Pre-Eclampsia Comorbid with HIV Infection Mimics the Release of sVCAM-1, sICAM-1, and sE-Selectin in African Women
Abstract
1. Introduction
2. Results
2.1. Patient Demographic and Clinical Characteristics
2.2. Plasma Concentration of Adhesion Markers
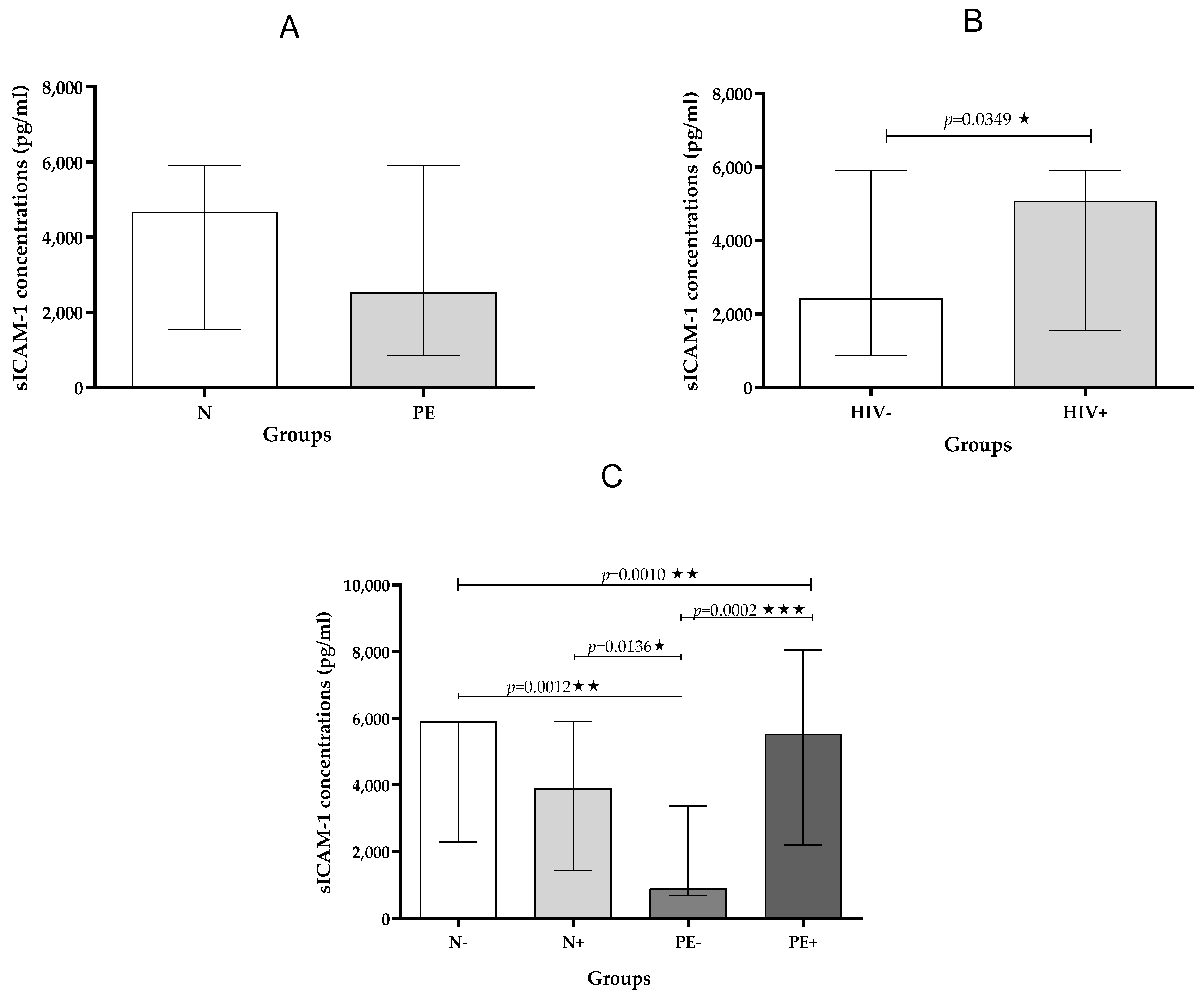
2.2.1. sICAM-1
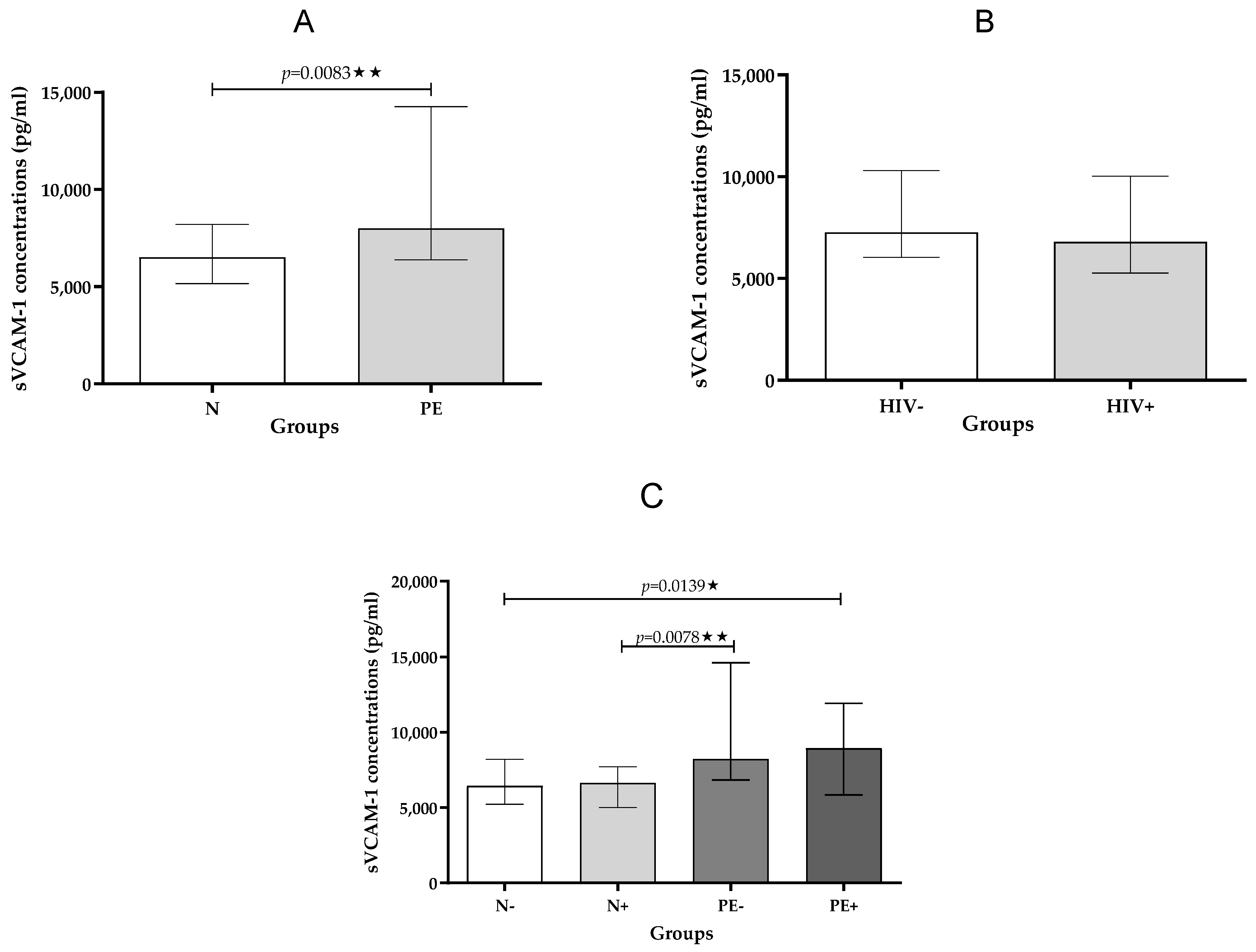
2.2.2. sVCAM-1
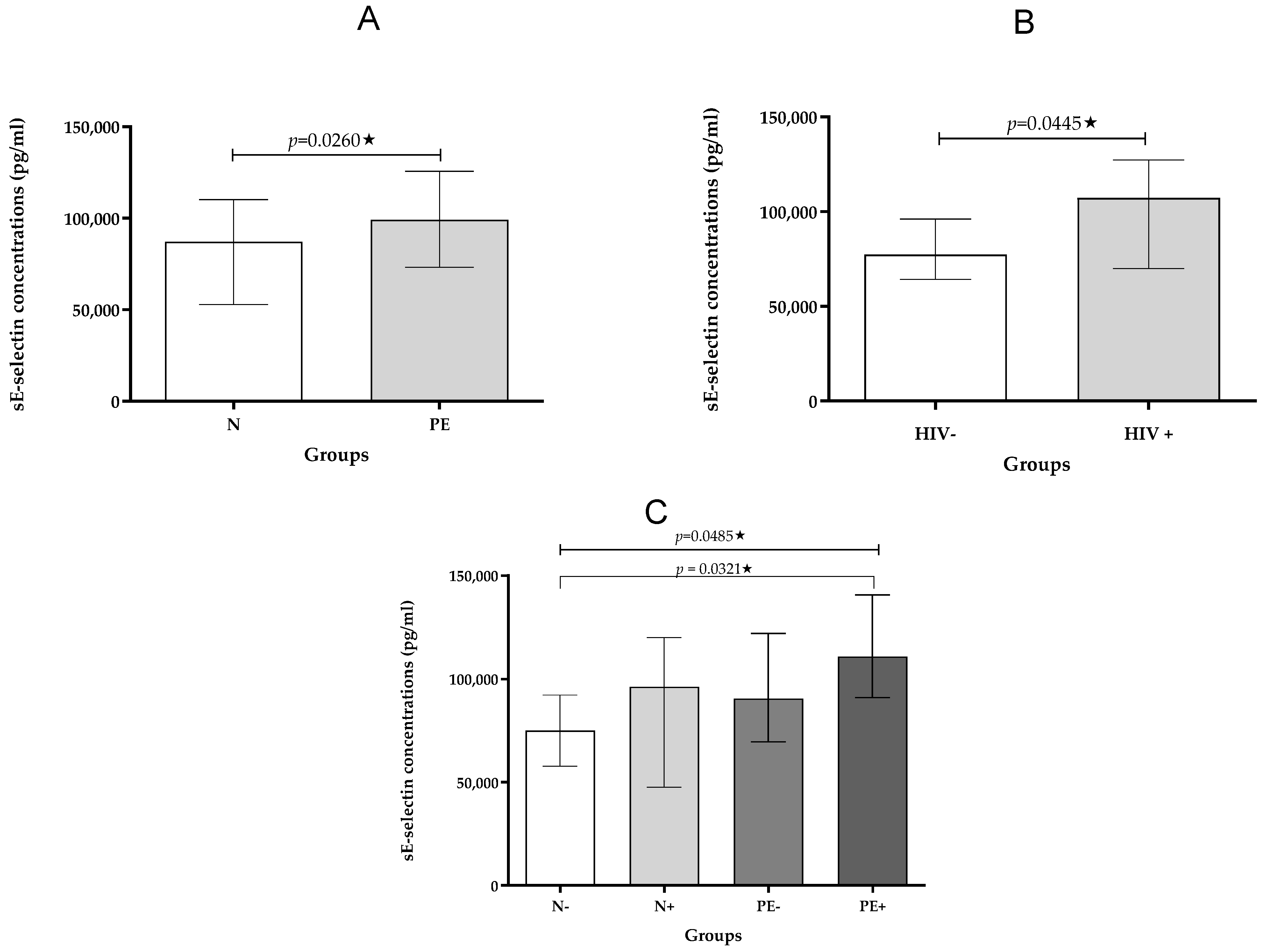
2.2.3. sE-Selectin
2.3. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Approval
4.2. Study Population
4.3. Exclusion Criteria
4.4. Sample Collection
4.5. Bio-Plex Immunoassays
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, A.; Rana, S.; Karumanchi, S.A. Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology 2009, 24, 147–158. [Google Scholar] [CrossRef]
- Moodley, J.; Soma-Pillay, P.; Buchmann, E.; Pattinson, R.C. Hypertensive disorders in pregnancy: 2019 National guideline. S. Afr. Med. J. 2019, 109, 12723. [Google Scholar]
- Naidoo, N.; Moodley, J.; Naicker, T. Maternal endothelial dysfunction in HIV-associated preeclampsia comorbid with COVID-19: A review. Hypertens. Res. 2021, 44, 386–398. [Google Scholar] [CrossRef]
- Woldesenbet, S.; Kufa, T.; Manda, S.; Ayalew, K.; Lombard, C.; Cheyip, M.; Puren, A. Association between viral suppression during the third trimester of pregnancy and unintended pregnancy among women on antiretroviral therapy: Results from the 2019 antenatal HIV Sentinel Survey, South Africa. PLoS ONE 2022, 17, e0265124. [Google Scholar] [CrossRef] [PubMed]
- Clouse, K.; Malope-Kgokong, B.; Bor, J.; Nattey, C.; Mudau, M.; Maskew, M. The South African National HIV Pregnancy Cohort: Evaluating continuity of care among women living with HIV. BMC Public Health 2020, 20, 1662. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Collier, J.; Bhagat, K. Infection, inflammation, and infarction: Does acute endothelial dysfunction provide a link? Lancet 1997, 349, 1391–1392. [Google Scholar] [CrossRef]
- Reglero-Real, N.; García-Weber, D.; Millán, J. Cellular barriers after extravasation: Leukocyte interactions with polarized epithelia in the inflamed tissue. Mediat. Inflamm. 2016, 2016, 7650260. [Google Scholar] [CrossRef]
