A Digital Image Analysis of the Morphology and Immunolocalization of Catalase and Caspase-3 in the Skin of Adult Male Rats After Treatment with Letrozole and Vitamin C
Abstract
1. Introduction
2. Results
2.1. Body Weight
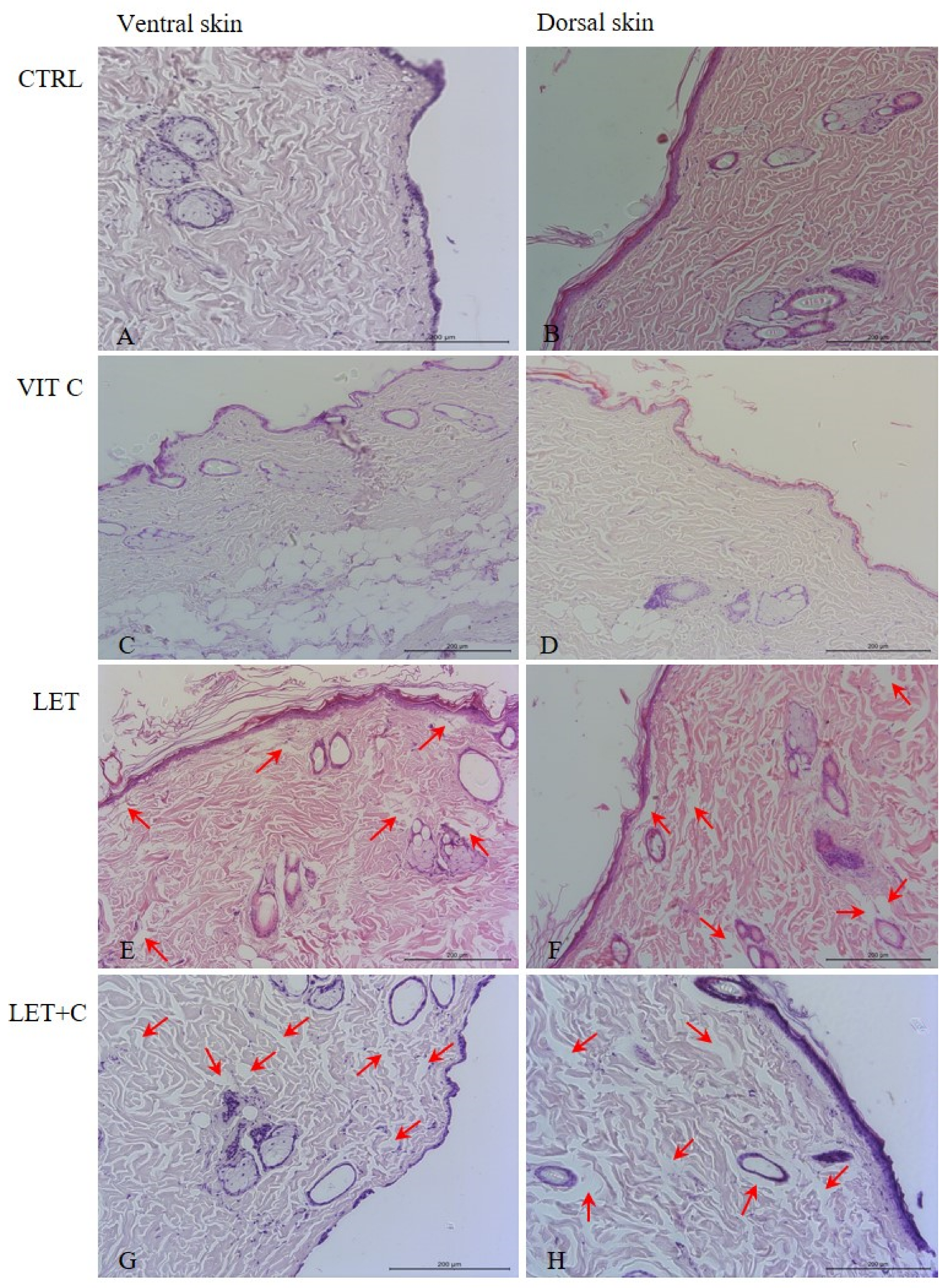
2.2. Morphology
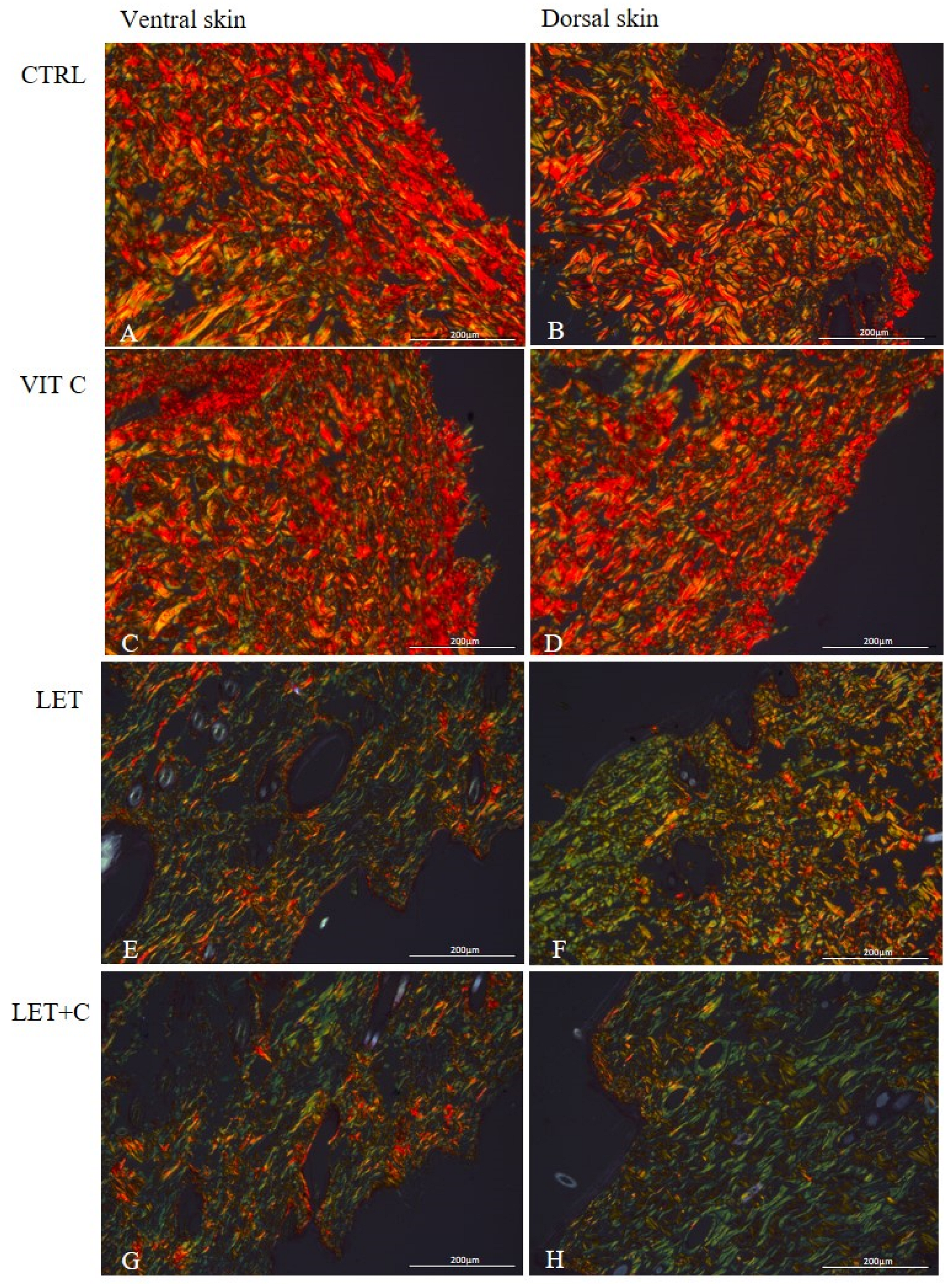
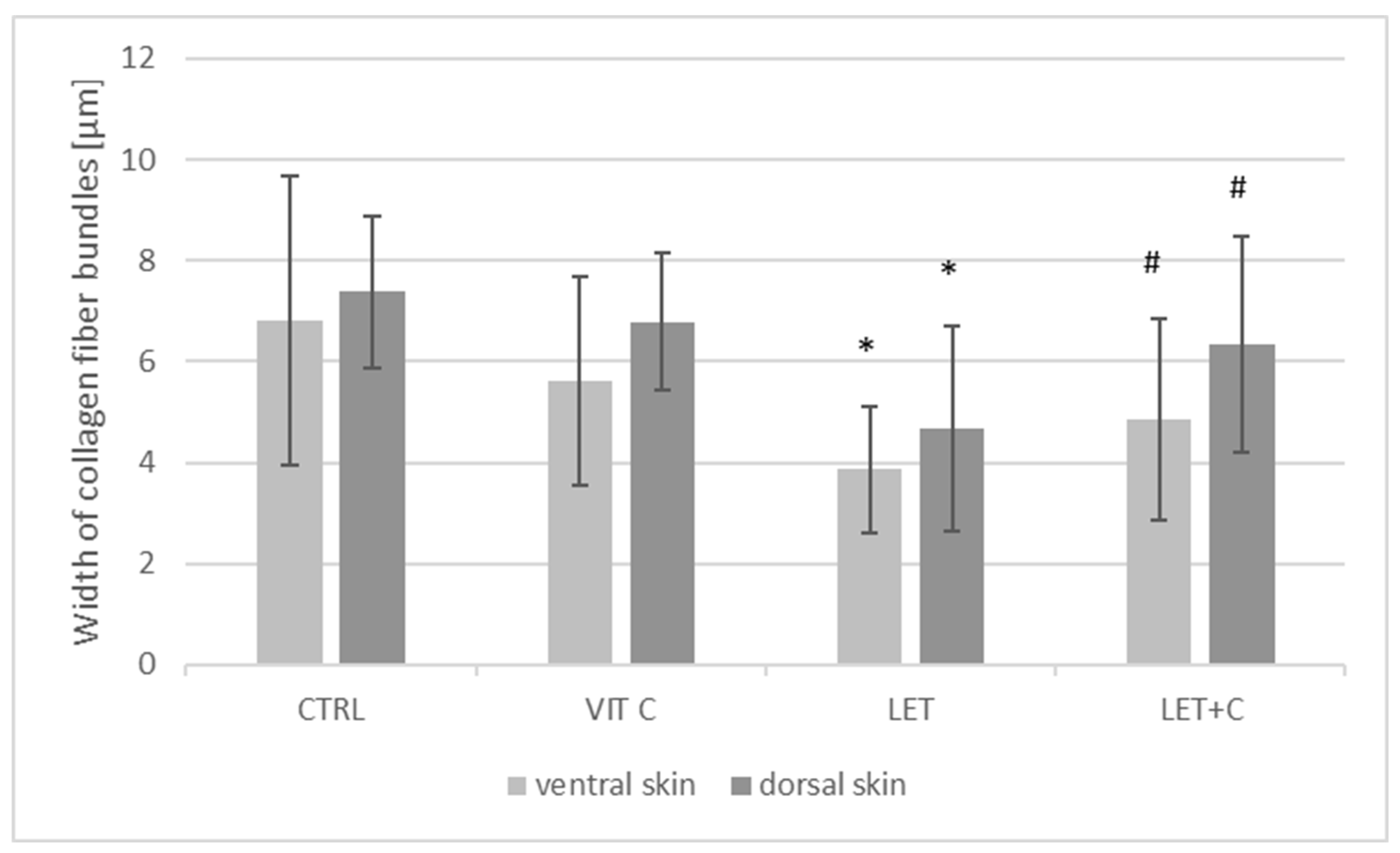
2.3. Morphometry
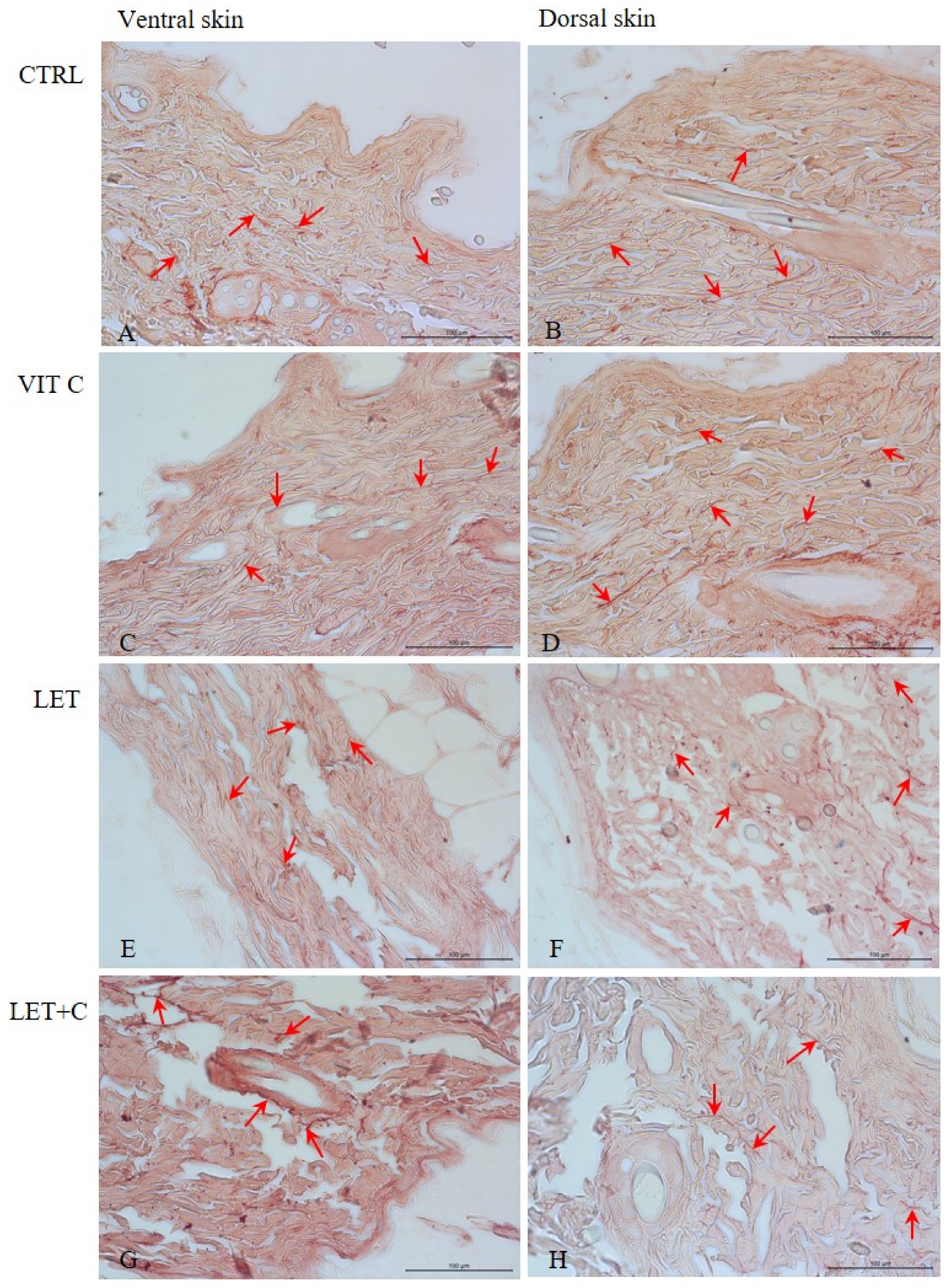
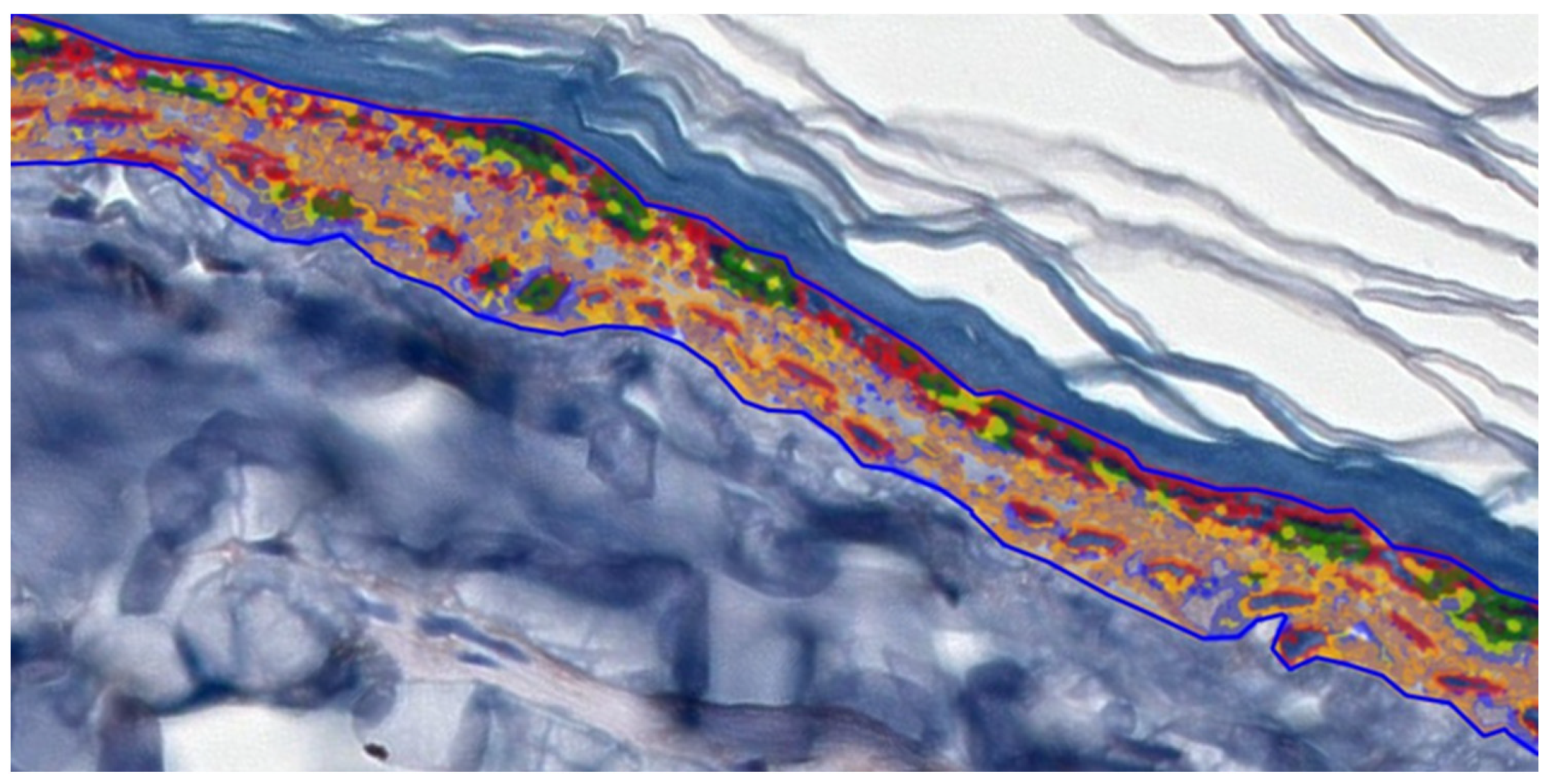
2.4. IHC Staining and Digital Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Tissues
4.2. Staining, Morphology, and Morphometry
4.3. IHC Staining
4.4. Digital Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| P450arom | cytochrome P450 aromatase |
| ER | estrogen receptor |
| TGFβ | transforming growth factor-beta |
| TIMPs | tissue inhibitors of matrix metalloproteinases |
| MMPs | matrix metalloproteinases |
| LET | letrozole |
| CTRL | control |
| VIT C | control supplemented with vitamin C |
| LET+C | letrozole supplemented with vitamin C |
| HE | hematoxylin and eosin staining |
| Me | median |
| X | mean |
| SD | standard deviation |
| rs | Spearman’s correlation |
| PI3K | phosphatidylinositol 3-kinase |
| MAPK | mitogen-activated protein kinase |
| ROS | reactive oxygen species |
References
- Pelletier, G.; Ren, L. Localization of sex steroid receptors in human skin. Histol. Histopathol. 2004, 19, 629–636. [Google Scholar] [PubMed]
- Zouboulis, C.C.; Chen, W.C.; Thornton, M.J.; Qin, K.; Rosenfield, R. Sexual hormones in human skin. Horm. Metab. Res. 2007, 39, 85–95. [Google Scholar] [CrossRef]
- Zomer, H.D.; Cooke, P.S. Targeting estrogen signaling and biosynthesis for aged skin repair. Front. Physiol. 2023, 31, 14. [Google Scholar] [CrossRef]
- Blakemore, J.; Naftolin, F. Aromatase: Contributions to physiology and disease in women and men. Physiology 2016, 31, 258–269. [Google Scholar] [CrossRef]
- Longcope, C. Methods and results of aromatization studies in vivo. Cancer Res. 1982, 42, 3307–3331. [Google Scholar]
- Abaffy, T.; Hiroaki, M. 19-hydroxy Steroids in the Aromatase Reaction: Review on Expression and Potential Functions. J. Endocr. Soc. 2021, 5, 7. [Google Scholar] [CrossRef]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45, 116–124. [Google Scholar] [CrossRef]
