Advances in the Biosynthetic Regulation and Functional Mechanisms of Glycine Betaine for Enhancing Plant Stress Resilience
Abstract
1. Introduction
2. Biosynthetic Pathways and Regulatory Mechanisms of GB in Plants
2.1. Choline Oxidation Pathway
2.2. Glycine Methylation Pathway
2.3. Mechanisms of GB Uptake and Transporters in Plants
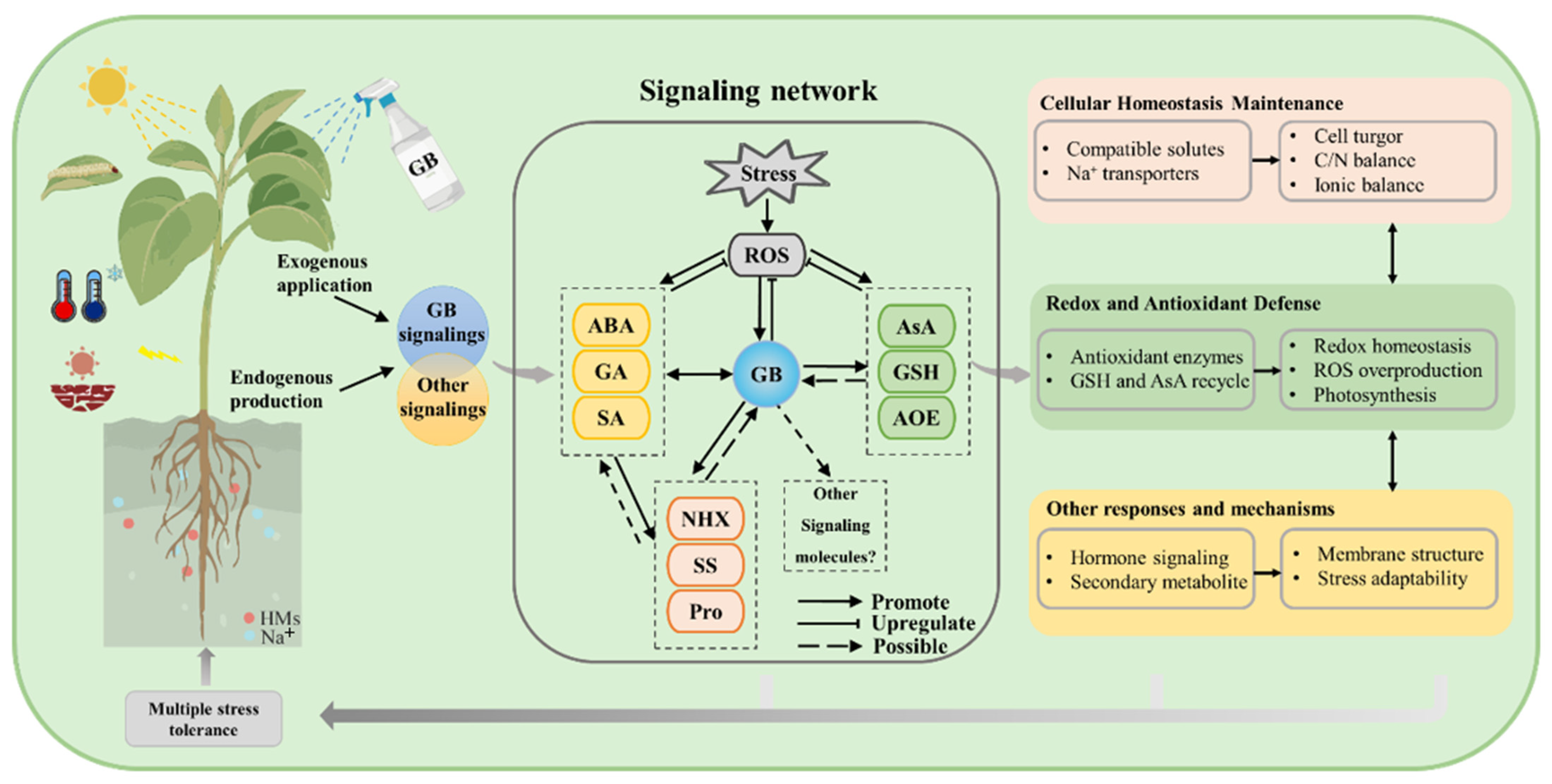
3. Functional Roles of GB in Plant Stress Tolerance Mechanisms
3.1. Maintenance of Cellular Homeostasis
3.1.1. Regulation of Osmotic Adjustment
3.1.2. Regulation of Ion Homeostasis
3.1.3. Regulation of Redox Homeostasis
3.1.4. Stabilization of Membrane Structures
3.2. Regulation of Metabolic Networks
3.3. Enhancement of Plant Tolerance to Abiotic Stress by GB
3.3.1. Drought Stress
3.3.2. Salt Stress
3.3.3. Temperature Stress
3.4. Enhancing Plant Resistance to Biotic Stress
4. Strategies for Applying GB to Enhance Plant Stress Resilience
4.1. Exogenous Application of GB
4.2. Genetic Engineering
5. Plant GB in Agriculture: Mechanisms, Challenges, and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Rhodes, D.; Hanson, A. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Banerjee, A.; Bhuyan, M.-H.-M.-B.; Roychoudhury, A.; Mahmud, J.-A. Targeting Glycinebetaine for Abiotic Stress Tolerance in Crop Plants: Physiological Mechanism, Molecular Interaction and Signaling. Phyton-Int. J. Exp. Bot. 2019, 88, 185–221. [Google Scholar] [CrossRef]
- Lutts, S. Exogenous glycinebetaine reduces sodium accumulation in salt-stressed rice plants. Int. Rice Res. Notes 2000, 25, 39–40. [Google Scholar] [CrossRef]
- Rathinasabapathi, B.; Burnet, M.; Russell, B.L.; Gage, D.A.; Liao, P.C.; Nye, G.J.; Scott, P.; Golbeck, J.H.; Hanson, A.D. Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: Prosthetic group characterization and cDNA cloning. Proc. Natl. Acad. Sci. USA 1997, 94, 3454–3458. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Takahashi, H.; Kitou, K.; Sahashi, K.; Tamagake, H.; Tanaka, Y.; Takabe, T. Suppressed expression of choline monooxygenase in sugar beet on the accumulation of glycine betaine. Plant Physiol. Biochem. 2015, 96, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.L.; Rathinasabapathi, B.; Hanson, A.D. Osmotic Stress Induces Expression of Choline Monooxygenase in Sugar Beet and Amaranth. Plant Physiol. 1998, 116, 859–865. [Google Scholar] [CrossRef]
- Hanke, G.; Mulo, P. Plant type ferredoxins and ferredoxin-dependent metabolism. Plant Cell Environ. 2013, 36, 1071–1084. [Google Scholar] [CrossRef]
- Carrillo, N.; Ceccarelli, E.A. Open questions in ferredoxin-NADP+ reductase catalytic mechanism. Eur. J. Biochem. 2003, 270, 1900–1915. [Google Scholar] [CrossRef]
- Chauhan, J.; Prathibha, M.D.; Singh, P.; Choyal, P.; Mishra, U.N.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B.; et al. Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress. 2023, 10, 100296. [Google Scholar] [CrossRef]
- Weigel, P.; Weretilnyk, E.A.; Hanson, A.D. Betaine aldehyde oxidation by spinach chloroplasts. Plant Physiol. 1986, 82, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yokota, S.; Muramoto, Y.; Tsutsui, K.; Oguri, Y.; Fukui, K.; Takabe, T. Expression of a betaine aldehyde dehydrogenase gene in rice, a glycinebetaine nonaccumulator, and possible localization of its protein in peroxisomes. Plant J. 1997, 11, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.M.; Moreau, R.A.; Yu, C.; Huang, A.H. Betaine accumulation and betaine-aldehyde dehydrogenase in spinach leaves. Plant Physiol. 1981, 67, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, K.; Katayama, M.; Takabe, T. Levels of Betaine and Betaine Aldehyde Dehydrogenase Activity in the Green Leaves, and Etiolated Leaves and Roots of Barley1. Plant Cell Physiol. 1990, 31, 797–803. [Google Scholar] [CrossRef]
- Li, P.; Cai, J.; Luo, X.; Chang, T.; Li, J.; Zhao, Y.; Xu, Y. Transformation of wheat Triticum aestivum with the HvBADH1 transgene from hulless barley improves salinity-stress tolerance. Acta Physiologiae Plantarum 2019, 41, 155. [Google Scholar] [CrossRef]
- Hasthanasombut, S.; Supaibulwatana, K.; Mii, M.; Nakamura, I. Genetic manipulation of Japonica rice using the OsBADH1 gene from Indica rice to improve salinity tolerance. Plant Cell Tissue Org. Culture 2011, 104, 79–89. [Google Scholar] [CrossRef]
- Breisch, J.; Bendel, M.; Averhoff, B. The choline dehydrogenase BetA of Acinetobacter baumannii: A flavoprotein responsible for osmotic stress protection. Environ. Microbiol. 2022, 24, 1052–1061. [Google Scholar] [CrossRef]
- Huang, H.; Song, D.; Zhang, W.; Fang, S.; Zhou, Q.; Zhang, H.; Liang, Z.; Li, Y. Choline Oxidase-Integrated Copper Metal-Organic Frameworks as Cascade Nanozymes for One-Step Colorimetric Choline Detection. J. Agric. Food Chem. 2022, 70, 5228–5236. [Google Scholar] [CrossRef]
- Shirasawa, K.; Takabe, T.; Takabe, T.; Kishitani, S. Accumulation of glycinebetaine in rice plants that overexpress choline monooxygenase from spinach and evaluation of their tolerance to abiotic stress. Ann. Bot. 2006, 98, 565–571. [Google Scholar] [CrossRef]
