AS1411 Aptamer-Conjugated Liposomal siRNA Targeting MTA2 Suppresses PI3K/AKT Signaling in Pancreatic Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Screening and Selection of the Optimal Aptamer for PDAC-Targeted Delivery
| Name | Target | Sequence (5′-3′) | 1 Kd (nM) | Reference |
|---|---|---|---|---|
| AS1411 | Nucleolin | GGTGGTGGTGGTG-TTGGTGGTGGTGG | 16.36 ± 10.30 | [36] |
| M17 | 2 MMP14 | AGGGCCCGACGTGACGGCACGTCGGATATCTCATGCGTGT | 4.98 ± 1.26 | [37] |
| XQ-2D | 3 TfR1 | ACTCATAGGGTTAGGGGCTGCT-GGCCAGATACTCAGATGGTAGGGTTACTATGAGC | 55.02 ± 0.4 | [38] |
| AP1153 | 4 CCKBR | CATGGTGCAGGTGTGGCTGGGATTCATTTGCCGGTGCTGGTGCGTCCGCGG-CCGCTAATCCTGTTC | 0.206 | [39] |
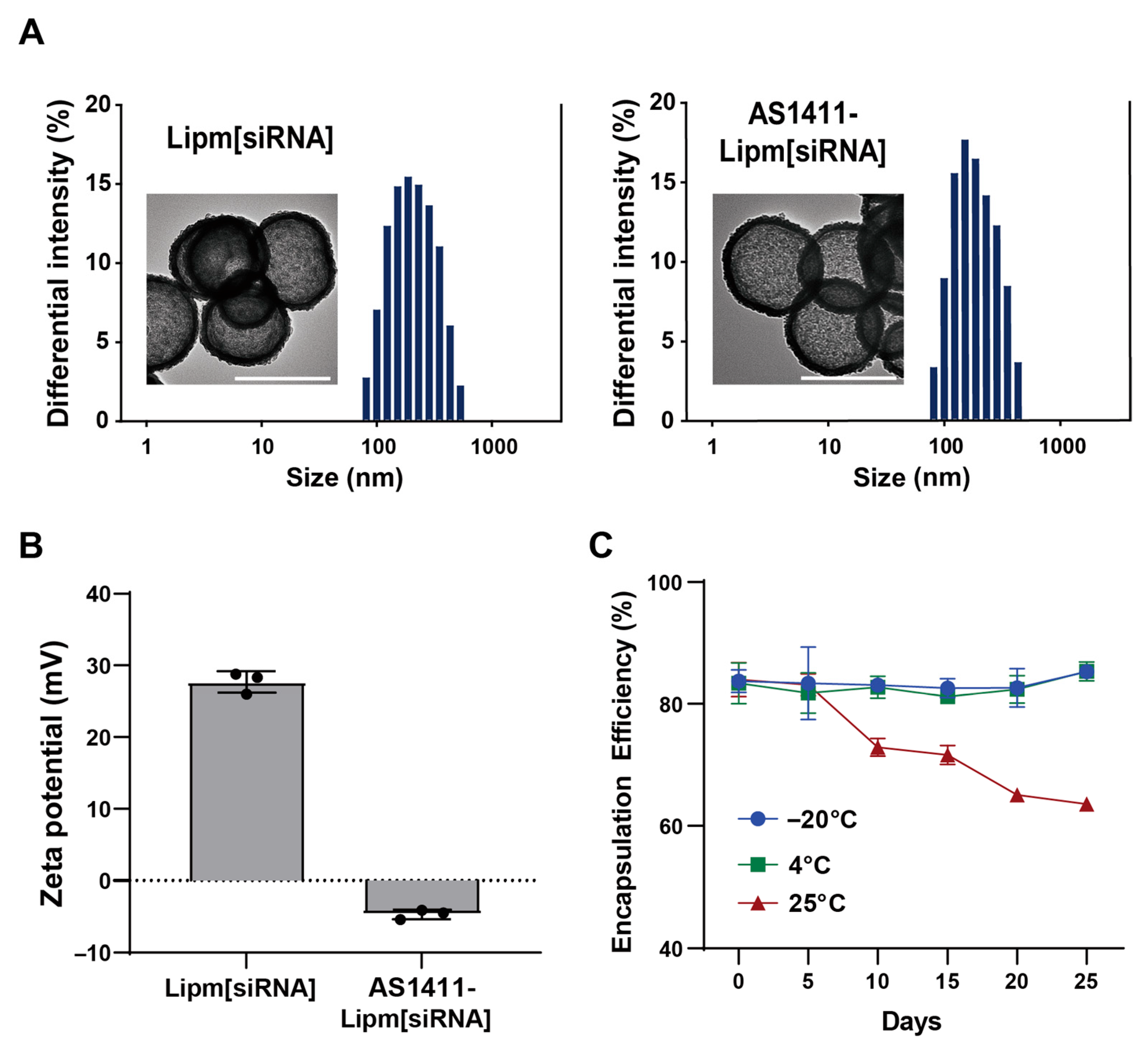
2.2. Preparation and Physicochemical Characterization of AS1411-Functionalized Liposomal siRNA Formulations
| Formulation | Particle Diameter (nM) | 1 PDI | Zeta Potential (mV) |
|---|---|---|---|
| Lipm | 138.2 ± 3.56 | 0.164 ± 0.001 | 27.3 ± 0.68 |
| AS1411-Lipm | 183.0 ± 0.513 | 0.171 ± 0.001 | −2.66 ± 2.33 |
| Lipm[siRNA] | 136.4 ± 6.60 | 0.168 ± 0.012 | 27.7 ± 1.49 |
| AS1411-Lipm[siRNA] | 185.9 ± 24.8 | 0.164 ± 0.016 | −4.71 ± 0.66 |
2.3. AS1411-Mediated Targeting Efficiency in Pancreatic Cancer Cells
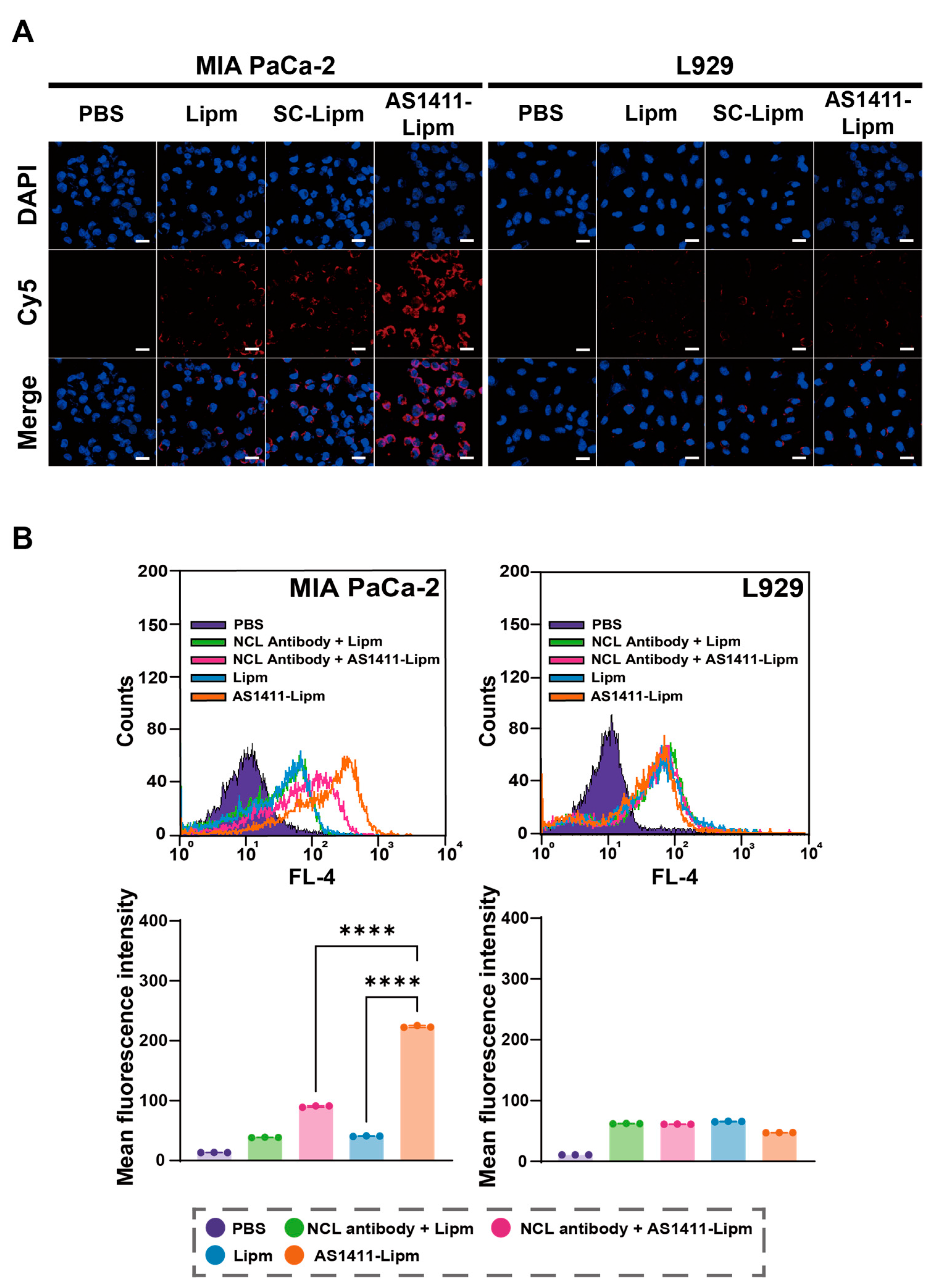
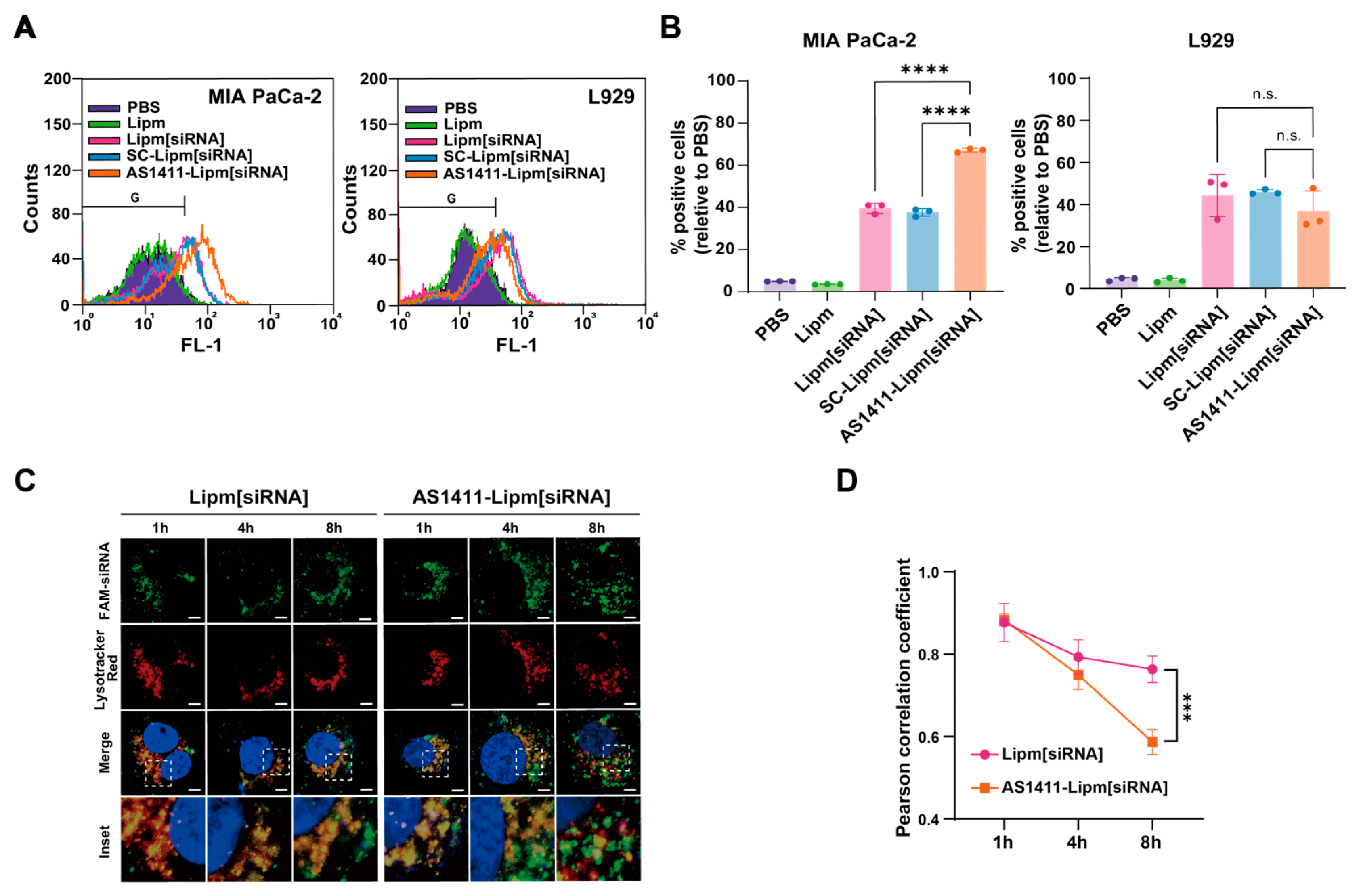
2.4. AS1411-Conjugated Liposomes Enhance Targeted siRNA Uptake and Promote Endosomal Escape in Nucleolin-Positive Pancreatic Cancer Cells
2.5. Targeted MTA2 Silencing via AS1411-Conjugated Liposomal siRNA Modulates PTEN Expression and PI3K/AKT Signaling
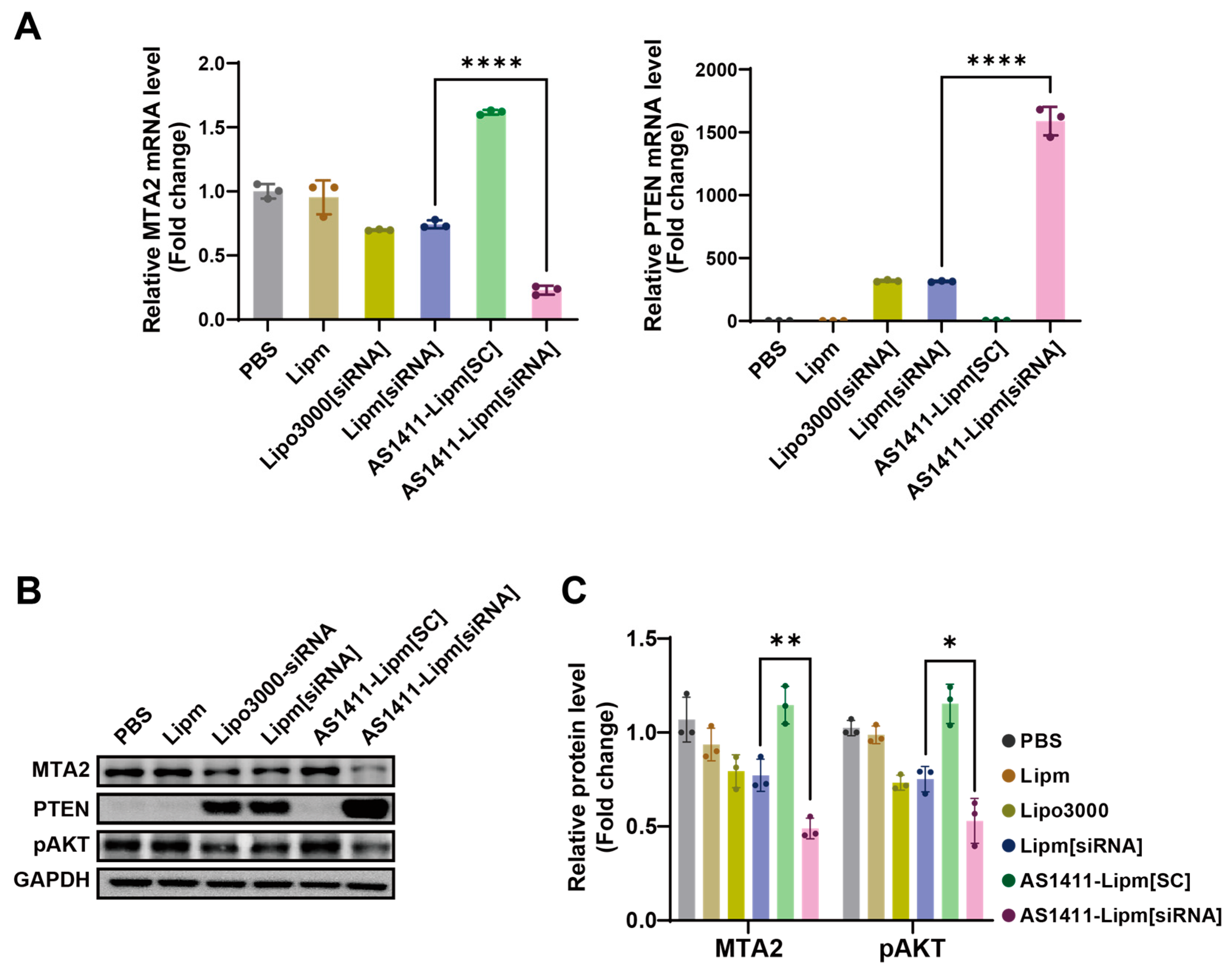
