Anti-HMGB1 Antibody Therapy Ameliorates Spinal Cord Ischemia–Reperfusion Injury in Rabbits
Abstract
1. Introduction
2. Results
2.1. Varied Neurological Outcomes in Rabbit SCI/R Injury
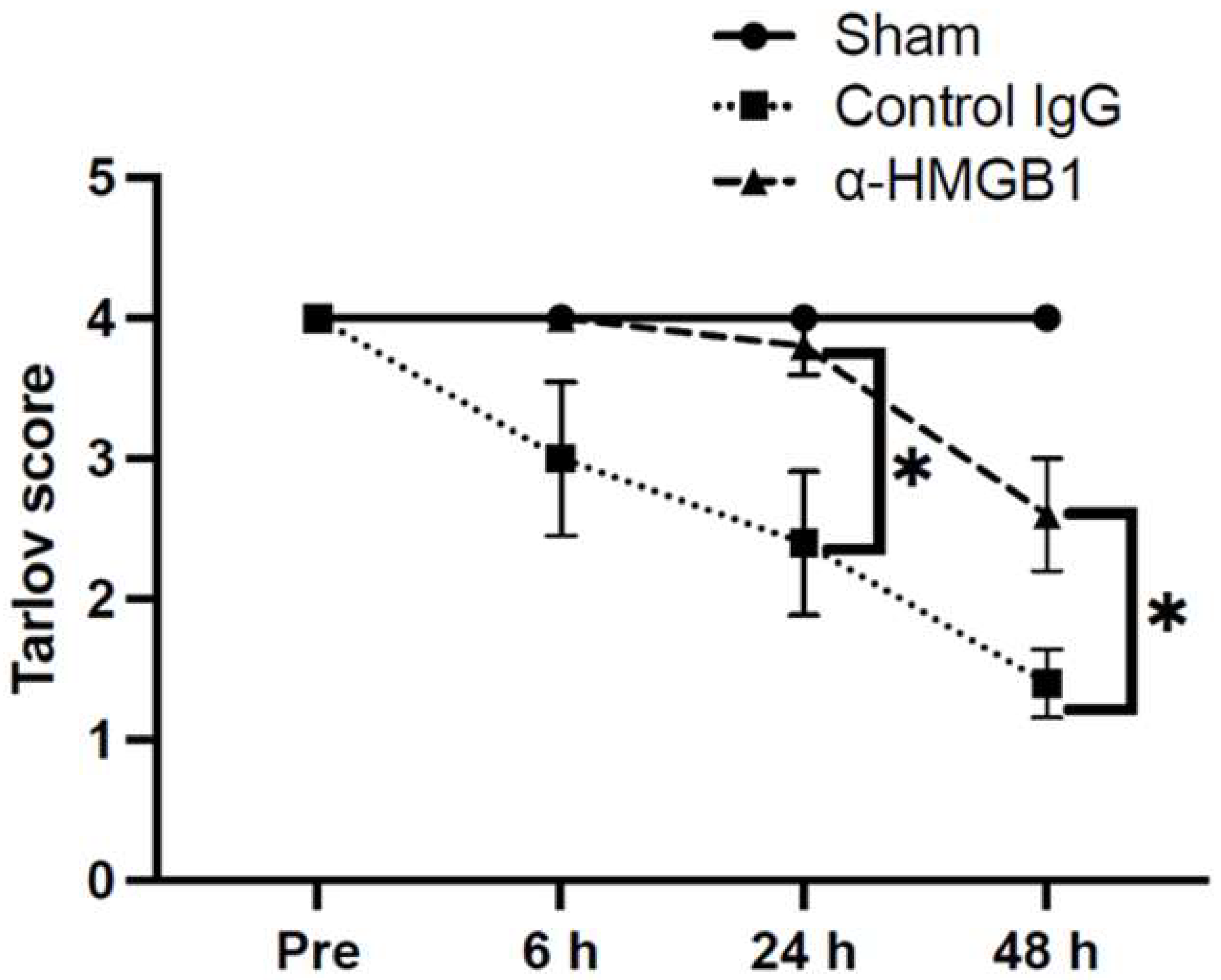
2.2. Anti-HMGB1 mAb Improves Neurological Deficits After SCI/R Injury
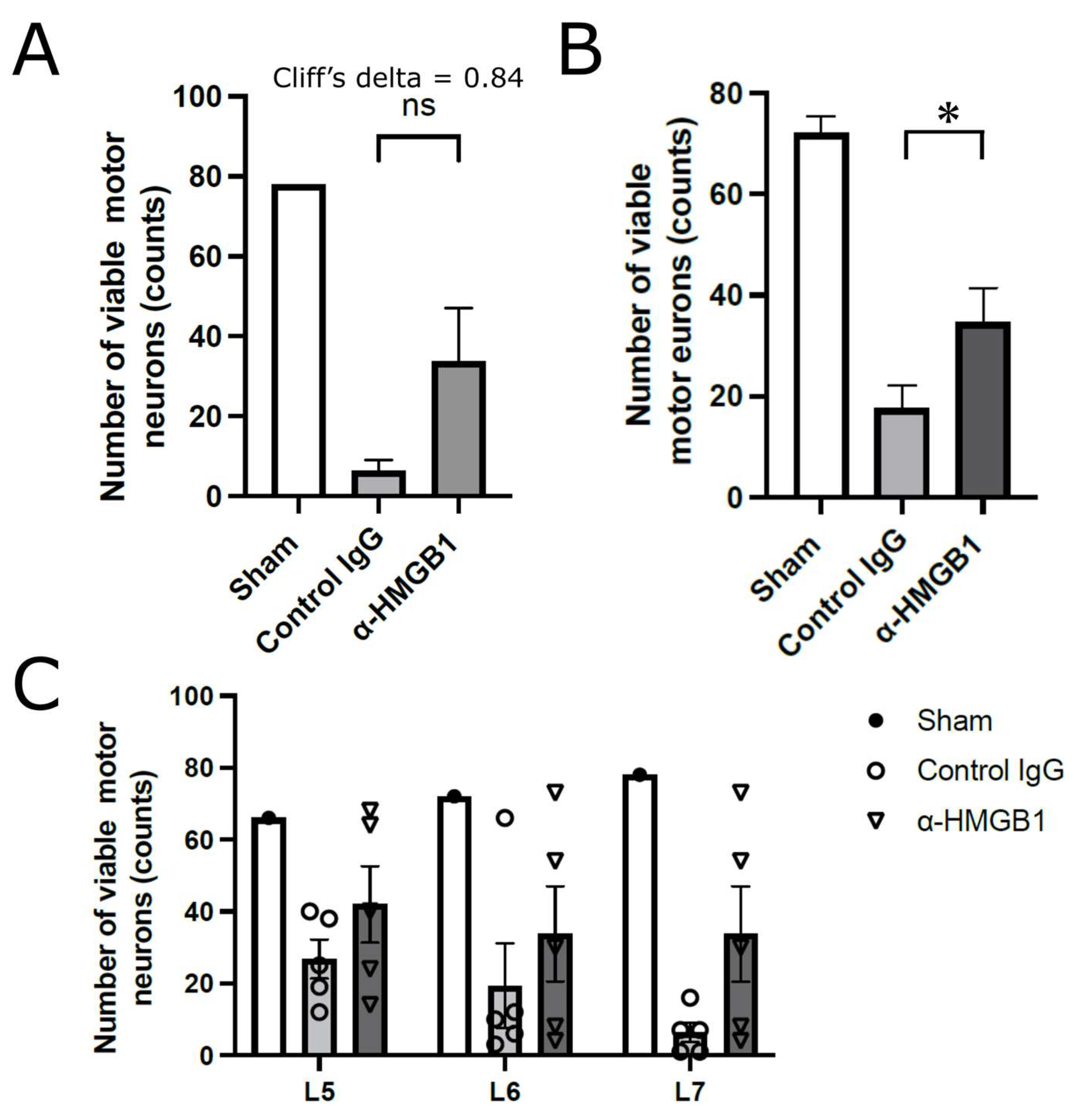
2.3. Histological Studies on the Effects of Anti-HMGB1 mAb
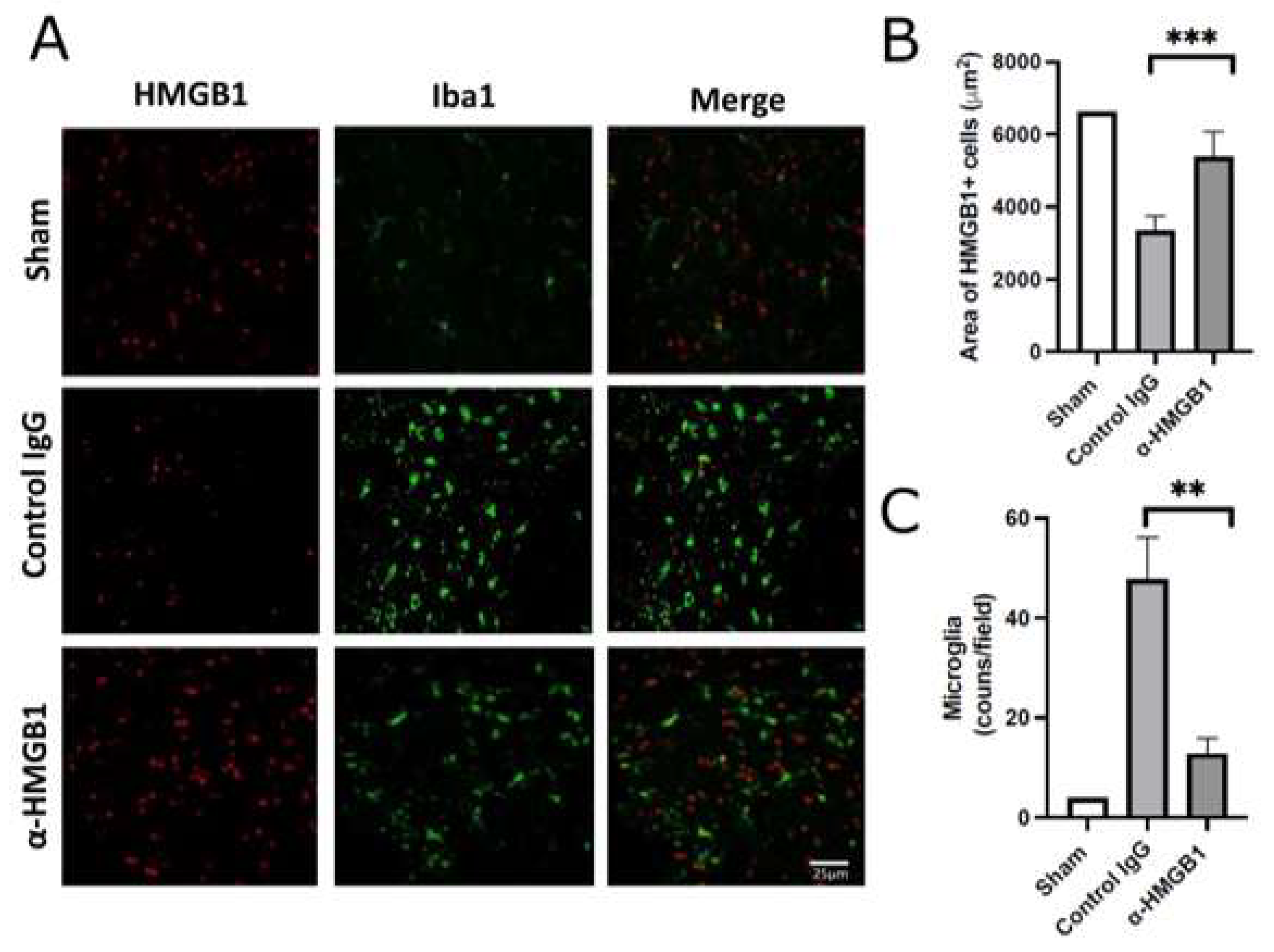
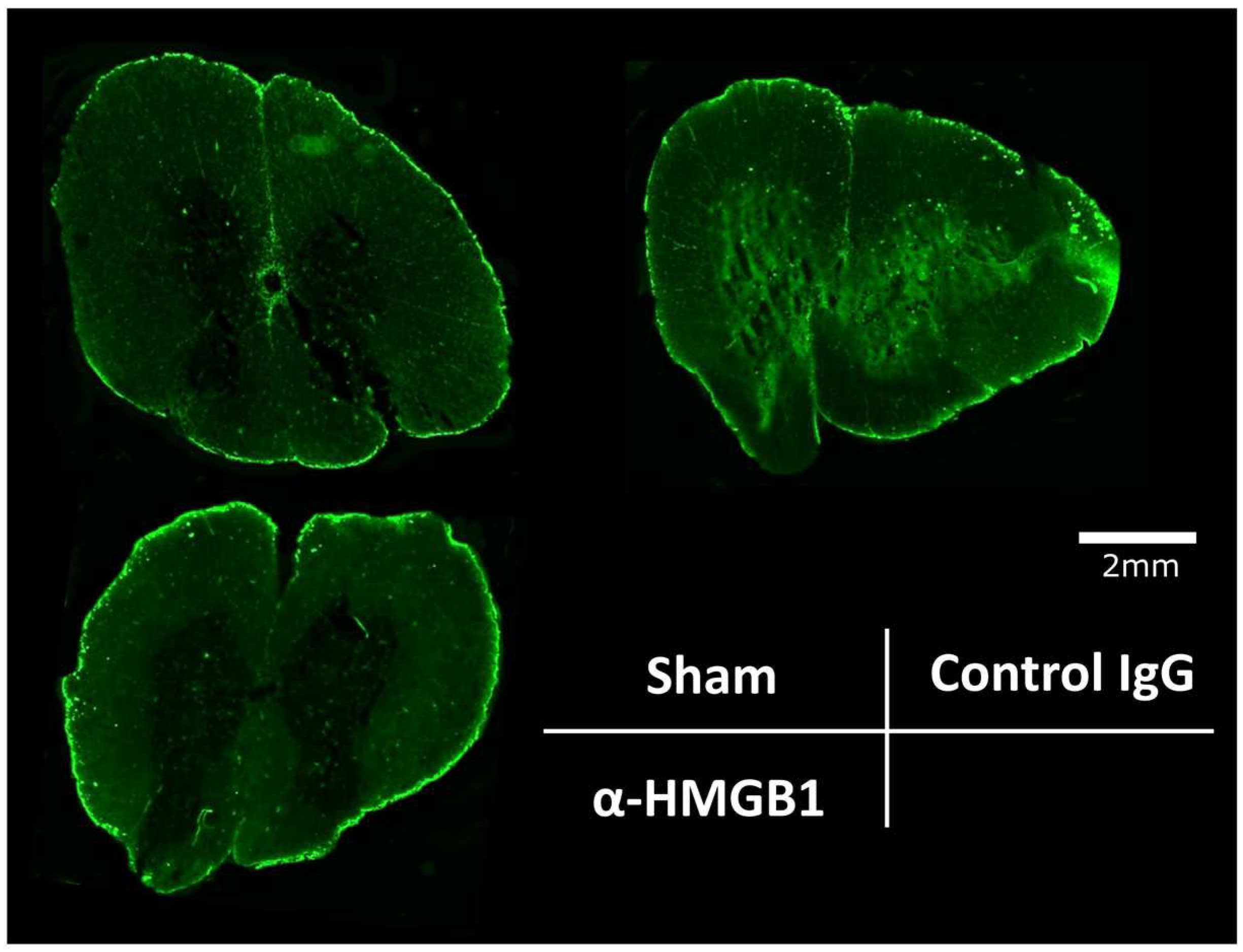
2.4. Anti-HMGB1 mAb Inhibits Microglia and Neutrophil Activation and HMGB1 Release
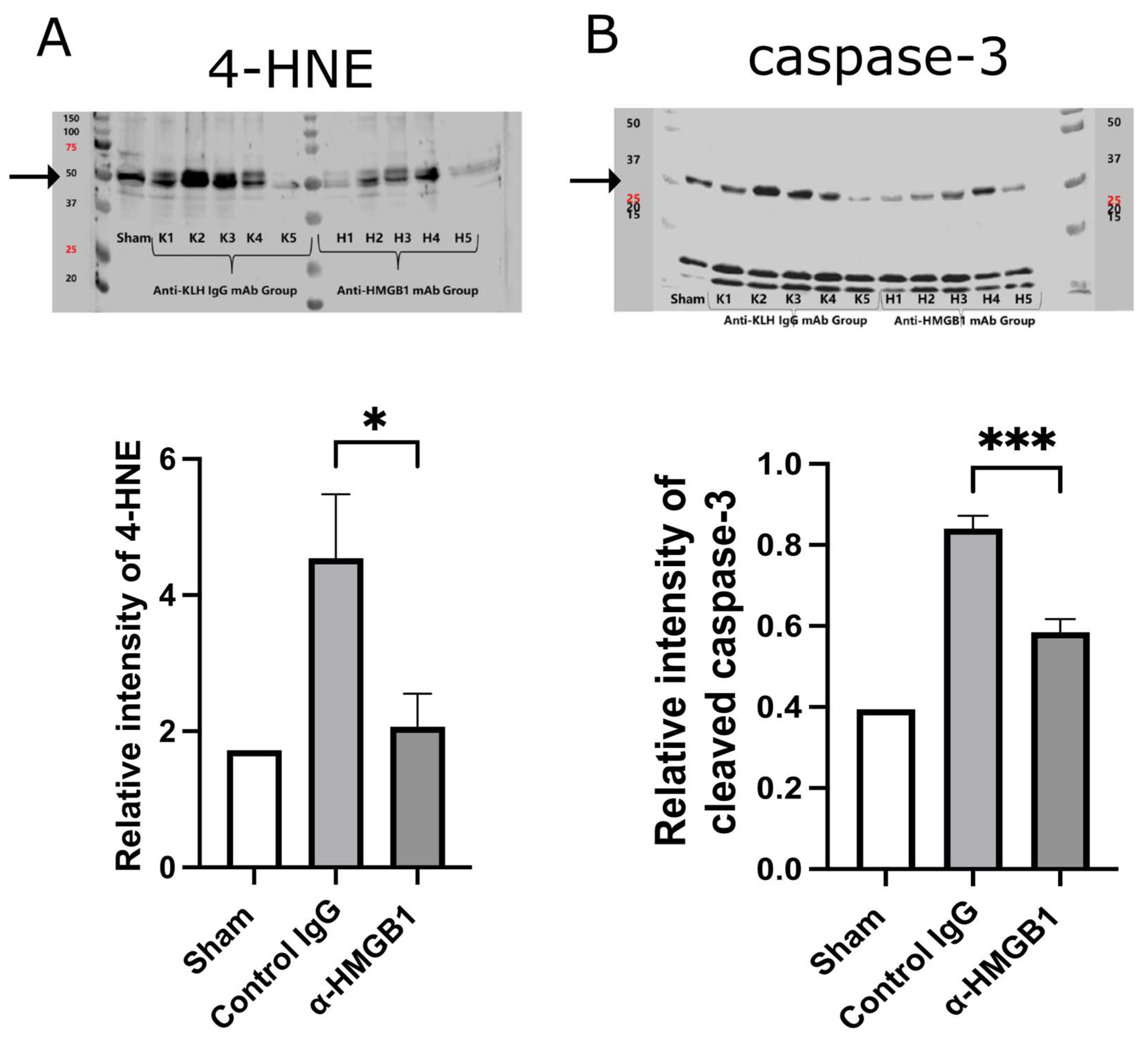
2.5. Caspase-3 and Oxidative Stress Are Reduced by Anti-HMGB1 mAb
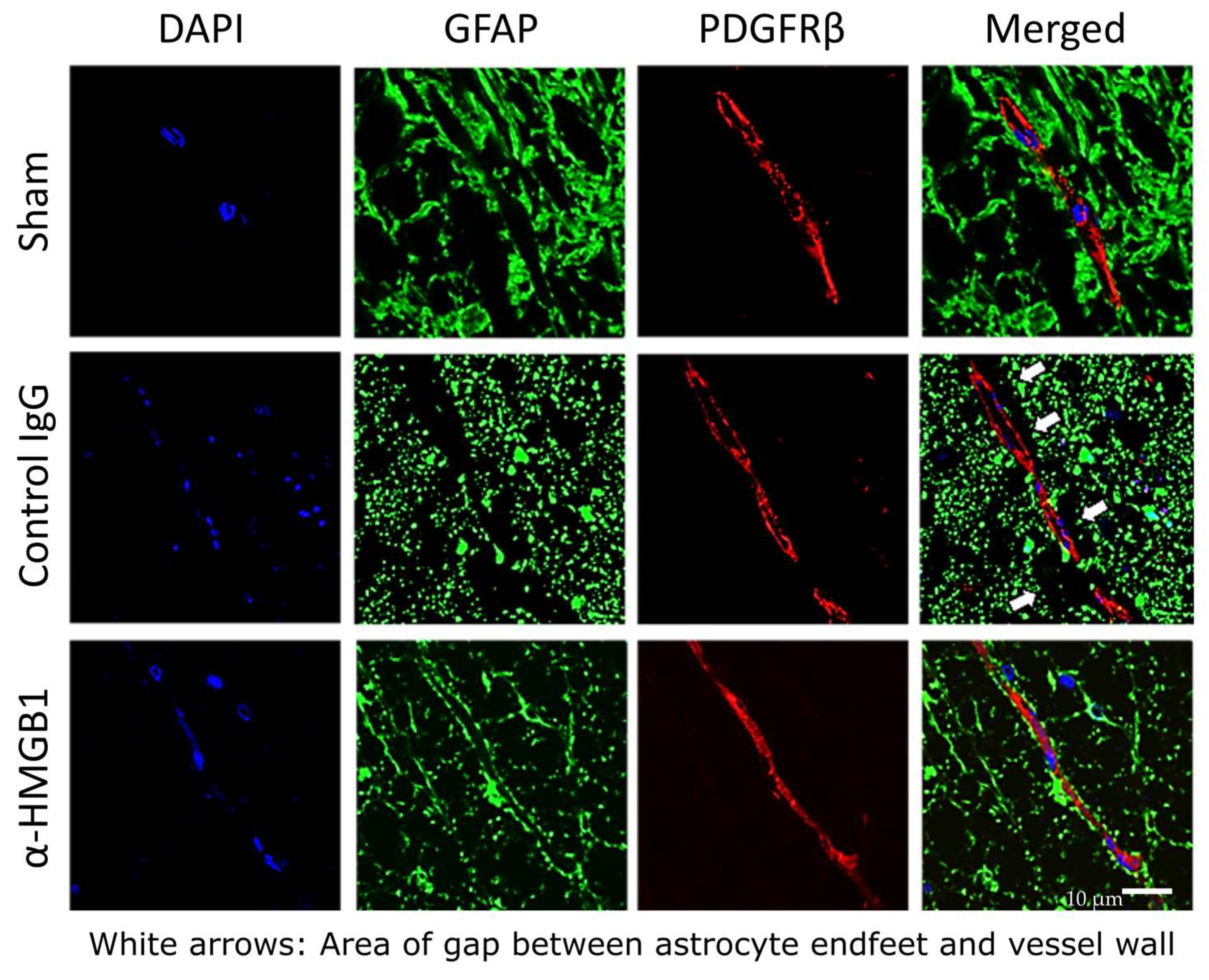
2.6. Anti-HMGB1 mAb Administration Preserves BSCB
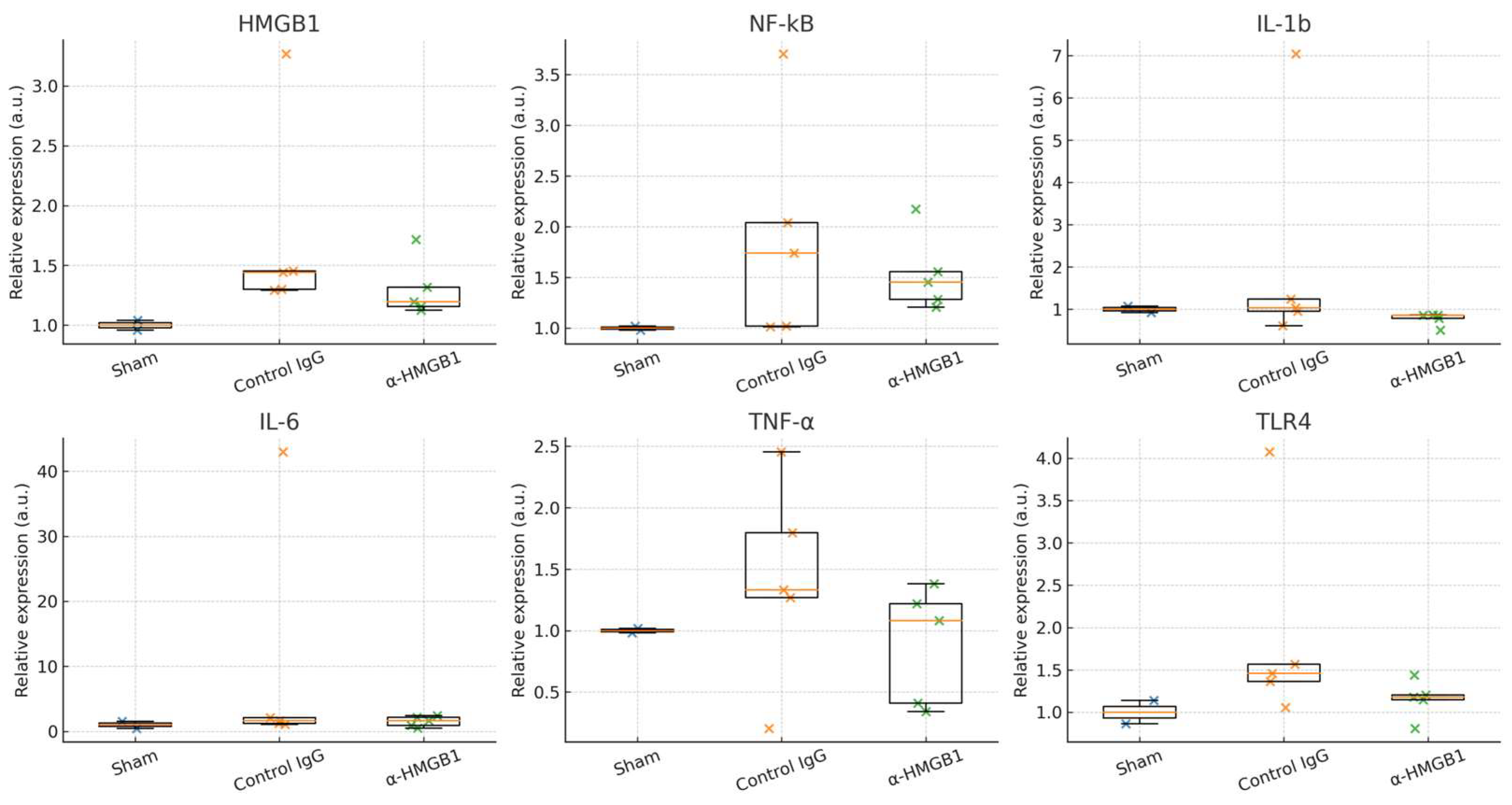
2.7. Determination of Inflammation-Related Molecules in the Spinal Cord Under I/R Injury
3. Discussion
4. Materials and Methods
4.1. Rabbit Model of SCI/R Injury Using Aortic Cross Clamp
4.2. Experimental Protocols
4.3. Hematoxylin and Eosin Staining
4.4. Immunofluorescence Staining
4.5. Western Blot Analysis
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Quantitative Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanaka, A.; Safi, H.J.; Estrera, A.L. Current strategies of spinal cord protection during thoracoabdominal aortic surgery. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 307–314. [Google Scholar] [CrossRef]
- Marturano, F.; Nisi, F.; Giustiniano, E.; Benedetto, F.; Piccioni, F.; Ripani, U. Prevention of spinal cord injury during thoracoabdominal aortic aneurysms repair: What the anaesthesiologist should know. J. Pers. Med. 2022, 12, 1629. [Google Scholar] [CrossRef]
- Alzghari, T.; An, K.R.; Harik, L.; Rahouma, M.; Dimagli, A.; Perezgorvas-Olaria, R.; Demetres, M.; Cancelli, G.; Soletti, G., Jr.; Lau, C.; et al. Spinal cord injury after open and endovascular repair of descending thoracic aneurysm and thoracoabdominal aortic aneurysm: An updated systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2023, 21, 409–417. [Google Scholar] [CrossRef]
