Efficient pecG-n (n = 1, 2) Basis Sets for Ga, Ge, As, Se, and Br Specialized for the Geometry Optimization of Molecular Structures
Abstract
1. Introduction
2. Results
3. Discussion
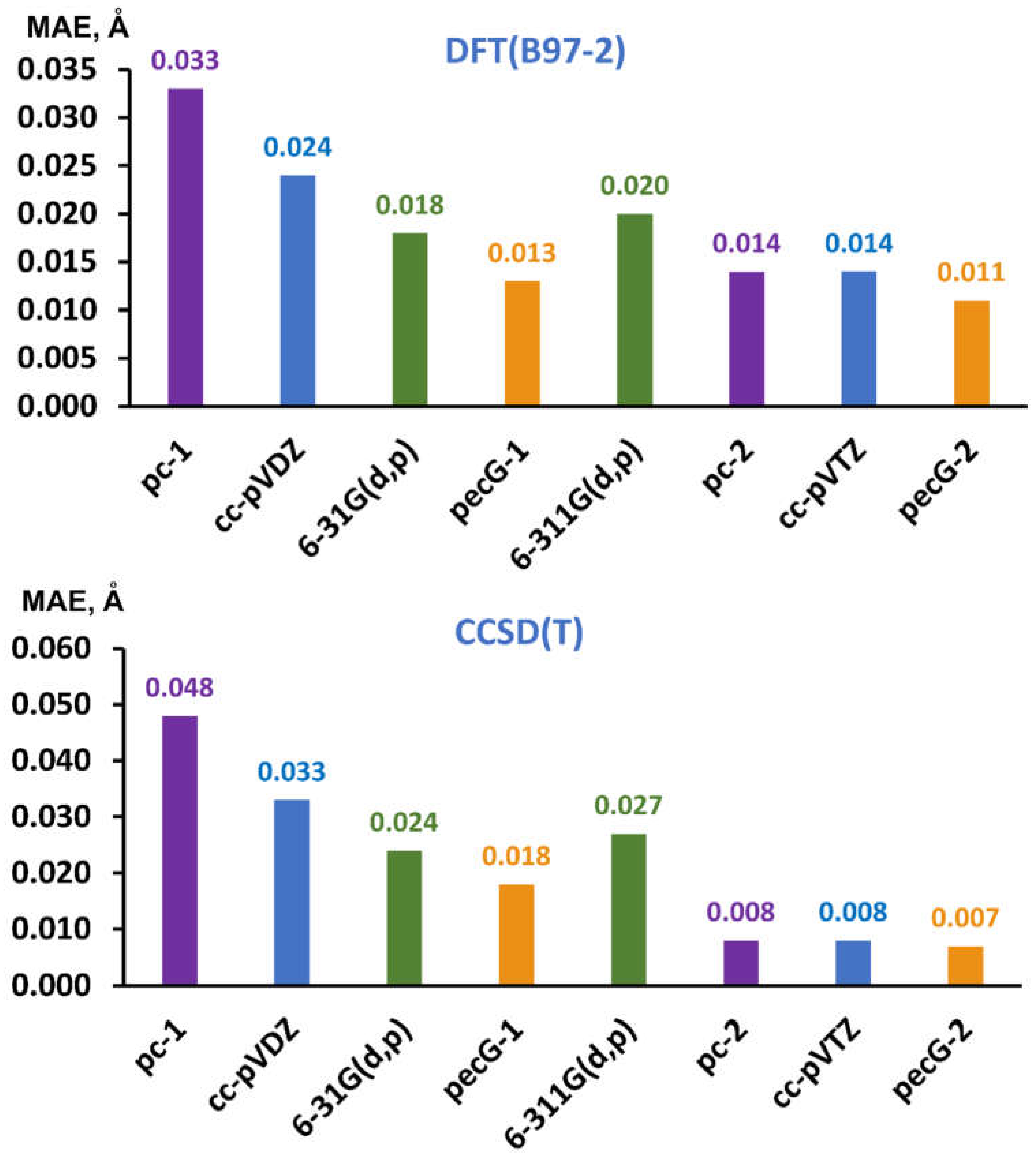
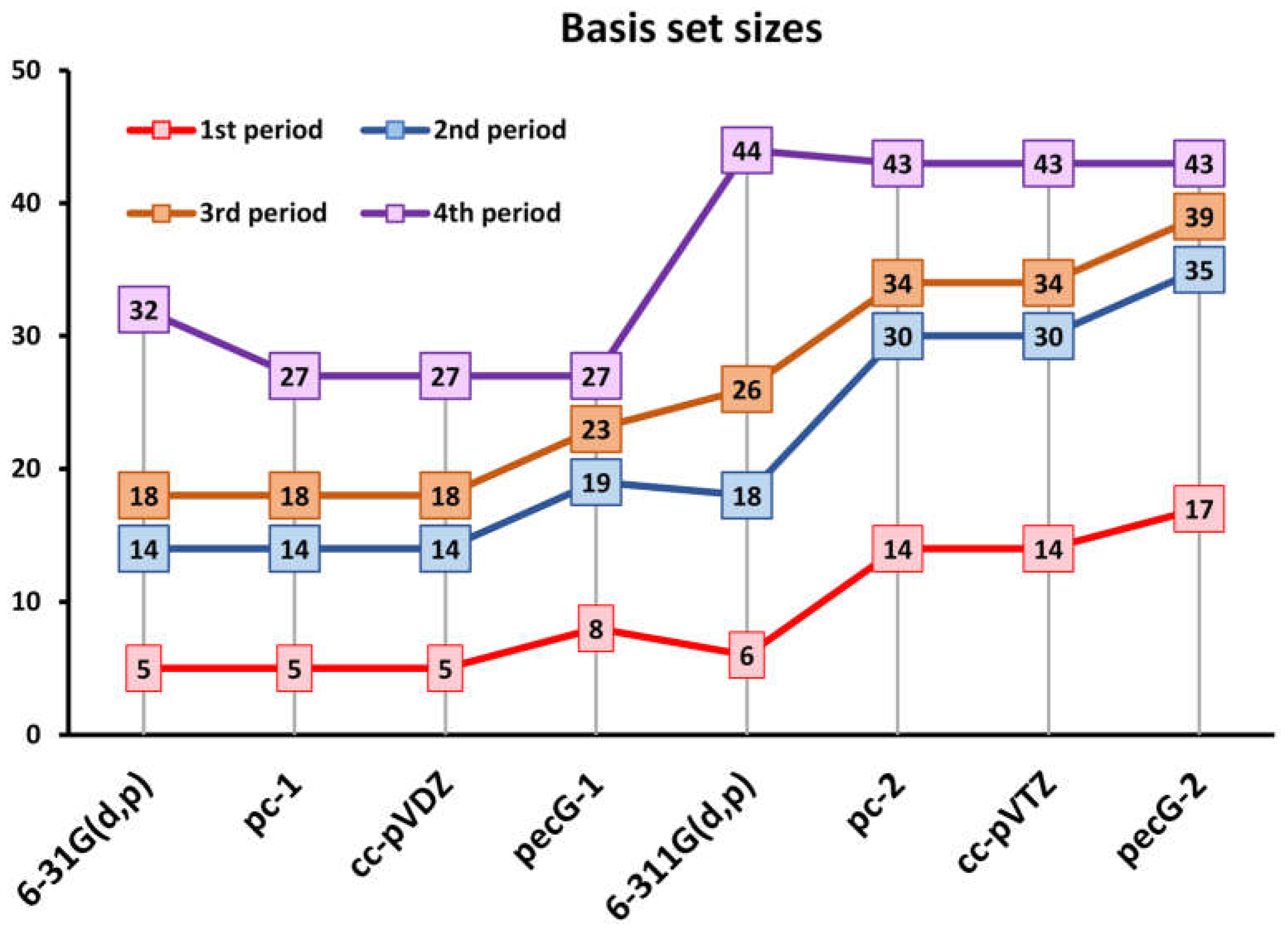
3.1. Theoretical Testing of the pecG-n (n = 1, 2) Basis Sets on the Equilibrium Bond Lengths
3.2. Testing the Performance of the pecG-n (n = 1, 2) Basis Sets by Comparison of the Calculated Bond Lengths with the Gas Phase Electron Diffraction Experiment
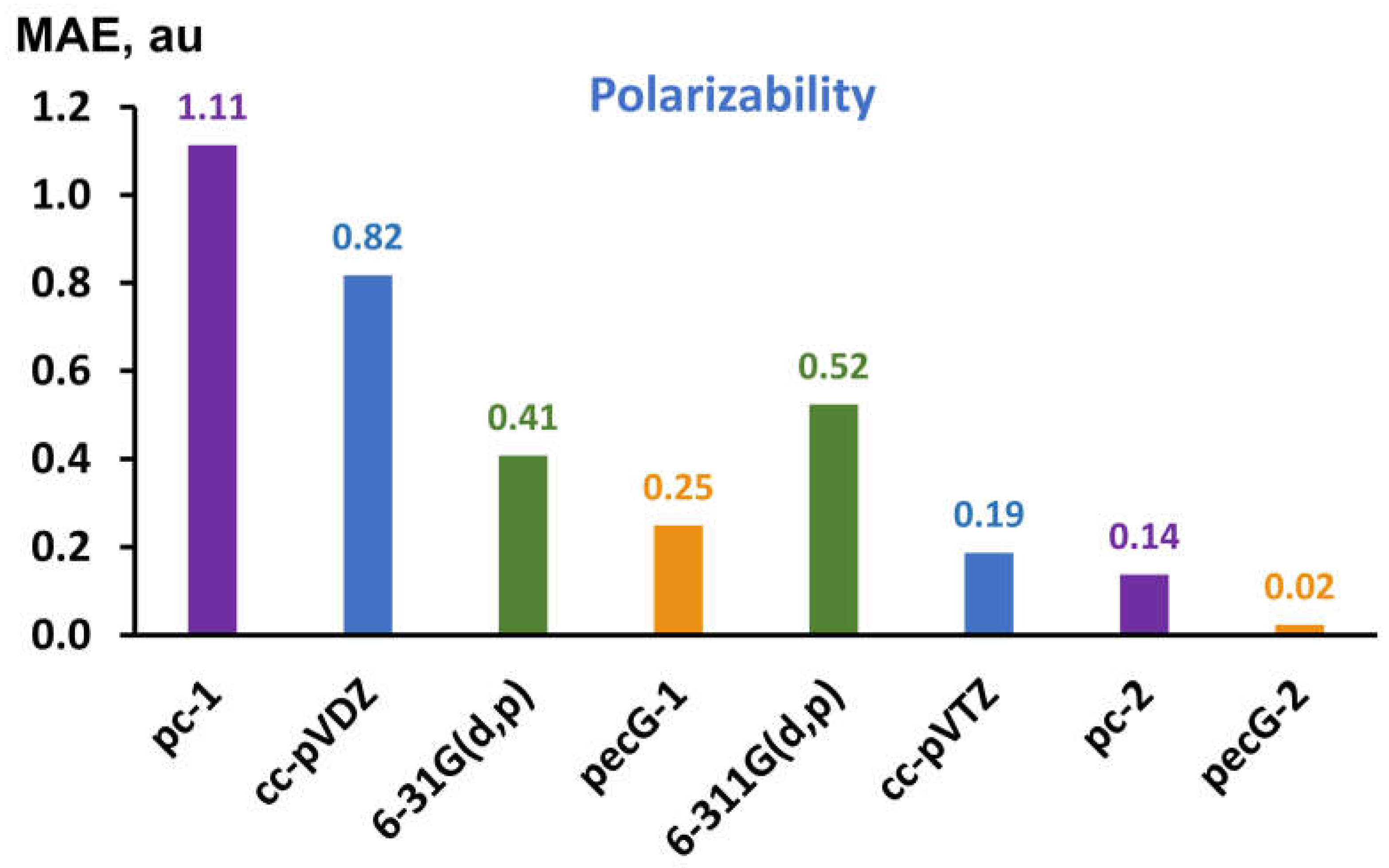
3.3. Testing the Performance of Equilibrium Geometries Obtained with the pecG-n (n = 1, 2) Basis Sets on Polarizability
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rusakov, Y.Y.; Nikurashina, Y.A.; Rusakova, I.L. On the Utmost Importance of the Geometry Factor of Accuracy in the Quantum Chemical Calculations of 31P NMR Chemical Shifts: New Efficient pecG-n (n = 1, 2) Basis Sets for the Geometry Optimization Procedure. J. Chem. Phys. 2024, 160, 084109. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. Getaway from the Geometry Factor Error in the Molecular Property Calculations: Efficient pecG-n (n = 1, 2) Basis Sets for the Geometry Optimization of Molecules Containing Light p Elements. J. Chem. Theory Comput. 2024, 20, 6661–6673. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Kumar, R. Structural and Electronic Properties of GaAs and GaP Semiconductors. AIP Conf. Proc. 2015, 1661, 050010. [Google Scholar] [CrossRef]
- Czelej, K.; Mansoor, M.; Sarsil, M.A.; Tas, M.; Sorkhe, Y.A.; Mansoor, M.; Mansoor, M.; Derin, B.; Ergen, O.; Timur, S.; et al. Atomistic Origins of Various Luminescent Centers and n-Type Conductivity in GaN: Exploring the Point Defects Induced by Cr, Mn, and O through an Ab Initio Thermodynamic Approach. Chem. Mater. 2024, 36, 6392–6409. [Google Scholar] [CrossRef]
- Singh, D.K.; Roul, B.K.; Nanda, K.K.; Krupanidhi, S.B. Group III-Nitrides and Their Hybrid Structures for Next-Generation Photodetectors. In Light-Emitting Diodes and Photodetectors—Advances and Future Directions; Casalino, M., Thirumalai, J., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Roy, R.; Hill, V.G.; Osborn, E.F. Polymorphism of Ga2O3 and the System Ga2O3−H2O. J. Am. Chem. Soc. 1952, 74, 719–722. [Google Scholar] [CrossRef]
- Shi, F.; Qiao, H. Preparations, Properties and Applications of Gallium Oxide Nanomaterials—A Review. Nano Sel. 2022, 3, 348–373. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Z.; Li, Y.; Chen, Z.; Zeng, H. Atomically Thin Arsenene and Antimonene: Semimetal-Semiconductor and Indirect-Direct Band-Gap Transitions. Angew. Chem. Int. Ed. 2015, 54, 3112–3115. [Google Scholar] [CrossRef]
- Moss, S.J.; Ledwith, A. The Chemistry of the Semiconductor Industry, 1st ed.; Blackie & Son Ltd.: New York, NY, USA, 1987; ISBN 0-412-01321-5. [Google Scholar]