- Mitsuya, S.; Yokota, Y.; Fujiwara, T.; Mori, N.; Takabe, T. OsBADH1 is possibly involved in acetaldehyde oxidation in rice plant peroxisomes. FEBS Lett. 2009, 583, 3625–3629. [Google Scholar] [CrossRef]
- Niu, X.; Tang, W.; Huang, W.; Ren, G.; Wang, Q.; Luo, D.; Xiao, Y.; Yang, S.; Wang, F.; Lu, B.R.; et al. RNAi-directed downregulation of OsBADH2 results in aroma (2-acetyl-1-pyrroline) production in rice (Oryza sativa L.). BMC Plant Biol. 2008, 8, 100. [Google Scholar] [CrossRef]
- Missihoun, T.D.; Willee, E.; Guegan, J.P.; Berardocco, S.; Shafiq, M.R.; Bouchereau, A.; Bartels, D. Overexpression of ALDH10A8 and ALDH10A9 Genes Provides Insight into Their Role in Glycine Betaine Synthesis and Affects Primary Metabolism in Arabidopsis thaliana. Plant Cell Physiol. 2015, 56, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hirji, R.; Adam, L.; Rozwadowski, K.L.; Hammerlindl, J.K.; Keller, W.A.; Selvaraj, G. Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: Metabolic limitations. Plant Physiol. 2000, 122, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Hibino, T.; Waditee, R.; Araki, E.; Ishikawa, H.; Aoki, K.; Tanaka, Y.; Takabe, T. Functional characterization of choline monooxygenase, an enzyme for betaine synthesis in plants. J. Biol. Chem. 2002, 277, 41352–41360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, W.; Yang, X.-H.; Zhang, H.-X. Plastid-expressed choline monooxygenase gene improves salt and drought tolerance through accumulation of glycine betaine in tobacco. Plant Cell Rep. 2008, 27, 1113–1124. [Google Scholar] [CrossRef]
- Nuccio, M.L.; Russell, B.L.; Nolte, K.D.; Rathinasabapathi, B.; Gage, D.A.; Hanson, A.D. The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase. Plant J. 1998, 16, 487–496. [Google Scholar] [CrossRef]
- Hayashi, H.; Alia; Mustardy, L.; Deshnium, P.; Ida, M.; Murata, N. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J. 1997, 12, 133–142. [Google Scholar] [CrossRef]
- Yilmaz, J.L.; Bulow, L. Enhanced stress tolerance in Escherichia coli and Nicotiana tabacum expressing a betaine aldehyde dehydrogenase/choline dehydrogenase fusion protein. Biotechnol. Prog. 2002, 18, 1176–1182. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Deng, X.P.; Kwak, S.S.; Chen, W.; Eneji, A.E. Enhanced tolerance of transgenic potato plants expressing choline oxidase in chloroplasts against water stress. Bot. Stud. 2013, 54, 30. [Google Scholar] [CrossRef]
- Li, Q.; Yin, H.; Li, D.; Zhu, H.; Zhang, Y.; Zhu, W. Isolation and characterization of CMO gene promoter from halophyte Suaeda liaotungensis K. J. Genet. Genom. 2007, 34, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Todaka, D.; Mizoi, J.; Yoshida, T.; Kidokoro, S.; Matsukura, S.; Takasaki, H.; Sakurai, T.; Yamamoto, Y.Y.; Yoshiwara, K.; et al. Identification of cis-acting promoter elements in cold- and dehydration-induced transcriptional pathways in Arabidopsis, rice, and soybean. DNA Res. 2012, 19, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Peng, L.; Zhang, Y. Plant DNA methylation responds to nutrient stress. Genes. 2022, 13, 992. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Liu, B.; Xu, Z.Y. Dynamic regulation of DNA methylation and histone modifications in response to abiotic stresses in plants. J. Integr. Plant Biol. 2022, 64, 2252–2274. [Google Scholar] [CrossRef]
- Nunez-Vazquez, R.; Desvoyes, B.; Gutierrez, C. Histone variants and modifications during abiotic stress response. Front. Plant Sci. 2022, 13, 984702. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Z.; Liu, L.; Duan, L. DNA methylation in plant responses and adaption to abiotic stresses. Int. J. Mol. Sci. 2022, 23, 6910. [Google Scholar] [CrossRef]
- Zi, N.; Ren, W.; Guo, H.; Yuan, F.; Liu, Y.; Fry, E. DNA Methylation Participates in Drought Stress Memory and Response to Drought in Medicago ruthenica. Genes 2024, 15, 1286. [Google Scholar] [CrossRef]
- McNeil, S.D.; Nuccio, M.L.; Ziemak, M.J.; Hanson, A.D. Enhanced synthesis of choline and glycine betaine in transgenic tobacco plants that overexpress phosphoethanolamine N-methyltransferase. Proc. Natl. Acad. Sci. USA 2001, 98, 10001–10005. [Google Scholar] [CrossRef]
- Hanson, A.D.; Rhodes, D. C Tracer Evidence for Synthesis of Choline and Betaine via Phosphoryl Base Intermediates in Salinized Sugarbeet Leaves. Plant Physiol. 1983, 71, 692–700. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Murata, N. The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem II complex. Photosynth. Res. 1995, 44, 243–252. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.J.; Saneoka, H.; Rhodes, D.; Joly, R.J.; Goldsbrough, P.B. Betaine aldehyde dehydrogenase in sorghum. Plant Physiol. 1996, 110, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, H.; Li, D.; Zhu, W.; Li, Q. Functional analysis of BADH gene promoter from Suaeda liaotungensis K. Plant Cell Rep. 2008, 27, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Nyyssola, A.; Kerovuo, J.; Kaukinen, P.; von Weymarn, N.; Reinikainen, T. Extreme halophiles synthesize betaine from glycine by methylation. J. Biol. Chem. 2000, 275, 22196–22201. [Google Scholar] [CrossRef]
