Metabolic Modulators in Depression: Emerging Molecular Mechanisms and Therapeutic Opportunities
Abstract
1. Introduction
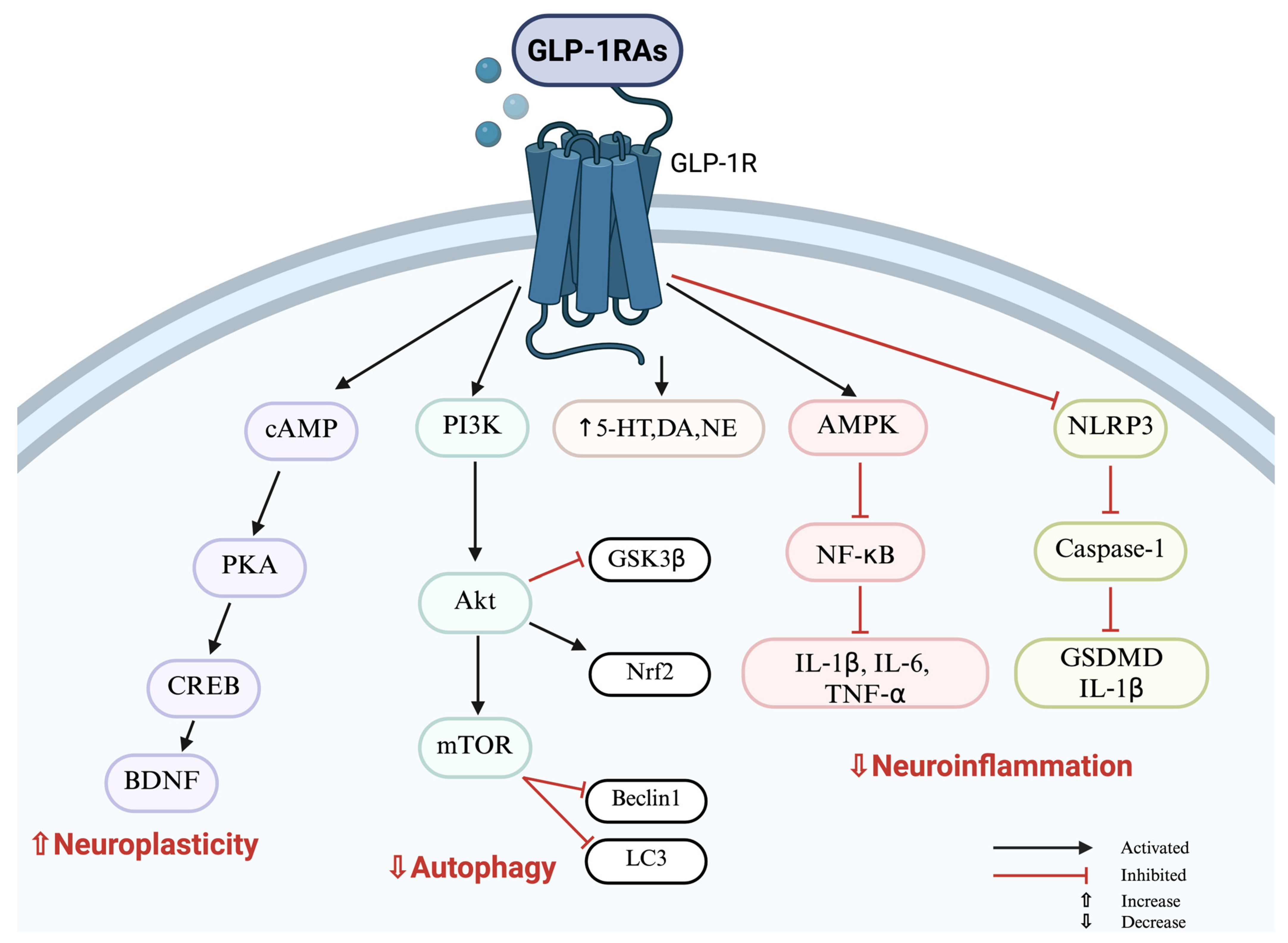
2. GLP-1 Receptor Agonists: Antidepressant Effect Beyond Weight Loss
2.1. Antidepressant Effects of GLP-1 Receptor Agonists: Evidence from Animal Models
| Species/ Strain/Cell Line | Animal Model/ Treatment | Results-Behavioral Tests | Samples | Methods | Results | References |
|---|---|---|---|---|---|---|
Male C57BL/6 mice (8–10 weeks old) | High Fat Diet (HFD; 12 weeks); Semaglutide (0.05 mg/Kg; i.p.)/Fluoxetine (20 mg/Kg; p.o.; 6 weeks; n = 15) | ↓ Immobility time in the tail suspension test and forced swim test; ↑ The alternation index in T Maze Spontaneous Alternation; ↑ Time spent in the open arm; ↓ time spent in the enclosed arms in elevated plus Maze Test | Hippocampus (HP), distal colon, feces, and serum | ELISA Immunostaining Western blot | ↓ Serum IL-1β and LPS ↑ Gut GLP-1 expression ↓ p-NF-κB, TNF-α, IL-6, IL-1β, nitrotyrosine in HP ↑ Brain 5-HT, 5-HTT, NMDAR1, NMDAR2, Glu2R, p-AMPK in HP ↑ GLP-1R expression HP | [52] |
| Male db/db mice (5 weeks old) | Diabetes-induced depression model; Exendin-4 (5 μg/kg; 4 days; i.p.; n = 9) | ↓ Immobility time in the tail suspension test and forced swim test; ↑ Total distance traveled in the open field test | Hippocampus (HP), Amygdala (AMY), Anterior cingulate cortex (ACC) | ELISA Immunostaining Western blot | ↑ Microglial GLP-1R expression HP ↓ Activation of microglia ↓ ASC, caspase-1, IL-1β, GSDMD, ROS | [75] |
| Male C57 BL/KsJ db/db mice (16 weeks old) | Diabetes-induced depression model; Semaglutide (0.05 mg/kg; 8 weeks; s.c.; n = 10) | ↑ Platform area crossing and time spent in the target quadrant in the Morris water maze test ↑ Total distance in the central zone, wall-climbing counts in the open field test | Hippocampus (HP) | qRT-PCR Western blot HE staining | ↑ Neurons in the CA1, CA3, and DG regions in HP ↑ BDNF in HP | [76] |
| Mice hippocampal neuronal cell line HT22 | Corticosterone (CORT; 200 μM) + high glucose (HG 50 mM) 48 h; GLP-1 (50 nM) | n.a. | Hippocampus (HP) | Flow Cytometry CLSM ELISA Western blot | ↓ Apoptosis and necrocytosis rates, LDH, glucose concentrations ↑ BDNF, 5-HT, DA, NE ↑ PKA, p-CREB, and p-Trkb | [77] |
Male Wistar rats (6 weeks old) | High Fat Diet (HFD; 12 weeks); Liraglutide (300 µg/kg/day;4 weeks; s.c.; n = 12) | ↑ Sucrose consumption in the sucrose preference test ↓ Immobility time in the forced swim test ↑ Crossed squares, ↓ time and latency to leave in the central zone in the open field test ↓ Time to reach the platform, ↑ time spent in the target quadrant in the Morris water maze test | Serum Hippocampus (HP) | RT-qPCR ELISA TEM | ↑ PI3K, Akt, mTOR, ↓ Beclin 1, LC3 HP gene expression ↑ PI3K, Akt, p-Akt, BDNF, p-mTOR, ↓ Beclin 1, LC3 proteins in HP ↓ Corticosterone in serum; TNF-α, IL-6 in HP | [78] |
| Male Wistar rats (2 months old) | Lipopolysaccharide (LPS; 0.25 mg/kg); Exendin-4 (0.1 μg/kg n = 10; 0.3/0.5 μg/kg; i.p.; n = 9) | ↔ Immobility, swimming, climbing time in the forced swim test ↔ Crossed squares in the open field test | Serum Hippocampus (HP) | ELISA Colorimetric Assay | ↓ IL-6, ↑ BDNF in HP ↓ TBARS serum | [79] |
Male Swiss albino mice (weighing 25–35 g) | Dexamethasone-induced depression (32 mcg/kg; 7 days); Liraglutide (200 mcg/kg; 28 days) | ↓ Immobility time in the tail suspension test and forced swim test ↑ Crossed squares in the open field test ↑ Sucrose consumption in the sucrose preference test | Hippocampus (HP) Prefrontal cortex (PFC) Brains left halves (BLH) | ELISA | ↑ 5-HT, DA, NE in HP, PFC ↓ NLRP, IL-1β; ↑Neuropeptide Y, IL-10 in BLH ↑ CREB, BDNF, PSD95; ↓ NMDAR2 in BLH | [80] |
| Male C57BL/6 mice (8–10 weeks old) | Chronic unpredictable mild stress (CUMS; 4 weeks); Liraglutide (300 μg/kg/day; i.p.; n = 10) | ↓ Immobility time in the tail suspension test and forced swim test | Serum Hippocampus (HP) Prefrontal cortex (PFC) | ELISA qRT-PCR Western blot | ↓ CORT in serum ↑ Nrf2, ↓ HMGB1 in HP and PFC ↓ IL-1β, IL-6, TNF-α; ↑ GSH, SOD in HP and PFC | [81] |
| Male C57BL/6N mice (8 weeks old) | Corticosterone (CORT; 35 μg/mL/d; 30 days); Liraglutide (20 nmol/kg; 15 days; i.p.) | ↑ Mobility time in the tail suspension test and forced swim test ↑ Exploration time in the open field test | Plasma Hippocampus (HP) | ELISA Western blot | ↓ ACTH in plasma ↑ GSK3β phosphorylation in HP ↑ DCX in HP | [82] |
Male ICR mice (7 weeks old) | Chronic mild stress (CMS; 4 weeks); Dulaglutide (0.3/0.6 mg/kg; 3 weeks; i.p.; n = 13–15) | ↓ Immobility time in the tail suspension test and forced swim test | Hippocampus (HP) | LC-MS/MS | Regulation PC, PE, PI, LPC and LPE, ↑ NAA,↓ L-glutamic acid, L-arginine, proline in HP ↑ succinic acid, creatine, gentisic acid, ADP, GMP, AMP | [67] |
| Male C57BL/6N mice (8 weeks old) | Chronic unpredictable mild stress (CUMS; 40 days); Lixisenatide (10/50 nmol/kg/d; i.n.; 25 days) | ↓ Immobility time in the tail suspension test and forced swim test ↑ Time spent in center area in open field test ↑ Time spent in open arm in elevated plus maze test | Olfactory bulbs (OB) Hippocampus (HP) | Western blot | ↑ CREB phosphorylation level ↑ DCX in HP DG region, OB | [83] |
