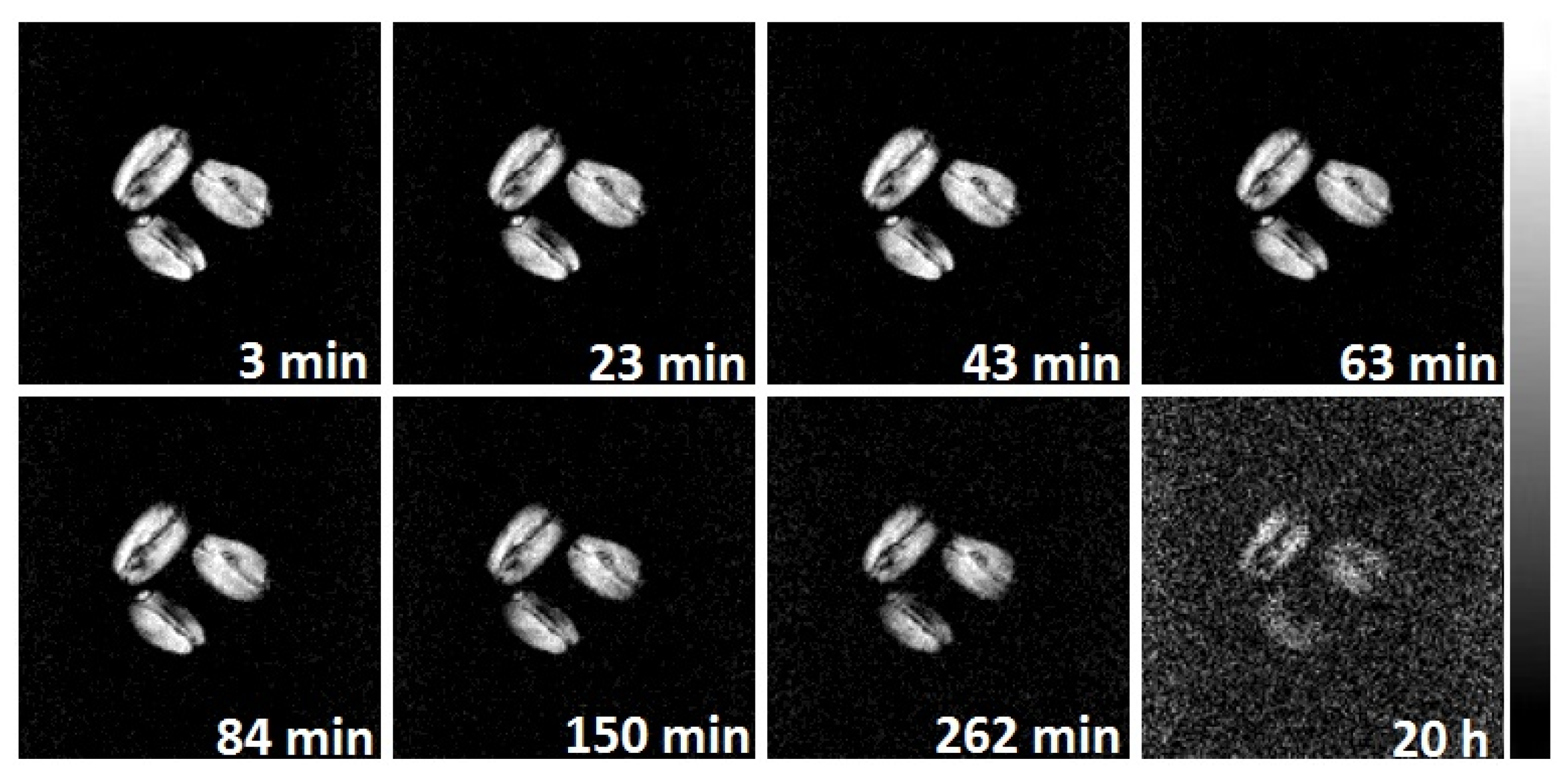
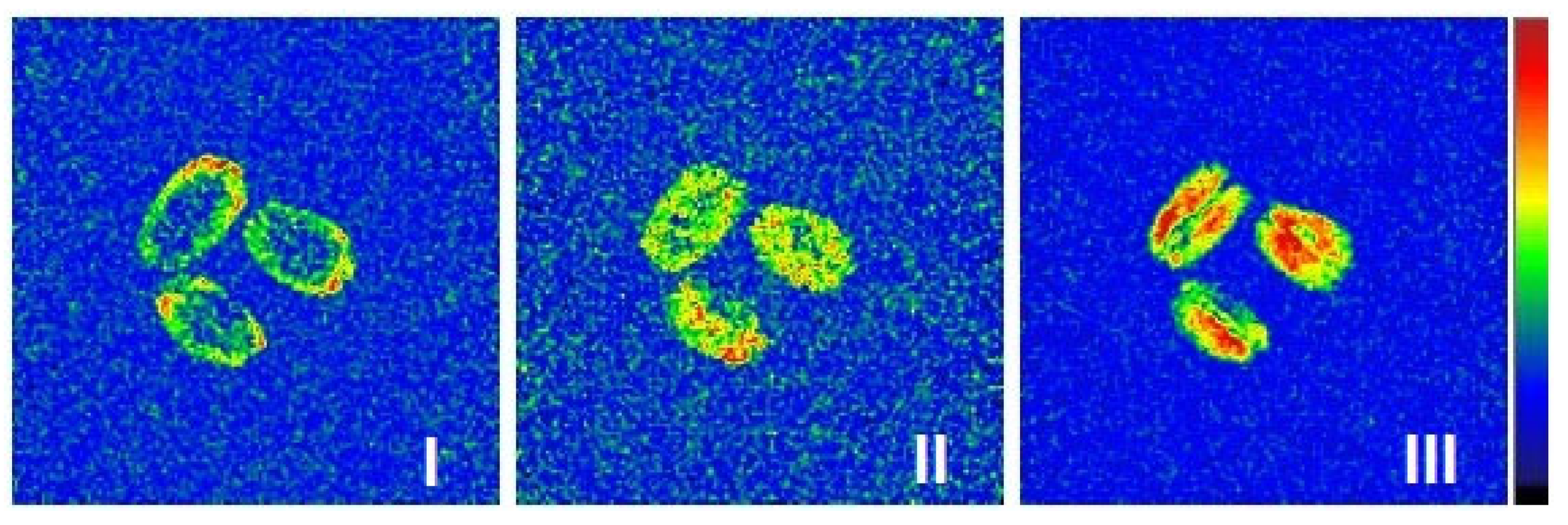
2.4. MRI Visualization
2.4.1. Moisture Visualization of Wet Grains
2.4.2. Moisture Visualization During Grains Drying
3. Discussion
4. Materials and Methods
4.1. Wheat Seeds
4.2. ATR-MIR
4.3. NMR Spectroscopy
4.4. Photoluminescence
4.5. MRI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Y.; Feng, G.; Zhang, H.; Hou, P.; Wang, C.; Chen, L.; Luo, B. Detection of Strigolactone-Treated wheat seeds via Dual-View hyperspectral data fusion and deep learning. Microchem. J. 2025, 215, 114295. [Google Scholar] [CrossRef]
- Song, M.; Zhou, S.; Hu, N.; Li, J.; Huang, Y.; Zhang, J.; Chen, X.; Du, X.; Niu, J.; Yang, X.; et al. Exogenous strigolactones alleviate drought stress in wheat (Triticum aestivum L.) by promoting cell wall biogenesis to optimize root architecture. Plant Physiol. Biochem. 2023, 204, 108121. [Google Scholar] [CrossRef]
- Barrozo, M.A.S. Air-Drying of Seeds: A Review. Dry. Technol. 2014, 32, 1127–1141. [Google Scholar] [CrossRef]
- Jittanit, W.; Srzednicki, G.; Driscoll, R. Corn, Rice, and Wheat Seed Drying by Two-Stage Concept. Dry. Technol. 2010, 28, 807–815. [Google Scholar] [CrossRef]
- Kudra, T. Energy Aspects in Drying. Dry. Technol. 2004, 22, 917–932. [Google Scholar] [CrossRef]
- Kudra, T.; Mujumdar, A.S. Advanced Drying Technologies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Hashim, N.; Onwude, D.I.; Maringgal, B. Technological advances in postharvest management of food grains. In Research and Technological Advances in Food Science Book; Chapter 15; Academic Press: London, UK, 2022; pp. 371–406. [Google Scholar] [CrossRef]
- Motevali, A.; Chayjan, R.A. Effect of various drying bed on thermodynamic characteristics. Case Stud. Therm. Eng. 2017, 10, 399–406. [Google Scholar] [CrossRef]
- Jimoh, K.A.; Hashim, N.; Shamsudin, R.; Man, H.C.; Jahari, M.; Onwude, D.I. Recent Advances in the Drying Process of Grains. Food Eng. Rev. 2023, 15, 548–576. [Google Scholar] [CrossRef]