- Waditee, R.; Tanaka, Y.; Aoki, K.; Hibino, T.; Jikuya, H.; Takano, J.; Takabe, T.; Takabe, T. Isolation and functional characterization of N-methyltransferases that catalyze betaine synthesis from glycine in a halotolerant photosynthetic organism Aphanothece halophytica. J. Biol. Chem. 2003, 278, 4932–4942. [Google Scholar] [CrossRef]
- Nyyssola, A.; Reinikainen, T.; Leisola, M. Characterization of glycine sarcosine N-methyltransferase and sarcosine dimethylglycine N-methyltransferase. Appl. Environ. Microbiol. 2001, 67, 2044–2050. [Google Scholar] [CrossRef]
- Waditee, R.; Bhuiyan, M.N.; Rai, V.; Aoki, K.; Tanaka, Y.; Hibino, T.; Suzuki, S.; Takano, J.; Jagendorf, A.T.; Takabe, T.; et al. Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance in Synechococcus and Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 1318–1323. [Google Scholar] [CrossRef]
- Lai, S.J.; Lai, M.C.; Lee, R.J.; Chen, Y.H.; Yen, H.E. Transgenic Arabidopsis expressing osmolyte glycine betaine synthesizing enzymes from halophilic methanogen promote tolerance to drought and salt stress. Plant Mol. Biol. 2014, 85, 429–441. [Google Scholar] [CrossRef]
- Rajan, L.A.; Joseph, T.C.; Thampuran, N.; James, R. Functional characterization and sequence analysis of choline dehydrogenase from Escherichia coli. Genet. Eng. Biotechnol. J. 2010, 2010, GEBJ-12. [Google Scholar]
- Zhao, F.; Luo, J.; Ibrahim, E.; Chen, L.; Shen, Y.; Ibrahim, M.; Alonazi, W.B.; Lu, J.; Luo, Y.; Wu, H. Engineering stress resistance: Advances in glycine betaine production for sustainable agriculture. Crop Health 2025, 3, 5. [Google Scholar] [CrossRef]
- Kleczkowski, L.A.; Givan, C.V. Serine formation in leaves by mechanisms other than the glycolate pathway. J. Plant Physiol. 1988, 132, 641–652. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Kleczkowski, L.A. The glycerate and phosphorylated pathways of serine synthesis in plants: The branches of plant glycolysis linking carbon and nitrogen metabolism. Front. Plant Sci. 2018, 9, 318, Erratum in Front. Plant Sci. 2018, 9, 984. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Lea, P.J.; Quick, W.P.; Leegood, R.C. Photorespiration: Metabolic pathways and their role in stress protection. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1517–1529. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Mastrolonardo, G.; Nacca, F.; Parisi, D.; Verlotta, A.; Fuggi, A. Nitrogen metabolism in durum wheat under salinity: Accumulation of proline and glycine betaine. Funct. Plant Biol. 2008, 35, 412–426. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and Temporal Profile of Glycine Betaine Accumulation in Plants Under Abiotic Stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef]
- Michel, V.; Yuan, Z.; Ramsubir, S.; Bakovic, M. Choline transport for phospholipid synthesis. Exp. Biol. Med. 2006, 231, 490–504. [Google Scholar] [CrossRef]
- Dettmer, J.; Ursache, R.; Campilho, A.; Miyashima, S.; Belevich, I.; O’Regan, S.; Mullendore, D.L.; Yadav, S.R.; Lanz, C.; Beverina, L.; et al. CHOLINE TRANSPORTER-LIKE1 is required for sieve plate development to mediate long-distance cell-to-cell communication. Nat. Commun. 2014, 5, 4276. [Google Scholar] [CrossRef]
- McNeil, S.D.; Rhodes, D.; Russell, B.L.; Nuccio, M.L.; Shachar-Hill, Y.; Hanson, A.D. Metabolic modeling identifies key constraints on an engineered glycine betaine synthesis pathway in tobacco. Plant Physiol. 2000, 124, 153–162. [Google Scholar] [CrossRef]
- Khan, M.S.; Yu, X.; Kikuchi, A.; Asahina, M.; Watanabe, K.N. Genetic engineering of glycine betaine biosynthesis to enhance abiotic stress tolerance in plants. Plant Biotechnol. 2009, 26, 125–134. [Google Scholar] [CrossRef]
- Yamada, N.; Sakakibara, S.; Tsutsumi, K.; Waditee, R.; Tanaka, Y.; Takabe, T. Expression and substrate specificity of betaine/proline transporters suggest a novel choline transport mechanism in sugar beet. J. Plant Physiol. 2011, 168, 1609–1616. [Google Scholar] [CrossRef]
- Yang, T.; Nian, Y.; Lin, H.; Li, J.; Lin, X.; Li, T.; Wang, R.; Wang, L.; Beattie, G.A.; Zhang, J.; et al. Structure and mechanism of the osmoregulated choline transporter BetT. Sci. Adv. 2024, 10, eado6229. [Google Scholar] [CrossRef]
- Kumar, V.; Shriram, V.; Hoque, T.S.; Hasan, M.M.; Burritt, D.J.; Hossain, M.A. Glycinebetaine-mediated abiotic oxidative-stress tolerance in plants: Physiological and biochemical mechanisms. Stress. Signal. Plants Genom. Proteom. Perspect. 2017, 2, 111–133. [Google Scholar] [CrossRef]
- Park, E.-J.; Jeknic, Z.; Chen, T.H. Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Sulpice, R.; Tsukaya, H.; Nonaka, H.; Mustardy, L.; Chen, T.H.; Murata, N. Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycine betaine. Plant J. 2003, 36, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Jeknic, Z.; PINO, M.T.; Murata, N.; CHEN, T.H.H. Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ. 2007, 30, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparros, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, M.; Jiang, X.; Sun, H.; Dang, X.; Cong, H.; Qiao, F. Glycinebetaine Biosynthesis in Response to Osmotic Stress Depends on Jasmonate Signaling in Watermelon Suspension Cells. Front. Plant Sci. 2018, 9, 1469. [Google Scholar] [CrossRef]