- Chen, Y.; Chen, C.; Kealhofer, R.; Liu, H.; Yuan, Z.; Jiang, L.; Suh, J.; Park, J.; Ko, C.; Choe, H.S.; et al. Black Arsenic: A Layered Semiconductor with Extreme In-Plane Anisotropy. Adv. Mater. 2018, 30, 1800754. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Tuinenga, C.; Guidez, E.B.; Lewis, C.; Shipman, J.; Roy, S.; Aikens, C.M.; Chikan, V. Synthesis and Characterization of Gallium-Doped CdSe Quantum Dots. J. Phys. Chem. C 2015, 119, 10749–10757. [Google Scholar] [CrossRef]
- Sallen, G.; Kunz, S.; Amand, T.; Bouet, L.; Kuroda, T.; Mano, T.; Paget, D.; Krebs, O.; Marie, X.; Sakoda, K.; et al. Nuclear Magnetization in Gallium Arsenide Quantum Dots at Zero Magnetic Field. Nat. Commun. 2014, 5, 3268. [Google Scholar] [CrossRef]
- van Riggelen-Doelman, F.; Wang, C.-A.; de Snoo, S.L.; Lawrie, W.I.L.; Hendrickx, N.W.; Rimbach-Russ, M.; Sammak, A.; Scappucci, G.; Déprez, C.; Veldhorst, M. Coherent Spin Qubit Shuttling through Germanium Quantum Dots. Nat. Commun. 2024, 15, 5716. [Google Scholar] [CrossRef]
- Franke, D.; Harris, D.K.; Chen, O.; Bruns, O.T.; Carr, J.A.; Wilson, M.W.B.; Bawendi, M.G. Continuous Injection Synthesis of Indium Arsenide Quantum Dots Emissive in the Short-Wavelength Infrared. Nat. Commun. 2016, 7, 12749. [Google Scholar] [CrossRef]
- Eybl, V.; Kotyzová, D.; Sýkora, J.; Topolčan, O.; Pikner, R.; Mihaljevič, M.; Brtko, J.; Glattre, E. Effects of Selenium and Tellurium on the Activity of Selenoenzymes Glutathione Peroxidase and Type I Iodothyronine Deiodinase, Trace Element Thyroid Level, and Thyroid Hormone Status in Rats. Biol. Trace Elem. Res. 2007, 117, 105–114. [Google Scholar] [CrossRef]
- Johansson, L.; Gafvelin, G.; Arnér, E.S.J. Selenocysteine in Proteins − Properties and Biotechnological Use. Biochim. Biophys. Acta 2005, 1726, 1–13. [Google Scholar] [CrossRef]
- Schwarz, K.; Foltz, C.M. Selenium as an Integral Part of Factor 3 Against Dietary Necrotic Liver Degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Behne, D.; Kyriakopoulos, A. Mammalian Selenium-Containing Proteins. Annu. Rev. Nutr. 2001, 21, 453–473. [Google Scholar] [CrossRef]
- Gribble, G.W. The Diversity of Naturally occurring Organobromine Compounds. Chem. Soc. Rev. 1999, 28, 335–346. [Google Scholar] [CrossRef]
- Slack, R.D.; Abramyan, A.M.; Tang, H.; Meena, S.; Davis, B.A.; Bonifazi, A.; Giancola, J.L.B.; Deschamps, J.R.; Naing, S.; Yano, H.; et al. A Novel Bromine-Containing Paroxetine Analogue Provides Mechanistic Clues for Binding Ambiguity at the Central Primary Binding Site of the Serotonin Transporter. ACS Chem. Neurosci. 2019, 10, 3946–3952. [Google Scholar] [CrossRef] [PubMed]
