Comparative Analysis of Plant Defense Activation by Four Biosurfactants: Mode of Action and Disease Control Potential
Abstract
1. Introduction
2. Results
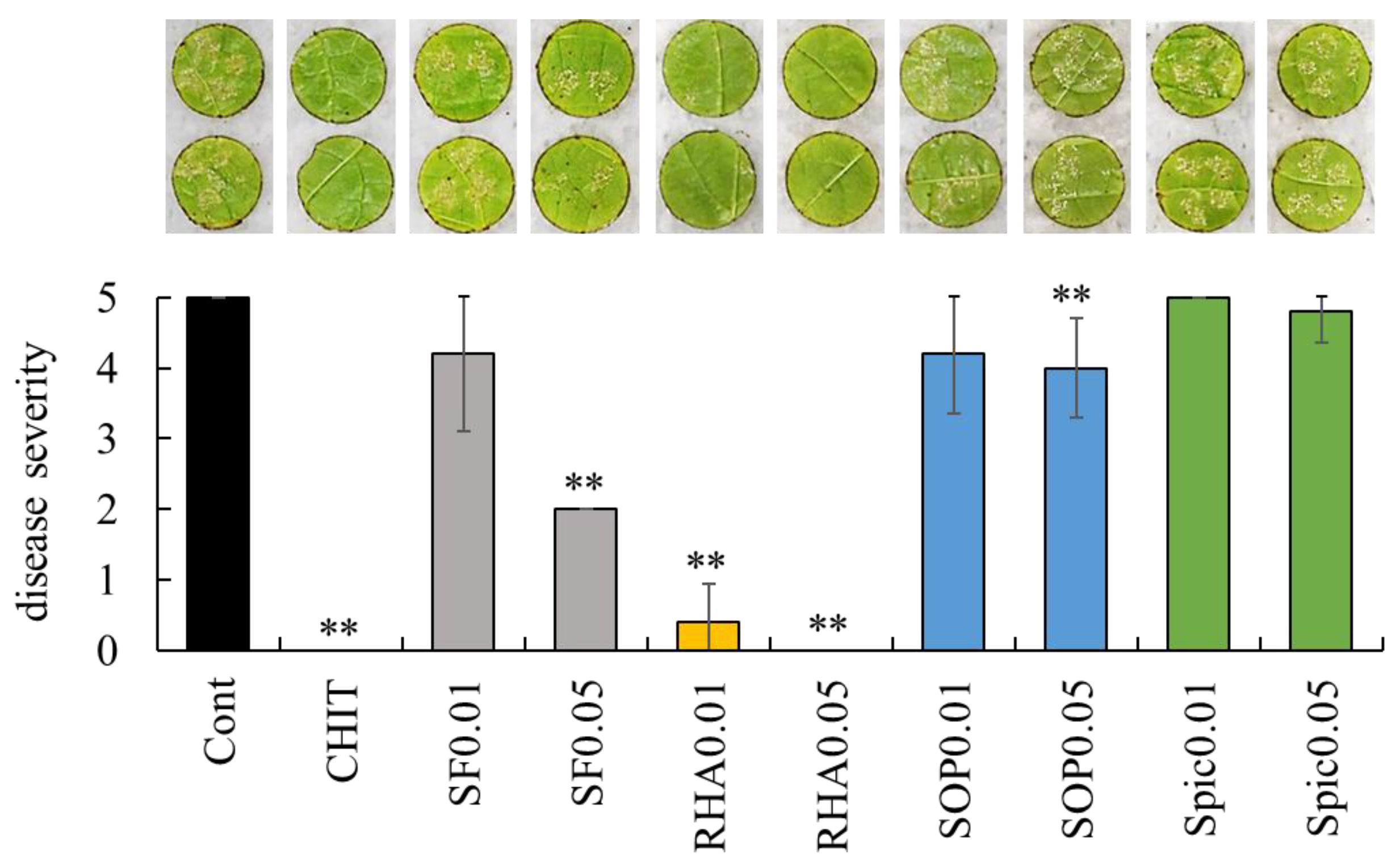
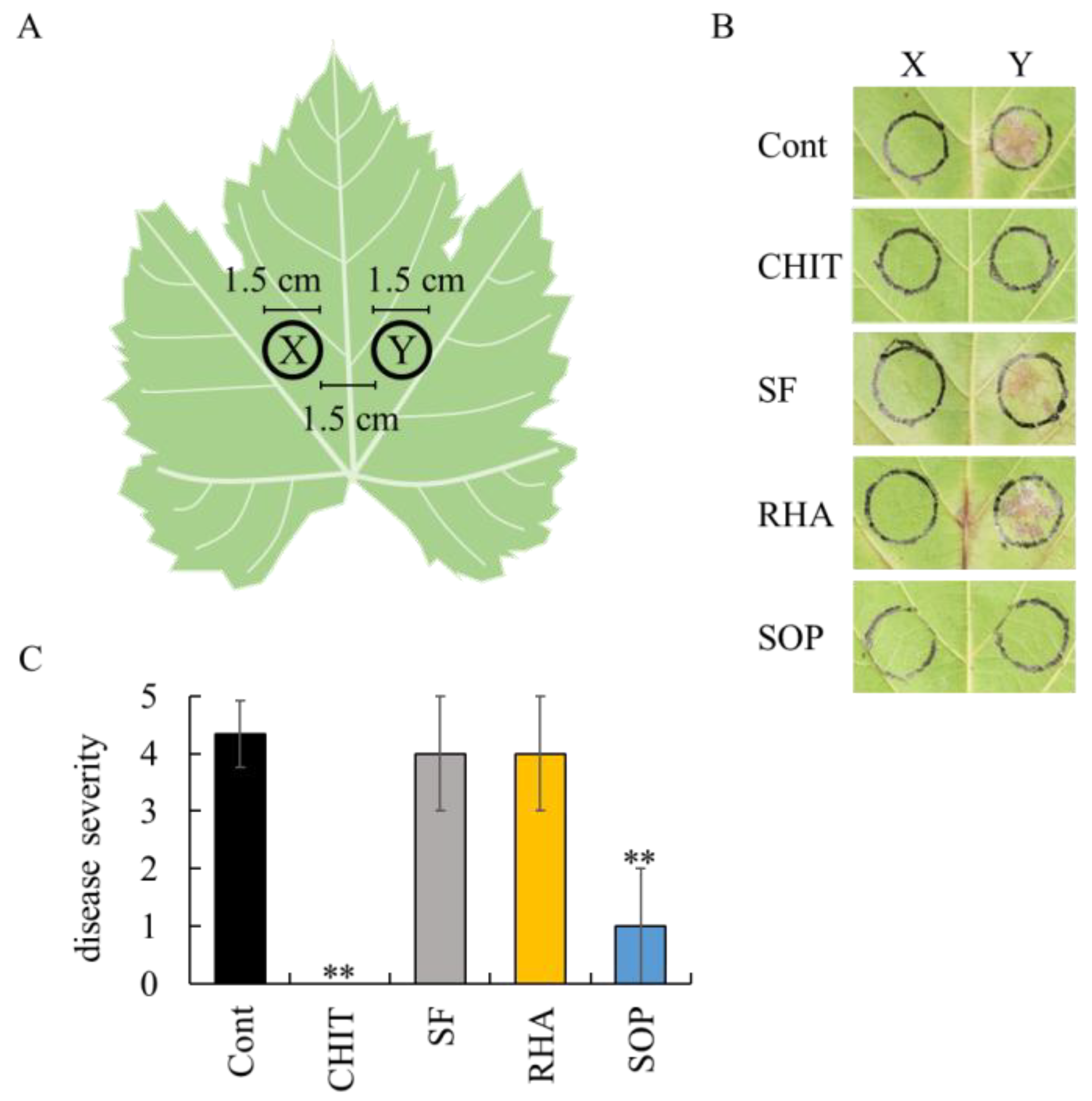
2.1. Effect of Surfactin, Rhamnolipid, Sophorolipid, and Spiculisporic Acid on Grape Downy Mildew in Grapevine Leaf Disks
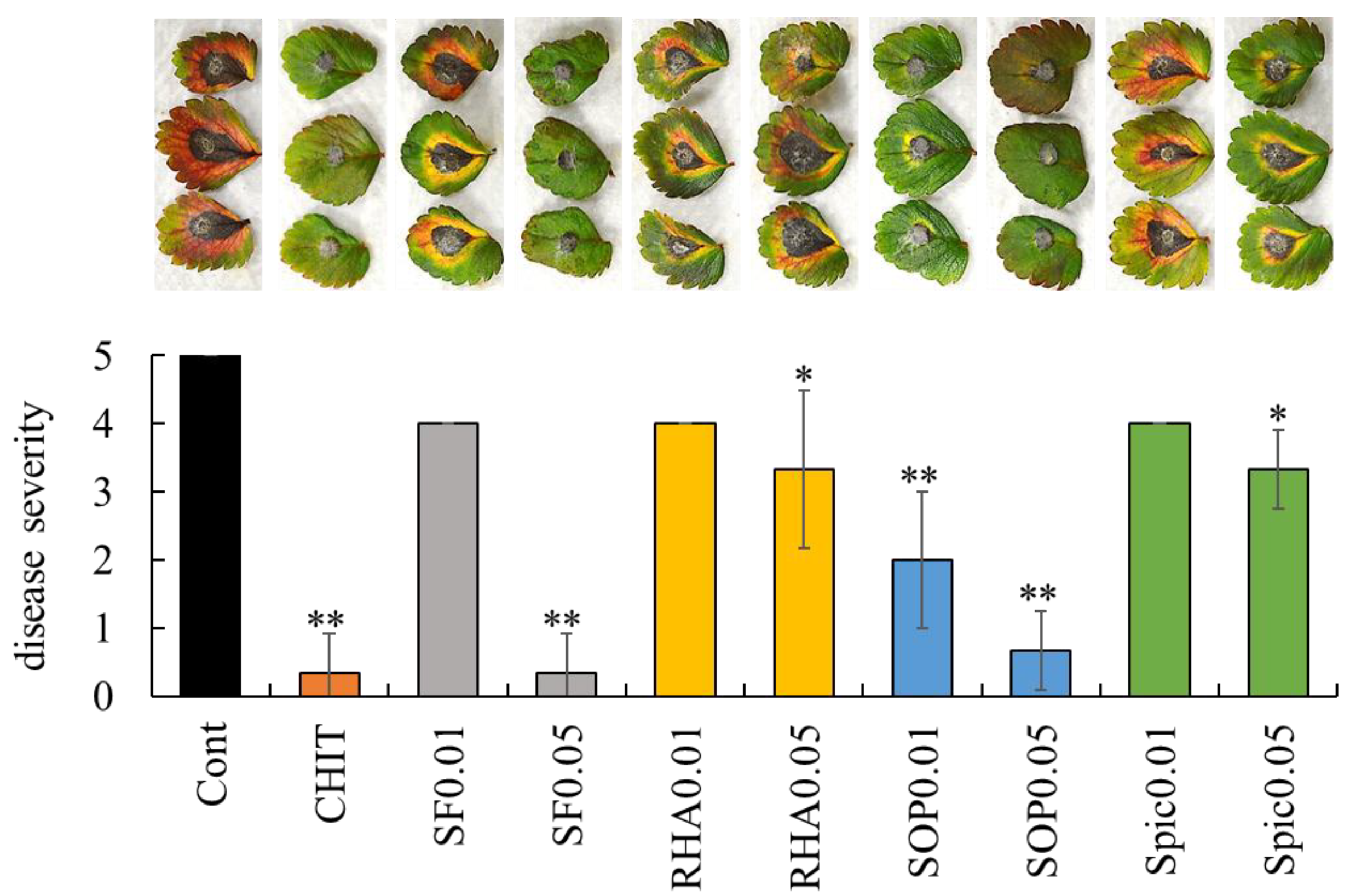
2.2. Effect of Surfactin, Rhamnolipid, Sophorolipid, and Spiculisporic Acid on Strawberry Anthracnose in Strawberry Leaflets
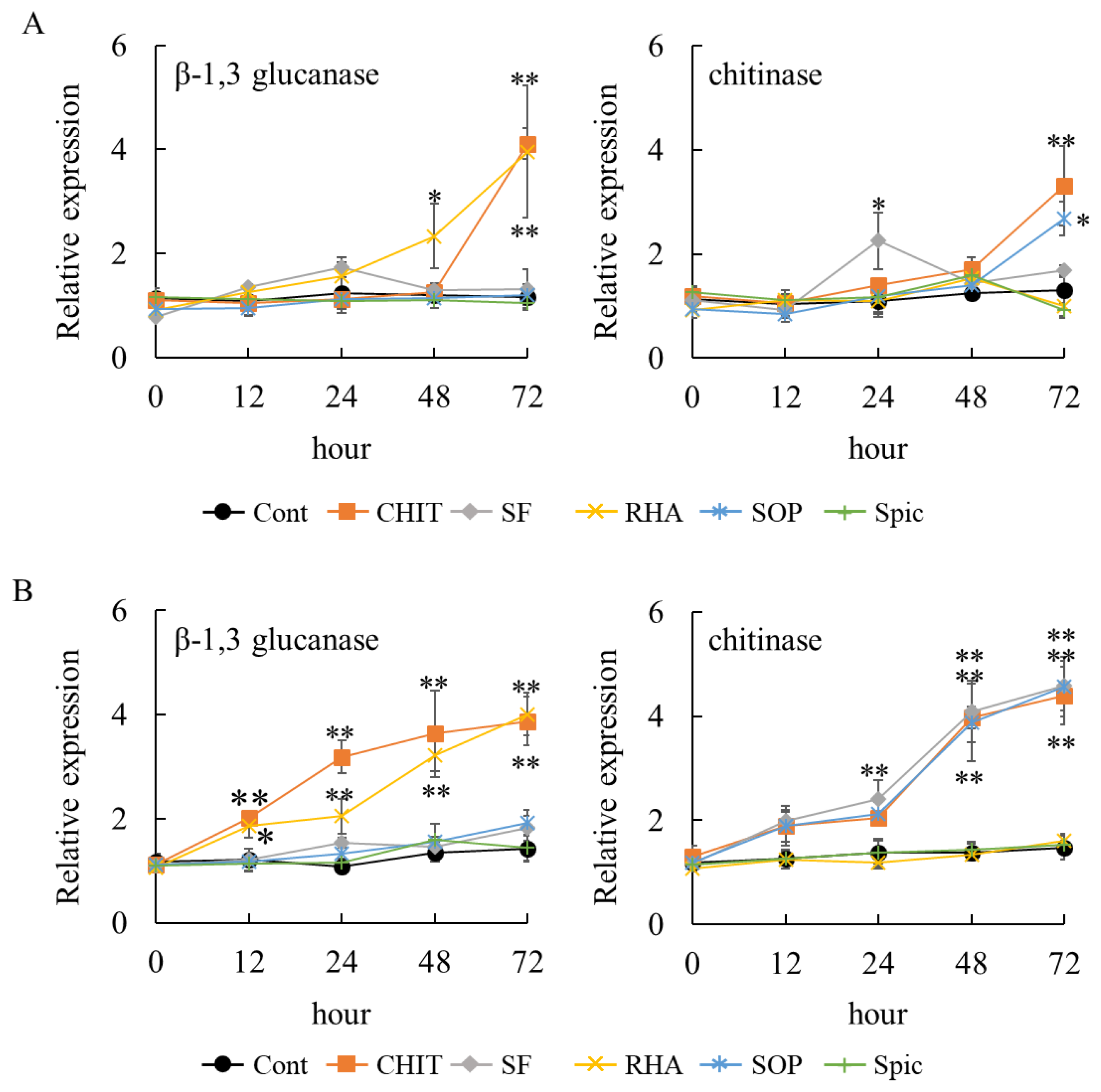
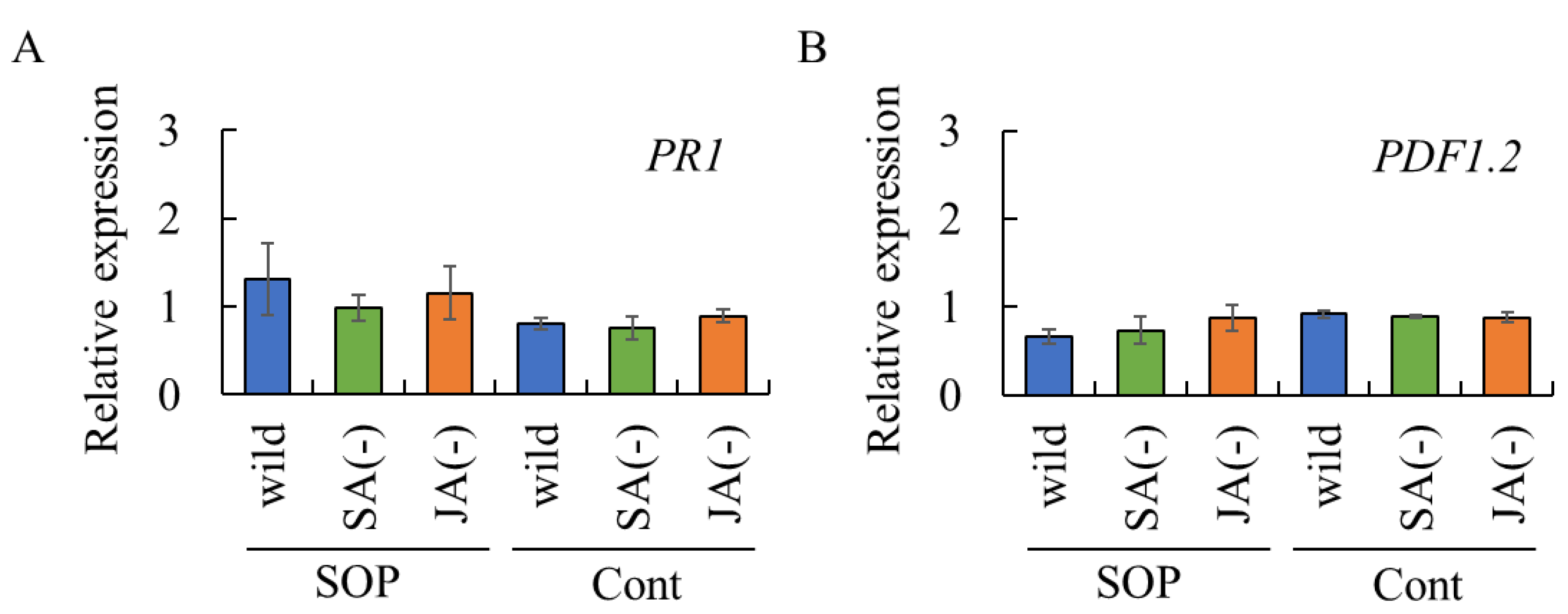
2.3. Effect of Surfactin, Rhamnolipid, Sophorolipid, and Spiculisporic Acid on Gene Expression of PR Proteins in Grapevine Leaves and Strawberry Leaflets
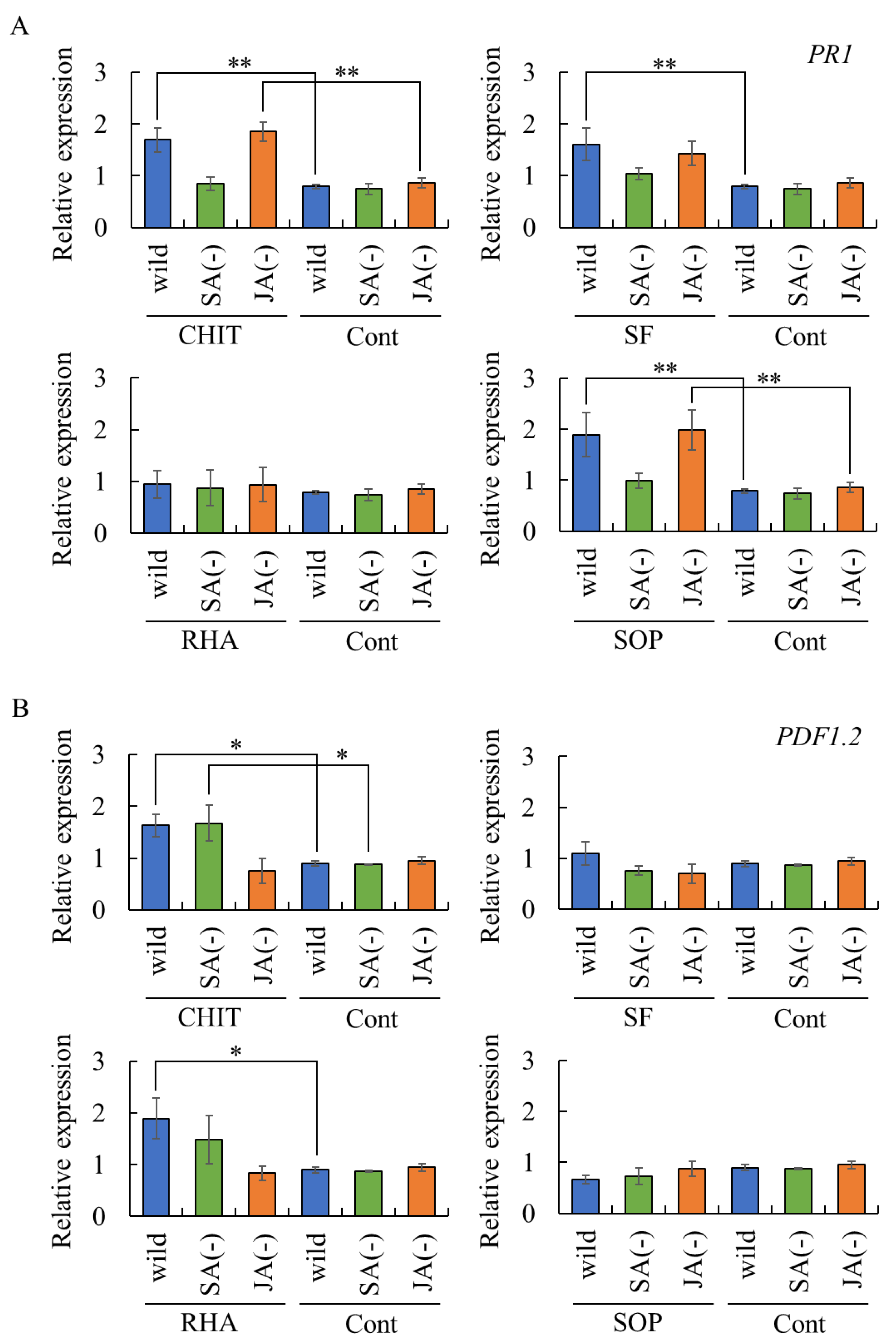
2.4. Assessment of Biosurfactant-Induced Resistance via SA- and/or JA-Dependent Defense Pathways
2.5. Assessment of Systemic Acquired Resistance Triggered by Biosurfactants
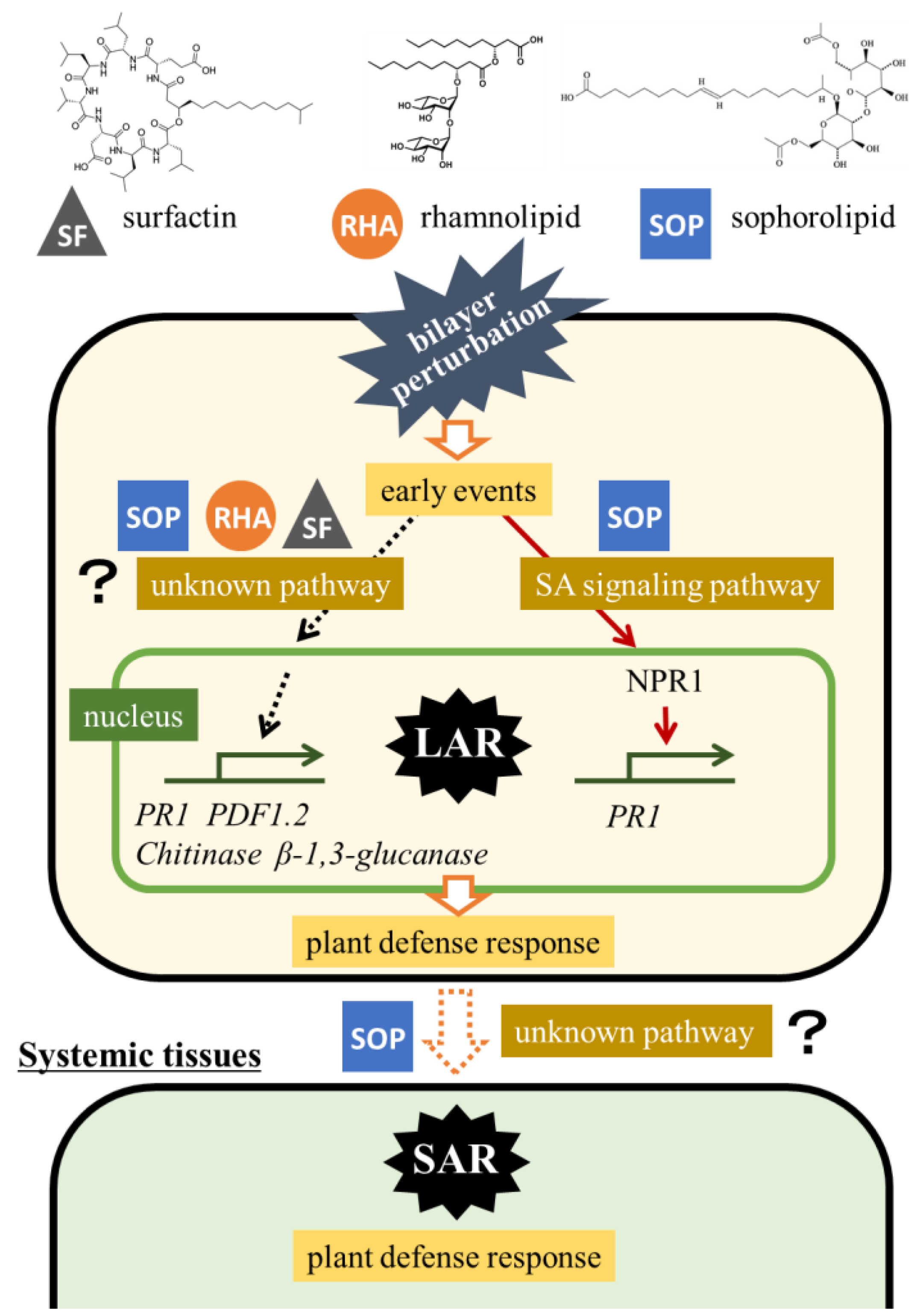
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials
4.3. In Vivo Bioassay for Downy Mildew Disease Severity in Grapevine Leaf Disks
4.4. In Vivo Bioassay for Anthracnose Disease Severity in Strawberry Leaves
4.5. Treatment of Arabidopsis Rosette Leaves with Each Biosurfactant
4.6. Total RNA Isolation
4.7. cDNA Synthesis
4.8. Real-Time RT-PCR