- Nikolakis, G.; Stratakis, C.A.; Kanaki, T.; Slominski, A.; Zouboulis, C.C. Skin steroidogenesis in health and disease. Rev. Endocr. Metab. Disord. 2016, 17, 247–258. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 16, 135–170. [Google Scholar]
- Thornton, M.J. Oestrogen functions in skin and skin appendages. Expert Opin. Ther. Targets 2005, 9, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Naftolin, F. Menopause and the skin: Old favorites and new innovations in cosmeceuticals for estrogen-deficient skin. Dermatol. Ther. 2021, 11, 53–69. [Google Scholar] [CrossRef]
- Hong, Y.; Rashid, R.; Chen, S. Binding Features of Steroidal and Nonsteroidal Inhibitors. Steroids 2011, 76, 802–806. [Google Scholar] [CrossRef]
- Elkhiat, Y. Aromatase inhibitors in the treatment of male infertility. Hum. Androl. 2011, 1, 35–38. [Google Scholar] [CrossRef]
- Peivandi, S.; Abbaspour, M.; Jafarpour, H.; Ebadi, A. Effect of letrozole on spermogram parameters and hormonal profile in infertile men: A clinical trial study. Endocr. Regul. 2019, 53, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Sigalos, J.T.; Pastuszak, A.W.; Khera, M. Hypogonadism: Therapeutic risks, benefits, and outcomes. Med. Clin. N. Am. 2018, 102, 361–372. [Google Scholar] [CrossRef]
- Hero, M.; Norjavaara, E.; Dunkel, L. Inhibition of estrogen biosynthesis with apotent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: A randomized controlled trial. J. Clin. Endocrinol. Metab. 2005, 90, 6396–6402. [Google Scholar] [CrossRef] [PubMed]
- Hero, M.; Wickman, S.; Dunkel, L. Treatment with the aromatase inhibitor letrozole during adolescence increases near-final height in boys with constitutional delay of puberty. Clin. Endocrinol. 2006, 64, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Isoir-Ingrez, M.; Falip, A.; Yousfi, N.; Arnaud-Sebillotte, L.; Biatry, B.; Leroy, F.; Wang, P.H.; Simonnet, J.T. Mastering the formulation of an unstable vitamin C (VC) as anti-aging skin care ingredient. Part I: A new approach. Int. J. Cosmet. Sci. 2025, 47, 597–603. [Google Scholar] [CrossRef]
- Darr, D.; Combs, S.; Dunston, S.; Manning, T.; Pinnell, S. Topical vitamin C protects porcine skin from ultraviolet radiation-induced damage. Br. J. Dermatol. 1992, 127, 247–253. [Google Scholar] [CrossRef]