- Anand, A.R.; Rachel, G.; Parthasarathy, D. HIV proteins and endothelial dysfunction: Implications in cardiovascular disease. Front. Cardiovasc. Med. 2018, 5, 185. [Google Scholar] [CrossRef]
- Krishnaswamy, G.; Kelley, J.; Yerra, L.; Smith, J.K.; Chi, D.S. Human endothelium as a source of multifunctional cytokines: Molecular regulation and possible role in human disease. J. Interferon Cytokine Res. 1999, 19, 91–104. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Soga, N.; Namba, N.; McAllister, S.; Cornelius, L.; Teitelbaum, S.L.; Dowdy, S.F.; Kawamura, J.; Hruska, K.A. Rho family GTPases regulate VEGF-stimulated endothelial cell motility. Exp. Cell Res. 2001, 269, 73–87. [Google Scholar] [CrossRef]
- Sánchez-Aranguren, L.C.; Prada, C.E.; Riaño-Medina, C.E.; Lopez, M. Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289, Correction in Nat. Rev. Nephrol. 2019, 15, 386. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Preeclampsia and the systemic inflammatory response. In Seminars in Nephrology; WB Saunders: Philadelphia, PA, USA, 2004; Volume 24, pp. 565–570. [Google Scholar]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ryu, H.M.; Yang, J.H.; Kim, M.Y.; Ahn, H.K.; Lim, H.J.; Shin, J.S.; Woo, H.J.; Park, S.Y.; Kim, Y.M.; et al. Maternal serum levels of VCAM-1, ICAM-1 and E-selectin in preeclampsia. J. Korean Med. Sci. 2004, 19, 688–692. [Google Scholar] [CrossRef]
- Kefaloyianni, E. Soluble forms of cytokine and growth factor receptors: Mechanisms of generation and modes of action in the regulation of local and systemic inflammation. FEBS Lett. 2022, 596, 589–606. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Gutterman, D.D. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid. Redox Signal. 2011, 15, 1517–1530. [Google Scholar] [CrossRef]
- Mazzuca, P.; Caruso, A.; Caccuri, F. Endothelial Cell Dysfunction in HIV-1 Infection. In Endothelial Dysfunction: Old Concepts and New Challenges; InTech: London, UK, 2018; p. 347. [Google Scholar] [CrossRef]