4.9. Possible Systemic Acquired Resistance in Grapevine Leaves
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JA | Jasmonate |
| PR protein | Pathogenesis-related protein |
| SA | Salicylic acid |
References
- Aoki, Y.; Kasai, Y.; Ikehara, S.; Sasada, T.; Suzuki, S. Monitoring of QoI fungicide resistance in Plasmopara viticola populations in Hokkaido. J. ASEV Jpn. 2019, 29, 113–118. (In Japanese) [Google Scholar]
- Aoki, Y.; Usujima, A.; Suzuki, S. High night temperature promotes downy mildew in grapevine via attenuating plant defense response and enhancing early Plasmopara viticola infection. Plant Prot. Sci. 2021, 57, 21–30. [Google Scholar] [CrossRef]
- Karačić, V.; Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Tamindžić, G.; Ivanović, M. Bacillus species: Excellent biocontrol agents against tomato diseases. Microorganisms 2024, 12, 457. [Google Scholar] [CrossRef]
- Soković, M.D.; Glamočlija, J.M.; Ćirić, A.D. Natural products from plants and fungi as fungicides. In Fungicides-Showcases of Integrated Plant Disease Management from Around the World; Nita, M., Ed.; IntechOpen: London, UK, 2013; pp. 185–232. [Google Scholar]
- Ranade, Y.; Pathak, P.; Chandrashekar, M.; Saha, S. Biological control of Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. by epiphytic bacteria isolated from Vitis vinifera (cv Thompson Seedless) grape berry. Biocontrol Sci. Technol. 2023, 33, 173–189. [Google Scholar] [CrossRef]