- Austria, R.; Semenzato, A.; Bettero, A. Stability of vitamin C derivatives in solution and topical formulations. J. Pharm. Biomed. Anal. 1997, 15, 795–801. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of vitamin C in skin diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Draelos, Z.; Bogdanowicz, P.; Saurat, J.H. Top weapons in skin aging and actives to target the consequences of skin cell senescence. J. Eur. Acad. Dermatol. Venereol. 2024, 38 (Suppl. S4), 15–22. [Google Scholar] [CrossRef]
- Rabat Sarpooshi, H.; Mortazavi, F.; Mojtaba, V.; Tabarayee, Y. The effects of topical vitamin C solution on burn wounds granulation: A randomized clinical trial. J. Biomed. 2016, 1, 8301. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Vitamin C: A Comprehensive Review of Its Role in Health, Disease Prevention, and Therapeutic Potential. Molecules 2025, 30, 748. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 1, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 11, 9613090. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Raj, D.; Brash, D.E.; Grossman, D. Keratinocyte apoptosis in epidermal development and disease. J. Investig. Dermatol. 2006, 126, 243–257. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 1, 5, Erratum in Cold Spring Harb. Perspect. Biol. 2015, 7, a026633. [Google Scholar] [CrossRef]
- Kondarewicz, A.; Kolasa, A.; Zawiślak, B.; Baranowska-Bosiacka, I.; Marchlewicz, M.; Wenda-Różewicka, L.; Wiszniewska, B. Testis Morphology in Rats Chronically Treated with Letrozole, an Aromatase Inhibitor. Folia Histochem. Cytobiol. 2011, 49, 677–684. [Google Scholar] [CrossRef]
- Misiakiewicz-Has, K.; Pilutin, A.; Wiszniewska, B. Influence of Hormonal Imbalance on the Integrity of Seminiferous Epithelium in the Testes of Adult Rats Chronically Exposed to Letrozole and Rats Exposed to Soya Isoflavones during the Prenatal Period, Lactation, and up to Sexual Maturity. Reprod. Biol. 2021, 21, 100562. [Google Scholar] [CrossRef]
- Pilutin, A.; Misiakiewicz-Has, K.; Rzeszotek, S.; Wiszniewska, B. Morphological and Morphometric Changes and Epithelial Apoptosis Are Induced in the Rat Epididymis by Long-Term Letrozole Treatment. Eur. J. Histochem. EJH 2021, 65, 3259. [Google Scholar] [CrossRef]