- Bouthenet, E.; Oh, K.-B.; Park, S.; Nagi, N.K.; Lee, H.-S.; Matthews, S.E. Synthesis and Antimicrobial Activity of Brominated Resorcinol Dimers. Bioorg. Med. Chem. Lett. 2011, 21, 7142–7145. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.I.; Lozano, R.D.; Martinez, E.A. A Transimpedance Circuit for Use with Selenium Photoelectric Cells. J. Phys. E Sci. Instrum. 1972, 5, 745. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; You, Q.; Huang, P.; Wang, Y.; Huang, Z.N.; Ge, Y.; Wu, L.; Dong, Z.; Dai, X.; et al. Enhanced Photodetection Properties of Tellurium-Selenium Roll-to-Roll Nanotube Heterojunctions. Nano Micro Small 2019, 15, 1900902. [Google Scholar] [CrossRef] [PubMed]
- Hadar, I.; Song, T.-B.; Ke, W.; Kanatzidis, M.G. Modern Processing and Insights on Selenium Solar Cells: The World’s First Photovoltaic Device. Adv. Energy Mater. 2019, 9, 1802766. [Google Scholar] [CrossRef]
- Lin, J.-H.; Zhang, H.; Cheng, X.-L.; Miyamoto, Y. Two-Dimensional Wide-Band-Gap Nitride Semiconductors: Single-Layer 1T-XN2 (X= S, Se, and Te). Phys. Rev. B 2016, 94, 195404. [Google Scholar] [CrossRef]
- Malik, M.A.; Ramasamy, K.; O’Brien, P. Selenium and Tellurium Containing Precursors for Semiconducting Materials. In Selenium and Tellurium Chemistry From Small Molecules to Biomolecules and Materials, 1st ed.; Woollins, J.D., Laitinen, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Johnson, J.A.; Saboungi, M.-L.; Thiyagarajan, P.; Csencsits, R.; Meisel, D. Selenium Nanoparticles: A Small-Angle Neutron Scattering Study. J. Phys. Chem. B 1999, 103, 59–63. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lampis, S.; Vallini, G.; Turner, R.J. Physical-Chemical Properties of Biogenic Selenium Nanostructures Produced by Stenotrophomonas maltophilia SeITE02 and Ochrobactrum sp. MPV1. Front. Microbiol. 2018, 9, 3178. [Google Scholar] [CrossRef]
- Mayers, B.; Jiang, X.; Sunderland, D.; Cattle, B.; Xia, Y. Hollow Nanostructures of Platinum with Controllable Dimensions Can Be Synthesized by Templating Against Selenium Nanowires and Colloids. J. Am. Chem. Soc. 2003, 125, 13364–13365. [Google Scholar] [CrossRef]
- Terasaki, O.; Yamazaki, K.; Thomas, J.M.; Ohsuna, T.; Watanabe, D.; Sanders, J.V.; Barry, J.C. Isolating Individual Chains of Selenium by Incorporation into the Channels of a Zeolite. Nature 1987, 330, 58–60. [Google Scholar] [CrossRef]
- Parise, J.B.; MacDougall, J.; Herron, N.; Farlee, R.; Sleight, A.W.; Wang, Y.; Bein, T.; Moller, K.; Moroney, L.M. Characterization of Selenium-Loaded Molecular Sieves A, X, Y, AIPO-5, and Mordenite. Inorg. Chem. 1988, 27, 221–228. [Google Scholar] [CrossRef]
- Helgaker, T.; Gauss, J.; Jørgensen, P.; Olsen, J. The Prediction of Molecular Equilibrium Structures by the Standard Electronic Wave Functions. J. Chem. Phys. 1997, 106, 6430–6440. [Google Scholar] [CrossRef]
- Faber, R.; Sauer, S.P.A. On the Discrepancy between Theory and Experiment for the F−F Spin−Spin Coupling Constant of Difluoroethyne. Phys. Chem. Chem. Phys. 2012, 14, 16440–16447. [Google Scholar] [CrossRef]
