Phylogenetic Determinants Behind the Ecological Traits of Relic Tree Family Juglandaceae, Their Root-Associated Symbionts, and Response to Climate Change
Abstract
1. Introduction
- Juglandaceae represent a specific pattern of dual mycorrhizal symbiotic interactions.
- Traits of root symbiosis of Juglandaceae reflect phylogenetic-related determinants.
- Dual mycorrhizal associations are undetected, not absent, among Juglandaceae trees.
- Dual symbiosis contributes to Juglandaceae resistance to unfavorable environments.
2. Results
2.1. Dual Mycorrhizal Symbiosis Among Juglandaceae Genera
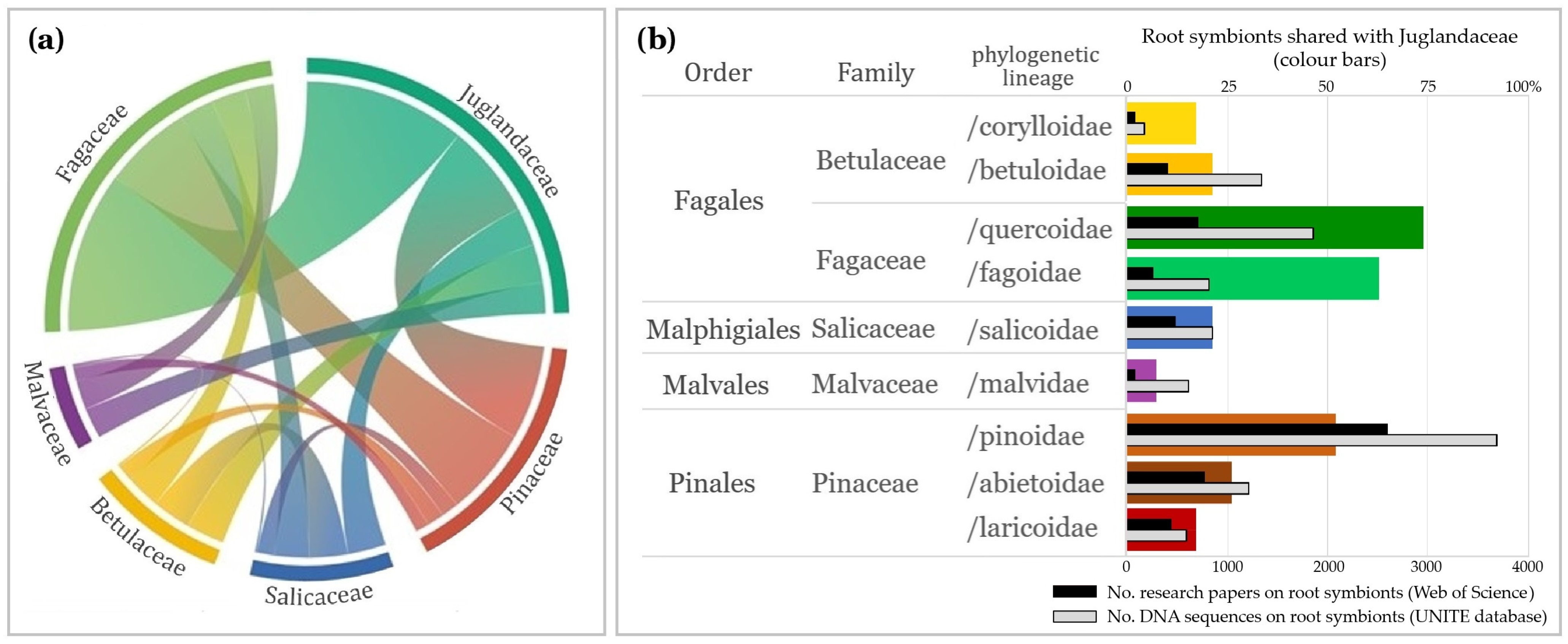
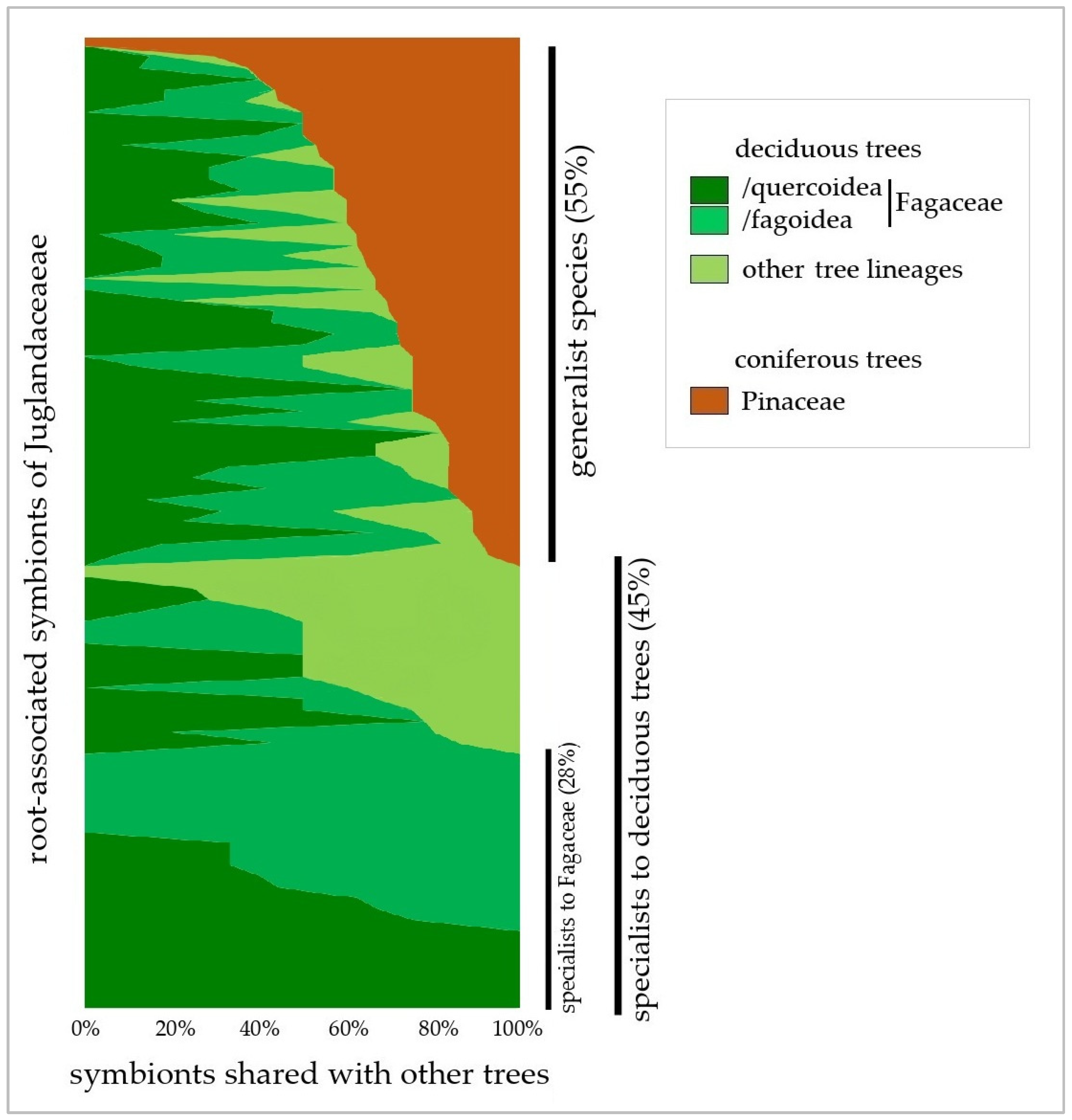
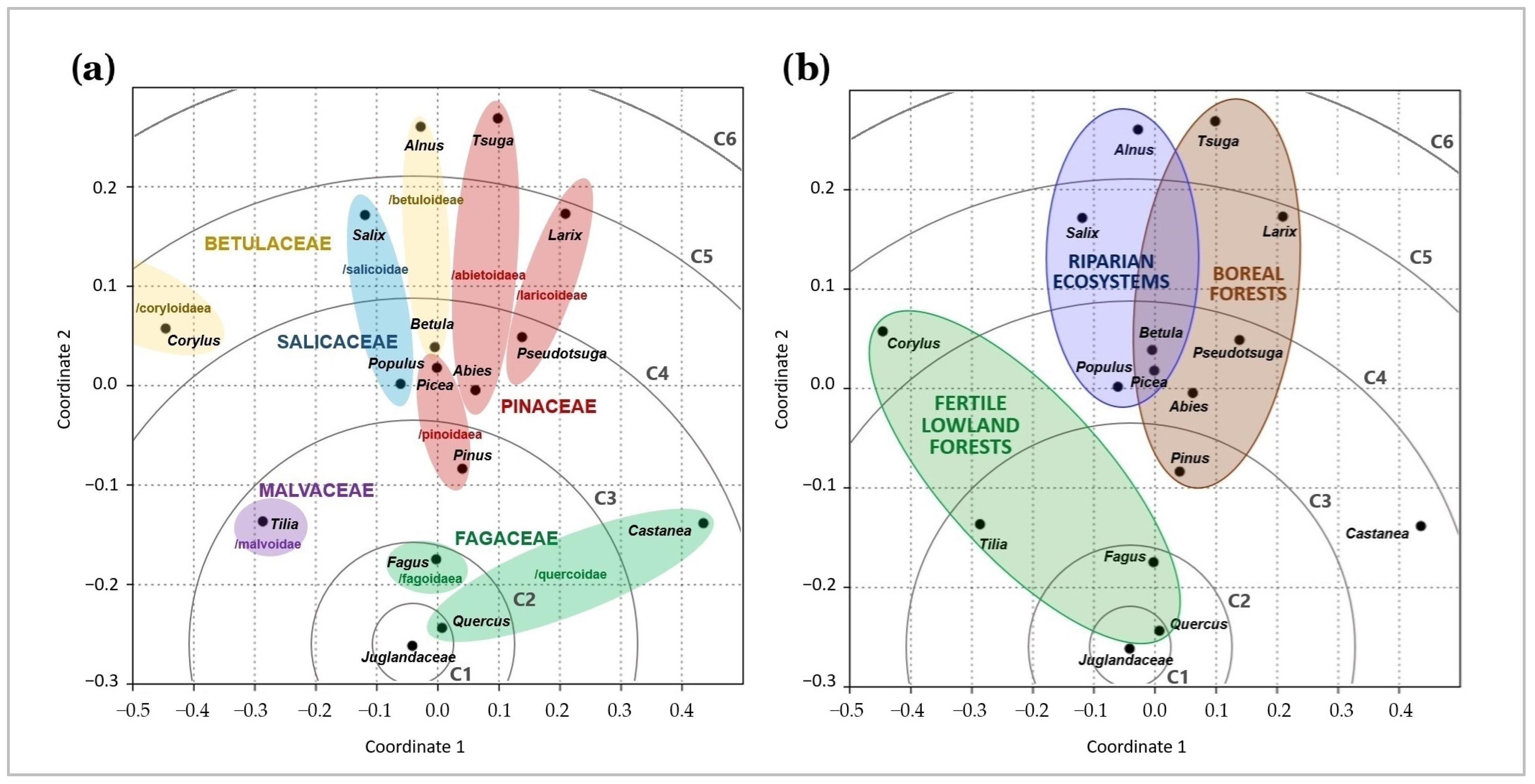
2.2. Diversity and Habitat Patterns Among Root Symbionts of Juglandaceae
3. Discussion
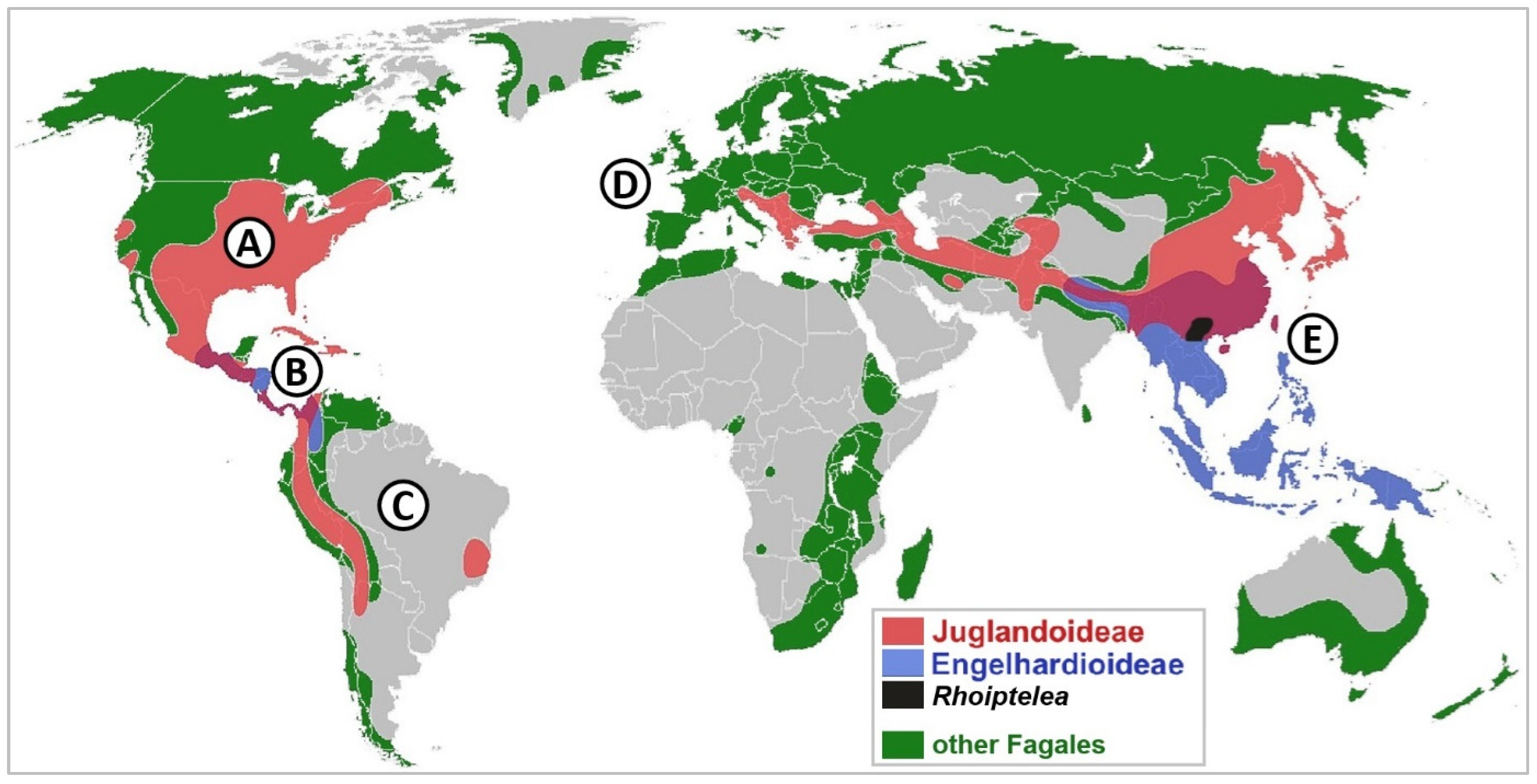
3.1. Global Distribution of Dual Mycorrhizal Trees and Mycorrhizal Association Types
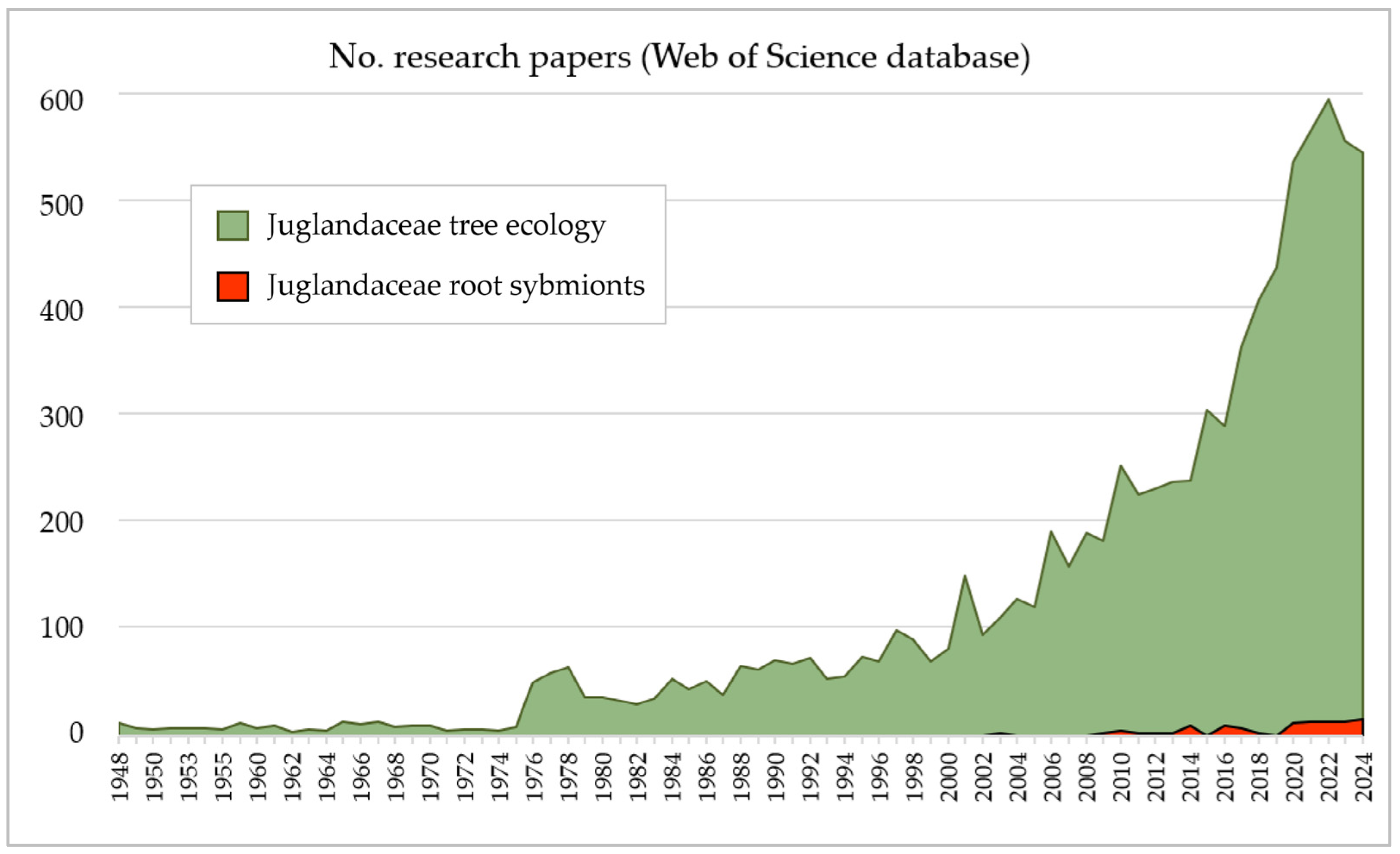
3.2. The History of Research on Dual Mycorrhizal Symbiosis Among Juglandaceae
3.3. Tree Phylogeny and Forest Habitat as Main Factors Shaping Root Symbiosis of Juglandaceae
3.4. Relevance of Dual Mycorrhiza for Juglandaceae Trees and Directions for Further Research
4. Materials and Methods
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, Y.G.; Fragnière, Y.; Meng, H.H.; Li, Y.; Bétrisey, S.; Corrales, A.; Manchester, S.; Deng, M.; Jasińska, A.K.; Văn Sâm, H.; et al. Global Biogeographic Synthesis and Priority Conservation Regions of the Relict Tree Family Juglandaceae. J. Biogeogr. 2020, 47, 643–657. [Google Scholar] [CrossRef]