- Sun, Z.-B.; Song, H.-J.; Liu, Y.-Q.; Ren, Q.; Wang, Q.-Y.; Li, X.-F.; Pan, H.-X.; Huang, X.-Q. The potential of microorganisms for the control of grape downy mildew—A review. J. Fungi 2024, 10, 702. [Google Scholar] [CrossRef]
- Ishiai, S.; Kondo, H.; Hattori, T.; Mikami, M.; Aoki, Y.; Enoki, S.; Suzuki, S. Hordenine is responsible for plant defense response through jasmonate-dependent defense pathway. Physiol. Mol. Plant Pathol. 2016, 96, 94–100. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shiraishi, S.; Suzuki, S. Are cyclic lipopeptides produced by Bacillus amyloliquefaciens S13-3 responsible for the plant defense response in strawberry against Colletotrichum gloeosporioides? Lett. Appl. Microbiol. 2015, 60, 379–386. [Google Scholar] [CrossRef]
- Romero Vega, G.; Gallo Stampino, P. Bio-based surfactants and biosurfactants: An overview and main characteristics. Molecules 2025, 30, 863. [Google Scholar] [CrossRef]
- Puyol McKenna, P.; Naughton, P.J.; Dooley, J.S.G.; Ternan, N.G.; Lemoine, P.; Banat, I.M. Microbial biosurfactants: Antimicrobial activity and potential biomedical and therapeutic exploits. Pharmaceuticals 2024, 17, 138. [Google Scholar] [CrossRef]
- Peypoux, F.; Bonmatin, J.; Wallach, J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 1999, 51, 553–563. [Google Scholar] [CrossRef]