- Temelso, B.; Valeev, E.F.; Sherrill, C.D. A Comparison of One-Particle Basis Set Completeness, Higher-Order Electron Correlation, Relativistic Effects, and Adiabatic Corrections for Spectroscopic Constants of BH, CH+, and NH+. J. Phys. Chem. A 2004, 108, 3068–3075. [Google Scholar] [CrossRef]
- Heckert, M.; Kállay, M.; Tew, D.P.; Klopper, W.; Gauss, J. Basis-set Extrapolation Techniques for the Accurate Calculation of Molecular Equilibrium Geometries Using Coupled-Cluster Theory. J. Chem. Phys. 2006, 125, 044108. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.K.; Woon, D.E.; Peterson, K.A.; Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. IX. The Atoms Gallium through Krypton. J. Chem. Phys. 1999, 110, 7667–7676. [Google Scholar] [CrossRef]
- Jensen, F. Polarization Consistent Basis Sets. VII. The Elements K, Ca, Ga, Ge, As, Se, Br, and Kr. J. Chem. Phys. 2012, 136, 114107. [Google Scholar] [CrossRef]
- Curtiss, L.A.; McGrath, M.P.; Blaudeau, J.-P.; Davis, N.E.; Binning, R.C.; Radom, L. Extension of Gaussian-2 Theory to Molecules Containing Third-Row Atoms Ga-Kr. J. Chem. Phys. 1995, 103, 6104–6113. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. An Efficient Method for Generating Property-Energy Consistent Basis Sets. New pecJ-n (n = 1, 2) Basis Sets for High-Quality Calculations of Indirect Nuclear Spin–Spin Coupling Constants Involving 1H, 13C, 15N, and 19F Nuclei. Phys. Chem. Chem. Phys. 2021, 23, 14925–14939. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. New Efficient pecS-n (n = 1, 2) Basis Sets for Quantum Chemical Calculations of 31P NMR Chemical Shifts. Phys. Chem. Chem. Phys. 2023, 25, 18728–18741. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. New pecJ-n (n = 1, 2) Basis Sets for High Quality Calculations of Indirect Nuclear Spin–Spin Coupling Constants Involving 31P and 29Si: The Advanced PEC method. Molecules 2022, 27, 6145. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. New pecJ-n (n = 1, 2) Basis Sets for Selenium Atom Purposed for the Calculations of NMR Spin–Spin Coupling Constants Involving Selenium. Int. J. Mol. Sci. 2023, 24, 7841. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. New pecS-n (n = 1, 2) Basis Sets for Quantum Chemical Calculations of the NMR Chemical Shifts of H, C, N, and O Nuclei. J. Chem. Phys. 2022, 156, 244112. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. An Unusual Way of Augmenting One-Electron Basis Sets: New aug-pecS-n (n = 1, 2) Basis Sets for H, C, N, and O Atoms for NMR Shielding Constant Calculations that Require Extra Diffuse Functions. J. Chem. Phys. 2025, 162, 164111. [Google Scholar] [CrossRef] [PubMed]