- Song, Y.G.; Walas, Ł.; Pietras, M.; Sâm, H.V.; Yousefzadeh, H.; Ok, T.; Farzaliyev, V.; Worobiec, G.; Worobiec, E.; Stachowicz-Rybka, R.; et al. Past, Present, and Future Suitable Areas for the Relict Tree Pterocarya fraxinifolia (Juglandaceae): Integrating Fossil Records, Niche Modeling, and Phylogeography for Conservation. Eur. J. For. Res. 2021, 140, 1323–1339. [Google Scholar] [CrossRef]
- Paź-Dyderska, S.; Jagodziński, A.M.; Dyderski, M.K. Possible Changes in Spatial Distribution of Walnut (Juglans regia L.) in Europe under Warming Climate. Reg. Environ. Change 2021, 21, 18. [Google Scholar] [CrossRef]
- Zhang, J.B.; Li, R.Q.; Xiang, X.G.; Manchester, S.R.; Lin, L.; Wang, W.; Wen, J.; Chen, Z.D. Integrated Fossil and Molecular Data Reveal the Biogeographic Diversification of the Eastern Asian-Eastern North American Disjunct Hickory Genus (Carya Nutt.). PLoS ONE 2013, 8, e70449. [Google Scholar] [CrossRef] [PubMed]
- Delavaux, C.S.; LaManna, J.A.; Myers, J.A.; Phillips, R.P.; Aguilar, S.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.J.; Baker, M.E.; Baltzer, J.L.; et al. Mycorrhizal Feedbacks Influence Global Forest Structure and Diversity. Commun. Biol. 2023, 6, 1066. [Google Scholar] [CrossRef] [PubMed]
- Leski, T.; Rudawska, M.; Kujawska, M.; Stasińska, M.; Janowski, D.; Karliński, L.; Wilgan, R. Both Forest Reserves and Managed Forests Help Maintain Ectomycorrhizal Fungal Diversity. Biol. Conserv. 2019, 238, 108206. [Google Scholar] [CrossRef]
- Kujawska, M.; Rudawska, M.; Wilgan, R.; Banach, J.; Leski, T. Comparable Ectomycorrhizal Fungal Species Richness but Low Species Similarity among Native Abies alba and Alien Abies grandis from Provenance Trials in Poland. For. Ecol. Manag. 2023, 546, 121355. [Google Scholar] [CrossRef]