- Maier, R.; Soberón-Chávez, G. Pseudomonas aeruginosa rhamnolipids: Biosynthesis and potential applications. Appl. Microbiol. Biotechnol. 2000, 54, 625–633. [Google Scholar] [CrossRef]
- Claus, S.; Van Bogaert, I.N. Sophorolipid production by yeasts: A critical review of the literature and suggestions for future research. Appl. Microbiol. Biotechnol. 2017, 101, 7811–7821. [Google Scholar] [CrossRef]
- Tabuchi, Y.; Nakamura, I.; Kobayashi, T. Accumulation of the open-ring acid of spiculisporic acid by Penicillium spiculisporum in shake culture. J. Ferment. Technol. 1977, 55, 37–42. [Google Scholar]
- Gama, Y.; Yasumoto, M.; Suzuki, H.; Ishigami, Y. Highly selective conversion of spiculisporic acid to ester, amide and imide derivatives. J. Jpn. Oil Chem. Soc. 1989, 38, 292–296. (In Japanese) [Google Scholar] [CrossRef]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.-L.; Thonart, P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Sanchez, L.; Courteaux, B.; Hubert, J.; Kauffmann, S.; Renault, J.H.; Clément, C.; Baillieul, F.; Dorey, S. Rhamnolipids elicit defense responses and induce disease resistance against biotrophic, hemibiotrophic, and necrotrophic pathogens that require different signaling pathways in Arabidopsis and highlight a central role for salicylic acid. Plant Physiol. 