- Wani, S.H.; Singh, N.B.; Haribhushan, A.; Mir, J.I. Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Singh, H.P.; Cha-Um, S. Foliar application of glycinebetaine regulates soluble sugars and modulates physiological adaptations in sweet potato (Ipomoea batatas) under water deficit. Protoplasma 2020, 257, 197–211. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol. J. 2004, 2, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Fang, P.; Zeng, W.; Ding, Y.; Zhuang, Z.; Peng, Y. Comparing transcriptome expression profiles to reveal the mechanisms of salt tolerance and exogenous glycine betaine mitigation in maize seedlings. PLoS ONE 2020, 15, e0233616. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef]
- Demiral, T.; Türkan, I. Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? J. Plant Physiol. 2004, 161, 1089–1100. [Google Scholar] [CrossRef]
- Li, N.; Gao, Y.; Pu, K.; Zhang, M.; Wang, T.; Li, J.; Xie, J. Glycine betaine enhances tolerance of low temperature combined with low light in pepper (Capsicum annuum L.) by improving the antioxidant capacity and regulating GB metabolism. Plant Physiol. Biochem. 2025, 222, 109705. [Google Scholar] [CrossRef]
- Renne, M.F.; Ernst, R. Membrane homeostasis beyond fluidity: Control of membrane compressibility. Trends Biochem. Sci. 2023, 48, 963–977. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Zhao, X.X.; Ma, Q.Q.; Liang, C.; Fang, Y.; Wang, Y.Q.; Wang, W. Effect of glycinebetaine on function of thylakoid membranes in wheat flag leaves under drought stress. Biol. Plantarum 2007, 51, 584–588. [Google Scholar] [CrossRef]
- Huang, W.; Han, S.; Wang, L.; Li, W. Carbon and nitrogen metabolic regulation in freshwater plant Ottelia alismoides in response to carbon limitation: A metabolite perspective. Front. Plant Sci. 2022, 13, 962622. [Google Scholar] [CrossRef] [PubMed]
- Mateo, A.; Funck, D.; Mühlenbock, P.; Kular, B.; Mullineaux, P.M.; Karpinski, S. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. Exp. Bot. 2006, 57, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Bhuiyan, N.H.; Selvaraj, G.; Wei, Y.; King, J. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. J. Exp. Bot. 2009, 60, 509–521. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Sha, S.; Cai, G.; Liu, S.; Ahmed, M.A. Roots to the rescue: How plants harness hydraulic redistribution to survive drought across contrasting soil textures. Adv. Biotechnol. 2024, 2, 43. [Google Scholar] [CrossRef]
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant 2016, 158, 225–235. [Google Scholar] [CrossRef]
- Karami, S.; Shiran, B.; Ravash, R. Molecular investigation of how drought stress affects chlorophyll metabolism and photosynthesis in leaves of C3 and C4 plant species: A transcriptome meta-analysis. Heliyon 2025, 11, e42368. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Li, S.; Chen, J.; Amin, A.S.; Wang, G.; Xiaojun, S.; Zain, M.; Gao, Y. Linking exogenous foliar application of glycine betaine and stomatal characteristics with salinity stress tolerance in cotton (Gossypium hirsutum L.) seedlings. BMC Plant Biol. 2021, 21, 146. [Google Scholar] [CrossRef]
- Gupta, P.; Rai, R.; Vasudev, S.; Yadava, D.K.; Dash, P.K. Ex-foliar application of glycine betaine and its impact on protein, carbohydrates and induction of ROS scavenging system during drought stress in flax (Linum usitatissimum). J. Biotechnol. 2021, 337, 80–89. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Zhang, T.; Chen, X.; Li, C.; Liu, Y.; Brestic, M.; Chen, T.H.H.; Yang, X. Glycinebetaine mitigated the photoinhibition of photosystem II at high temperature in transgenic tomato plants. Photosynth. Res. 2021, 147, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zeng, W.; Chen, F.; Ji, X.; Zhuang, Z.; Jin, B.; Wang, J.; Jia, L.; Peng, Y. Transcriptome expression profiles reveal response mechanisms to drought and drought-stress mitigation mechanisms by exogenous glycine betaine in maize. Biotechnol. Lett. 2022, 44, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Mohammad, F.; Shakeel, A.; Corpas, F.J. Glycine betaine: A multifaceted protectant against salt stress in Indian mustard through ionic homeostasis, ROS scavenging and osmotic regulation. Physiol. Plant 2024, 176, e14530. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Sofy, M.R.; Elhawat, N.; Tarek, A. Glycine betaine counters salinity stress by maintaining high K+/Na+ ratio and antioxidant defense via limiting Na+ uptake in common bean (Phaseolus vulgaris L.). Ecotoxicol. Environ. Saf. 2020, 200, 110732. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Q.; Zhang, Y.; Zhang, M.; Li, Z. Glycine betaine increases salt tolerance in maize (Zea mays L.) by regulating Na+ homeostasis. Front. Plant Sci. 2022, 13, 978304. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J. Plant Physiol. 2008, 165, 813–824. [Google Scholar] [CrossRef]
- Ghorbani, A.; Ghasemi-Omran, V.O.; Chen, M. The Effect of Glycine Betaine on Nitrogen and Polyamine Metabolisms, Expression of Glycoside-Related Biosynthetic Enzymes, and K/Na Balance of Stevia under Salt Stress. Plants 2023, 12, 1628. [Google Scholar] [CrossRef]