Male CD-1 mice (7 weeks old) | Streptozotocin (STZ; 40 mg/kg; 5 days); Semaglutide (0.21/0.42/0.03/0.06 mg/kg; 2 weeks; s.c.; n = 8) | ↑ Crossed squares, ↑ time spent in the central zone in the open field test ↓ Immobility time in the forced swim test | Perftontal cortex (PFC) Serum | ELISA HPLC | ↓ GFAP, NSE, GAL3 in PFC ↓ CRP in serum | [84] |
Male ICR mice (weighing 18–22 g) | Repeated restraint stress (RRS; 30 days); Geniposide (50/100mg/kg); Fluoxetine (20 mg/kg); 15 days; i.g.; n = 12) | ↑ Sucrose consumption in the sucrose preference test ↑ Crossed squares in the open field test ↓ Immobility time in the tail suspension test and forced swim test | Hippocampus (HP) | ELISA Immunostaining Western blot | ↓ Apoptosis of neurons in HP ↓ cleaved Caspase-3, Bax/Bcl-2 ratio) ↓ IL-1β, TNF-α in HP ↑ GLP-1R expression, p-GSK3β, p-AKT in HP | [85] |
| Male Sprague Dawley rats (18–20 months old) | Surgery-induced trauma Exendin-4 (5 µg/kg/day; 14 days; i.p.; n = 30) | ↑ Platform area crossing and time spent in the target quadrant in the Morris water maze test ↑ Total distance traveled, time spent in center area in the open field test | Hippocampus (HP) | Western blot Immunostaining | ↓ NF-κB p65, IL-1β in HP ↓ Iba-1, ↑ SYN, p-GSK-3β in HP ↑ GLP-1/GLP-1R expression in HP | [86] |
2.2. Antidepressant Effects of GLP-1 Receptor Agonists: Evidence from Human Studies
3. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors and Their Emerging Role in Mood Regulation
| Species/Strain | Model of Depression/Treatment | Results-Behavioral Tests | Samples | Methods | Results | References |
|---|---|---|---|---|---|---|
Male Sprague–Dawley rats (6–8 weeks old) | DM-induced depressive-like behavior; Dapagliflozin (1 mg/kg/day intragastric/500 ng/mL microinjection; n = 8) | ↑ Total distance traveled, time spent in center area in the field test ↓ Immobility time in the forced swim test | Lateral habenula (LHb) | Western blot Immunostaining Chromatography | Intragastric administration: ↓ activity of LHb via ↓ c-Fos Microinjection into the LHb: ↑ 5-HT in the DRN Intragastric administration: ↑ p-AMPK, p-GABABR2 expression in the LHb | [124] |
Male Wistar rats (30–33 days old) | Chronic unpredictable stress (CUS; 35 days); Dapagliflozin (1 mg/kg/day; 4 weeks; p.o.; n = 10) | ↑ Sucrose consumption in the sucrose preference test ↓ Immobility time in the forced swim test | Hippocampus (HP) Serum Cortex | ELISA qRT-PCR Immunostaining | ↑ 5-HT, DA, NE, BDNF in HP ↓ IL-1β, IL-18 in serum ↓ p-NF-κB p65, NLRP3, caspase-1 activity, IL-1β, IL-18 in HP ↓ ET-1, ↑ ETBR in HP ↑ BBB integrity: ↓ TNF-α in HP and cortex, ↑ ZO-1 cortex | [125] |
Male Wistar rats (weighing 180–200 g) | Reserpine-induced depression (Res; 0.2 mg/kg/day; 14 days; i.p.); Escitalopram (10 mg/kg/day i.p.) Empagliflozin (10 mg/kg/day i.p.; n = 13) | ↓ Immobility time in the tail suspension test and forced swim test ↑ Crossed squares in the open field test | Hippocampus (HP) | Western blot ELISA qRT-PCR Immunostaining | ↑ 5-HT, DA, NE in HP ↑ p-AMPK, Beclin1, LC3B;↓ mTOR in HP ↑ GSH; ↓ MDA, NLRP3 caspase-1, IL-1β, IL-18, TNF-α in HP ↑ p-PKCζ, p-NF-kB p65, BDNF, p-CREB in HP | [126] |
Male Sprague Dawley rats (8 weeks old) | Chronic unpredictable mild stress (CUMS; 28 days); Empagliflozin (10 mg/kg/day; 28 days; p.o.) | ↓ Immobility time in the forced swim test ↓ Latency time; ↑ frequency of rearing, time and grooming in the open field test ↑ Grooming time in the splash test | Hippocampus (HP) Serum | ELISA Immunostaining | ↑ 5-HT, NE, GSH in HP ↓ Serum corticosterone ↓ MDA, IL-1β, IL-18, NF-α, NF-κB, NLRP3, iba-1 in HP ↓ Hippocampal apoptosis (↓ cytochrome c) | [127] |
Male adult Wistar rats (weighing 150–200 g) | Chronic unpredictable mild stress (CUMS; 7 weeks); Canagliflozin (20 mg/kg; 3 weeks) | ↓ Latency time; ↑ frequency of grooming in the open field test ↓ Immobility time in the tail suspension test and forced swim test | Colon Serum Hippocampus (HP) | Western blot ELISA Immunostaining | ↑Gut integrity (↑ E-cadherin, β-catenin, claudin-1, ZO-1, Goblet cells) ↓ Serum corticosterone ↓ SGLT2 receptors, IL-1β, IL-6, NF-κB, IDO in HP ↓ TNF-α, GFAP, IbA, CD86 in HP ↑ p-AMPK, Beclin-1 and LC3-II/I; ↓ p-mTor in HP | [128] |
| Male adult BALB/C mice (weighing 24–30 g) | Single-prolonged stress (SPS); Dapagliflozin (1 mg/kg/day; 7 days; oral gavage; n = 9) | ↓ Immobility time in the tail suspension test and forced swim test | Prefrontal cortex (PFC) Serum | Real-time PCR ELISA | ↓ Crh, IL-1β, BDNF, Bax mRna expression in PFC ↓ Serum corticosterone | [129] |
4. PPARα Agonists: Exploring the Neuroprotective and Metabolic Pathways
5. Angiotensin Receptor Blockers and Angiotensin Receptor-Neprilysin Inhibitors and Their Impact on Depressive Disorders
6. Concluding Remarks and Future Perspectives
7. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luitel, N.P.; Lamichhane, B.; Pokhrel, P.; Upadhyay, R.; Taylor Salisbury, T.; Akerke, M.; Gautam, K.; Jordans, M.J.D.; Thornicroft, G.; Kohrt, B.A. Prevalence of depression and associated symptoms among patients attending primary healthcare facilities: A cross-sectional study in Nepal. BMC Psychiatry 2024, 24, 356. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 31 March 2023).