- Etz, C.D.; Weigang, E.; Hartert, M.; Lonn, L.; Mestres, C.A.; Di Bartolomeo, R.; Bachet, J.E.; Carrel, T.P.; Grabenwoger, M.; Schepens, M.A.A.M.; et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: A position paper of the vascular domain of the European Association for Cardio-Thoracic Surgery. Eur. J. Cardiothorac. Surg. 2015, 47, 943–957. [Google Scholar] [CrossRef]
- Jacobs, M.J.; Schurink, G.W.; Mees, B.M. Spinal Cord Ischaemia after Complex Procedures. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 279–280. [Google Scholar] [CrossRef]
- Cheung, A.T.; Weiss, S.J.; McGarvey, M.L.; Stecker, M.M.; Hogan, M.S.; Escherich, A.; Bavaria, J.E. Interventions for reversing delayed-onset postoperative paraplegia after thoracic aortic reconstruction. Ann. Thorac. Surg. 2002, 74, 413–421. [Google Scholar] [CrossRef]
- Etz, C.D.; Luehe, M.; Kari, F.A.; Bodian, C.A.; Smego, D.; Plestis, K.A.; Griepp, R.B. Paraplegia after extensive thoracic and thoracoabdominal aortic aneurysm repair: Does critical spinal cord ischemia occur postoperatively? J. Thorac. Cardiovasc. Surg. 2008, 135, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Kakinohana, M.; Kida, K.; Minamishima, S.; Atochin, D.N.; Huang, P.L.; Kaneki, M.; Ichinose, F. Delayed paraplegia after spinal cord ischemic injury requires caspase-3 activation in mice. Stroke 2011, 42, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.D.; Puskas, F.; Meng, X.Z.; Lee, J.H.; Cleveland, J.C.; Weyant, M.J.; Fullerton, D.A.; Reece, B. The evolution of chemokine release supports a bimodal mechanism of spinal cord ischemia and reperfusion injury. Circulation 2012, 126, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Sakurai, M.; Abe, K.; Sadahiro, M.; Tabayashi, K.; Itoyama, Y. Apoptosis of motor neurons with induction of caspases in the spinal cord after ischemia. Stroke 1998, 29, 1007–1013. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current mechanistic concepts in ischemia and reperfusion injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tracey, K.J. Targeting HMGB1 in inflammation. Biochim. Biophys. Acta 2010, 1799, 149–156. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Shaikh, M.F.; Chakraborti, A.; Kumari, Y.; Aledo-Serrano, A.; Aleksovska, K.; Alvim, M.K.M.; Othman, I. HMGB1: A common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front. Neurosci. 2018, 12, 628. [Google Scholar] [CrossRef]