2012, 160, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Mian, G.; Musetti, R.; Belfiore, N.; Boscaro, D.; Lovat, L.; Tomasi, D. Chitosan application reduces downy mildew severity on grapevine leaves by positively affecting gene expression pattern. Physiol. Mol. Plant Pathol. 2023, 125, 102025. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Aziz, A.; Heyraud, A.; Lambert, B. Oligogalacturonide signal transduction, induction of defense-related responses and protection of grapevine against Botrytis cinerea. Planta 2004, 218, 767–774. [Google Scholar] [CrossRef]
- Derckel, J.P.; Baillieul, F.; Manteau, S.; Audran, J.C.; Haye, B.; Lambert, B.; Legendre, L. Differential induction of grapevine defenses by two strains of Botrytis cinerea. Phytopathology 1999, 89, 197–203. [Google Scholar] [CrossRef]
- Uknes, S.; Dincher, S.; Friedrich, L.; Negrotto, D.; Williams, S.; Thompson-Taylor, H.; Potter, S.; Ward, E.; Ryals, J. Regulation of pathogenesis-related protein-1a gene expression in tobacco. Plant Cell 1993, 5, 159–169. [Google Scholar] [PubMed]
- Manners, J.M.; Penninckx, I.A.; Vermaere, K.; Kazan, K.; Brown, R.L.; Morgan, A.; Maclean, D.J.; Curtis, M.D.; Cammue, B.P.; Broekaert, W.F. The promoter of the plant defensin gene PDF1.2 from Arabidopsis is systemically activated by fungal pathogens and responds to methyl jasmonate but not to salicylic acid. Plant Mol. Biol. 1998, 38, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef]