- Ganjavi, A.S.; Oraei, M.; Gohari, G.; Akbari, A.; Faramarzi, A. Glycine betaine functionalized graphene oxide as a new engineering nanoparticle lessens salt stress impacts in sweet basil (Ocimum basilicum L.). Plant Physiol. Biochem. 2021, 162, 14–26. [Google Scholar] [CrossRef]
- Hanif, S.; Zia, M. Glycine betaine capped ZnO NPs eliminate oxidative stress to coriander plants grown under NaCl presence. Plant Physiol. Biochem. 2023, 197, 107651. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Ban, S.; Han, L.; Li, L.; Zhang, Y.; Zhang, Y.; Zhu, W. Effects of exogenous glycine betaine on growth and development of tomato seedlings under cold stress. Front. Plant Sci. 2024, 15, 1332583. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, T.; Liu, Y.; Wang, J.; Wang, Q.; Zhu, W. Effect of Exogenous Glycine Betaine on the Germination of Tomato Seeds under Cold Stress. Int. J. Mol. Sci. 2022, 23, 10474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, M.; Hu, J.; Zhang, X.; Wang, K.; Ashraf, M. Modulation Role of Abscisic Acid (ABA) on Growth, Water Relations and Glycinebetaine Metabolism in Two Maize (Zea mays L.) Cultivars under Drought Stress. Int. J. Mol. Sci. 2012, 13, 3189–3202. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Sun, Y.; Ge, W.; Wei, B.; Cheng, S.; Ji, S. Exogenous glycine betaine treatment alleviates low temperature-induced pericarp browning of ‘Nanguo’ pears by regulating antioxidant enzymes and proline metabolism. Food Chem. 2020, 306, 125626. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2020, 22, 117. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Quan, J.; Li, X.; Li, Z.; Wu, M.; Zhu, B.; Hong, S.B.; Shi, J.; Zhu, Z.; Xu, L.; Zang, Y. Transcriptomic Analysis of Heat Stress Response in Brassica rapa L. ssp. pekinensis with Improved Thermotolerance through Exogenous Glycine Betaine. Int. J. Mol. Sci. 2023, 24, 6429. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Li, D.; Li, C.; Wei, D.; Li, S.; Liu, Y.; Chen, T.H.H.; Yang, X. Comparative effects of glycinebetaine on the thermotolerance in codA- and BADH-transgenic tomato plants under high temperature stress. Plant Cell Rep. 2020, 39, 1525–1538. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; YavaŞ, İ.; Alhammad, B.A.; Shami, A.; Hasanuzzaman, M.; Kalderis, D. Glycine Betaine Accumulation, Significance and Interests for Heavy Metal Tolerance in Plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using Exogenous Melatonin, Glutathione, Proline, and Glycine Betaine Treatments to Combat Abiotic Stresses in Crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef] [PubMed]
- Sofy, A.R.; Dawoud, R.A.; Sofy, M.R.; Mohamed, H.I.; Hmed, A.A.; El-Dougdoug, N.K. Improving Regulation of Enzymatic and Non-Enzymatic Antioxidants and Stress-Related Gene Stimulation in Cucumber mosaic cucumovirus-Infected Cucumber Plants Treated with Glycine Betaine, Chitosan and Combination. Molecules 2020, 25, 2341. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Jamwal, V.L.; Sharma, A.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Marraiki, N.; Ahmad, P. Evaluation of the role of rhizobacteria in controlling root-knot nematode infection in Lycopersicon esculentum plants by modulation in the secondary metabolite profiles. AoB Plants 2019, 11, plz069. [Google Scholar] [CrossRef]
- Khanna, K.; Sharma, A.; Ohri, P.; Bhardwaj, R.; Abd_Allah, E.F.; Hashem, A.; Ahmad, P. Impact of Plant Growth Promoting Rhizobacteria in the Orchestration of Lycopersicon esculentum Mill. Resistance to Plant Parasitic Nematodes: A Metabolomic Approach to Evaluate Defense Responses Under Field Conditions. Biomolecules 2019, 9, 676. [Google Scholar] [CrossRef]
- Niu, X.; Hu, Y.; Wang, X.; Li, R.; Li, W.; Shao, Y. Glycine betaine and MiWRKY53 enhance antioxidant capacity and disease resistance against Colletotrichum gloeosporioides in mango fruit. Postharvest Biol. Technol. 2025, 224, 113464. [Google Scholar] [CrossRef]
- Shan, T.; Jin, P.; Zhang, Y.; Huang, Y.; Wang, X.; Zheng, Y. Exogenous glycine betaine treatment enhances chilling tolerance of peach fruit during cold storage. Postharvest Biol. Technol. 2016, 114, 104–110. [Google Scholar] [CrossRef]
- Dong, X.; Ma, X.; Zhao, Z.; Ma, M. Exogenous betaine enhances salt tolerance of Glycyrrhiza uralensis through multiple pathways. BMC Plant Biol. 2024, 24, 165. [Google Scholar] [CrossRef]
- Min, K.; Cho, Y.; Kim, E.; Lee, M.; Lee, S.-R. Exogenous Glycine Betaine Application Improves Freezing Tolerance of Cabbage (Brassica oleracea L.) Leaves. Plants 2021, 10, 2821. [Google Scholar] [CrossRef]
- Hussain, N.; Yasmeen, A.; Yousaf, M. Antioxidant status and their enhancements strategies for water stress tolerance in chickpea. Braz. J. Biol. 2021, 82, e237809. [Google Scholar] [CrossRef]
- Wang, G.-Y.; Ahmad, S.; Wang, B.-W.; Shi, L.-B.; Wang, Y.; Shi, C.-Q.; Zhou, X.-B. Exogenous Glycinebetaine Regulates the Contrasting Responses in Leaf Physiochemical Attributes and Growth of Maize under Drought and Flooding Stresses. Biology 2024, 13, 360. [Google Scholar] [CrossRef]