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, X.D.; Shapiro, M.D.; Lip, G.Y.H.; Tilg, H.; Valenti, L.; Somers, V.K.; Byrne, C.D.; Targher, G.; Yang, W.; et al. Global burden of metabolic diseases, 1990–2021. Metabolism 2024, 160, 155999. [Google Scholar] [CrossRef]
- Garus-Pakowska, A. Metabolic Diseases-A Challenge for Public Health in the 21st Century. Int. J. Environ. Res. Public Health 2023, 20, 6789. [Google Scholar] [CrossRef]
- Hernández-Cacho, A.; García-Gavilán, J.F.; Atzeni, A.; Konstanti, P.; Belzer, C.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Mela, V.; et al. Multi-omics approach identifies gut microbiota variations associated with depression. NPJ Biofilms Microbiomes 2025, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Arone, A.; Palermo, S.; Annuzzi, E.; Cappellato, G.; Chiarantini, I.; Prete, L.D.; Dell’Osso, L. The Wicked Relationship between Depression and Metabolic Syndrome. Clin. Neuropsychiatry 2023, 20, 100–108. [Google Scholar]
- Gumuskaya, P.O.; Altun, O.; Yildirim, E.; Yuztas, N.K.; Ozsoy, N.; Kalyon, S.; Irak, L.; Ozcan, M.; Altun, Z.O.; Demir, P.S.; et al. The Association Between Depression and Antidiabetic Treatments in Type 2 Diabetes Patients with Both Good and Poor Glycemic Control. J. Clin. Med. 2025, 14, 3460. [Google Scholar] [CrossRef]
- Li, S.; Yang, D.; Zhou, X.; Chen, L.; Liu, L.; Lin, R.; Li, X.; Liu, Y.; Qiu, H.; Cao, H.; et al. Neurological and metabolic related pathophysiologies and treatment of comorbid diabetes with depression. CNS Neurosci. Ther. 2024, 30, e14497. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. EXCLI J. 2023, 22, 274–294. [Google Scholar] [PubMed]
- Ghetia, S.M.; Shastry, C.S. Validation of diabetes associated depression by streptozotocin induced rat model. Plant Arch. 2021, 21 (Suppl. 1), 1688–1691. [Google Scholar] [CrossRef]
- Borgland, S.L. Can treatment of obesity reduce depression or vice versa? J. Psychiatry Neurosci. 2021, 46, E313–E318. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Ming, J.; Li, C. Association between dyslipidemia and depression: A cross-sectional analysis of NHANES data from 2007 to 2018. BMC Psychiatry 2024, 24, 893. [Google Scholar] [CrossRef]
- Enko, D.; Brandmayr, W.; Halwachs-Baumann, G.; Schnedl, W.J.; Meinitzer, A.; Kriegshäuser, G. Prospective plasma lipid profiling in individuals with and without depression. Lipids Health Dis. 2018, 17, 149. [Google Scholar] [CrossRef]
- Engel, D.F.; de Oliveira, J.; Lopes, J.B.; Santos, D.B.; Moreira, E.L.G.; Farina, M.; Rodrigues, A.L.S.; de Souza Brocardo, P.; de Bem, A.F. Is there an association between hypercholesterolemia and depression? Behavioral evidence from the LDLr(-/-) mouse experimental model. Behav. Brain Res. 2016, 311, 31–38. [Google Scholar] [CrossRef]
- Zemdegs, J.; Quesseveur, G.; Jarriault, D.; Pénicaud, L.; Fioramonti, X.; Guiard, B.P. High-fat diet-induced metabolic disorders impairs 5-HT function and anxiety-like behavior in mice. Br. J. Pharmacol. 2016, 173, 2095–2110. [Google Scholar] [CrossRef]
- Yu, H.; Yu, B.; Qin, X.; Shan, W. A unique inflammation-related mechanism by which high-fat diets induce depression-like behaviors in mice. J. Affect. Disord. 2023, 339, 180–193. [Google Scholar] [CrossRef]
- Qiu, W.; Cai, A.; Li, L.; Feng, Y. Association of depression trajectories and subsequent hypertension and cardiovascular disease: Findings from the CHARLS cohort. J. Hypertens. 2024, 42, 432–440. [Google Scholar] [CrossRef]
- Duman, H.; Duman, H.; Puşuroğlu, M.; Yılmaz, A.S. Anxiety disorders and depression are associated with resistant hypertension. Adv. Clin. Exp. Med. 2024, 33, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Cosci, F.; Chouinard, P.G. The monoamine hypothesis of depression revisited: Could it mechanistically novel antidepressant strategies? In Neurobiology of Depression, 1st ed.; Quevedo, J., Carvalho, A.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 63–73. [Google Scholar]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Pastis, I.; Santos, M.G.; Paruchuri, A. Exploring the role of inflammation in major depressive disorder: Beyond the monoamine hypothesis. Front. Behav. Neurosci. 2024, 17, 1282242. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Kaleem, M.; Kharwade, R.; Almutairy, A.F.; Shahzad, N.; Ali Mujtaba, M.; Taha, M.; Pise, A.; Zafar, A.; Mahmood, D. Depression unveiled: Insights into etiology and animal models for behavioral assessment, exploring the multifactorial nature and treatment of depression. Brain Res. 2025, 1847, 149313. [Google Scholar] [CrossRef]
- Tsai, W.H.; Sung, F.C.; Chiu, L.T.; Shih, Y.H.; Tsai, M.C.; Wu, S.I. Decreased Risk of Anxiety in Diabetic Patients Receiving Glucagon-like Peptide-1 Receptor Agonist: A Nationwide, Population-Based Cohort Study. Front. Pharmacol. 2022, 13, 765446, Erratum in Front. Pharmacol. 2022, 13, 886343. [Google Scholar] [CrossRef]
- Mui, J.V.; Li, L.; Chou, O.H.I.; Azfar, N.; Lee, A.; Hui, J.; Lee, S.; Tse, G.; Zhou, J. Comparing sodium-glucose cotransporter 2 inhibitors and dipeptidyl peptidase-4 inhibitors on new-onset depression: A propensity score-matched study in Hong Kong. Acta Diabetol. 2023, 60, 917–927. [Google Scholar] [CrossRef]
- Geraets, A.F.J.; Köhler, S.; Muzambi, R.; Schalkwijk, C.G.; Oenema, A.; Eussen, S.J.P.M.; Dagnelie, P.C.; Stehouwer, C.D.A.; Schaper, N.C.; Henry, R.M.A.; et al. The association of hyperglycaemia and insulin resistance with incident depressive symptoms over 4 years of follow-up: The Maastricht Study. Diabetologia 2020, 63, 2315–2328. [Google Scholar] [CrossRef]
- Coppola, T.; Daziano, G.; Legroux, I. Béraud-Dufour S, Blondeau N, Lebrun, P. Unlocking Therapeutic Synergy: Tailoring Drugs for Comorbidities such as Depression and Diabetes through Identical Molecular Targets in Different Cell Types. Cells 2023, 12, 2768. [Google Scholar] [CrossRef]
- Khawagi, W.Y.; Al-Kuraishy, H.M.; Hussein, N.R.; Al-Gareeb, A.I.; Atef, E.; Elhussieny, O.; Alexiou, A.; Papadakis, M.; Jabir, M.S.; Alshehri, A.A.; et al. Depression and type 2 diabetes: A causal relationship and mechanistic pathway. Diabetes Obes. Metab. 2024, 26, 3031–3044. [Google Scholar] [CrossRef]
- Bădescu, S.V.; Tătaru, C.; Kobylinska, L.; Georgescu, E.L.; Zahiu, D.M.; Zăgrean, A.M.; Zăgrean, L. The association between Diabetes mellitus and Depression. J. Med. Life 2016, 9, 120–125. [Google Scholar] [PubMed]
- Yao, L.; Yang, C.; Zhang, W.; Li, S.; Li, Q.; Chen, L.; Lui, S.; Kemp, G.J.; Biswal, B.B.; Shah, N.J.; et al. A multimodal meta-analysis of regional structural and functional brain alterations in type 2 diabetes. Front. Neuroendocrinol. 2021, 62, 100915. [Google Scholar] [CrossRef] [PubMed]
- Oktem, E.O.; Sayman, D.; Ayyildiz, S.; Oktem, Ç.; Ipek, L.; Ayyildiz, B.; Aslan, F.; Altindal, E.U.; Yagci, N.; Dikici, R.; et al. Cognitive Function Deficits Associated With Type 2 Diabetes and Retinopathy: Volumetric Brain MR Imaging Study. Brain Behav. 2025, 15, e70387. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, D.; Dai, Z.; Li, X. Association Between Systemic Immune-Inflammation Index and Diabetic Depression. Clin. Interv. Aging 2021, 16, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Beverly, E.A.; Gonzalez, J.S. The Interconnected Complexity of Diabetes and Depression. Diabetes Spectr. 2025, 38, 23–31. [Google Scholar] [CrossRef]
- Matera, E.; Cristofano, G.; Furente, F.; Marzulli, L.; Tarantini, M.; Margari, L.; Piarulli, F.M.; De Giacomo, A.; Petruzzelli, M.G. Glucose and Lipid Profiles Predict Anthropometric Changes in Drug-Naïve Adolescents Starting Treatment with Risperidone or Sertraline: A Pilot Study. Biomedicines 2022, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Dębski, J.; Przybyłowski, J.; Skibiak, K.; Czerwińska, M.; Walędziak, M.; Różańska-Walędziak, A. Depression and Obesity-Do We Know Everything about It? A Narrative Review. Nutrients 2024, 16, 3383. [Google Scholar] [CrossRef]