- Pilutin, A.; Misiakiewicz-Has, K.; Kolasa-Wołosiuk, A.; Trybek, G.; Urban, F.; Marchlewicz, M.; Leszczy’nski, B.; Wróbel, A.; Wiszniewska, B. Morphology and Serum and Bone Tissue Calcium and Magnesium Concentrations in the Bones of Male Rats Chronically Treated with Letrozole, a Nonsteroidal Cytochrome P450 Aromatase Inhibitor. Connect. Tissue Res. 2021, 62, 454–463. [Google Scholar] [CrossRef]
- Pilutin, A.; Rzeszotek, S.; Wilk, A.; Klimaszewska, K.; Łukasiewicz, J.; Mafuta, R.L.; Nagendran, T.; Ndambara, R.; Wiszniewska, B. Effects of Letrozole Treatment and Vitamin C Supplementation on Morphology, Endoplasmic Reticulum Stress, Programmed Cell Death, and Oxidative Stress in the Small Intestine of Adult Male Rats. Curr. Issues Mol. Biol. 2024, 46, 1943–1954. [Google Scholar] [CrossRef]
- Verdier-Sevrain, S.; Bonte’, F.; Gilchrest, B. Biology of estrogens in skin: Implications for skin aging. Exp. Dermatol. 2006, 15, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Calvin, M. Oestrogens and wound healing. Maturitas 2000, 34, 195–210. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Ashworth, J.J. Potential role of estrogensin wound healing. Am. J. Clin. Dermatol. 2003, 4, 737–743. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogenic control of mitochondrial function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef]
- Kovács, T.; Szabó-Meleg, E.; Ábrahám, I.M. Estradiol-induced epigenetically mediated mechanisms and regulation of gene expression. Int. J. Mol. Sci. 2020, 21, 3177. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Paramanik, V. Neuromodulating roles of estrogen and phytoestrogens in cognitive therapeutics through epigenetic modifications during aging. Front. Aging Neurosci. 2022, 14, 945076. [Google Scholar] [CrossRef] [PubMed]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef]
- Taheri, M.; Ghafouri-Fard, S.; Najafi, S.; Kallenbach, J.; Keramatfar, E.; Atri Roozbahani, G.; Heidari Horestani, M.; Hussen, B.M.; Baniahmad, A. Hormonal regulation of telomerase activity and hTERT expression in steroid-regulated tissues and cancer. Cancer Cell Int. Biomed. 2022, 22, 258. [Google Scholar] [CrossRef]