- Chungloo, D.; Tisarum, R.; Samphumphuang, T.; Pipatsitee, P.; Sotesaritkul, T.; Cha-um, S. Exogenous glycine betaine alleviates water-deficit stress in Indian pennywort (Centella asiatica) under greenhouse conditions. Protoplasma 2024, 261, 625–639. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Wang, Y.-N.; Shi, G.-L.; Liu, R.; Yang, A.; Ge, S.; Meng, H. Effects of exogenous betaine on physiological responses of peach tree under water stress. Chin. J. Appl. Ecol. 2007, 18, 542–548. [Google Scholar]
- Shemi, R.; Wang, R.; Gheith, E.-S.M.; Hussain, H.A.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Role of exogenous-applied salicylic acid, zinc and glycine betaine to improve drought-tolerance in wheat during reproductive growth stages. BMC Plant Biol. 2021, 21, 574. [Google Scholar] [CrossRef] [PubMed]
- Manaf, H.H. Beneficial effects of exogenous selenium, glycine betaine and seaweed extract on salt stressed cowpea plant. Ann. Agric. Sci. 2016, 61, 41–48. [Google Scholar] [CrossRef]
- Hu, L.; Hu, T.; Zhang, X.; Pang, H.; Fu, J. Exogenous glycine betaine ameliorates the adverse effect of salt stress on perennial ryegrass. J. Am. Soc. Hortic. Sci. 2012, 137, 38–46. [Google Scholar] [CrossRef]
- Dong, X.; Liu, Y.; Ma, X.; Wang, S.; Yang, H.; Gao, X.; Wang, G.; Wang, H. Disclosing the effect of exogenous betaine on growth of Suaeda salsa (L.) Pall in the Liaohe coastal wetland, North China. Mar. Pollut. Bull. 2024, 198, 115852. [Google Scholar] [CrossRef]
- Zhao, Y.; Aspinall, D.; Paleg, L. Protection of membrane integrity in Medicago sativa L. by glycinebetaine against the effects of freezing. J. Plant Physiol. 1992, 140, 541–543. [Google Scholar] [CrossRef]
- Tarabih, M.; El-Eryan, E. Glycine Betaine and Proline with Thinning Technique for Resistance Abiotic Stress of Cristalina Cactus Pear. Pak. J. Biol. Sci. 2020, 23, 68–80. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, T.; Liu, M.; Xu, X.; Wang, J.; Sun, G.; He, S.; Liao, L.; Xiong, B.; Wang, X.; et al. Effects of Exogenous Application of Glycine Betaine Treatment on ‘Huangguoggan’ Fruit during Postharvest Storage. Int. J. Mol. Sci. 2023, 24, 14316. [Google Scholar] [CrossRef]
- Chen, W.; Li, P.; Chen, T. Glycinebetaine increases chilling tolerance and reduces chilling-induced lipid peroxidation in Zea mays L. Plant Cell Environ. Plant Cell Environ. 2000, 23, 609–618. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Strasser, R. Exogenous glycine betaine and proline play a protective role in heat-stressed barley leaves (Hordeum vulgare L.): A chlorophyll a fluorescence study. Plant Biosyst. 2012, 146, 1037–1043. [Google Scholar] [CrossRef]
- Sorwong, A.; Sakhonwasee, S. Foliar application of glycine betaine mitigates the effect of heat stress in three marigold (Tagetes erecta) cultivars. Horticult. J. 2015, 84, 161–171. [Google Scholar] [CrossRef]
- Wang, G.-P.; Wang, J.-Z.; Xue, X.-M.; Lu, C.; Chen, R.; Wang, L.-P.; Han, X.-P. Foliar spraying of glycine betaine lowers photosynthesis inhibition of Malus hupehensis leaves under drought and heat stress. Int. J. Agric. Biol. 2020, 23, 1121–1128. [Google Scholar]
- Mo, X.; Qian, J.; Liu, P.; Zeng, H.; Chen, G.; Wang, Y. Exogenous Betaine Enhances the Protrusion Vigor of Rice Seeds under Heat Stress by Regulating Plant Hormone Signal Transduction and Its Interaction Network. Antioxidants 2022, 11, 1792. [Google Scholar] [CrossRef]
- Rasheed, R.; Wahid, A.; Farooq, M.; Hussain, I.; Basra, S.M.A. Role of proline and glycinebetaine pretreatments in improving heat tolerance of sprouting sugarcane (Saccharum sp.) buds. Plant Growth Regul. 2011, 65, 35–45. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Wang, J.; Zhang, W.; Meng, Q.; Chen, T.H.; Murata, N.; Yang, X. Glycinebetaine enhances the tolerance of tomato plants to high temperature during germination of seeds and growth of seedlings. Plant Cell Environ. 2011, 34, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Zhang, H.; Zhao, Y.; Zhao, H.; Liu, H. Glycine betaine application in grain filling wheat plants alleviates heat and high light-induced photoinhibition by enhancing the psbA transcription and stomatal conductance. Acta Physiol. Plant 2014, 36, 2195–2202. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Abid, M.; Rizwan, M.; Ali, B.; Tanveer, A.; Ahmad, I.; Azam, M.; Ghani, M.A. Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ. Sci. Pollut. Res. Int. 2020, 27, 1101–1111. [Google Scholar] [CrossRef]
- Jabeen, N.; Abbas, Z.; Iqbal, M.; Rizwan, M.; Jabbar, A.; Farid, M.; Ali, S.; Ibrahim, M.; Abbas, F. Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch. Agron. Soil Sci. 2016, 62, 648–662. [Google Scholar] [CrossRef]
- Kumar, P.; Tokas, J.; Singal, H. Amelioration of chromium VI toxicity in Sorghum (Sorghum bicolor L.) using glycine betaine. Sci. Rep. 2019, 9, 16020. [Google Scholar] [CrossRef]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. Int. 2015, 22, 10669–10678. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Bharwana, S.A.; Rizwan, M.; Ishaque, W.; Farid, M.; Mahmood, K.; Iqbal, Z. Cadmium stress in cotton seedlings: Physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S. Afr. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- He, X.; Richmond, M.E.; Williams, D.V.; Zheng, W.; Wu, F. Exogenous glycinebetaine reduces cadmium uptake and mitigates cadmium toxicity in two tobacco genotypes differing in cadmium tolerance. Int. J. Mol. Sci. 2019, 20, 1612. [Google Scholar] [CrossRef]