- Rusakov, Y.Y.; Nikurashina, Y.A.; Rusakova, I.L. Going Beyond the Routine Consideration of Solvent Effects on 31P NMR Shielding Constants: A Meticulous Basis Set Study and New aug-pecS-n (n = 1 and 2) Basis Sets for Phosphorus Atoms. Phys. Chem. Chem. Phys. 2025, 27, 6730–6743. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry. V. Systematic Optimization of Exchange-Correlation Functionals. J. Chem. Phys. 1997, 107, 8554–8560. [Google Scholar] [CrossRef]
- Wilson, P.J.; Bradley, T.J.; Tozer, D.J. Hybrid Exchange-Correlation Functional Determined from Thermochemical Data and Ab Initio Potentials. J. Chem. Phys. 2001, 115, 9233–9242. [Google Scholar] [CrossRef]
- Raffenetti, R.C. General Contraction of Gaussian Atomic Orbitals: Core, Valence, Polarization, and Diffuse Basis Sets; Molecular Integral Evaluation. J. Chem. Phys. 1973, 58, 4452–4458. [Google Scholar] [CrossRef]
- Cremer, D. Møller–Plesset Perturbation Theory: From Small Molecule Methods to Methods for Thousands of Atoms. WIREs Comput. Mol. Sci. 2011, 1, 509–530. [Google Scholar] [CrossRef]
- Gauss, J.; Stanton, J.F. Electron-correlated approaches for the calculation of NMR chemical shifts. In Advances in Chemical Physics, 1st ed.; Prigogine, I., Rice, S.A., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2002; Volume 123, pp. 355–422. [Google Scholar]
- Jensen, F. Introduction to Computational Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2007. [Google Scholar]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* Basis Set for Third-Row Atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Morino, Y.; Ukaji, T.; Ito, T. Molecular Structure Determination by Gas Electron Diffraction at High Temperatures. I. Arsenic. Bull. Chem. Soc. Jpn. 1966, 39, 64–71. [Google Scholar] [CrossRef]
- Samdal, S.; Barnhart, D.M.; Hedberg, K. An Electron-Diffraction Investigation of Gaseous AsBr3 I. Molecular Structure at 373 K and 466 K II. Quadratic Potential Constants from Amplitudes. J. Mol. Struct. 1976, 35, 67–80. [Google Scholar] [CrossRef]
- Konaka, S.; Kimura, M. Determination of the Molecular Structures of AsF3 and AsCl3 by Gas Electron Diffraction. Bull. Chem. Soc. Jpn. 1970, 43, 1693–1703. [Google Scholar] [CrossRef]
- Tamagawa, K.; Kimura, M. Molecular Structure of Bromoform as Determined by a Joint Analysis of Electron Diffraction and Microwave Data. Bull. Chem. Soc. Jpn. 1979, 52, 2747–2751. [Google Scholar] [CrossRef]
- Réffy, B.; Kolonits, M.; Hargittai, M. Gallium Tribromide: Molecular Geometry of Monomer and Dimer from Gas-Phase Electron Diffraction. J. Mol. Struct. 1998, 445, 139–148. [Google Scholar] [CrossRef]
- Haaland, A.; Hammel, A.; Martinsen, K.-G.; Tremmel, J.; Volden, H.V. Molecular Structures of Monomeric Gallium Trichloride, Indium Trichloride and Lead Tetrachloride by Gas Electron Diffraction. J. Chem. Soc. Dalton Trans. 1992, 14, 2209–2214. [Google Scholar] [CrossRef]
- Schultz, G.Y.; Tremmel, J.; Hargittai, I.; Kagramanov, N.D.; Maltsev, A.K.; Nefedov, O.M. Electron Diffraction Study of the Molecular Structure of Germanium Dibromide. J. Mol. Struct. 1982, 82, 107–113. [Google Scholar] [CrossRef]