- Kim, J.B.; Choi, J.S.; Yu, Y.M.; Nam, K.; Piao, C.S.; Kim, S.W.; Lee, M.H.; Han, P.L.; Park, J.S.; Lee, J.K. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 2006, 26, 6413–6421. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Mori, S.; Takahashi, H.K.; Tomono, Y.; Wake, H.; Kanke, T.; Sato, Y.; Hiraga, N.; Adachi, N.; Yoshino, T.; et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007, 21, 3904–3916. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, K.; Wake, H.; Teshigawara, K.; Mori, S.; Nishibori, M. Anti-high mobility group box-1 (HMGB1) antibody inhibits hemorrhage-induced brain injury and improved neurological deficits in rats. Sci. Rep. 2017, 7, 46243. [Google Scholar] [CrossRef]
- Okuma, Y.; Liu, K.; Wake, H.; Zhang, J.; Maruo, T.; Date, I.; Yoshino, T.; Ohtsuka, A.; Otani, N.; Tomura, S.; et al. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann. Neurol. 2012, 72, 373–384. [Google Scholar] [CrossRef]
- Zhang, J.; Takahashi, H.K.; Liu, K.; Wake, H.; Liu, R.; Maruo, T.; Date, I.; Yoshino, T.; Ohtsuka, A.; Mori, S.; et al. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke 2011, 42, 1420–1428. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Fan, X.; Yuan, F.; Dai, J.; Hu, J. Dexmedetomidine Preconditioning Ameliorates Inflammation and Blood-Spinal Cord Barrier Damage After Spinal Cord Ischemia-Reperfusion Injury by Down-Regulation High Mobility Group Box 1-Toll-Like Receptor 4-Nuclear Factor κB Signaling Pathway. Spine 2019, 44, E74–E81. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, Q.; Zhou, Y.; Gou, X.; Hou, L.; Chen, S.; Zhu, Z.; Xiong, L. Ethyl pyruvate attenuates spinal cord ischemic injury with a wide therapeutic window through inhibiting high-mobility group box 1 release in rabbits. Anesthesiology 2009, 110, 1279–1286. [Google Scholar] [CrossRef]