- Stepien, P. Effects of the exogenous glycinebetaine on photosynthetic apparatus in cucumber leaves challenging Al stress. In Proceedings of the 18th International Conference on Heavy Metals in the Environment, Ghent, Belgium, 12–15 September 2016. [Google Scholar] [CrossRef]
- Ji, Y.; Ren, Y.; Han, C.; Zhu, W.; Gu, J.; He, J. Application of exogenous glycinebetaine alleviates lead toxicity in pakchoi (Brassica chinensis L.) by promoting antioxidant enzymes and suppressing Pb accumulation. Environ. Sci. Pollut. R. 2022, 29, 25568–25580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, K.; Liu, T.; Zhang, Y.; Tang, Z.; Dong, J.; Wang, F. The characterization and expression analysis under stress conditions of PCST1 in Arabidopsis. Plant Signal Behav. 2022, 17, 2134675. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, Y.; Wang, L.; Zheng, Y.; Jin, P. Amino acid metabolomic analysis involved in flavor quality and cold tolerance in peach fruit treated with exogenous glycine betaine. Food Res. Int. 2022, 157, 111204. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Niu, L.; Cai, Q.; Wei, J.; Shang, L.; Yang, X.; Ma, R. Improved salt-tolerance of transgenic soybean by stable over-expression of AhBADH gene from Atriplex hortensis. Plant Cell Rep. 2023, 42, 1291–1310. [Google Scholar] [CrossRef]
- Shahbazi, M.; Tohidfar, M.; Aliniaeifard, S.; Yazdanpanah, F.; Bosacchi, M. Transgenic tobacco co-expressing flavodoxin and betaine aldehyde dehydrogenase confers cadmium tolerance through boosting antioxidant capacity. Protoplasma 2022, 259, 965–979. [Google Scholar] [CrossRef]
- Imran, M.; Shafiq, S.; Tang, X. CRISPR-Cas9-mediated editing of BADH2 gene triggered fragrance revolution in rice. Physiol. Plant 2023, 175, e13871. [Google Scholar] [CrossRef]
- Phitaktansakul, R.; Kim, K.W.; Aung, K.M.; Maung, T.Z.; Min, M.H.; Somsri, A.; Lee, W.; Lee, S.B.; Nam, J.; Kim, S.H.; et al. Multi-omics analysis reveals the genetic basis of rice fragrance mediated by betaine aldehyde dehydrogenase 2. J. Adv. Res. 2022, 42, 303–314. [Google Scholar] [CrossRef]
- Hui, S.; Li, H.; Mawia, A.M.; Zhou, L.; Cai, J.; Ahmad, S.; Lai, C.; Wang, J.; Jiao, G.; Xie, L.; et al. Production of aromatic three-line hybrid rice using novel alleles of BADH2. Plant Biotechnol J. 2022, 20, 59–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fitzgerald, T.L.; Waters, D.L.E.; Henry, R.J. The effect of salt on betaine aldehyde dehydrogenase transcript levels and 2-acetyl-1-pyrroline concentration in fragrant and non-fragrant rice (Oryza sativa). Plant Sci. 2008, 175, 539–546. [Google Scholar] [CrossRef]
- Prodhan, Z.H.; Islam, S.A.; Alam, M.S.; Li, S.; Jiang, M.; Tan, Y.; Shu, Q. Impact of OsBadh2 Mutations on Salt Stress Response in Rice. Plants 2022, 11, 2829. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-P.; Xie, R.-H.; Yang, F.; Zhou, W.; Jiang, X.-Q.; Hu, H.-F.; Yang, C.; Xie, Y.-Y.; Xiao, S. When agriculture meets biotechnology: A route for future agricultural innovation. Adv. Biotechnol. 2024, 2, 40. [Google Scholar] [CrossRef] [PubMed]

| Stress Type | Species | Exogenous GB Concentration | Resistance Effect | Reference |
|---|---|---|---|---|
| Drought stress | Chickpea (Cicer arietinum L.) | 0.17 mM | Increased antioxidant enzyme activity. | [119] |
| Drought stress | Flax (Linum usitatissimum) | 50–100 mM | Enhancement on induction of protein, carbohydrate, and ROS scavenging systems. | [91] |
| Drought stress | Maize (Zea mays L.) | 0.5–10 mM | Improves water retention and reduces osmotic potential, mitigating drought-induced water loss. | [120] |
| Drought stress | Indian pennywort (Centella asiatica) | 25–50 mM | Overall physiological, morphological, and secondary metabolite traits were enhanced. | [121] |
| Drought stress | Peach (potted Prunus persica L.) | 0.85–4 mM | Decreased leaf plasma membrane permeability, H2O2, Pro, and soluble sugar contents, increased leaf ASA-POD activity and soluble protein content. | [122] |
| Drought stress | Sweet potato (Ipomoea batatas) | 50–100 mM | Increased sugar content and photosynthetic ability, controlled cellular osmotic potential, and maintained storage root yield. | [70] |
| Drought stress | Tobacco (Nicotiana tabacum) | 20 mM | Improved plant growth, osmotic adjustment, photosynthesis, and antioxidant enzyme activities. | [56] |
| Drought stress | Wheat (Triticum aestivum) | 98.2 mM | Decreased contents of H2O2, O2− and MDA, increased antioxidant enzymes, proline, and soluble sugar contents. | [123] |
| Salt stress | Cowpeas (Vigna unguiculata) | 5–10 mM | Increased soluble sugar contents and antioxidant enzymes. | [124] |
| Salt stress | Chinese licorice (Glycyrrhiza uralensis Fisch.) | 10–40 mM | Enhances antioxidant defense, osmoregulation, and salt excretion capacity. | [117] |
| Salt stress | Maize (Zea mays L.) | 0.1 mM | Improved photosynthesis and antioxidant activity. | [97] |
| Salt stress | Perennial ryegrass (Lolium perenne) | 20–50 mM | Increased fresh weight and relative water content; reduced electrolyte leakage and malondialdehyde content. | [125] |