- Lynfield, Y.L. Effect of pregnancy on the human hair cycle. J. Investig. Dermatol. 1960, 35, 323–327. [Google Scholar] [CrossRef]
- Ai-Min, Y.; Na, C.; Yi-Fei, S.; Gui-Min, H. Letrozole for Female Infertility. Front. Endocrinol. 2021, 16, 676133. [Google Scholar] [CrossRef] [PubMed]
- Misiakiewicz-Has, K.; Zawiślak, A.; Pilutin, A.; Kolasa-Wołosiuk, A.; Szumilas, P.; Duchnik, E.; Wiszniewska, B. Morphological and Functional Changes in Skin of Adult Male Rats Chronically Treated with Letrozole, a Nonsteroidal Inhibitor of Cytochrome P450 Aromatase. Acta Histochem. Cytochem. 2020, 53, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Zouloboulis, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostic of skin aging. Clin. Dermatol. 2011, 29, 3–14. [Google Scholar] [CrossRef]
- Markiewicz, M.; Asano, Y.; Znoyko, S.; Gong, Y.; Watson, D.K.; Trojanowska, M. Distinct effects of gonadectomy in male and female mice on collagen fibrillogenesis in the skin. J. Dermatol. Sci. 2007, 47, 217–226. [Google Scholar] [CrossRef]
- Garavello-Freitas, I.; Baranauskas, V.; Joazeiro, P.P.; Padovani, C.R.; Dal Pai-Silva, M.; da Cruz-Höfling, M.A. Low-power laser irradiation improves histomorphological parameters and bone matrix organization during tibia wound healing in rats. J. Photochem. Photobiol. B 2003, 70, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method. for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Dayan, D.; Hiss, Y.; Hirshberg, A.; Bubis, J.J.; Wolman, M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry 1989, 93, 27–29. [Google Scholar] [CrossRef]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthet. Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Stevenson, S.; Thornton, J. Effect of estrogens on skin aging and the potential role of SERMs. Clin. Interv. Aging 2007, 2, 283–297. [Google Scholar]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Gref, R.; Deloménie, C.; Maksimenko, A.; Gouadon, F.; Percoco, G.; Lati, E.; Desmaële, D.; Zouhiri, F.; Couvreur, P. Vitamin C–squalene bioconjugate promotes epidermal thickening and collagen production in human skin. Sci. Rep. 2020, 10, 16883. [Google Scholar] [CrossRef]