- Siow, R.C. Culture of human endothelial cells from umbilical veins. Hum. Cell Cult. Protoc. 2012, 806, 265–274. [Google Scholar]
- Krauss, T.; Kuhn, W.; Lakoma, C.; Augustin, H.G. Circulating endothelial cell adhesion molecules as diagnostic markers for the early identification of pregnant women at risk for development of preeclampsia. Am. J. Obstet. Gynecol. 1997, 177, 443–449. [Google Scholar] [CrossRef]
- Wojtowicz, A.; Zembala-Szczerba, M.; Babczyk, D.; Kołodziejczyk-Pietruszka, M.; Lewaczyńska, O.; Huras, H. Early-and late-onset preeclampsia: A comprehensive cohort study of laboratory and clinical findings according to the new ISHHP criteria. Int. J. Hypertens. 2019, 2019, 4108271. [Google Scholar] [CrossRef]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; LaMarca, B. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef]
- Lyall, F.; Greer, I.A. Pre-eclampsia: A multifaceted vascular disorder of pregnancy. J. Hypertens. 1994, 12, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Ogalde, M.V.H.; Broughton Pipkin, F.; Escher, G.; Kurlak, L.O. Maternal, Fetal, and Placental Selectins in Women With Pre-eclampsia; Association With the Renin-Angiotensin-System. Front. Med. 2020, 7, 270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gearing, A.J.; Newman, M. Circulating adhesion molecules in disease. Immunol. Today 1993, 14, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.K.; Lee, S.; Ramos, R.A.; Lobb, R.; Rosa, M.; Chi-Rosso, G.; Wright, S.D. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1, αmβ2) on human neutrophils. J. Exp. Med. 1991, 173, 1493–1500. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Tzavara, V.; Vlachoyiannopoulos, P.G.; Kordossis, T.; Galaris, D.; Travlou, A.; Dafni, U.; Moutsopoulos, H.M. Evidence for non-adaptive immune response in HIV infection. Eur. J. Clin. Investig. 1997, 27, 846–849. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Chan, P.Y.; Aruffo, A. VLA-4 integrin mediates lymphocyte migration on the inducible endothelial cell ligand VCAM-1 and the extracellular matrix ligand fibronectin. J. Biol. Chem. 1993, 268, 24655–24664. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Roos, J.W.; Hildreth, J.E. Increased infectivity of HIV type 1 particles bound to cell surface and solid-phase ICAM-1 and VCAM-1 through acquired adhesion molecules LFA-1 and VLA-4. AIDS Res. Hum. Retroviruses 2000, 16, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Card, C.M.; Abrenica, B.; McKinnon, L.R.; Ball, T.B.; Su, R.C. Endothelial cells promote productive HIV infection of resting CD4+ T cells by an integrin-mediated cell adhesion-dependent mechanism. AIDS Res. Hum. Retroviruses 2022, 38, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef]
- Naicker, T.; Govender, N.; Abel, T.; Naidoo, N.; Moodley, M.; Pillay, Y.; Singh, S.; Khaliq, O.P.; Moodley, J. HIV associated preeclampsia: A multifactorial appraisal. Int. J. Mol. Sci. 2021, 22, 9157. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Jamaluddin, M.S.; Lin, P.H.; Yao, Q.; Chen, C. Non-nucleoside reverse transcriptase inhibitor efavirenz increases monolayer permeability of human coronary artery endothelial cells. Atherosclerosis 2010, 208, 104–111. [Google Scholar] [CrossRef]
- Mondal, D.; Pradhan, L.; Ali, M.; Agrawal, K.C. HAART Drugs Induce Oxidative Stress in Human Endothelial Cells and Increase Endothelial Recruitment of Mononuclear Cells: Exacerbation by Inflammatory Cytokines and Amelioration by Antioxidants. Cardiovasc Toxicol. 2004, 4, 287–302. [Google Scholar] [CrossRef]
- Lee, T.W.; Tsang, V.W.; Birch, N.P. Synaptic plasticity-associated proteases and protease inhibitors in the brain linked to the processing of extracellular matrix and cell adhesion molecules. Neuron Glia Biol. 2008, 4, 223–234. [Google Scholar] [CrossRef]
- Zong, D.; Lu, X.; Conklin, B.S.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. HIV protease inhibitor ritonavir induces cytotoxicity of human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1560–1566. [Google Scholar] [CrossRef]