- Sundareswaran, S.; Ray Choudhury, P.; Vanitha, C.; Yadava, D.K. Seed Quality: Variety Development to Planting—An Overview. In Seed Science and Technology; Dadlani, M., Yadava, D.K., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Shabanov, V.F.; Anshits, A.G. Sorption Drying of Wheat Seeds Using Kieserite as a Solid Desiccan. AgriEngineering 2024, 6, 2023. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Solovyov, L.A.; Shabanov, V.F.; Anshits, A.G. The Preparation and Contact Drying Performance of Encapsulated Microspherical Composite Sorbents Based on Fly Ash Cenospheres. Molecules 2024, 29, 2391. [Google Scholar] [CrossRef]
- Jia, C.; Wang, L.; Li, R.; Liu, C. Experimental study on drying characteristics of wheat by low-field nuclear magnetic resonance. Dry. Technol. 2017, 35, 1258–1265. [Google Scholar] [CrossRef]
- Chang, K.; Li, J.; Jin, Y.; Liu, C. Development of Grain Dryer Control Technology from the Perspective of Low Carbon and Intelligentization. Appl. Sci. 2024, 14, 10587. [Google Scholar] [CrossRef]
- Yang, S.; Cao, Y.; Li, C.; Castagnini, J.M.; Barba, F.J.; Shan, C.; Zhou, J. Enhancing grain drying methods with hyperspectral imaging technology: A visualanalysis. Curr. Res. Food Sci. 2024, 8, 100695. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tian, X.; Yang, B.; Ma, J.; Shan, J.; Xing, F. Analysis of the impact of drying on common wheat quality and safety. Heliyon 2024, 10, e33163. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.J.; Hemdane, S.; Delcour, J.A.; Courtin, C.M. Dry heat treatment affects wheat bran surface properties and hydration kinetics. Food Chem. 2016, 203, 513–520. [Google Scholar] [CrossRef]
- Gordon, A.J.; Ford, R.A. The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References; Wiley: New York, NY, USA, 1972; 560p. [Google Scholar]
- Donkers, P.A.J.; Beckert, S.; Pel, L.; Stallmach, F.; Steiger, M.; Adan, O.C.G. Water Transport in MgSO4·7H2O During Dehydration in View of Thermal Storage. J. Phys. Chem. C 2015, 119, 28711–28720. [Google Scholar] [CrossRef]