- Kujawska, M.B.; Rudawska, M.; Wilgan, R.; Leski, T. Similarities and Differences among Soil Fungal Assemblages in Managed Forests and Formerly Managed Forest Reserves. Forests 2021, 12, 353. [Google Scholar] [CrossRef]
- Tedersoo, L.; Smith, M.E. Lineages of Ectomycorrhizal Fungi Revisited: Foraging Strategies and Novel Lineages Revealed by Sequences from Belowground. Fungal Biol. Rev. 2013, 27, 83–99. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global Diversity and Geography of Soil Fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Buée, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. 454 Pyrosequencing Analyses of Forest Soils Reveal an Unexpectedly High Fungal Diversity. New Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Emerson, B.C.; Gillespie, R.G. Phylogenetic Analysis of Community Assembly and Structure over Space and Time. Trends Ecol. Evol. 2008, 23, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Martiny, J.B.H.; Bohannan, B.J.M.; Brown, J.H.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Horner-Devine, M.C.; Kane, M.; Krumins, J.A.; Kuske, C.R.; et al. Microbial Biogeography: Putting Microorganisms on the Map. Nat. Rev. Microbiol. 2006, 4, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and Community Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Aučina, A.; Rudawska, M.; Wilgan, R.; Janowski, D.; Skridaila, A.; Dapkūnienė, S.; Leski, T. Functional Diversity of Ectomycorrhizal Fungal Communities along a Peatland–Forest Gradient. Pedobiologia 2019, 74, 15–23. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Li, S.; Chen, T.; Chen, R.; Yin, L.; Yao, X.; Chen, G. Effects of C:N Imbalance on Soil Microbial Physiology in Subtropical Tree Plantations Associated with Ectomycorrhizal and Arbuscular Mycorrhizal Fungi. Geoderma 2022, 422, 115932. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Winston, G.C.; Goulden, M.L.; Treseder, K.K. Environmental Filtering Affects Soil Fungal Community Composition More than Dispersal Limitation at Regional Scales. Fungal Ecol. 2014, 12, 14–25. [Google Scholar] [CrossRef]
- Wilgan, R.; Leski, T.; Kujawska, M.; Karliński, L.; Janowski, D.; Rudawska, M. Ectomycorrhizal Fungi of Exotic Carya Ovata in the Context of Surrounding Native Forests on Central European Sites. Fungal Ecol. 2020, 44, 100908. [Google Scholar] [CrossRef]
- Wilgan, R.; Kujawska, M.B.; Leski, T. Impact of Invasive Alien Tree Species on Symbiotic Soil Fungal Communities in Pine-Dominated Forest Ecosystems in Central Europe. Geoderma 2024, 452, 117111. [Google Scholar] [CrossRef]
- Wilgan, R.; Leski, T. Ectomycorrhizal Assemblages of Invasive Quercus rubra L. and Non-Invasive Carya Nutt. Trees under Common Garden Conditions in Europe. Forests 2022, 13, 676. [Google Scholar] [CrossRef]
- Teste, F.P.; Jones, M.D.; Dickie, I.A. Dual-mycorrhizal Plants: Their Ecology and Relevance. New Phytol. 2019, 225, 1835–1851. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Tedersoo, L. Resolving the Mycorrhizal Status of Important Northern Hemisphere Trees. Plant Soil 2020, 454, 3–34. [Google Scholar] [CrossRef]