- de Carvalho-Ferreira, J.P.; Masquio, D.C.; da Silveira Campos, R.M.; Dal Molin Netto, B.; Corgosinho, F.C.; Sanches, P.L.; Tock, L.; Tufik, S.; de Mello, M.T.; Finlayson, G.; et al. Is there a role for leptin in the reduction of depression symptoms during weight loss therapy in obese adolescent girls and boys? Peptides 2015, 65, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Molina, K.; Baskin, M.L.; Long, D.; Carson, T.L. Psychological and behavioral pathways between perceived stress and weight change in a behavioral weight loss intervention. J. Behav. Med. 2021, 44, 822–832. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Popoviciu, M.S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef]
- Farkas, E.; Szilvásy-Szabó, A.; Ruska, Y.; Sinkó, R.; Rasch, M.G.; Egebjerg, T.; Pyke, C.; Gereben, B.; Knudsen, L.B.; Fekete, C. Distribution and ultrastructural localization of the glucagon-like peptide-1 receptor (GLP-1R) in the rat brain. Brain Struct. Funct. 2021, 226, 225–245. [Google Scholar] [CrossRef]
- Zhang, J.; He, H.; Qiao, Y.; Zhou, T.; He, H.; Yi, S.; Zhang, L.; Mo, L.; Li, Y.; Jiang, W.; et al. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia 2020, 68, 2674–2692. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Perlman, G.; Kim, N.; Wu, C.Y.; Daher, V.; Zhou, A.; Mathers, E.H.; Anita, N.Z.; Lanctôt, K.L.; Herrmann, N.; et al. Depression in type 2 diabetes: A systematic review and meta-analysis of blood inflammatory markers. Psychoneuroendocrinology 2021, 134, 105448. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, B.; Yuan, P.; Cheng, L.; Sun, H.; Gui, J.; Pan, Y.; Huang, D.; Chen, H.; Jiang, L. Role of PKA/CREB/BDNF signaling in PM2.5-induced neurodevelopmental damage to the hippocampal neurons of rats. Ecotoxicol. Environ. Saf. 2021, 214, 112005. [Google Scholar] [CrossRef]
- Wan, J.; Ma, L.; Jiao, X.; Dong, W.; Lin, J.; Qiu, Y.; Wu, W.; Liu, Q.; Chen, C.; Huang, H.; et al. Impaired synaptic plasticity and decreased excitability of hippocampal glutamatergic neurons mediated by BDNF downregulation contribute to cognitive dysfunction in mice induced by repeated neonatal exposure to ketamine. CNS Neurosci. Ther. 2024, 30, e14604. [Google Scholar] [CrossRef]
- Martin, H.; Bullich, S.; Martinat, M.; Chataigner, M.; Di Miceli, M.; Simon, V.; Clark, S.; Butler, J.; Schell, M.; Chopra, S.; et al. Insulin modulates emotional behavior through a serotonin-dependent mechanism. Mol. Psychiatry 2024, 29, 1610–1619. [Google Scholar] [CrossRef]
- Zemdegs, J.; Martin, H.; Pintana, H.; Bullich, S.; Manta, S.; Marqués, M.A.; Moro, C.; Layé, S.; Ducrocq, F.; Chattipakorn, N.; et al. Metformin Promotes Anxiolytic and Antidepressant-Like Responses in Insulin-Resistant Mice by Decreasing Circulating Branched-Chain Amino Acids. J. Neurosci. 2019, 39, 5935–5948. [Google Scholar] [CrossRef]
- Peach, J.T.; Funk, D.; Frothingham, L.; McMilin, C.R.; Bothner, B.; Miles, M.P. Branched chain amino acid supplementation drives metabolomic shifts downstream of serotonin in endurance runners. Med. Sci. Sports Exerc. 2021, 53, 283. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, C.; Yang, Y.; Hu, J.; Yi, X.; Huang, J.; Li, H.; Liu, X.; Xue, K.; Chen, X. The Role of Mitochondrial Energy Metabolism in the Mechanism of Exercise Improving Depression. Curr. Issues Mol. Biol. 2025, 47, 382. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wu, Z.; Guo, Y.; Bullich, S.; Manta, S.; Marqués, M.A.; Moro, C.; Layé, S.; Ducrocq, F.; Chattipakorn, N.; et al. Roles of microglia in adult hippocampal neurogenesis in depression and their therapeutics. Front. Immunol. 2023, 14, 1193053. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fu, S.; Li, X.; Shi, M.; Qian, J.; Zhao, S.; Yuan, P.; Ding, L.; Xia, X.; Zheng, J.C. Microglial glutaminase 1 mediates chronic restraint stress-induced depression-like behaviors and synaptic damages. Signal Transduct. Target. Ther. 2023, 8, 452. [Google Scholar] [CrossRef]
- Odaira-Satoh, T.; Nakagawasai, O.; Takahashi, K.; Shimada, M.; Nemoto, W.; Tan-No, K. AMPK activation improves depression-like symptoms in olfactory bulbectomized mice by regulating microglia M1/M2 polarization in the hippocampus. Brain Behav. Immun. Health 2025, 46, 101008. [Google Scholar] [CrossRef]
- de Paiva, I.H.R.; da Silva, R.S.; Mendonça, I.P.; de Souza, J.R.B.; Peixoto, C.A. Semaglutide Attenuates Anxious and Depressive-Like Behaviors and Reverses the Cognitive Impairment in a Type 2 Diabetes Mellitus Mouse Model via the Microbiota-Gut-Brain Axis. J. Neuroimmune Pharmacol. 2024, 19, 36. [Google Scholar] [CrossRef]
- Yang, L.; Guo, C.; Zheng, Z.; Dong, Y.; Xie, Q.; Lv, Z.; Li, M.; Lu, Y.; Guo, X.; Deng, R.; et al. Stress dynamically modulates neuronal autophagy to gate depression onset. Nature 2025, 641, 427–437 , Erratum in Nature 2025, 641, E12. [Google Scholar] [CrossRef]
- Panczyszyn-Trzewik, P.; Misztak, P.; Nowak, G.; Sowa-Kucma, M. Association of A-Klotho with regulation of Keap1/Nrf2/Interleukin-1 pathway and AMPA receptor trafficking in the brain of suicide victims. J. Physiol. Pharmacol. 2024, 75, 241–252. [Google Scholar] [CrossRef]
- Pańczyszyn-Trzewik, P.; Czechowska, E.; Stachowicz, K.; Sowa-Kućma, M. The Importance of α-Klotho in Depression and Cognitive Impairment and Its Connection to Glutamate Neurotransmission-An Up-to-Date Review. Int. J. Mol. Sci. 2023, 24, 15268. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xiao, L.; Wang, H.; Wang, G. Neurogenic hypothesis of positive psychology in stress-induced depression: Adult hippocampal neurogenesis, neuroinflammation, and stress resilience. Int. Immunopharmacol. 2021, 97, 107653. [Google Scholar] [CrossRef]
- Li, E.; Yin, H.; Su, M.; Li, Q.; Zhao, Y.; Zhang, L.; Guo, J.; Lai, X.; Xue, X.; Tang, C. Inhibition of ferroptosis alleviates chronic unpredictable mild stress-induced depression in mice via tsRNA-3029b. Brain Res. Bull. 2023, 204, 110773. [Google Scholar] [CrossRef]
- Athanassi, A.; Breton, M.; Chalençon, L.; Brunelin, J.; Didier, A.; Bath, K.; Mandairon, N. Chronic unpredictable mild stress alters odor hedonics and adult olfactory neurogenesis in mice. Front. Neurosci. 2023, 17, 1224941. [Google Scholar] [CrossRef]
- Guan, W.; Ni, M.X.; Gu, H.J.; Yang, Y. CREB: A Promising Therapeutic Target for Treating Psychiatric Disorders. Curr. Neuropharmacol. 2024, 22, 2384–2401. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.H.; Pedro, L.C.; Manosso, L.M.; Gonçalves, C.L.; Réus, G.Z. Pre- and Post-Synaptic protein in the major depressive Disorder: From neurobiology to therapeutic targets. Neuroscience 2024, 556, 14–24. [Google Scholar] [CrossRef]
- Falaschi, V.; Palego, L.; Marazziti, D.; Betti, L.; Musetti, L.; Maglio, A.; Dell’Oste, V.; Sagona, S.; Felicioli, A.; Carpita, B.; et al. Variation of Circulating Brain-Derived Neurotrophic Factor (BDNF) in Depression: Relationships with Inflammatory Indices, Metabolic Status and Patients’ Clinical Features. Life 2023, 13, 1555. [Google Scholar] [CrossRef]
- van Velzen, L.S.; Wijdeveld, M.; Black, C.N.; an Tol, M.J.; van der Wee, N.J.A.; Veltman, D.J.; Penninx, B.W.J.H.; Schmaal, L. Oxidative stress and brain morphology in individuals with depression, anxiety and healthy controls. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 76, 140–144. [Google Scholar] [CrossRef]