- Glidewell, C.; Rankin, D.W.H.; Robiette, A.G.; Sheldrick, G.M.; Beagley, B.; Cradock, S. Molecular Structures of Digermyl Ether and Digermyl Sulphide in the Gas Phase, studied by Electron Diffraction. J. Chem. Soc. A. 1970, 315–317. [Google Scholar] [CrossRef]
- Boyd, A.S.F.; Laurenson, G.S.; Rankin, D.W.H. The Molecular Structure of Difluorophosphine Selenide, Determined Using a Combination of Gas Electron Diffraction and Liquid-Crystal NMR Data. J. Mol. Struct. 1981, 71, 217–226. [Google Scholar] [CrossRef]
- Shlykov, S.A.; Titov, A.V.; Oberhammer, H.; Giricheva, N.I.; Girichev, G.V. The Molecular Structure of Selenium Dibromide as Determined by Combined Gas-Phase Electron Diffraction–Mass Spectrometric Experiments and Quantum Chemical Calculations. Phys. Chem. Chem. Phys. 2008, 10, 6438–6445. [Google Scholar] [CrossRef]
- Fernholt, L.; Haaland, A.; Seip, R.; Kniep, R.; Korte, L. The Molecular Structure of Selenium Dichloride, SeCl2, Determined by Gas Electron Diffraction. Z. Naturforschung B 1983, 38, 1072–1073. [Google Scholar] [CrossRef]
- Almenningen, A.; Fernholt, L.; Seip, H.M. The Molecular Structure of Gaseous Disilyl Selenide, (SiH3)2Se. Acta Chem. Scand. 1968, 22, 51–58. [Google Scholar] [CrossRef]
- Kuchitsu, K. Effect of Molecular Vibrations on Gas Electron Diffraction. I. Probability Distribution Function and Molecular Intensity for Diatomic Molecules. Bull. Chem. Soc. Jpn. 1967, 40, 498–504. [Google Scholar] [CrossRef]
- Drake, J.E.; Hemmings, R.T.; Hencher, J.L.; Mustoe, F.M.; Shen, Q. Electron Diffraction Determination of the Molecular Structures of Difluoro(dimethyl)germane and Trifluoro(methyl)germane in the Vapour Phase. J. Chem. Soc. Dalton Trans. 1976, 5, 394–398. [Google Scholar] [CrossRef]
- Cossairt, B.M.; Cummins, C.C.; Head, A.R.; Lichtenberger, D.L.; Berger, R.J.F.; Hayes, S.A.; Mitzel, N.W.; Wu, G. On the Molecular and Electronic Structures of AsP3 and P4. J. Am. Chem. Soc. 2010, 132, 8459–8465. [Google Scholar] [CrossRef]
- Jacob, E.J. Structure of Bromochlorofluoromethane by Electron Diffraction. J. Mol. Struct. 1979, 52, 63–69. [Google Scholar] [CrossRef]
- Bartell, L.S. Effects of Anharmonicity of Vibration on the Diffraction of Electrons by Free Molecules. J. Chem. Phys. 1955, 23, 1219–1222. [Google Scholar] [CrossRef]
- Stanton, J.F. Why CCSD (T) Works: A Different Perspective. Chem. Phys. Lett. 1997, 281, 130–134. [Google Scholar] [CrossRef]
- Scuseria, G.E.; Lee, T.J. Comparison of Coupled‒Cluster Methods which Include the Effects of Connected Triple Excitations. J. Chem. Phys. 1990, 93, 5851–5855. [Google Scholar] [CrossRef]
- Valeev, E.F.; Crawford, T.D. Simple Coupled‒Cluster Singles and Doubles Method with Perturbative Inclusion of Triples and Explicitly Correlated Geminals: The CCSD(T)‒R12 Model. J. Chem. Phys. 2008, 128, 244113. [Google Scholar] [CrossRef]