- Gong, G.; Yuan, L.B.; Hu, L.; Wu, W.; Yin, L.; Hou, J.L.; Liu, Y.H.; Zhou, L.S. Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and HMGB1. Acta Pharmacol. Sin. 2012, 33, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Cao, Z.; Liu, Y. Glycyrrhizin protects spinal cord and reduces inflammation in spinal cord ischemia-reperfusion injury. Int. J. Neurosci. 2013, 123, 745–751. [Google Scholar] [CrossRef]
- Simon, F.; Oberhuber, A. Ischemia and reperfusion injury of the spinal cord: Experimental strategies to examine postischemic paraplegia. Neural Regen. Res 2016, 11, 414–415. [Google Scholar] [CrossRef]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.M.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101, 296–301. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Qiu, J.; Nishimura, M.; Wang, Y.; Sims, J.R.; Qiu, S.; Savitz, S.I.; Salomone, S.; Moskowitz, M.A. Early release of HMGB-1 from neurons after the onset of brain ischemia. J. Cereb. Blood Flow Metab. 2008, 28, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Fossati, S.; Bianchi, M.E.; Patrone, M.; Pedrazzi, M.; Sparatore, B.; Moroni, F.; Chiarugi, A. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J. Neurochem. 2007, 103, 590–603. [Google Scholar] [CrossRef]
- Xue, J.; Suarez, J.S.; Minaai, M.; Li, S.; Gaudino, G.; Pass, H.I.; Carbone, M.; Yang, H. HMGB1 as a Therapeutic Target in Disease. J. Intern. Med. 2013, 276, 150–163. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195, Erratum in Nature 2010, 467, 622. [Google Scholar] [CrossRef]
- Hayakawa, K.; Pham, L.D.; Katusic, Z.S.; Arai, K.; Lo, E.H. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc. Natl. Acad. Sci. USA 2012, 109, 7505–7510. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Lim, C.M.; Yu, Y.M.; Lee, J.K. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J. Neurosci. Res. 2008, 86, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lv, J.; Wang, Z.; Wang, L.; Qin, Z.; Demg, C.; Liu, J.; Sun, L. Inhibition of HMGB1 Attenuates Spinal Cord Edema by Reducing the Expression of Na+-K+-Cl− Cotransporter-1 and Na+/H+ Exchanger-1 in Both Astrocytes and Endothelial Cells After Spinal Cord Injury in Rats. J. Neuroinflamm. 