- Virijevic, K.; Spasojevic, N.; Stefanovic, B.; Ferizovic, H.; Jankovic, M.; Vasiljevic, P.; Dronjak, S. Chronic mild stress-induced dysregulation of MAPK and PI3K/AKT signaling in the hippocampus and medial prefrontal cortex of WKY female rats. Neurosci. Lett. 2024, 825, 137709. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, S.; Wang, J.; Zhu, G. cAMP-PKA cascade: An outdated topic for depression? Biomed. Pharmacother. 2022, 150, 113030. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, J.; Zhang, H.; Perry, S.W.; Yin, B.; Tan, X.; Chai, T.; Liang, W.; Huang, Y.; Li, Y.; et al. The gut microbiome modulates gut-brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression. Mol. Psychiatry 2021, 26, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, Y.; Liu, M.; Li, L.; Zheng, Y. Neuroprotection vs. Neurotoxicity: Dual Impact Brain Lipids Depression. Int. J. Mol. Sci. 2025, 26, 2722. [Google Scholar]
- Jin, M.; Zhang, S.; Huang, B.; Li, L.; Liang, H.; Ni, A.; Han, L.; Liang, P.; Liu, J.; Shi, H.; et al. Dulaglutide treatment reverses depression-like behavior and hippocampal metabolomic homeostasis in mice exposed to chronic mild stress. Brain Behav. 2024, 14, e3448. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Zheng, P.; Zhou, C.; Hu, C.; Hou, X.; Wang, H.; Xie, P.; Xu, G. Plasma lipidomics reveals potential lipid markers of major depressive disorder. Anal. Bioanal. Chem. 2016, 408, 6497–6507. [Google Scholar] [CrossRef]
- Fan, L.; Yang, L.; Li, X.; Teng, T.; Xiang, Y.; Liu, X.; Jiang, Y.; Zhu, Y.; Zhou, X.; Xie, P. Proteomic and metabolomic characterization of amygdala in chronic social defeat stress rats. Behav. Brain Res. 2021, 412, 113407. [Google Scholar] [CrossRef]
- Du, Y.; Wei, J.; Zhang, Z.; Yang, X.; Wang, M.; Wang, Y.; Qi, X.; Zhao, L.; Tian, Y.; Guo, W.; et al. Plasma Metabolomics Profiling of Metabolic Pathways Affected by Major Depressive Disorder. Front. Psychiatry 2021, 12, 644555. [Google Scholar] [CrossRef]
- Yu, M.; Jia, H.; Zhou, C.; Yang, Y.; Zhao, Y.; Yang, M.; Zou, Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 2017, 138, 231–239. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Nagy, C.; Wakid, M.; Turecki, G.; Mechawar, N. Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol. Psychiatry 2016, 21, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Polcyn, R.; Matzelle, D.; Banik, N.L. New Insights into the Role of Neuron-Specific Enolase in Neuro-Inflammation, Neurodegeneration, and Neuroprotection. Brain Sci. 2018, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- García-Revilla, J.; Boza-Serrano, A.; Espinosa-Oliva, A.M.; Soto, M.S.; Deierborg, T.; Ruiz, R.; de Pablos, R.M.; Burguillos, M.A.; Venero, J.L. Galectin-3, a rising star in modulating microglia activation under conditions of neurodegeneration. Cell Death Dis. 2022, 13, 628. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Qi, J.; Zhang, K.; Jiang, Y.; Feng, B.; Lv, T.; Yang, L.; Yang, Q.; Zhao, M.; et al. Glucagon-like Peptide 1 Receptor Activation Inhibits Microglial Pyroptosis via Promoting Mitophagy to Alleviate Depression-like Behaviors in Diabetic Mice. Nutrients 2022, 15, 38. [Google Scholar] [CrossRef]
- Lai, S.; Kang, Z.; Sun, J.; Wang, Z.; Xu, Y.; Xing, S.; Feng, M.; Wang, Y.; Liu, H. Semaglutide and High-Intensity Interval Exercise Attenuate Cognitive Impairment in Type 2 Diabetic Mice via BDNF Modulation. Brain Sci. 2025, 15, 480. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Liu, X.X.; An, Z.G.; Luo, X.; Zhang, L.L.; Yan, P.; Jin, L.; Cai, R.; Yi, Q.Z. GLP-1 plays a protective role in hippocampal neuronal cells by activating cAMP-CREB-BDNF signaling pathway against CORT+HG-induced toxicity. Heliyon 2023, 9, e18491. [Google Scholar] [CrossRef] [PubMed]
- Magdy, Y.M.; Kamar, S.A.; Habib, M.Z.; Rady, H.Y.; Rabei, M.R.; Khedr, S. Liraglutide improves depressive and cognitive deficits in a high-fat diet rat model of obesity: The role of hippocampal autophagy and the PI3K/Akt/mTOR pathway. Psychopharmacology 2025. [Google Scholar] [CrossRef]
- Géa, L.P.; da Rosa, E.D.; Panizzutti, B.S.; de Aguiar, É.Z.; de Oliveira, L.F.; Ferrari, P.; Piato, A.; Gomez, R.; Colombo, R.; Rosa, A.R. Reduction of hippocampal IL-6 levels in LPS-injected rats following acute exendin-4 treatment. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1303–1311. [Google Scholar] [CrossRef]
- Abdelkawy, Y.S.; Elharoun, M.; Sheta, E.; Abdel-Raheem, I.T.; Nematalla, H.A. Liraglutide and Naringenin relieve depressive symptoms in mice by enhancing Neurogenesis and reducing inflammation. Eur. J. Pharmacol. 2024, 971, 176525. [Google Scholar] [CrossRef]
- Sun, J.; Fu, X.; Liu, Y.; Wang, T.; Zhao, X.; Cui, R.; Yang, W. Exploring the Effect and Mechanism of Liraglutide in Treating Depression Based on Network Pharmacology and Experimental Analysis. J. Cell Mol. Med. 2025, 29, e70630. [Google Scholar] [CrossRef]
- Weina, H.; Yuhu, N.; Christian, H.; Birong, L.; Feiyu, S.; Le, W. Liraglutide attenuates the depressive- and anxiety-like behaviour in the corticosterone induced depression model via improving hippocampal neural plasticity. Brain Res. 2018, 1694, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xue, P.; Wu, B.; Yang, F.; Wu, X. Intranasal treatment of lixisenatide attenuated emotional and olfactory symptoms via CREB-mediated adult neurogenesis in mouse depression model. Aging 2021, 13, 3898–3908. [Google Scholar] [CrossRef]
- Piątkowska-Chmiel, I.; Wicha-Komsta, K.; Pawłowski, K.; Syrytczyk, A.; Kocki, T.; Dudka, J.; Herbet, M. Beyond Diabetes: Semaglutide’s Role in Modulating Mood Disorders through Neuroinflammation Pathways. Cell Mol. Neurobiol. 2025, 45, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, H.; Fang, F.; Qin, T.; Xiao, W.; Wang, Z.; Ma, S. Geniposide improves repeated restraint stress-induced depression-like behavior in mice by ameliorating neuronal apoptosis via regulating GLP-1R/AKT signaling pathway. Neurosci. Lett. 2018, 676, 19–26. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.; Cao, X.; Ma, H.; White, P.F.; Xu, X.; Jiang, Y.; Sun, X.; Cui, Y. Exendin-4 improves behaviorial deficits via GLP-1/GLP-1R signaling following partial hepatectomy. Brain Res. 2019, 1706, 116–124. [Google Scholar] [CrossRef]
- Do, D.; Lee, T.; Inneh, A.; Patel, U. Glucagon-Like Peptide-1 Use and Healthcare Resource Utilization for Depression and Anxiety Among Adults with Type 2 Diabetes: 2019 to 2023. J. Behav. Heal. Serv. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Misra, A.; Bloomgarden, Z. Impact of treatment with GLP-1RAs on suicide attempts in adults persons with type 2 diabetes: A retrospective comparative effectiveness study based on a global TriNetX health research database. J. Diabetes 2024, 16, e13547. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, I.K.; Osler, M.; Jørgensen, M.B.; Rungby, J.; Wium-Andersen, M.K. Diabetes, antidiabetic medications and risk of depression—A population-based cohort and nested case-control study. Psychoneuroendocrinology 2022, 140, 105715. [Google Scholar] [CrossRef]
- Kornelius, E.; Huang, J.Y.; Lo, S.C.; Huang, C.N.; Yang, Y.S. The risk of depression, anxiety, and suicidal behavior in patients with obesity on glucagon like peptide-1 receptor agonist therapy. Sci. Rep. 2024, 14, 24433. [Google Scholar] [CrossRef]
- Tobaiqy, M.; Elkout, H. Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: A pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database. Int. J. Clin. Pharm. 2024, 46, 488–495. [Google Scholar] [CrossRef]