| Salt stress | Suaeda salsa (L.) | 10–50 mM | Increased soluble sugars and elevated activity of Na+, K+-ATPase (enhancing osmotic stability). | [126] |
| Cold stress | Alfalfa (Medicago sativa) | 200 mM | Decreased ion leakage from shoot tissues. | [127] |
| Cold stress | Cabbage (Brassica oleracea L.) | 30 mM | Freezing tolerance is enhanced by reducing membrane leakage, MDA, and ROS without affecting leaf growth. | [118] |
| Cold stress | Cristalina Cactus Pear (OPUNTIA ficus-indica (L.) Mill.) | 5 mM | Improved fruit quality through enhanced size, composition, and nutritional content. | [128] |
| Cold stress | ‘Huangguogan’(Citrus reticulata Blanco) | 10–20 mM | Antioxidant system activation reduces ROS and lipid peroxidation. | [129] |
| Cold stress | Maize (Zea mays L.) | 2.5 mM | Prevented chlorosis; reduced lipid peroxidation of membrane. | [130] |
| Cold stress | Peach (Prunus persica Batsch.) | 10 mM | Activating arginine/GABA metabolism and inhibiting polyamine degradation. | [116] |
| Cold stress | Pears (Pyrus communis L.) | 10 mM | Decreased membrane lipid peroxidation, maintained membrane integrity, increased activities and expression of APX, CAT, SOD, and Pro content. | [105] |
| Cold stress | Tomato (Lycopersicon esculentum) | 10 mM | Minimizes cold-induced seed damage by modulating oxidants, metabolites, and hormones to promote germination. | [103] |
| Heat stress | Barley (Hordeum vulgare) | 10 mM | Enhances PSII stability by increasing antenna connectivity, protecting the oxygen-evolving complex. | [131] |
| Heat stress | Mrigold (Tagetes erecta) | 0.5–1 mM | Improves heat tolerance by protecting the photosynthetic apparatus, increasing stomatal conductance, and enhancing ROS scavenging. | [132] |
| Heat stress | Pingyi Tiancha (Malus hupehensis (Pamp.) Rehder) | 10 mM | Improved water status and enhanced antioxidant enzyme activity may underlie GB-induced enhancement of photosynthesis under stress. | [133] |
| Heat stress | Rice (Oryza sativa L.) | 10 mM | Boosting antioxidant enzymes, lowering MDA and ROS, and improving osmoregulation to ease heat stress. | [134] |
| Heat stress | Sugarcane (Saccharum sp.) | 20 mM | Increasing K+ and Ca2+ levels, supporting tissue differentiation and dry weight accumulation. | [135] |
| Heat stress | Tomato (Lycopersicon esculentum) | 0.1–5 mM | Enhanced expression of heat-shock genes and accumulation of HSPs. | [136] |
| Heat stress | Wheat (Triticum aestivum) | 100 mM | Maintenance of higher chlorophyll content, PSII photochemical activity, and net photosynthetic rate. | [137] |
| Heavy metal stress (Cr) | Cauliflower (Brassica oleracea L.) | 1 mM | Increased dry biomass and improved antioxidative enzyme activities. | [138] |
| Heavy metal stress (Cr) | Mung bean (Vigna radiata) | 50–100 mM | Improved plant growth; alleviated chromium stress. | [139] |
| Heavy metal stress (Cr) | Sorghum (Sorghum bicolor L.) | 50–100 mM | Increases antioxidant enzyme activity due to a decrease in chromium uptake or reduction in EL. | [128,140] |
| Heavy metal stress (Cr) | Wheat (Triticum aestivum) | 100 mM | Improved growth, chlorophyll contents, and biomass and protein levels under chromium stress. | [141] |
| Heavy metal stress (Cd) | Cotton (Gossypium hirsutum Linn.) | 1 mM | Cd toxicity is mitigated by enhanced antioxidant enzyme activity. | [142] |
| Heavy metal stress (Cd) | Tobacco (Nicotiana tabacum) | 0.5 mM | Reduced MDA content, induced stomatal closure, improved leaf/root ultrastructure, increased the chl content, Fv/Fm, SOD, POD, CAT, and APX activities. | [143] |
| Heavy metal stress (Al) | Cucumber (Cucumis sativus L.) | 100 mM | Accumulating in chloroplasts, GB mitigates Al stress by protecting the photosynthetic apparatus, enhancing electron transport, gas exchange, and CO2 fixation. | [144] |
| Heavy metal stress (Pb) | Pakchoi (Brassica campestris L.) | 0.5–2 mM | Increased dry biomass, mineral nutrient, and pigment contents, antioxidative enzyme activities, and reduced Pb contents. | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, J.; Liu, Y.; Zhang, K.; Zhu, F.; Xie, Y. Advances in the Biosynthetic Regulation and Functional Mechanisms of Glycine Betaine for Enhancing Plant Stress Resilience. Int. J. Mol. Sci. 2025, 26, 7971. https://doi.org/10.3390/ijms26167971
Chen J, Zhang J, Liu Y, Zhang K, Zhu F, Xie Y. Advances in the Biosynthetic Regulation and Functional Mechanisms of Glycine Betaine for Enhancing Plant Stress Resilience. International Journal of Molecular Sciences. 2025; 26(16):7971. https://doi.org/10.3390/ijms26167971
Chicago/Turabian StyleChen, Jiaxu, Jing Zhang, Yihang Liu, Kailu Zhang, Fuyuan Zhu, and Yanjie Xie. 2025. "Advances in the Biosynthetic Regulation and Functional Mechanisms of Glycine Betaine for Enhancing Plant Stress Resilience" International Journal of Molecular Sciences 26, no. 16: 7971. https://doi.org/10.3390/ijms26167971
APA StyleChen, J., Zhang, J., Liu, Y., Zhang, K., Zhu, F., & Xie, Y. (2025). Advances in the Biosynthetic Regulation and Functional Mechanisms of Glycine Betaine for Enhancing Plant Stress Resilience. International Journal of Molecular Sciences, 26(16), 7971. https://doi.org/10.3390/ijms26167971