- Otzen, D.E. Biosurfactants and surfactants interacting with membranes and proteins: Same but different? Biochim. Biophys. Acta Biomembr. 2017, 1859, 639–649. [Google Scholar] [CrossRef]
- Zipfel, C.; Robatzek, S.; Navarro, L.; Oakeley, E.J.; Jones, J.D.; Felix, G.; Boller, T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 2004, 428, 764–767. [Google Scholar] [CrossRef]
- Gorin, P.A.J.; Spencer, J.F.T.; Tulloch, A.P. Hydroxy fatty acid glycosides of sophorose from Torulopsis magnoliae. Can. J. Chem. 1961, 39, 846–855. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fukuoka, T. Sophorolipids, commercialized glycolipid biosurfactants: Derivatives, component analysis, and applications. J. Am. Oil. Chem. Soc. 2025, 102, 251–260. [Google Scholar] [CrossRef]
- De O Caretta, T.; I Silveira, V.A.; Andrade, G.; Macedo, F., Jr.; Celligoi, M.A.P.C. Antimicrobial activity of sophorolipids produced by Starmerella bombicola against phytopathogens from cherry tomato. J. Sci. Food Agric. 2022, 102, 1245–1254. [Google Scholar] [CrossRef]
- Hipólito, A.; Silva, R.A.A.; Caretta, T.O.; Silveira, V.A.I.; Amador, I.R.; Panagio, L.A.; Borsato, D.; Celligoi, M.A.P.C. Evaluation of the antifungal activity of sophorolipids from Starmerella bombicola against food spoilage fungi. Biocatal. Agric. Biotechnol. 2020, 29, 101797. [Google Scholar] [CrossRef]