- Purvis, G.D.; Bartlett, R.J., III. A Full Coupled‒Cluster Singles and Doubles Model: The Inclusion of Disconnected Triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Gauss, J.; Stanton, J.F. Analytic CCSD(T) Second Derivatives. Chem. Phys. Lett. 1997, 276, 70–77. [Google Scholar] [CrossRef]
- Scuseria, G.E.; Janssen, C.L.; Schaefer, H.F. An Efficient Reformulation of the Closed‒Shell Coupled Cluster Single and Double Excitation (CCSD) Equations. J. Chem. Phys. 1988, 89, 7382–7387. [Google Scholar] [CrossRef]
- Koch, H.; Sánchez de Merás, A.; Helgaker, T.; Christiansen, O. The integral-direct coupled cluster singles and doubles model. J. Chem. Phys. 1996, 104, 4157–4165. [Google Scholar] [CrossRef]
- Monkhorst, H.J. Calculation of Properties with the Coupled-Cluster Method. Int. J. Quantum Chem. Symp. 1977, 12, 421–432. [Google Scholar] [CrossRef]
- Christiansen, O. Coupled Cluster Theory with Emphasis on Selected New Developments. Theor. Chem. Acc. 2006, 116, 106–123. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Musiał, M. Coupled-Cluster Theory in Quantum Chemistry. Rev. Mod. Phys. 2007, 79, 291–352. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for Mixing Exact Exchange with Density Functional Approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof Exchange-Correlation Functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Dyall, K.G. Relativistic and Nonrelativistic Finite Nucleus Optimized Triple-Zeta Basis Sets for the 4p, 5p and 6p Elements. Theor. Chem. Acc. 2002, 108, 335–340. [Google Scholar] [CrossRef]
- Dyall, K.G. Relativistic Quadruple-Zeta and Revised Triple-Zeta and Double-Zeta Basis Sets for the 4p, 5p, and 6p Elements. Theor. Chem. Acc. 2006, 115, 441–447. [Google Scholar] [CrossRef]
- Loboda, O.; Ingrosso, F.; Ruiz-Lopez, M.F.; Reis, H.; Millot, C. Dipole and quadrupole polarizabilities of the water molecule as a function of geometry. J. Comput. Chem. 2016, 37, 2125–2132. [Google Scholar] [CrossRef]
- Loboda, O.; Ingrosso, F.; Ruiz-López, M.F.; Szalewicz, K.; Millot, C. Geometry-dependent distributed polarizability models for the water molecule. J. Chem. Phys. 2016, 144, 034304. [Google Scholar] [CrossRef] [PubMed]
- Avila, G. Ab initio dipole polarizability surfaces of water molecule: Static and dynamic at 514.5 nm. J. Chem. Phys. 2005, 122, 144310. [Google Scholar] [CrossRef] [PubMed]
- Lao, K.U.; Jia, J.; Maitra, R.; DiStasio, R.A. On the geometric dependence of the molecular dipole polarizability in water: A benchmark study of higher-order electron correlation, basis set incompleteness error, core electron effects, and zero-point vibrational contributions. J. Chem. Phys. 2018, 149, 204303. [Google Scholar] [CrossRef]