- Christou, C.; Agapiou, A.; Kokkinfta, R. Use of FTIR spectroscopy and chemometrics for the classification of carobs origin. J. Adv. Res. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hssaini, L.; Razouk, R.; Charafi, J.; Houmanat, K.; Hanine, H. Fig seeds: Combined approach of lipochemical assessment using gas chromatography and FTIR-ATR spectroscopy using chemometrics. Vib. Spectrosc. 2021, 114, 103251. [Google Scholar] [CrossRef]
- Grasso, R.; Gulino, M.; Giuffrida, F.; Agnello, M.; Musumeci, F.; Scordino, A. Non-destructive evaluation of watermelon seeds germination by using Delayed Luminescence. J. Photochem. Photobiol. B Biol. 2018, 187, 126–130. [Google Scholar] [CrossRef]
- Wang, Y. Principles of Magnetic Resonance Imaging: Physics Concepts, Pulse Sequences, & Biomedical Applications; CreateSpace Independent Publishing Platform (Amazon): Scotts Valley, CA, USA, 2012; 345p. [Google Scholar]
- Morozov, E.V.; Il’ichev, A.V.; Bouznik, V.M. Magnetic Resonance Imaging Study of Water Absorption of Polymer Composite Materials Subjected to Mechanical and Temperature Impact. Russ. J. Phys. Chem. B 2023, 17, 1361–1369. [Google Scholar] [CrossRef]
- Morozov, E.; Novikov, M.; Bouznik, V.; Yurkov, G. NMR imaging of 3D printed biocompatible polymer scaffolds interacting with water. Rapid Prototyp. J. 2019, 25, 1007–1016. [Google Scholar] [CrossRef]
- Morozov, E.V.; Bouznik, V.M.; Bespalov, A.S.; Grashchenkov, D.V. Magnetic Resonance Imaging of Water Absorption by Highly Porous Ceramic Materials. Dokl. Chem. 2019, 484, 44–47. [Google Scholar] [CrossRef]
- Deene, Y.D. New Developments in NMR: NMR and MRI of Gels, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2020; 458p. [Google Scholar]