2014, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, Y.; Liu, W.; Zeng, F.F.; Shi, J.; Li, J.; Chen, H.; Tu, C.; Xu, Y.; Tan, Z.; et al. HMGB1 promotes the release of sonic hedgehog from astrocyte. J. Neurochem. 2006, 98, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, M.; He, F.; Zhou, S.; Zhu, L. Targeting the NLRP3 inflammasome to attenuate spinal cord injury in mice. J. Neuroinflamm. 2017, 14, 207. [Google Scholar] [CrossRef]
- Müller, N.; Scheld, M.; Voelz, C.; Gasterich, N.; Zhao, W.; Behrens, V.; Weiskirchen, R.; Baazm, M.; Clarner, T.; Beyer, C.; et al. Lipocalin-2 deficiency diminishes canonical NLRP3 inflammasome formation and IL-1β production in the subacute phase of spinal cord injury. Int. J. Mol. Sci. 2023, 24, 8689. [Google Scholar] [CrossRef]
- Zhu, C.S.; Wang, W.; Qiang, X.; Chen, W.; Lan, X.; Li, J.; Wang, H. Endogenous Regulation and Pharmacological Modulation of Sepsis-Induced HMGB1 Release and Action: An Updated Review. Cells 2021, 10, 2220. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Rowland, J.W.; Hawryluk, G.W.J.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Xing, Y.; Xiao, Y.Z.; Zhao, J.J.; Zhao, K.; Xiao, C.L. The role of oxidative stress in spinal cord ischemia reperfusion injury: Mechanisms and therapeutic implications. Front. Cell. Neurosci. 2025, 19, 1590493. [Google Scholar] [CrossRef]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef]
- Singh, A.; Tetreault, T.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar]
- Lee, B.B.; Cripps, R.A.; Fitzharris, M.; Wing, P.C. The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord 2014, 52, 110–116. [Google Scholar] [CrossRef]
- Uezono, N.; Zhu, Y.; Fujimoto, Y.; Yasui, T.; Matsuda, T.; Nakajo, M.; Abematsu, M.; Setoguchi, T.; Mori, S.; Takahashi, H.K.; et al. Prior Treatment with Anti-High Mobility Group Box-1 Antibody Boosts Human Neural Stem Cell Transplantation-Mediated Functional Recovery After Spinal Cord Injury. Stem Cells 2018, 36, 737–750. [Google Scholar] [CrossRef]