2.6. Targeted Delivery of MTA2 siRNA via AS1411 Liposomes Elicits Potent Anticancer Effects in Pancreatic Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Aptamer Screening via Flow Cytometry
4.3. Preparation of AS1411-Conjugated Liposomal siRNA
4.3.1. Preparation of AS1411-Aptamer-Conjugated Micelles
4.3.2. Preparation of siRNA-Encapsulated Cationic Liposomes
4.3.3. Post-Insertion of Aptamer-Conjugated Micelles into Liposomes
4.4. Physicochemical Characterization and Stability Evaluation
4.5. Evaluation of Intracellular Delivery Efficiency of Cy5-Labeled Liposomes
4.6. Evaluation of siRNA Uptake Efficiency and Endosomal Escape Assay
4.7. Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
4.8. Western Blot Analysis
4.9. Apoptosis Assay
4.10. Wound Healing Assay
4.11. Cell Viability Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS1411-Lipm[siRNA] | AS1411 aptamer-conjugated liposomes encapsulating MTA2 siRNA |
| CCKBR | Cholecystokinin B receptor |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| DLS | Dynamic light scattering |
| DOPE | 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOTAP | 1,2-Dioleoyl-3-trimethylammonium-propane |
| ELS | Enhanced permeability and retention |
| Kd | Dissociation constant |
| Lipm | Naked liposomes |
| Lipm[SC] | Scrambled double-stranded RNA-encapsulated liposomes |
| Lipm[siRNA] | MTA2 siRNA liposomes |
| MFI | Mean fluorescence intensity |
| MMP14 | Matrix metalloproteinase 14 |
| MTA2 | Metastasis tumor-associated protein 2 |
| N/P ratio | Nitrogen-to-phosphate molar ratio |
| NCL | Nucleolin |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDI | Polydispersity index |