- Koptyug, I.V.; Sagdeev, R.Z. Applications of NMR tomography to mass transfer studies. Russ. Chem. Rev. 2002, 71, 789–835. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, P.; Xing, H.; Li, L.; Jia, C.; Chen, S.; Wang, L. Water migration and diffusion mechanism in the wheat drying. Dry. Technol. 2020, 39, 738–751. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Jayas, D.S.; Gruwel, M.L.; White, N.D. A Magnetic Resonance Imaging Study of Wheat Drying Kinetics. Biosyst. Eng. 2007, 97, 189–199. [Google Scholar] [CrossRef]
- Vinogradova, I.S.; Falaleev, O.V. Free water in bean seeds: The imbibition process observed by micro-magnetic resonance imaging. J. Struct. Chem. 2016, 57, 812–814. [Google Scholar] [CrossRef]
- Vinogradova, I.S.; Falaleev, O.V. Formation of the vascular system of developing bean (Phaseolus limensis L.) seeds according to nuclear magnetic resonance microtomography. Russ. J. Dev. Biol. 2012, 43, 25–34. [Google Scholar] [CrossRef]
- Gorgulu, S.T.; Dogan, M.; Severcan, F. The Characterization and Differentiation of Higher Plants by Fourier Transform Infrared Spectroscopy. Appl. Spectrosc. 2007, 61, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Tunklova, B.; Sera, M.; Sramkova, P.; Durcanyova, S.; Sery, M.; Kovacik, D.; Zahoranova, A.; Hnilicka, F. Growth stimulation of durum wheat and common buckwheat by non-thermal atmospheric pressure plasma. Plants. 2023, 12, 4172. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Gill, B.S. Changes in physicochemical, nutritional characteristics and ATR–FTIR molecular interactions of cereal grains during germination. J Food Sci Technol. 2021, 58, 2313–2324. [Google Scholar] [CrossRef]
- Demir, P.; Onde, S.; Severcan, F. Phylogeny of cultivated and wild wheat species using ATR–FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Calucci, L.; Galleschi, L.; Geppi, M.; Mollica, G. Structure and dynamics of flour by solid state NMR: Effects of hydration and wheat aging. Biomacromolecules 2004, 5, 1536–1544. [Google Scholar] [CrossRef]
- Webb, G.A.; Belton, P.; Gil, A.M.; Delgadillo, I. Magnetic Resonance in Food Science: A View to the Future; The Royal Society of Chemistry: Cambridge, UK, 2001; pp. 43–53. [Google Scholar] [CrossRef]