- Al-Mutar, D.M.K.; Noman, M.; Abduljaleel Alzawar, N.S.; Azizullah; Li, D.; Song, F. Cyclic lipopeptides of Bacillus amyloliquefaciens DHA6 are the determinants to suppress watermelon Fusarium wilt by direct antifungal activity and host defense modulation. J. Fungi 2023, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Héloir, M.C.; Zhang, X.; Geissler, M.; Trouvelot, S.; Jacquens, L.; Henkel, M.; Su, X.; Fang, X.; Wang, Q. Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol. Plant Pathol. 2019, 20, 1037–1050. [Google Scholar] [CrossRef]
- Rodrigues, A.I.; Gudiña, E.J.; Abrunhosa, L.; Malheiro, A.R.; Fernandes, R.; Teixeira, J.A.; Rodrigues, L.R. Rhamnolipids inhibit aflatoxins production in Aspergillus flavus by causing structural damages in the fungal hyphae and down-regulating the expression of their biosynthetic genes. Int. J. Food Microbiol. 2021, 348, 109207. [Google Scholar] [CrossRef] [PubMed]
- Fungicide Resistance Action Committee. CAA Working Group Report. Available online: https://www.frac.info/frac-teams/working-groups/caa-fungicides#open-tour (accessed on 25 June 2025).
- Lorenzo, O.; Chico, J.M.; Sánchez-Serrano, J.J.; Solano, R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 2004, 16, 1938–1950. [Google Scholar] [CrossRef]
- Furuya, S.; Mochizuki, M.; Saito, S.; Kobayashi, H.; Takayanagi, T.; Suzuki, S. Monitoring of QoI fungicide resistance in Plasmopara viticola populations in Japan. Pest Manag. Sci. 2010, 66, 1268–1272. [Google Scholar] [CrossRef]