| PEG-DSPE2000 | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine–polyethylene glycol 2000 |
| PFA | Paraformaldehyde |
| PTEN | Phosphatase and tensin homolog |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| siRNA | Small interfering RNA |
| TEM | Transmission electron microscopy |
| TfR1 | Transferrin receptor 1 |
References
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012, 13, 283–296. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Mehra, S.; Deshpande, N.; Nagathihalli, N. Targeting PI3K Pathway in Pancreatic Ductal Adenocarcinoma: Rationale and Progress. Cancers 2021, 13, 4434. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Springfeld, C.; Ferrone, C.R.; Katz, M.H.G.; Philip, P.A.; Hong, T.S.; Hackert, T.; Buchler, M.W.; Neoptolemos, J. Neoadjuvant therapy for pancreatic cancer. Nat. Rev. Clin. Oncol. 2023, 20, 318–337. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Underwood, P.W.; Korc, M.; Trevino, J.G.; Munshi, H.G.; Rana, A. The Current Treatment Paradigm for Pancreatic Ductal Adenocarcinoma and Barriers to Therapeutic Efficacy. Front. Oncol. 2021, 11, 688377. [Google Scholar] [CrossRef]
- Fang, Y.T.; Yang, W.W.; Niu, Y.R.; Sun, Y.K. Recent advances in targeted therapy for pancreatic adenocarcinoma. World J. Gastrointest. Oncol. 2023, 15, 571–595. [Google Scholar] [CrossRef]
- Pecot, C.V.; Calin, G.A.; Coleman, R.L.; Lopez-Berestein, G.; Sood, A.K. RNA interference in the clinic: Challenges and future directions. Nat. Rev. Cancer 2011, 11, 59–67. [Google Scholar] [CrossRef]