- El Nokab, M.E.H.; Alassmy, Y.A.; Abduljawad, M.M.; Al-shamrani, K.M.; Alnafisah, M.S.; Asgar Pour, Z.; Tucker, C.L.; Sebakhy, K.O. Solid-State NMR Spectroscopy: Towards Structural Insights into Starch-Based Materials in the Food Industry. Polymers 2022, 14, 4686. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Z.; Li, M.; Bian, K.; Guan, E. Heat treatment of wheat for improving moisture diffusion and the effects on wheat milling characteristics. J. Cereal Sci. 2023, 114, 103806. [Google Scholar] [CrossRef]
- Mann, J.; Schiedt, B.; Baumann, A.; Conde-Petit, B.; Vilgis, T.A. Effect of heat treatment on wheat dough rheology and wheat protein solubility. Food Sci. Technol. Int. 2013, 20, 341–351. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Vernon-Carter, E.J.; Alvarez-Ramirez, J.; Carrera-Tarela, Y. Effects of dry heat treatment temperature on the structure of wheat flour and starch in vitro digestibility of bread. Int. J. Biol. Macromol. 2021, 166, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Srikiatden, J.; Roberts, J.S. Moisture Transfer in Solid Food Materials: A Review of Mechanisms, Models, and Measurements. Int. J. Food Prop. 2007, 10, 739–777. [Google Scholar] [CrossRef]
- Thorpe, G.R.; Alberto Ochoa Tapia, J.; Whitaker, S. The diffusion of moisture in food grains—I. The development of a mass transport equation. J. Stored Prod. Res. 1991, 27, 1–9. [Google Scholar] [CrossRef]
- Yu, P.; Zhu, W.; Shen, C.; Qiao, Y.; Zhang, W.; Zhu, Y.; Gong, J.; Cai, J. Current Status of Grain Drying Technology and Equipment Development: A Review. Foods 2025, 14, 2426. [Google Scholar] [CrossRef] [PubMed]
- Baidhe, E.; Clementson, C.L. A review of the application of modeling and simulation to drying systems for improved grain and seed quality. Comput. Electron. Agric. 2024, 222, 109094. [Google Scholar] [CrossRef]