- Nishida, K.; Chrétien, B.; Dolladille, C.; Ebina, T.; Aleksic, B.; Cabé, N.; Savey, V.; Onoue, T.; Yatsuya, H. Psychiatric and psychological adverse effects associated with dulaglutide, semaglutide, and liraglutide: A vigibase study. Clin. Nutr. 2025, 51, 252–265. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Z.; Yan, M.; Liu, S.; Xiao, S. Depression and suicide/self-injury signals for weight loss medications: A disproportionality analysis of semaglutide, liraglutide, and tirzepatide in FAERS database. J. Affect. Disord. 2025, 389, 119670. [Google Scholar] [CrossRef] [PubMed]
- Stojkovska, A.; Rus Prelog, P.; Kokalj Palandacic, A. Association of semaglutide use with depressive symptoms and suicidal behavior in a patient with type 2 diabetes: A case report. J. Int. Med. Res. 2025, 53, 3000605251349393. [Google Scholar] [CrossRef]
- Li, J.R.; Cao, J.; Wei, J.; Geng, W. Case Report: Semaglutide-associated depression: A report of two cases. Front. Psychiatry 2023, 14, 1238353. [Google Scholar] [CrossRef] [PubMed]
- Eren-Yazicioglu, C.Y.; Kara, B.; Sancak, S.; Uysal, S.P.; Yazici, D.; Okuroglu, N.; Whitton, A.E.; Rutherford, A.V.; Yapici-Eser, H. Effect of Exenatide Use on Cognitive and Affective Functioning in Obese Patients With Type 2 Diabetes Mellitus: Exenatide Use Mediates Depressive Scores Through Increased Perceived Stress Levels. J. Clin. Psychopharmacol. 2021, 41, 428–435. [Google Scholar] [CrossRef]
- Saisho, Y. SGLT2 Inhibitors: The Star in the Treatment of Type 2 Diabetes? Diseases 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Katranski, J.; Liang, S.; Morris, D.; Suppiah, V.; Lim, C.X. Psychiatric adverse events linked to glucagon-like peptide 1 analogues: A disproportionality analysis in American, Canadian and Australian adverse event databases. Pharm. Weekbl. 2025, 1–9. [Google Scholar] [CrossRef]
- Asanuma, H. Effect of sodium-glucose cotransporter 2 inhibitors on blood pressure in patients with type 2 diabetes and cardiovascular diseases. Hypertens. Res. 2024, 47, 2309–2311. [Google Scholar] [CrossRef]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef]
- Nguyen, T.; Wen, S.; Gong, M.; Yuan, X.; Xu, D.; Wang, C.; Jin, J.; Zhou, L. Dapagliflozin Activates Neurons in the Central Nervous System and Regulates Cardiovascular Activity by Inhibiting SGLT-2 in Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 2781–2799. [Google Scholar] [CrossRef]
- He, S.; Huang, X.; Zheng, J.; Zhang, Y.; Ruan, X. An NTS-CeA projection modulates depression-like behaviors in a mouse model of chronic pain. Neurobiol. Dis. 2022, 174, 105893. [Google Scholar] [CrossRef]
- Mannekote Thippaiah, S.; Wang, M.; Ransdell, M.; Dwivedi, Y. The Clinical Impact of Habenular Dysfunction on Depression and Suicidality: A Literature Review and Discussion on the Implications for Psychiatric Practice. Curr. Behav. Neurosci. Rep. 2025, 12, 4. [Google Scholar] [CrossRef]
- Otsubo, H.; Kondoh, T.; Shibata, M.; Torii, K.; Ueta, Y. Induction of Fos expression in the rat forebrain after intragastric administration of monosodium L-glutamate, glucose and NaCl. Neuroscience 2011, 196, 97–103. [Google Scholar] [CrossRef]
- Kim, Y.; Morath, B.; Hu, C.; Byrne, L.K.; Sutor, S.L.; Frye, M.A.; Tye, S.J. Antidepressant actions of lateral habenula deep brain stimulation differentially correlate with CaMKII/GSK3/AMPK signaling locally and in the infralimbic cortex. Behav. Brain Res. 2016, 306, 170–177. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, S.; Yao, K.; Meng, Q.; Lu, Y.; Ren, Y.; Li, L.; Zhao, J. Breakdown of the blood-brain barrier in depressed mice induced by chronic unpredictable mild stress. J. Psychiatr. Res. 2024, 180, 138–146. [Google Scholar] [CrossRef]
- Taene, A.; Khalili-Tanha, G.; Esmaeili, A.; Mobasheri, L.; Kooshkaki, O.; Jafari, S.; Shokouhifar, A.; Sarab, G.A. The Association of Major Depressive Disorder with Activation of NLRP3 Inflammasome, Lipid Peroxidation, and Total Antioxidant Capacity. J. Mol. Neurosci. 2020, 70, 65–70. [Google Scholar] [CrossRef]
- Gulati, A.; Hornick, M.G.; Briyal, S.; Lavhale, M.S. A novel neuroregenerative approach using ET(B) receptor agonist, IRL-1620, to treat CNS disorders. Physiol. Res. 2018, 67 (Suppl. 1), S95–S113. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, Y.; Ren, P.; Liu, X.; Chen, Y.; Ying, Q.; Zhou, J. Autophagy-Unlocking New Dimensions in the Pathology and Treatment of Depression. Cells 2025, 14, 795. [Google Scholar] [CrossRef]
- Pierone, B.C.; Pereira, C.A.; Garcez, M.L.; Kaster, M.P. Stress and signaling pathways regulating autophagy: From behavioral models to psychiatric disorders. Exp. Neurol. 2020, 334, 113485. [Google Scholar] [CrossRef]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A.E.; Zemskov, E.A.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef] [PubMed]
- Odaira, T.; Nakagawasai, O.; Takahashi, K.; Nemoto, W.; Sakuma, W.; Lin, J.R.; Tan-No, K. Mechanisms underpinning AMP-activated protein kinase-related effects on behavior and hippocampal neurogenesis in an animal model of depression. Neuropharmacology 2019, 150, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Ge, M.; Wang, J.; Kuang, D.; Wei, H.; Wang, Z.; Hu, Z.; Zhao, C.; Jin, Q.; Chen, M.; et al. Causal association between kynurenine and depression investigated using two-sample mendelian randomization. Sci. Rep. 2024, 14, 1821. [Google Scholar] [CrossRef]
- Chen, L.M.; Bao, C.H.; Wu, Y.; Liang, S.H.; Wang, D.; Wu, L.Y.; Huang, Y.; Liu, H.R.; Wu, H.G. Tryptophan-kynurenine metabolism: A link between the gut and brain for depression in inflammatory bowel disease. J. Neuroinflammation. 2021, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Lialios, P.; Alimperti, S. Role of E-cadherin in epithelial barrier dysfunction: Implications for bacterial infection, inflammation, and disease pathogenesis. Front. Cell Infect. Microbiol. 2025, 15, 1506636. [Google Scholar] [CrossRef] [PubMed]
- Gąssowska-Dobrowolska, M.; Chlubek, M.; Kolasa, A.; Tomasiak, P.; Korbecki, J.; Skowrońska, K.; Tarnowski, M.; Masztalewicz, M.; Baranowska-Bosiacka, I. Microglia and Astroglia-The Potential Role in Neuroinflammation Induced by Pre- and Neonatal Exposure to Lead (Pb). Int. J. Mol. Sci. 2023, 24, 9903. [Google Scholar] [CrossRef]
- Sowa-Kućma, M.; Styczeń, K.; Siwek, M.; Misztak, P.; Nowak, R.J.; Dudek, D.; Rybakowski, J.K.; Nowak, G.; Maes, M. Lipid Peroxidation and Immune Biomarkers Are Associated with Major Depression and Its Phenotypes, Including Treatment-Resistant Depression and Melancholia. Neurotox. Res. 2018, 33, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, C.; Jin, P.; Sun, W.; Yu, E. Agomelatine prevented depression in the chronic restraint stress model through enhanced catalase activity and halted oxidative stress. PLoS ONE 2024, 19, e0289248. [Google Scholar] [CrossRef]
- Elfakharany, S.A.; Eskaros, S.S.; Azhary, N.M.E.; Abdelmonsif, D.A.; Zeitoun, T.M.; Ammar, G.A.G.; Hatem, Y.A. Neuroprotective Role of Selenium Nanoparticles Against Behavioral, Neurobiochemical and Histological Alterations in Rats Subjected to Chronic Restraint Stress. Mol. Neurobiol. 2024, 61, 10159–10181. [Google Scholar] [CrossRef]
- Domínguez-Borràs, J.; Vuilleumier, P. Amygdala function in emotion, cognition, and behavior. Handb. Clin. Neurol. 2022, 187, 359–380. [Google Scholar]
- Aubry, A.V.; Durand-de Cuttoli, R.; Karpman, E.; Fisher-Foye, R.L.; Parise, L.F.; Cathomas, F.; Burnett, C.J.; Yang, Y.; Yuan, C.; LaBanca, A.R.; et al. A crucial role for the cortical amygdala in shaping social encounters. Nature 2025, 639, 1006–1015. [Google Scholar] [CrossRef]
- Klug, M.; Enneking, V.; Borgers, T.; Jacobs, C.M.; Dohm, K.; Kraus, A.; Grotegerd, D.; Opel, N.; Repple, J.; Suslow, T.; et al. Persistence of amygdala hyperactivity to subliminal negative emotion processing in the long-term course of depression. Mol. Psychiatry 2024, 29, 1501–1509. [Google Scholar] [CrossRef]