- Tian, Z.; Liang, G.; Cui, K.; Liang, Y.; Wang, Q.; Lv, S.; Cheng, X.; Zhang, L. Insight Into the Prospects for RNAi Therapy of Cancer. Front. Pharmacol. 2021, 12, 644718. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Chang, H.; Nam, G.; Ko, Y.; Kim, S.H.; Roberts, T.M.; Ryu, J.H. RNAi-Based Approaches for Pancreatic Cancer Therapy. Pharmaceutics 2021, 13, 1638. [Google Scholar] [CrossRef]
- Si, W.; Liu, X.; Wei, R.; Zhang, Y.; Zhao, Y.; Cui, L.; Hong, T. MTA2-mediated inhibition of PTEN leads to pancreatic ductal adenocarcinoma carcinogenicity. Cell Death Dis. 2019, 10, 206. [Google Scholar] [CrossRef]
- Chen, D.W.; Fan, Y.F.; Li, J.; Jiang, X.X. MTA2 expression is a novel prognostic marker for pancreatic ductal adenocarcinoma. Tumour Biol. 2013, 34, 1553–1557. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, A.; Miyata, K.; Kataoka, K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv. Drug Deliv. Rev. 2016, 104, 61–77. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Stealth nanoparticles: High density but sheddable PEG is a key for tumor targeting. J. Control Release 2010, 145, 178–181. [Google Scholar] [CrossRef]
- Torchilin, V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009, 71, 431–444. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- DuFort, C.C.; DelGiorno, K.E.; Carlson, M.A.; Osgood, R.J.; Zhao, C.; Huang, Z.; Thompson, C.B.; Connor, R.J.; Thanos, C.D.; Scott Brockenbrough, J.; et al. Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase. Biophys. J. 2016, 110, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Stylianopoulos, T.; Boucher, Y.; Jain, R.K. Delivery of molecular and nanoscale medicine to tumors: Transport barriers and strategies. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 281–298. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Gao, Y.; Li, X.; Tian, F.; Zhang, Y.; Fu, M.; Li, P.; Wang, Y.; Wang, F. Current Transport Systems and Clinical Applications for Small Interfering RNA (siRNA) Drugs. Mol. Diagn. Ther. 2018, 22, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.J.; Laber, D.A.; Miller, D.M.; Thomas, S.D.; Trent, J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009, 86, 151–164. [Google Scholar] [CrossRef]
- Reyes-Reyes, E.M.; Teng, Y.; Bates, P.J. A new paradigm for aptamer therapeutic AS1411 action: Uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 2010, 70, 8617–8629. [Google Scholar] [CrossRef]
- Mosafer, J.; Teymouri, M.; Abnous, K.; Tafaghodi, M.; Ramezani, M. Study and evaluation of nucleolin-targeted delivery of magnetic PLGA-PEG nanospheres loaded with doxorubicin to C6 glioma cells compared with low nucleolin-expressing L929 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 123–133. [Google Scholar] [CrossRef]
- Kozani, P.S.; Kozani, P.S.; Malik, M.T. AS1411-functionalized delivery nanosystems for targeted cancer therapy. Explor. Med. 2021, 2, 146–166. [Google Scholar] [CrossRef]
- Baek, S.E.; Lee, K.H.; Park, Y.S.; Oh, D.K.; Oh, S.; Kim, K.S.; Kim, D.E. RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo. J. Control Release 2014, 196, 234–242. [Google Scholar] [CrossRef]
- Kim, D.M.; Kim, M.; Park, H.B.; Kim, K.S.; Kim, D.E. Anti-MUC1/CD44 Dual-Aptamer-Conjugated Liposomes for Cotargeting Breast Cancer Cells and Cancer Stem Cells. ACS Appl. Bio Mater. 2019, 2, 4622–4633. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.S.; Kim, W.; Lee, J.H.; Jun, B.H.; Kim, K.S.; Kim, D.E. Aptamer-conjugated nano-liposome for immunogenic chemotherapy with reversal of immunosuppression. J. Control Release 2022, 348, 893–910. [Google Scholar] [CrossRef]