- Khavkin, J.; Ellis, D.A. Aging skin: Histology, physiology, and pathology. Facial Plast. Surg. Clin. N. Am. 2011, 19, 229–234. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Yaar, M.; Gilchrest, B. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeong, J.H.; Lee, I.H.; Lee, J.; Jung, J.H.; Park, H.Y.; Lee, D.H.; Chae, Y.S. Effect of High-dose Vitamin C Combined With Anti-cancer Treatment on Breast Cancer Cells. Anticancer Res. 2019, 39, 751–758. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Sadi, G.; Yılmaz, Ö.; Güray, T. Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu–Zn SOD and catalase. Mol. Cell Biochem. 2008, 309, 109–116. [Google Scholar] [CrossRef]
- Krzastek, S.; Smith, R. Non-testosterone management of male hypogonadism: An examination of the existing literature. Trans. Androl. Ur. 2019, 9, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 18, 13. [Google Scholar] [CrossRef]
- Strehlow, K.; Rotter, S.; Wassmann, S.; Adam, O.; Grohé, C.; Laufs, K.; Böhm, M.; Nickenig, G. Modulation of antioxidant enzyme expression and function by estrogen. Circ. Res. 2003, 25, 170–177. [Google Scholar] [CrossRef]
- Griñan-Lison, C.; Blaya-Cánovas, J.L.; López-Tejada, A.; Ávalos-Moreno, M.; Navarro-Ocón, A.; Cara, F.E.; González-González, A.; Lorente, J.A.; Marchal, J.A.; Granados-Principal, S. Antioxidants for the Treatment of Breast Cancer: Are We There Yet? Antioxidants 2021, 10, 205. [Google Scholar] [CrossRef]
- Iffat, N. Effect of Ascorbic Acid Supplementation on Liver Function Tests in Hepatitis C Patients. OJIM 2020, 10, 263–279. [Google Scholar]
- Al-Niaimi, F.; Chiang, N.Y.Z. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. J. Clin. Aesthet. Dermatol. 2017, 10, 14–17. [Google Scholar]
- Shumyantseva, V.V.; Makhova, A.A.; Bulko, T.V.; Kuzikov, A.V.; Shich, E.V.; Suprun, E.V.; Kukes, V.; Usanov, S.; Archakov, A. The dose-dependent influence of antioxidant vitamins on electrochemically-driven cytochrome P450 3A4 catalysis. Oxid. Antioxid. Med. Sci. 2013, 2, 113–117. [Google Scholar] [CrossRef]