- Hu, X.; Wu, P.; Zhang, S.; Chen, S.; Wang, L. Moisture conversion and migration in single-wheat kernel during isothermal drying process by LF-NMR. Dry. Technol. 2018, 37, 803–812. [Google Scholar] [CrossRef]
- GOST 13586.5-2015; Grain. Method of Moisture Content Determination. Standartinform: Moscow, Russia, 2019; 12p. (In Russian)
- ISO 712-1:2024; Cereals and Cereal Products Determination of Moisture Content Reference Method. ISO: Geneva, Switzerland. Available online: https://www.iso.org/ru/standard/85395.html (accessed on 13 February 2025).
- TU 20.13.41-001-23877036-2017; Agrochemical Magnesium Sulfate Grades: Fine-Crystalline Epsomite, Granulated Epsomite, Fine-Crystalline Kieserite, Granulated Kieserite. South Ural Magnesium Compounds Plant: Kuvandyk, Russia, 2017; 22p. (In Russian)
- GOST 12038-84; Seeds of Agricultural Crops. Germination Methods. Publishing of standards: Moscow, Russia, 2004; 29p. (In Russian)
- ISTA. International Rules for Seed Testing (Bassersdorf: International Seed Testing Association). 2005. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing.html (accessed on 13 February 2025).
- ISO 24333:2009; Cereals and Cereal Products—Sampling. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/42165.html (accessed on 13 February 2025).
- GOST 13586.3-2015; Grain. Acceptance Rules and Sampling Methods. Standartinform: Moscow, Russia, 2019; 17p. (In Russian)
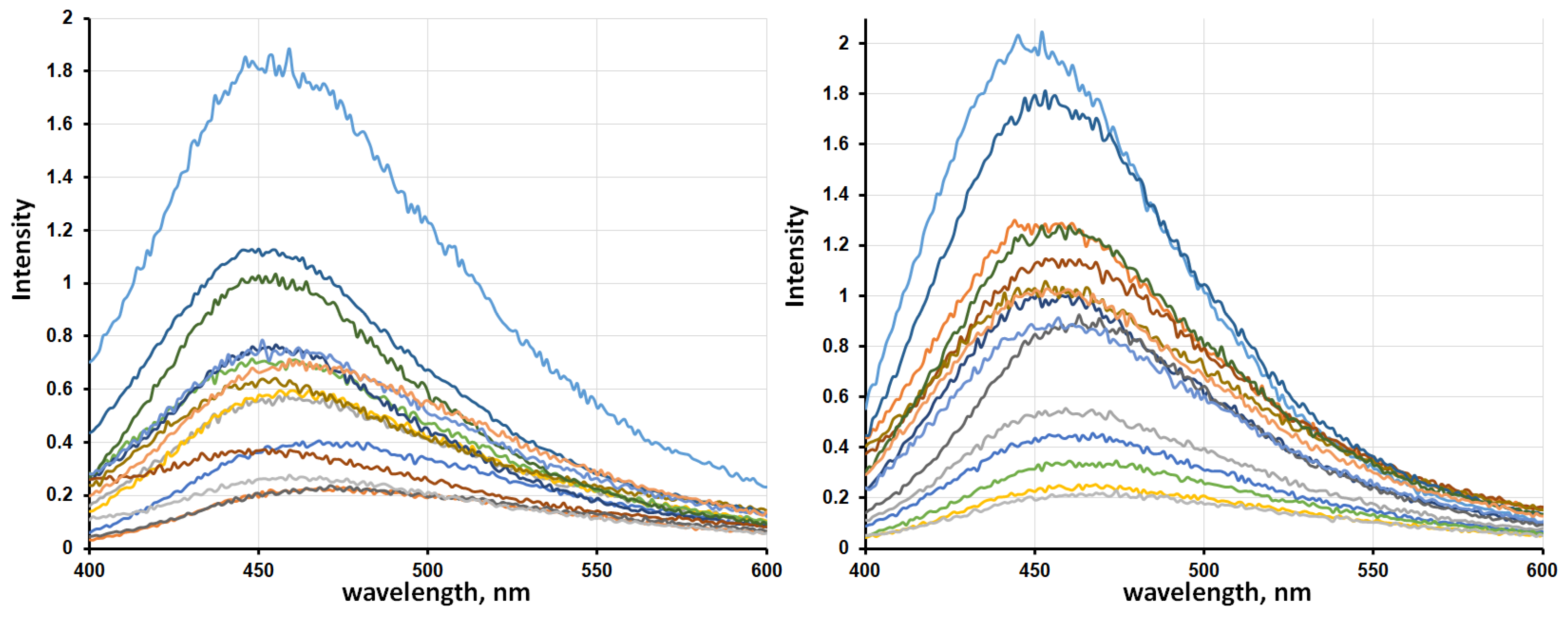
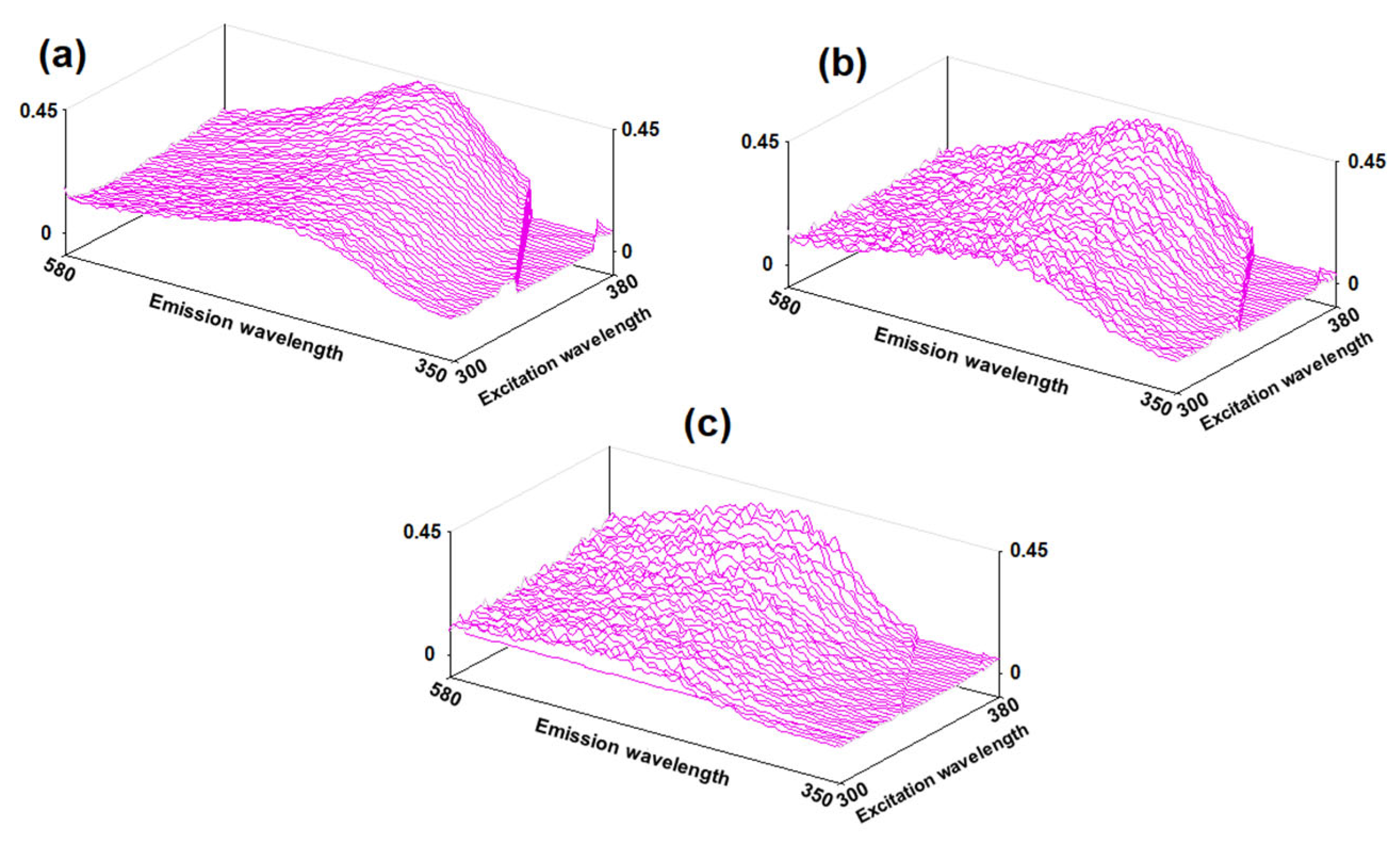




| Sorption Drying | Thermal Drying | ||||||
|---|---|---|---|---|---|---|---|
| N * | λmax, nm | Imax | Half Weight, nm | N * | λmax, nm | Imax | Half Weight, nm |
| 1 | 468 | 0.405 | 120 | 1 | 467 | 0.456 | 103 |
| 2 | 473 | 0.234 | 128 | 2 | 455 | 1.277 | 106 |
| 3 | 458 | 0.580 | 109 | 3 | 459 | 0.555 | 102 |
| 4 | 460 | 0.596 | 112 | 4 | 468 | 0.253 | 112 |
| 5 | 459 | 1.886 | 106 | 5 | 452 | 2.047 | 86 |
| 6 | 460 | 0.708 | 113 | 6 | 474 | 0.347 | 109 |
| 7 | 454 | 0.374 | 126 | 7 | 458 | 1.442 | 84 |
| 8 | 455 | 0.769 | 98 | 8 | 458 | 0.999 | 97 |