- Park, J.Y.; Cho, Y.L.; Chae, J.R.; Moon, S.H.; Cho, W.G.; Choi, Y.J.; Lee, S.J.; Kang, W.J. Gemcitabine-Incorporated G-Quadruplex Aptamer for Targeted Drug Delivery into Pancreas Cancer. Mol. Ther. Nucleic Acids 2018, 12, 543–553. [Google Scholar] [CrossRef]
- Huang, X.; Zhong, J.; Ren, J.; Wen, D.; Zhao, W.; Huan, Y. A DNA aptamer recognizing MMP14 for in vivo and in vitro imaging identified by cell-SELEX. Oncol. Lett. 2019, 18, 265–274. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Z.; Bai, H.; Fu, T.; Yang, C.; Hu, X.; Liu, Q.; Champanhac, C.; Teng, I.T.; Ye, M.; et al. DNA Aptamer Selected against Pancreatic Ductal Adenocarcinoma for in vivo Imaging and Clinical Tissue Recognition. Theranostics 2015, 5, 985–994. [Google Scholar] [CrossRef]
- Clawson, G.A.; Abraham, T.; Pan, W.; Tang, X.; Linton, S.S.; McGovern, C.O.; Loc, W.S.; Smith, J.P.; Butler, P.J.; Kester, M.; et al. A Cholecystokinin B Receptor-Specific DNA Aptamer for Targeting Pancreatic Ductal Adenocarcinoma. Nucleic Acid. Ther. 2017, 27, 23–35. [Google Scholar] [CrossRef]
- Esposito, C.L.; Nuzzo, S.; Catuogno, S.; Romano, S.; de Nigris, F.; de Franciscis, V. STAT3 Gene Silencing by Aptamer-siRNA Chimera as Selective Therapeutic for Glioblastoma. Mol. Ther. Nucleic Acids 2018, 10, 398–411. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, Y.; Qian, K.; Yang, P.; Zhou, L.; Xu, M.; Sheng, D.; Wang, T.; Li, Y.; Yang, X.; et al. siRNA-Encapsulated Biomimetic Liposomes Effectively Inhibit Tumor Cells’ Hexosamine Biosynthesis Pathway for Attenuating Hyaluronan Barriers to Pancreatic Cancer Chemotherapy. ACS Nano 2025, 19, 7928–7947. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Hillaireau, H.; Vergnaud, J.; Mura, S.; Delomenie, C.; Sauvage, F.; Ismail, S.; Fattal, E. Aptamer-guided siRNA-loaded nanomedicines for systemic gene silencing in CD-44 expressing murine triple-negative breast cancer model. J. Control Release 2018, 271, 98–106. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, M.; Hua, T.C.; Jin, H.; Lee, J.; Kim, D.-E. AS1411 Aptamer-Conjugated Liposomal siRNA Targeting MTA2 Suppresses PI3K/AKT Signaling in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2025, 26, 8467. https://doi.org/10.3390/ijms26178467
Kwak M, Hua TC, Jin H, Lee J, Kim D-E. AS1411 Aptamer-Conjugated Liposomal siRNA Targeting MTA2 Suppresses PI3K/AKT Signaling in Pancreatic Cancer Cells. International Journal of Molecular Sciences. 2025; 26(17):8467. https://doi.org/10.3390/ijms26178467
Chicago/Turabian StyleKwak, Minseo, Truong Chinh Hua, Hyesoo Jin, Jongsam Lee, and Dong-Eun Kim. 2025. "AS1411 Aptamer-Conjugated Liposomal siRNA Targeting MTA2 Suppresses PI3K/AKT Signaling in Pancreatic Cancer Cells" International Journal of Molecular Sciences 26, no. 17: 8467. https://doi.org/10.3390/ijms26178467
APA StyleKwak, M., Hua, T. C., Jin, H., Lee, J., & Kim, D.-E. (2025). AS1411 Aptamer-Conjugated Liposomal siRNA Targeting MTA2 Suppresses PI3K/AKT Signaling in Pancreatic Cancer Cells. International Journal of Molecular Sciences, 26(17), 8467. https://doi.org/10.3390/ijms26178467