- Cohen, M.M., Jr. Perspectives on RAGE signaling and its role in cardiovascular disease. Am. J. Med. Genet. A 2013, 161, 2750–2755. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhou, P.; Sun, Y.; Meng, X.; Dai, Z.; Sun, G.; Sun, X. Protective Effects and Target Network Analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion Injury: A Comprehensive Overview of Experimental Studies. Cells 2018, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Liang, L.; Wu, Y.; Zhong, J.; Sun, X. Anti-high mobility group box-1 antibody attenuated vascular smooth muscle cell phenotypic switching and vascular remodelling after subarachnoid haemorrhage in rats. Neurosci. Lett. 2019, 708, 134338. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Matsumoto, M.; Yamashita, A.; Ohtake, K.; Ishida, K.; Morimoto, Y.; Sakabe, T. The temporal profile of the reaction of microglia, astrocytes, and macrophages in the delayed onset paraplegia after transient spinal cord ischemia in rabbits. Anesth. Analg. 2003, 96, 1777–1784. [Google Scholar] [CrossRef]
- Moore, W.M., Jr.; Hollier, L.H. The influence of severity of spinal cord ischemia in the etiology of delayed-onset paraplegia. Ann. Surg. 1991, 213, 427–431. [Google Scholar] [CrossRef]
- Kawanishi, Y.; Okada, K.; Tanaka, H.; Yamashita, T.; Nakagiri, K.; Okita, Y. The adverse effect of back-bleeding from lumbar arteries on spinal cord pathophysiology in a rabbit model. J. Thorac. Cardiovasc. Surg. 2007, 133, 1553–1558. [Google Scholar] [CrossRef]
- Nakamichi, T. Glutamate neurotoxicity during spinal cord ischemia--the neuroprotective effects of adenosine. Jpn. J. Thorac. Cardiovasc. Surg. 1998, 46, 354–360. [Google Scholar] [CrossRef]
- Akuzawa, S.; Kazui, T.; Shi, E.; Yamashita, K.; Bashar, A.H.M.; Terada, H. Interleukin-1 receptor antagonist attenuates the severity of spinal cord ischemic injury in rabbits. J. Vasc. Surg. 2008, 48, 694–700. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell death and disease related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Uchida, K. 4-Hydroxy-2-nonenal: A product and mediator of oxidative stress. Prog. Lipid Res. 2003, 42, 318–343. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Font. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef]