- Aoki, T.; Aoki, Y.; Ishiai, S.; Otoguro, M.; Suzuki, S. Impact of Bacillus cereus NRKT on grape ripe rot disease through resveratrol synthesis in berry skin. Pest Manag. Sci. 2017, 73, 174–180. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, Y.; Asada, T.; Nojiri, M.; Suzuki, S. Comparative Analysis of Plant Defense Activation by Four Biosurfactants: Mode of Action and Disease Control Potential. Int. J. Mol. Sci. 2025, 26, 8313. https://doi.org/10.3390/ijms26178313
Aoki Y, Asada T, Nojiri M, Suzuki S. Comparative Analysis of Plant Defense Activation by Four Biosurfactants: Mode of Action and Disease Control Potential. International Journal of Molecular Sciences. 2025; 26(17):8313. https://doi.org/10.3390/ijms26178313
Chicago/Turabian StyleAoki, Yoshinao, Takayuki Asada, Masutoshi Nojiri, and Shunji Suzuki. 2025. "Comparative Analysis of Plant Defense Activation by Four Biosurfactants: Mode of Action and Disease Control Potential" International Journal of Molecular Sciences 26, no. 17: 8313. https://doi.org/10.3390/ijms26178313
APA StyleAoki, Y., Asada, T., Nojiri, M., & Suzuki, S. (2025). Comparative Analysis of Plant Defense Activation by Four Biosurfactants: Mode of Action and Disease Control Potential. International Journal of Molecular Sciences, 26(17), 8313. https://doi.org/10.3390/ijms26178313