- Kamel, A.S.; Wahid, A.; Abdelkader, N.F.; Ibrahim, W.W. Boosting amygdaloid GABAergic and neurotrophic machinery via dapagliflozin-enhanced LKB1/AMPK signaling in anxious demented rats. Life Sci. 2022, 310, 121002. [Google Scholar] [CrossRef]
- Dong, D.; Liu, X.; Ma, L.; Hao, Y.L.; Zhang, L.; Song, M.; Xu, Z.; Zhao, H. Dapagliflozin inhibits the activity of lateral habenula to alleviate diabetes mellitus-induced depressive-like behavior. Exp. Neurol. 2023, 366, 114448. [Google Scholar] [CrossRef]
- Muhammad, R.N.; Ahmed, L.A.; Abdul Salam, R.M.; Ahmed, K.A.; Attia, A.S. Crosstalk Among NLRP3 Inflammasome, ETBR Signaling, and miRNAs in Stress-Induced Depression-Like Behavior: A Modulatory Role for SGLT2 Inhibitors. Neurotherapeutics 2021, 18, 2664–2681. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, R.N.; Albahairy, M.A.; Abd El Fattah, M.A.; Ibrahim, W.W. Empagliflozin-activated AMPK elicits neuroprotective properties in reserpine-induced depression via regulating dynamics of hippocampal autophagy/inflammation and PKCζ-mediated neurogenesis. Psychopharmacol 2024, 241, 2565–2584. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Michel, H.E.; Menze, E.T.; Tadros, M.G.; Wahdan, S.A. The potential neuroprotective effect of empagliflozin against depressive-like behavior induced by chronic unpredictable mild stress in rats: Involvement of NLRP3 inflammasome. Eur. J. Pharmacol. 2025, 998, 177525. [Google Scholar] [CrossRef] [PubMed]
- Khedr, L.H.; Eladawy, R.M.; Nassar, N.N.; Saad, M.A.E. Canagliflozin attenuates chronic unpredictable mild stress induced neuroinflammation via modulating AMPK/mTOR autophagic signaling. Neuropharmacology 2023, 223, 109293. [Google Scholar] [CrossRef]
- Amawi, H.; Makhlouf, T.; Hammad, A.M.; Alsheyab, S.; Alhazaimeh, R.; Hall, F.S.; Khan, J.T.; Al-Trad, B.; Tiwari, A.K. Sodium-glucose cotransporter-2 inhibitor, dapagliflozin, reverses depressive-like behavior in a mouse model of post-traumatic stress disorder. Brain Res. Bull. 2025, 228, 111414. [Google Scholar] [CrossRef]
- Luan, X.; Wang, X.; Shi, Y.; Zhang, X.; Wang, Y.; Zhou, M.; Wu, Z.; Liu, Z.; Li, X.; Zhang, L.; et al. Abnormalities of lipid metabolism in the progression and treatment of depression. Front. Psychiatry 2025, 16, 1589663. [Google Scholar] [CrossRef]
- Xu, D.R.; Gao, X.; Zhao, L.B.; Liu, S.D.; Tang, G.; Zhou, C.J.; Chen, Y. Association between triglyceride and depression: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0311625. [Google Scholar] [CrossRef]
- Seminotti, B.; Grings, M.; Glänzel, N.M.; Vockley, J.; Leipnitz, G. Peroxisome proliferator-activated receptor (PPAR) agonists as a potential therapy for inherited metabolic disorders. Biochem. Pharmacol. 2023, 209, 115433. [Google Scholar] [CrossRef]
- Warden, A.; Truitt, J.; Merriman, M.; Ponomareva, O.; Jameson, K.; Ferguson, L.B.; Mayfield, R.D.; Harris, R.A. Localization of PPAR isotypes in the adult mouse and human brain. Sci. Rep. 2016, 6, 27618. [Google Scholar] [CrossRef]
- Scheggi, S.; Pinna, G.; Braccagni, G.; De Montis, M.G.; Gambarana, C. PPARα Signaling: A Candidate Target in Psychiatric Disorder Management. Biomolecules 2022, 12, 723. [Google Scholar] [CrossRef]
- Chen, C.; Shen, J.H.; Xu, H.; Chen, P.; Chen, F.; Guan, Y.X.; Jiang, B.; Wu, Z.H. Hippocampal PPARα is involved in the antidepressant-like effects of venlafaxine in mice. Brain Res. Bull. 2019, 153, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, H.; Wang, Y.J.; Wang, J.L.; Zhu, Q.; Wu, F.; Zhang, W.; Jiang, B. Hippocampal PPARα is a novel therapeutic target for depression and mediates the antidepressant actions of fluoxetine in mice. Br. J. Pharmacol. 2018, 175, 2968–2987. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, J.H.; Liu, L.; Liu, Y.; Tang, W.Q.; Ji, C.H.; Guan, W.; Zhao, X.Y.; Sun, Y.F.; Xu, D.W.; et al. Hippocampal PPARα Plays a Role in the Pharmacological Mechanism of Vortioxetine, a Multimodal-Acting Antidepressant. Front. Pharmacol. 2021, 12, 673221. [Google Scholar] [CrossRef]
- Matrisciano, F.; Pinna, G. PPAR-α Hypermethylation in the Hippocampus of Mice Exposed to Social Isolation Stress Is Associated with Enhanced Neuroinflammation and Aggressive Behavior. Int. J. Mol. Sci. 2021, 22, 10678. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, Y.J.; Wang, H.; Song, L.; Huang, C.; Zhu, Q.; Wu, F.; Zhang, W. Antidepressant-like effects of fenofibrate in mice via the hippocampal brain-derived neurotrophic factor signalling pathway. Br. J. Pharmacol. 2017, 174, 177–194. [Google Scholar] [CrossRef]
- Scheggi, S.; Melis, M.; De Felice, M.; Aroni, S.; Muntoni, A.L.; Pelliccia, T.; Gambarana, C.; De Montis, M.G.; Pistis, M. PPARα modulation of mesolimbic dopamine transmission rescues depression-related behaviors. Neuropharmacology 2016, 110, 251–259. [Google Scholar] [CrossRef]
- Ni, Y.F.; Wang, H.; Gu, Q.Y.; Wang, F.Y.; Wang, Y.J.; Wang, J.L.; Jiang, B. Gemfibrozil has antidepressant effects in mice: Involvement of the hippocampal brain-derived neurotrophic factor system. J. Psychopharmacol. 2018, 32, 469–481. [Google Scholar] [CrossRef]
- Zandifar, A.; Badrfam, R.; Shamabadi, A.; Jalilevand, S.; Pourmirbabaei, S.; Torkamand, F.; Sahebolzamani, E.; Akhondzadeh, S. Efficacy of Gemfibrozil as an Adjunct to Sertraline in Major Depressive Disorder, A Double-Blind, Randomized, and Placebo-Controlled Clinical Trial. Iran. J. Psychiatry 2021, 16, 52–59. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, J.; Liu, Q.Z.; Wang, L.L.; Shang, J. Simvastatin and Bezafibrate ameliorate Emotional disorder Induced by High fat diet in C57BL/6 mice. Sci. Rep. 2017, 7, 2335. [Google Scholar] [CrossRef]
- Yang, R.; Wang, P.; Chen, Z.; Hu, W.; Gong, Y.; Zhang, W.; Huang, C. WY-14643, a selective agonist of peroxisome proliferator-activated receptor-α, ameliorates lipopolysaccharide-induced depressive-like behaviors by preventing neuroinflammation and oxido-nitrosative stress in mice. Pharmacol. Biochem. Behav. 2017, 153, 97–104. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, C.; Zhu, Q.; Tong, L.J.; Zhang, W. WY14643 produces anti-depressant-like effects in mice via the BDNF signaling pathway. Psychopharmacology 2015, 232, 1629–1642. [Google Scholar] [CrossRef]
- Zhou, J.J.; Zhao, J.; Gao, S.Y.; Gao, Y.Y.; Chen, C.; Ding, Y.; Wu, Z.H.; Chen, P.J. Administration of chiglitazar reverses chronic stress-induced depressive-like symptoms in mice via activation of hippocampal PPARα and BDNF. Front. Pharmacol. 2025, 16, 1587399. [Google Scholar] [CrossRef]
- Schaare, H.L.; Blöchl, M.; Kumral, D.; Uhlig, M.; Lemcke, L.; Valk, S.L.; Villringer, A. Associations between mental health, blood pressure and the development of hypertension. Nat. Commun. 2023, 14, 1953. [Google Scholar] [CrossRef]
- Bergantin, L.B. Depression Rises the Risk of Hypertension Incidence: Discussing the Link through the Ca2+/cAMP Signalling. Curr. Hypertens. Rev. 2020, 16, 73–78. [Google Scholar] [CrossRef]