- Hileman, C.O.; Funderburg, N.T. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr. HIV/AIDS Rep. 2017, 14, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.; Israr, M.; Alam, S.; Dinello, D.; Kishel, J.; Jia, R.; Meyers, C. HIV nucleoside reverse transcriptase inhibitors efavirenz and tenofovir change the growth and differentiation of primary gingival epithelium. HIV Med. 2014, 15, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Papasavvas, E.; Azzoni, L.; Pistilli, M.; Hancock, A.; Reynolds, G.; Gallo, C.; Ondercin, J.; Kostman, J.R.; Mounzer, K.; Shull, J.; et al. Increased soluble vascular cell adhesion molecule-1 plasma levels and soluble intercellular adhesion molecule-1 during antiretroviral therapy interruption and retention of elevated soluble vascular cellular adhesion molecule-1 levels following resumption of antiretroviral therapy. AIDS 2008, 22, 1153–1161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conti-Ramsden, F.; Bramham, K.; de Marvao, A. Long-term cardiovascular disease after pre-eclampsia: Time to move from epidemiology to action. Eur. Heart J.-Qual. Care Clin. Outcomes 2024, 10, 1–3. [Google Scholar] [CrossRef]
- Francisci, D.; Giannini, S.; Baldelli, F.; Leone, M.; Belfiori, B.; Guglielmini, G.; Malincarne, L.; Gresele, P. HIV type 1 infection, and not short-term HAART, induces endothelial dysfunction. AIDS 2009, 23, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; De Placido, G.; Nikas, Y.; Alviggi, C. Pathogenesis of endometriosis: Natural immunity dysfunction or autoimmune disease? Trends Mol. Med. 2003, 9, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, S.A.; Moodley, J.; Naicker, T. Current Updates on Pre-eclampsia: Maternal and Foetal Cardiovascular Diseases Predilection, Science or Myth? Future cardiovascular disease risks in mother and child following pre-eclampsia. Curr. Hypertens. Rep. 2021, 23, 16. [Google Scholar] [CrossRef]
- Hoffman, M.; Ipp, H.; Phatlhane, D.V.; Erasmus, R.T.; Zemlin, A.E. E-Selectin and markers of HIV disease severity, inflammation and coagulation in HIV-infected treatment-naïve individuals. Afr. Health Sci. 2018, 18, 1066–1075. [Google Scholar] [CrossRef]
- Magee, L.A.; Smith, G.N.; Bloch, C.; Côté, A.M.; Jain, V.; Nerenberg, K.; von Dadelszen, P.; Helewa, M.; Rey, E. Guideline No. 426: Hypertensive disorders of pregnancy: Diagnosis, prediction, prevention, and management. J. Obstet. Gynaecol. Can. 2022, 44, 547–571. [Google Scholar] [CrossRef]