- Coumau, C.; Csajka, C. A Systematic Review and Classification of the Effects of P-glycoprotein Inhibitors and Inducers in Humans, Using Digoxin, Fexofenadine, and Dabigatran as Probe Drugs. Clin. Pharmacokinet. 2025, 64, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Luca, A.; Avena, P.; De Amicis, F.; Casaburi, I.; Sirianni, R.; Pezzi, V. Estrogen Receptors-Mediated Apoptosis in Hormone-Dependent Cancers. Int. J. Mol. Sci. 2022, 23, 1242. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Chen, S.; Klyne, D.M.; Harrich, D.; Ding, W.; Yang, S.; Han, F.Y. Low back pain and osteoarthritis pain: A perspective of estrogen. Bone Res. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Lewoniewska, S.; Oscilowska, I.; Forlino, A.; Palka, J. Understanding the Role of Estrogen Receptor Status in PRODH/POX-Dependent Apoptosis/Survival in Breast Cancer Cells. Biology 2021, 10, 1314. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 21, 1501. [Google Scholar] [CrossRef]
- Raymond, A.A.; Méchin, M.C.; Nachat, R.; Toulza, E.; Tazi-Ahnini, R.; Serre, G. Nine procaspases are expressed in normal human epidermis, but only caspase-14 is fully processed. Br. J. Dermatol. 2007, 156, 420–427. [Google Scholar] [CrossRef]

| Groups | ||||
|---|---|---|---|---|
| CTRL | VIT C | LET | LET+C | |
| Elastic fibers [%] | ||||
| Dorsal | ||||
| X ± SD | 0.38 ± 0.37 | 0.30 ± 0.20 | 0.15 ± 0.05 | 0.17 ± 0.13 |
| Me | 0.15 | 0.23 | 0.15 | 0.15 |
| Ventral | ||||
| X ± SD | 0.21 ± 0.12 | 0.19 ± 0.09 | 0.21 ± 0.16 | 0.34 ± 0.10 |
| Me | 0.16 | 0.19 | 0.16 | 0.39 |
| Collagen fibers [µm] | ||||
| Dorsal | ||||
| X ± SD | 7.38 ± 1.51 | 6.79 ± 1.35 | 4.68 ± 2.04 | 6.33 ± 2.14 |
| Me | 6.83 | 6.54 | 4.27 * | 6.21 # |
| Ventral | ||||
| X ± SD | 6.82 ± 2.87 | 5.61 ± 2.08 | 3.86 ± 1.26 | 4.84 ± 1.99 |
| Me | 6.19 | 4.97 | 3.77 * | 4.55 # |
| Catalase [µm2] | ||||
| Dorsal | ||||
| X ± SD | 102.85 ± 145.96 | 772.76 ± 286.18 | 554.12 ± 251.59 | 11.03 ± 21.19 |
| Me | 29.40 | 767.7 * | 441.39 | 0.88 # |
| Ventral | ||||
| X ± SD | 196.18 ± 146.86 | 626.84 ± 133.57 | 650.14 ± 550.99 | 196.34 ± 144.37 |
| Me | 149.25 | 594.85 * | 488.87 * | 145.97 # |
| Caspase-3 [µm2] | ||||
| Dorsal | ||||
| X ± SD | 48.79 ± 51.16 | 277.48 ± 76.46 | 114.25 ± 91.64 | 377.40 ± 315.65 |
| Me | 32.85 | 291.15 * | 71.94 | 235.00 |
| Ventral | ||||
| X ± SD | 464.30 ± 187.87 | 398.21 ± 175.58 | 27.09 ± 27.30 | 563.57 ± 351.82 |
| Me | 412.85 | 348.78 | 21.87 * | 503.76 # |
| Catalase | Caspase-3 | |||
|---|---|---|---|---|