- Talma, N.; Kok, W.F.; de Veij Mestdagh, C.F.; Shanbhag, N.C.; Bouma, H.R.; Henning, R.H. Neuroprotective hypothermia—Why keep your head cool during ischemia and reperfusion. Biochim. Biophys. Acta 2016, 1860, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, M.; Wang, D.; Ousaka, D.; Wake, H. High mobility group box-1 and blood-brain barrier disruption. Cells 2020, 9, 2650. [Google Scholar] [CrossRef]
- Mo, Y.; Chan, K. Review: The role of HMGB1 in spinal cord injury. Front. Immunol. 2023, 13, 1094925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and dysfunction of the blood-brain barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Hayashi, T.M.; Abe, K.; Sadahiro, M.; Tabayashi, K. Delayed and selective motor neuron death after transient spinal cord ischemia: A role of apoptosis? J. Thorac. Cardiovasc. Surg. 1998, 115, 1310–1315. [Google Scholar] [CrossRef][Green Version]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010, 6, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Sichita, T.; Sakaguchi, R.; Suzuki, M.; Yoshimura, A. Post-ischemic inflammation in the brain. Front. Immunol. 2012, 3, 132. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Morioka, N.; Abe, H.; Zhang, F.F.; Hisaoka-Nakashima, K.; Liu, K.; Nishibori, M.; Nakata, Y. Neuropathic pain in rats with a partial sciatic nerve ligation is alleviated by intravenous injection of monoclonal antibody to high mobility group box-1. PLoS ONE 2013, 8, e73640. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.Y.; Xue, M.; Wang, Y.; Huang, Z.H.; Huang, C. Electroacupuncture Alleviates Spared Nerve Injury-Induced Neuropathic Pain And Modulates HMGB1/NF-κB Signaling Pathway In The Spinal Cord. J. Pain Res. 2019, 12, 2851–2863. [Google Scholar] [CrossRef]
- Matsuura, W.; Harada, S.; Liu, K.; Nishibori, M.; Tokuyama, S. Evidence of a role for spinal HMGB1 in ischemic stress-induced mechanical allodynia in mice. Brain Res. 2018, 1687, 1–10. [Google Scholar] [CrossRef]
- Ministry of the Environment, Japan. Standards Relating to the Care and Keeping and Reducing Pain of Laboratory Animals (Notice of the Ministry of the Environment No. 88 of 2006; Latest Revision: No. 84 of 2013). Available online: https://www.env.go.jp/nature/dobutsu/aigo/2_data/laws/nt_h25_84_en.pdf (accessed on 31 August 2025).