| Normotensive HIV-Negative | Normotensive HIV-Positive | Pre-Eclampsia HIV-Negative | Pre-Eclampsia HIV-Positive | p-Value | |
|---|---|---|---|---|---|
| Maternal Age (years) | 23.00 (19.00–27.00) | 28.00 (23.00–35.00) | 28.00 (23.00–37.00) | 29.00 (26.00–30.50) | 0.2735 |
| Gestational Age (weeks) | 27.00 (39.00–40.00) | 27.00 (37.00–40.00) | 24.00 (27.00–23.00) | 23.00 (26.50–22.00) | 0.0001 *** |
| Systolic BP (mmHg) | 124 (114.5–127.5) | 115 (106.5–121.0) | 162 (123.0–173.5) | 165 (155.5–177.0) | 0.0001 *** |
| Diastolic BP (mmHg) | 75.00 (65.50–86.00) | 70.00 (67.50–75.00) | 98.00 (78.00–110.0) | 106.0 (96.50–117.5) | 0.0001 *** |
| Weight (kg) | 75.00 (70.50–86.90) | 75.00 (68.00–80.25) | 68.50 (63.35–83.55) | 72.00 (65.55–85.00) | 0.6618 |
| BMI (kg/m2) | 30.54 (27.62–34.00) | 27.69 (24.29–31.62) | 28.53 (24.27–32.16) | 34.30 (25.50–37.44)) | 0.045 * |
| Baby weight (kg) | 3.20 (3.02–3.76) | 3.34 (2.90–3.49) | 2.53 (1.50–2.80) | 2.60 (1.80–2.86) | 0.001 ** |
| Normotensive HIV-Negative | Normotensive HIV-Positive | Pre-Eclampsia HIV-Negative | Pre-Eclampsia HIV-Positive | p Value | |
|---|---|---|---|---|---|
| sICAM-1 pg/mL | 5904 (2290–5904) | 3905 (1434–5904) | 900.0 (693.2–3374) | 5533 (2210–8054) | 0.0010 ** |
| sVCAM-1 pg/mL | 6443 (5223–8205) | 6641 (5507–7790) | 8227 (6832–145,610) | 8949 (5831–11,915) | 0.0139 * |
| sE-selectin pg/mL | 75,039 (57,769–92,196) | 96,231 (47,572–119,905) | 90,514 (69,581–121,906) | 110,685 (91,056–140,562) | 0.0485 * |
| Normotensive | Pre-Eclampsia | p Value | |
|---|---|---|---|
| sICAM-1 pg/mL | 4685 (1557–5904) | 2543 (866–5904) | ns |
| sVCAM-1 pg/mL | 6515 (5162–8205) | 8010 (6382–14,269) | 0.0083 ** |
| sE-selectin pg/mL | 87,133 (52,948–110,126) | 99,190 (73,146–125,653) | 0.0260 * |
| HIV-Negative | HIV-Positive | p Value | |
|---|---|---|---|
| sICAM-1 pg/mL | 2432 (866–5904) | 5082 (1537–5904) | 0.0349 * |
| sVCAM-1 pg/mL | 7274 (6047–10,302) | 6803 (5274–10,031) | ns |
| sE-selectin pg/mL | 77,279 (64,159–96,092) | 107,249 (69,949–127,279) | 0.0445 * |