| rs | rs | |||
| Ventral Skin | Dorsal Skin | Ventral Skin | Dorsal Skin | |
| VIT C:CTRL | −0.50000 | −0.50000 | 1.00000 | 0.866025 |
| LET:CTRL | 0.500000 | −1.00000 | 0.500000 | 0.866025 |
| LET:LET+C | −0.500000 | 1.00000 | −0.50000 | 1.00000 |
| No. | Group | Description | Treatment | Body Weight [g] |
|---|---|---|---|---|
| 1. | CTRL | Control group | A bread pellet | 500.83 |
| 2. | VIT C | Group supplemented with vitamin C | A bread pellet + vitamin C, at a dose of 500 mg/L of water | 563.33 |
| 3. | LET | Letrozole-treated group | A bread pellet with LET at a dose of 1 mg/kg b. w./day | 439.17 |
| 4. | LET+C | Letrozole-treated and vitamin C-supplemented group | A pellet with LET at a dose of 1 mg/kg b. w./day + water with vitamin C at a dose of 500 mg/L | 467.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilutin, A.; Łukasiewicz, J.; Rzeszotek, S.; Misiakiewicz-Has, K.; Wilk, A. A Digital Image Analysis of the Morphology and Immunolocalization of Catalase and Caspase-3 in the Skin of Adult Male Rats After Treatment with Letrozole and Vitamin C. Int. J. Mol. Sci. 2025, 26, 8645. https://doi.org/10.3390/ijms26178645
Pilutin A, Łukasiewicz J, Rzeszotek S, Misiakiewicz-Has K, Wilk A. A Digital Image Analysis of the Morphology and Immunolocalization of Catalase and Caspase-3 in the Skin of Adult Male Rats After Treatment with Letrozole and Vitamin C. International Journal of Molecular Sciences. 2025; 26(17):8645. https://doi.org/10.3390/ijms26178645
Chicago/Turabian StylePilutin, Anna, Julia Łukasiewicz, Sylwia Rzeszotek, Kamila Misiakiewicz-Has, and Aleksandra Wilk. 2025. "A Digital Image Analysis of the Morphology and Immunolocalization of Catalase and Caspase-3 in the Skin of Adult Male Rats After Treatment with Letrozole and Vitamin C" International Journal of Molecular Sciences 26, no. 17: 8645. https://doi.org/10.3390/ijms26178645
APA StylePilutin, A., Łukasiewicz, J., Rzeszotek, S., Misiakiewicz-Has, K., & Wilk, A. (2025). A Digital Image Analysis of the Morphology and Immunolocalization of Catalase and Caspase-3 in the Skin of Adult Male Rats After Treatment with Letrozole and Vitamin C. International Journal of Molecular Sciences, 26(17), 8645. https://doi.org/10.3390/ijms26178645