| Clamp Time (min) | Modified Tarlov Score After I/R | ||
|---|---|---|---|
| 6 h After I/R | 24 h After I/R | 48 h After I/R | |
| 30 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| 12 | 0 | 0 | 0 |
| 11 | 4 | 3 ± 1 | 1.5 ± 0.5 |
| 10 | 4 | 4 | 4 |
| 9 | 4 | 4 | 4 |
| 8 | 4 | 4 | 4 |
| SpO2 (%) | HR (bpm) | BT (°C) | SBP (mmHg) | DBP (mmHg) | |
|---|---|---|---|---|---|
| Pre-ischemia | |||||
| Sham | 100 | 284 | 38.0 | 54 | 40 |
| Control IgG | 100 | 270.4 ± 11.2 | 38.4 ± 0.2 | 73.8 ± 3.5 | 49.0 ± 7.3 |
| α-HMGB1 | 100 | 278.0 ± 4.9 | 38.3 ± 0.1 | 66.0 ± 5.8 | 52.2 ± 3.1 |
| Post-ischemia | |||||
| Sham | 100 | 259 | 37.4 | 36 | 30 |
| Control IgG | 99.8 ± 0.2 | 250.2 ± 6.8 | 37.6 ± 0.7 | 55.8 ± 0.3 | 42.0 ± 0.8 |
| α-HMGB1 | 100 | 249.2 ± 7.0 | 37.5 ± 0.1 | 62.8 ± 5.2 | 43.0 ± 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muraoka, G.; Fujii, Y.; Liu, K.; Qiao, H.; Wang, D.; Ousaka, D.; Oozawa, S.; Kasahara, S.; Nishibori, M. Anti-HMGB1 Antibody Therapy Ameliorates Spinal Cord Ischemia–Reperfusion Injury in Rabbits. Int. J. Mol. Sci. 2025, 26, 8643. https://doi.org/10.3390/ijms26178643
Muraoka G, Fujii Y, Liu K, Qiao H, Wang D, Ousaka D, Oozawa S, Kasahara S, Nishibori M. Anti-HMGB1 Antibody Therapy Ameliorates Spinal Cord Ischemia–Reperfusion Injury in Rabbits. International Journal of Molecular Sciences. 2025; 26(17):8643. https://doi.org/10.3390/ijms26178643
Chicago/Turabian StyleMuraoka, Genya, Yasuhiro Fujii, Keyue Liu, Handong Qiao, Dengli Wang, Daiki Ousaka, Susumu Oozawa, Shingo Kasahara, and Masahiro Nishibori. 2025. "Anti-HMGB1 Antibody Therapy Ameliorates Spinal Cord Ischemia–Reperfusion Injury in Rabbits" International Journal of Molecular Sciences 26, no. 17: 8643. https://doi.org/10.3390/ijms26178643
APA StyleMuraoka, G., Fujii, Y., Liu, K., Qiao, H., Wang, D., Ousaka, D., Oozawa, S., Kasahara, S., & Nishibori, M. (2025). Anti-HMGB1 Antibody Therapy Ameliorates Spinal Cord Ischemia–Reperfusion Injury in Rabbits. International Journal of Molecular Sciences, 26(17), 8643. https://doi.org/10.3390/ijms26178643