- Jørgensen, M.W.; Faber, R.; Ligabue, A.; Sauer, S.P.A. Benchmarking correlated methods for frequency-dependent polarizabilities: Aromatic molecules with the CC3, CCSD, CC2, SOPPA, SOPPA(CC2), and SOPPA(CCSD) methods. J. Chem. Theory Comput. 2020, 16, 3006–3018. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016; Available online: https://gaussian.com/glossary/g09 (accessed on 20 July 2025).
- Stanton, J.F.; Gauss, J.; Cheng, L.; Harding, M.E.; Matthews, D.A.; Szalay, P.G.; Asthana, A.; Auer, A.A.; Bartlett, R.J.; Benedikt, U.; et al. CFOUR, a Quantum Chemical Program Package. Available online: http://www.cfour.de (accessed on 20 July 2025).
- DIRAC, a Relativistic ab Initio Electronic Structure Program, Release DIRAC14 (2014), Written by T. Saue, L. Visscher, H.J. Aa. Jensen, and R. Bast, with Contributions from V. Bakken, K.G. Dyall, S. Dubillard, U. Ekström, E. Eliav, T. Enevoldsen, E. Faßhauer, T. Fleig, O. Fossgaard, A.S.P. Gomes, T. Helgaker, J. Henriksson, M. Iliaš, Ch. R. Jacob, S. Knecht, S. Komorovský, O. Kullie, C.V. Larsen, J.K. Lærdahl, Y.S. Lee, H.S. Nataraj, P. Norman, G. Olejniczak, J. Olsen, Y.C. Park, J.K. Pedersen, M. Pernpointner, R. di Remigio, K. Ruud, P. Sałek, B. Schimmelpfennig, J. Sikkema, A.J. Thorvaldsen, J. Thyssen, J. van Stralen, S. Villaume, O. Visser, T. Winther, and S. Yamamoto. Available online: https://www.diracprogram.org (accessed on 20 July 2025).
- Lévy-Leblond, J.M. Nonrelativistic Particles and Wave Equations. Commun. Math. Phys. 1967, 6, 286–311. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, W.; Kutzelnigg, W. Comparison of Restricted, Unrestricted, Inverse, and Dual Kinetic Balances for Four-Component Relativistic Calculations. Theor. Chem. Acc. 2011, 129, 423–436. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusakov, Y.Y.; Rusakova, I.L. Efficient pecG-n (n = 1, 2) Basis Sets for Ga, Ge, As, Se, and Br Specialized for the Geometry Optimization of Molecular Structures. Int. J. Mol. Sci. 2025, 26, 8197. https://doi.org/10.3390/ijms26178197
Rusakov YY, Rusakova IL. Efficient pecG-n (n = 1, 2) Basis Sets for Ga, Ge, As, Se, and Br Specialized for the Geometry Optimization of Molecular Structures. International Journal of Molecular Sciences. 2025; 26(17):8197. https://doi.org/10.3390/ijms26178197
Chicago/Turabian StyleRusakov, Yuriy Yu., and Irina L. Rusakova. 2025. "Efficient pecG-n (n = 1, 2) Basis Sets for Ga, Ge, As, Se, and Br Specialized for the Geometry Optimization of Molecular Structures" International Journal of Molecular Sciences 26, no. 17: 8197. https://doi.org/10.3390/ijms26178197
APA StyleRusakov, Y. Y., & Rusakova, I. L. (2025). Efficient pecG-n (n = 1, 2) Basis Sets for Ga, Ge, As, Se, and Br Specialized for the Geometry Optimization of Molecular Structures. International Journal of Molecular Sciences, 26(17), 8197. https://doi.org/10.3390/ijms26178197