- Ren, L.; Lu, X.; Danser, A.H.J. Revisiting the Brain Renin-Angiotensin System-Focus on Novel Therapies. Curr. Hypertens. Rep. 2019, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Haliga, R.E.; Cojocaru, E.; Sîrbu, O.; Hrițcu, I.; Alexa, R.E.; Haliga, I.B.; Șorodoc, V.; Coman, A.E. Immunomodulatory Effects of RAAS Inhibitors: Beyond Hypertension and Heart Failure. Biomedicines 2025, 13, 1779. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Hamad, R.S.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E. Correction to “Role of brain renin-angiotensin system in depression: A new perspective”. CNS Neurosci. Ther. 2024, 30, e14884. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; You, M.J.; Yang, B.; Jang, K.B.; Yoo, J.; Choi, H.J.; Lee, S.H.; Bang, M.; Kwon, M.S. Chronically infused angiotensin II induces depressive-like behavior via microglia activation. Sci. Rep. 2020, 10, 22082. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakagawasai, O.; Kurokawa, K.; Miyagawa, K.; Mochida-Saito, A.; Takeda, H.; Tsuji, M. Activation of Hippocampal ACE2 Prevents the Dysbiosis-induced Depression-like Behavior in Mice by Enhanced Neurogenesis and Neuroprotection via Mas Receptor. J. Neuroimmune Pharmacol. 2025, 20, 71. [Google Scholar] [CrossRef]
- Han, W.; Wei, Z.; Dang, R.; Guo, Y.; Zhang, H.; Geng, C.; Wang, C.; Feng, Q.; Jiang, P. Angiotensin-II and angiotensin-(1-7) imbalance affects comorbidity of depression and coronary heart disease. Peptides 2020, 131, 170353. [Google Scholar] [CrossRef]
- Gonçalves, S.C.A.; Gregório, J.F.; Ferraz, K.S.; Magalhães, G.S.; Zebral, S.A.; Fontes, M.A.P.; Rodrigues-Machado, M.D.G.; Sinisterra, R.D.; Haibara, A.S.; Kangussu, L.M.; et al. Oral or intranasal angiotensin-(1-7) improves anxiety and depression-like behaviors in mice subjected to allergic pulmonary inflammation. Behav. Brain Res. 2025, 494, 115744. [Google Scholar] [CrossRef]
- Karasaki, K.; Kokubo, H.; Bumdelger, B.; Kaji, N.; Sakai, C.; Ishida, M.; Yoshizumi, M. Angiotensin II Type 1 Receptor Blocker Prevents Abdominal Aortic Aneurysm Progression in Osteoprotegerin-Deficient Mice via Upregulation of Angiotensin (1-7). J. Am. Heart Assoc. 2023, 12, e027589. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, Z.; Zhou, X.; Wang, L.; Zhou, F.; Yao, D.; Zhou, B.; Becker, B. The central renin-angiotensin system: A genetic pathway, functional decoding, and selective target engagement characterization in humans. Proc. Natl. Acad. Sci. USA 2024, 121, e2306936121. [Google Scholar] [CrossRef]
- Min, S.; Mazurka, R.; Pizzagalli, D.A.; Whitton, A.E.; Milev, R.V.; Bagby, R.M.; Kennedy, S.H.; Harkness, K.L. Stressful Life Events and Reward Processing in Adults: Moderation by Depression and Anhedonia. Depress. Anxiety 2024, 2024, 8853631. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.; Ramkiran, S.; Schnellbächer, G.J.; Rajkumar, R.; Collee, M.; Khudeish, N.; Veselinović, T.; Shah, N.J.; Neuner, I. Phenomena of hypo- and hyperconnectivity in basal ganglia-thalamo-cortical circuits linked to major depression: A 7T fMRI study. Mol. Psychiatry 2025, 30, 158–167. [Google Scholar] [CrossRef]
- Erdem, S.; Özaçmak, H.S.; Turan, İ.; Ergenç, M. The protective effect of angiotensin II type I receptor blocker (valsartan) on behavioral impairment, NLRP3, BDNF, and oxidative stress in the brain tissue of ovariectomized female rats. Physiol. Rep. 2024, 12, e70003. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Wang, T.; Zhao, S.; Yi, S.; Zhang, T.; Yang, Y.; Zhang, F.; Zhang, D.; Xu, X.; Xu, J.; et al. Higher Blood-brain barrier permeability in patients with major depressive disorder identified by DCE-MRI imaging. Psychiatry Res. Neuroimaging 2024, 337, 111761. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Ma, B.; Ding, Y.; Liu, X.; He, C.; Wang, B.; Yuan, M. Trifluoperazine regulates blood-brain barrier permeability via the MLCK/p-MLC pathway to promote ischemic stroke recovery. iScience 2024, 27, 109156. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, J.; Qin, H.; Ding, Y.; Liu, X.; He, C.; Wang, B.; Yuan, M. Irbesartan suppresses lipopolysaccharide (LPS)-induced blood-brain barrier (BBB) dysfunction by inhibiting the activation of MLCK/MLC. Int. Immunopharmacol. 2021, 98, 107834. [Google Scholar] [CrossRef]
- Gouveia, F.; Fonseca, C.; Silva, A.; Camins, A.; Teresa Cruz, M.; Ettcheto, M.; Fortuna, A. Intranasal irbesartan reverts cognitive decline and activates the PI3K/AKT pathway in an LPS-induced neuroinflammation mice model. Int. Immunopharmacol. 2024, 128, 111471. [Google Scholar] [CrossRef]
- Ayyub, M.; Najmi, A.K.; Akhtar, M. Protective Effect of Irbesartan an Angiotensin (AT1) Receptor Antagonist in Unpredictable Chronic Mild Stress Induced Depression in Mice. Drug Res. 2017, 67, 59–64. [Google Scholar] [CrossRef]
- Qian, C.; Xiang, X.; Yao, H.; Li, P.; Cheng, B.; Wei, D.; An, W.; Lu, Y.; Chu, M.; Wei, P.; et al. Reconstruction of TrkB complex assemblies and localizing antidepressant targets using Artificial Intelligence. bioRxiv 2023. bioRxiv: 2023.02.21.529454. [Google Scholar] [CrossRef]
- Diniz, C.R.A.F.; Casarotto, P.C.; Fred, S.M.; Biojone, C.; Castrén, E.; Joca, S.R.L. Antidepressant-like effect of losartan involves TRKB transactivation from angiotensin receptor type 2 (AGTR2) and recruitment of FYN. Neuropharmacology 2018, 135, 163–171. [Google Scholar] [CrossRef]
- Colbourne, L.; Luciano, S.; Harrison, P.J. Onset and recurrence of psychiatric disorders associated with anti-hypertensive drug classes. Transl. Psychiatry 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Huang, X.; Jiang, F.; Xiao, Z. Impact of angiotensin receptor-neprilysin inhibition (ARNI) in improving ejection fraction and left and right ventricular remodeling in heart failure. Curr. Probl. Cardiol. 2024, 49, 102464. [Google Scholar] [CrossRef]
- Malik, J.; Shahid, A.W.; Shah, M.; Rana, G.; Kamal, A.; Naeem, H. Outcome of angiotensin receptor-neprilysin inhibitor on anxiety and depression in heart failure with reduced ejection fraction vs. heart failure with preserved ejection fraction. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 629–634. [Google Scholar] [PubMed]
- Dereli, S.; Kılınçel, O.; Çerik, İ.B.; Kaya, A. Impact of sacubitril/valsartan treatment on depression and anxiety in heart failure with reduced ejection fraction. Acta Cardiol. 2020, 75, 774–782. [Google Scholar] [CrossRef]
- Neijenhuis, R.M.L.; Nederend, M.; van Groningen, A.E.; Jongbloed, M.R.M.; Vliegen, H.W.; Jukema, J.W.; Kiès, P.; Egorova, A.D. Sacubitril/valsartan is associated with improvements in quality of life in adult congenital heart disease patients with systemic right ventricular failure. Open Heart 2025, 12, e003009. [Google Scholar] [CrossRef] [PubMed]
- Sharafshah, A.; Lewandrowski, K.U.; Gold, M.S.; Fuehrlein, B.; Ashford, J.W.; Thanos, P.K.; Wang, G.J.; Hanna, C.; Cadet, J.L.; Gardner, E.L.; et al. In Silico Pharmacogenomic Assessment of Glucagon-like Peptide-1 (GLP1) Agonists and the Genetic Addiction Risk Score (GARS) Related Pathways: Implications for Suicidal Ideation and Substance Use Disorder. Curr. Neuropharmacol. 2025, 23, 974–995. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyndał, K.; Pańczyszyn-Trzewik, P.; Sowa-Kućma, M. Metabolic Modulators in Depression: Emerging Molecular Mechanisms and Therapeutic Opportunities. Int. J. Mol. Sci. 2025, 26, 8755. https://doi.org/10.3390/ijms26178755
Dyndał K, Pańczyszyn-Trzewik P, Sowa-Kućma M. Metabolic Modulators in Depression: Emerging Molecular Mechanisms and Therapeutic Opportunities. International Journal of Molecular Sciences. 2025; 26(17):8755. https://doi.org/10.3390/ijms26178755
Chicago/Turabian StyleDyndał, Kinga, Patrycja Pańczyszyn-Trzewik, and Magdalena Sowa-Kućma. 2025. "Metabolic Modulators in Depression: Emerging Molecular Mechanisms and Therapeutic Opportunities" International Journal of Molecular Sciences 26, no. 17: 8755. https://doi.org/10.3390/ijms26178755
APA StyleDyndał, K., Pańczyszyn-Trzewik, P., & Sowa-Kućma, M. (2025). Metabolic Modulators in Depression: Emerging Molecular Mechanisms and Therapeutic Opportunities. International Journal of Molecular Sciences, 26(17), 8755. https://doi.org/10.3390/ijms26178755