| Gestational Age (Weeks) | Baby Weight (kg) | |||
|---|---|---|---|---|
| r | p | r | p | |
| sICAM-1 | ||||
| Normotensive pregnancy | –0.1546 | 0.4414 | 0.2473 | 0.2136 |
| Pre-eclamptic pregnancy | –0.1652 | 0.4008 | –0.3912 | 0.0395 * |
| HIV-positive | 0.1664 | 0.4165 | 0.0830 | 0.6996 |
| HIV-negative | –0.0206 | 0.9169 | –0.2789 | 0.1769 |
| sVCAM-1 | ||||
| Normotensive pregnancy | 0.2655 | 0.1232 | –0.0898 | 0.6078 |
| Pre-eclampsia | –0.1114 | 0.5371 | –0.1340 | 0.4572 |
| HIV-positive | 0.3973 | 0.0221 * | 0.4106 | 0.0269 * |
| HIV-negative | 0.1418 | 0.4239 | 0.0824 | 0.6707 |
| sE-selectin | ||||
| Normotensive pregnancy | 0.3517 | 0.0613 | –0.1198 | 0.5358 |
| Pre-eclamptic pregnancy | 0.0436 | 0.8253 | 0.0273 | 0.8900 |
| HIV-positive | 0.0792 | 0.6944 | 0.0096 | 0.9636 |
| HIV-negative | 0.4121 | 0.0293 * | 0.2890 | 0.1612 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibiya, S.; Mthembu, M.H.; Singh, S.; Naicker, T.; Mkhwanazi, N.P. Pre-Eclampsia Comorbid with HIV Infection Mimics the Release of sVCAM-1, sICAM-1, and sE-Selectin in African Women. Int. J. Mol. Sci. 2025, 26, 8383. https://doi.org/10.3390/ijms26178383
Sibiya S, Mthembu MH, Singh S, Naicker T, Mkhwanazi NP. Pre-Eclampsia Comorbid with HIV Infection Mimics the Release of sVCAM-1, sICAM-1, and sE-Selectin in African Women. International Journal of Molecular Sciences. 2025; 26(17):8383. https://doi.org/10.3390/ijms26178383
Chicago/Turabian StyleSibiya, Samukelisiwe, Mbuso H. Mthembu, Shoohana Singh, Thajasvarie Naicker, and Nompumelelo P. Mkhwanazi. 2025. "Pre-Eclampsia Comorbid with HIV Infection Mimics the Release of sVCAM-1, sICAM-1, and sE-Selectin in African Women" International Journal of Molecular Sciences 26, no. 17: 8383. https://doi.org/10.3390/ijms26178383
APA StyleSibiya, S., Mthembu, M. H., Singh, S., Naicker, T., & Mkhwanazi, N. P. (2025). Pre-Eclampsia Comorbid with HIV Infection Mimics the Release of sVCAM-1, sICAM-1, and sE-Selectin in African Women. International Journal of Molecular Sciences, 26(17), 8383. https://doi.org/10.3390/ijms26178383