| 9 | 471 | 0.237 | 78 | 9 | 463 | 0.926 | 89 |
| 10 | 455 | 0.641 | 109 | 10 | 453 | 1.056 | 111 |
| 11 | 450 | 1.129 | 104 | 11 | 453 | 1.815 | 93 |
| 12 | 455 | 1.036 | 93 | 12 | 457 | 1.277 | 98 |
| 13 | 458 | 0.753 | 113 | 13 | 457 | 0.914 | 99 |
| 14 | 460 | 0.691 | 119 | 14 | 460 | 1.025 | 101 |
| 15 | 459 | 0.279 | 122 | 15 | 474 | 0.231 | 118 |
| Mean | 459 | 0.688 | 110 | Mean | 461 | 0.970 | 100 |
| S | 1.65 | 0.11 | 3.25 | S | 1.61 | 0.13 | 2.23 |
| ±Δ | 3.53 | 0.23 | 6.95 | ±Δ | 3.44 | 0.28 | 4.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanenko, T.Y.; Fomenko, E.V.; Morozov, E.V.; Matsulev, A.N.; Lutoshkin, M.A.; Shestakov, N.P.; Shabanov, V.F. Determination of Spectral Characteristics and Moisture Distribution in Wheat Grains After Sorption, Thermal, and Natural Drying. Int. J. Mol. Sci. 2025, 26, 8952. https://doi.org/10.3390/ijms26188952
Ivanenko TY, Fomenko EV, Morozov EV, Matsulev AN, Lutoshkin MA, Shestakov NP, Shabanov VF. Determination of Spectral Characteristics and Moisture Distribution in Wheat Grains After Sorption, Thermal, and Natural Drying. International Journal of Molecular Sciences. 2025; 26(18):8952. https://doi.org/10.3390/ijms26188952
Chicago/Turabian StyleIvanenko, Timur Yu., Elena V. Fomenko, Evgeny V. Morozov, Aleksander N. Matsulev, Maxim A. Lutoshkin, Nicolay P. Shestakov, and Vasiliy F. Shabanov. 2025. "Determination of Spectral Characteristics and Moisture Distribution in Wheat Grains After Sorption, Thermal, and Natural Drying" International Journal of Molecular Sciences 26, no. 18: 8952. https://doi.org/10.3390/ijms26188952
APA StyleIvanenko, T. Y., Fomenko, E. V., Morozov, E. V., Matsulev, A. N., Lutoshkin, M. A., Shestakov, N. P., & Shabanov, V. F. (2025). Determination of Spectral Characteristics and Moisture Distribution in Wheat Grains After Sorption, Thermal, and Natural Drying. International Journal of Molecular Sciences, 26(18), 8952. https://doi.org/10.3390/ijms26188952