- Dickie, I.A.; Koide, R.T.; Fayish, A.C. Vesicular-Arbuscular Mycorrhizal Infection of Quercus Rubra Seedlings. New Phytol. 2001, 151, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Fernández Bidondo, L.; Colombo, R.P.; Recchi, M.; Silvani, V.A.; Pérgola, M.; Martínez, A.; Godeas, A.M. Detection of Arbuscular Mycorrhizal Fungi Associated with Pecan (Carya illinoinensis) Trees by Molecular and Morphological Approaches. MycoKeys 2018, 42, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Rudawska, M.; Leski, T.; Wilgan, R.; Karliński, L.; Kujawska, M.; Janowski, D. Mycorrhizal Associations of the Exotic Hickory Trees, Carya laciniosa and Carya cordiformis, Grown in Kórnik Arboretum in Poland. Mycorrhiza 2018, 28, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How Much Does Climate Change Threaten European Forest Tree Species Distributions? Glob. Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Wilgan, R. Dual and Tripartite Symbiosis of Invasive Woody Plants. In Symbiotic Soil Microorganisms; Shrivastava, N., Mahajan, S., Varma, A., Eds.; Springer: Cham, Switzerland, 2021; pp. 87–97. ISBN 9783030519162. [Google Scholar]
- Adjoud-Sadadou, D.; Halli-Hargas, R. Dual Mycorrhizal Symbiosis: An Asset for Eucalypts out of Australia? Can. J. For. Res. 2017, 47, 500–505. [Google Scholar] [CrossRef]
- Karliński, L.; Rudawska, M.; Kieliszewska-Rokicka, B.; Leski, T. Relationship between Genotype and Soil Environment during Colonization of Poplar Roots by Mycorrhizal and Endophytic Fungi. Mycorrhiza 2010, 20, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Fruleux, A.; Duclercq, J.; Dubois, F.; Decocq, G. First Report of Ectomycorrhizae in Prunus serotina in the Exotic Range. Plant Soil 2022, 484, 171–181. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.; Arnold, A.E.; Ferrer, A.; Turner, B.L.; Dalling, J.W. Variation in Ectomycorrhizal Fungal Communities Associated with Oreomunnea mexicana (Juglandaceae) in a Neotropical Montane Forest. Mycorrhiza 2016, 26, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Schlaeppi, K.; Bender, S.F.; Mascher, F.; Russo, G.; Patrignani, A.; Camenzind, T.; Hempel, S.; Rillig, M.C.; van der Heijden, M.G.A. High-Resolution Community Profiling of Arbuscular Mycorrhizal Fungi. New Phytol. 2016, 212, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Wright, W. The Empire of the Hittites. Old Testam. Stud. 1888, 4, 1. [Google Scholar]
- Ma, W.Y.; Qin, Q.Y.; Zou, Y.N.; Kuča, K.; Giri, B.; Wu, Q.S.; Hashem, A.; Al-Arjani, A.B.F.; Almutairi, K.F.; Abd-Allah, E.F.; et al. Arbuscular Mycorrhiza Induces Low Oxidative Burst in Drought-Stressed Walnut through Activating Antioxidant Defense Systems and Heat Shock Transcription Factor Expression. Front. Plant Sci. 2022, 13, 1089420. [Google Scholar] [CrossRef] [PubMed]
- Durney, C.; Boussageon, R.; El-Mjiyad, N.; Wipf, D.; Courty, P.E. Arbuscular Mycorrhizal Symbiosis with Rhizophagus irregularis DAOM197198 Modifies the Root Transcriptome of Walnut Trees. Mycorrhiza 2024, 34, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Mortier, E.; Mounier, A.; Kreplak, J.; Martin-Laurent, F.; Recorbet, G.; Lamotte, O. Evidence That a Common Arbuscular Mycorrhizal Network Alleviates Phosphate Shortage in Interconnected Walnut Sapling and Maize Plants. Front. Plant Sci. 2023, 14, 1206047. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Qin, Q.Y.; Ma, W.Y.; Zhou, L.J.; Wu, Q.S.; Xu, Y.J.; Kuča, K.; Hashem, A.; Al-Arjani, A.B.F.; Almutairi, K.F.; et al. Metabolomics Reveals Arbuscular Mycorrhizal Fungi-Mediated Tolerance of Walnut to Soil Drought. BMC Plant Biol. 2023, 23, 1206047. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Zarif, N.; Li, H.; Cui, D.; Yu, J.; Duan, J.; Li, C.; Wang, Q. Effects of Low Light, Interspecific Competition, and Their Combination on Flavonoid Exudation Patterns and Rhizosphere Fungal Community in Juglans mandshurica and Fraxinus mandshurica Roots. Plant Soil 2024, 511, 835–853. [Google Scholar] [CrossRef]
- Yin, L.; Dijkstra, F.A.; Phillips, R.P.; Zhu, B.; Wang, P.; Cheng, W. Arbuscular Mycorrhizal Trees Cause a Higher Carbon to Nitrogen Ratio of Soil Organic Matter Decomposition via Rhizosphere Priming than Ectomycorrhizal Trees. Soil Biol. Biochem. 2021, 157, 108246. [Google Scholar] [CrossRef]
- Fajardo, L.; Cáceres, A.; Arrindell, P. Arbuscular Mycorrhizae, a Tool to Enhance the Recovery and Re-Introduction of Juglans venezuelensis Manning, an Endemic Tree on the Brink of Extinction. Symbiosis 2014, 64, 63–71. [Google Scholar] [CrossRef]
- Bonito, G.; Brenneman, T.; Vilgalys, R. Ectomycorrhizal Fungal Diversity in Orchards of Cultivated Pecan (Carya illinoinensis; Juglandaceae). Mycorrhiza 2011, 21, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.; Mangan, S.A.; Turner, B.L.; Dalling, J.W. An Ectomycorrhizal Nitrogen Economy Facilitates Monodominance in a Neotropical Forest. Ecol. Lett. 2016, 19, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, J.; Meng, M.; Guo, X.; Wu, Y.; Liu, X.; Zhao, K.; Ding, L.; Shao, Y.; Fu, W. Forest-Type Shift and Subsequent Intensive Management Affected Soil Organic Carbon and Microbial Community in Southeastern China. Eur. J. For. Res. 2017, 136, 689–697. [Google Scholar] [CrossRef]
- Tonn, N.; Ibáñez, I. Plant-Mycorrhizal Fungi Associations along an Urbanization Gradient: Implications for Tree Seedling Survival. Urban Ecosyst. 2017, 20, 823–837. [Google Scholar] [CrossRef]
- Ge, Z.W.; Brenneman, T.; Bonito, G.; Smith, M.E. Soil PH and Mineral Nutrients Strongly Influence Truffles and Other Ectomycorrhizal Fungi Associated with Commercial Pecans (Carya illinoinensis). Plant Soil 2017, 418, 493–505. [Google Scholar] [CrossRef]
- Ilyas, S.; Razaq, A.; Khalid, A.N. Molecular Investigations to Determine the Ectomycorrhizal Habit of Lactarius sanguifluus Associated with Coniferous and Deciduous Vegetation of Galyat, Khyber Pakhtunkhwa, Pakistan. Int. J. Agric. Biol. 2013, 15, 857–863. [Google Scholar]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal Lifestyle in Fungi: Global Diversity, Distribution, and Evolution of Phylogenetic Lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef] [PubMed]
- Frene, J.P.; Lawson, S.S.; Lue Sue, N.D.; Crawford, R.H.; Gardner, T.G. Effects of Tree Species Identity on Soil Microbial Communities in Juglans Nigra and Quercus Rubra Plantations. Front. Microbiol. 2024, 15, 1442026. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhuang, J.; Tian, Y.; Wan, S.; Ding, S.; Zhang, M. The Effect of Arbuscular Mycorrhizal Fungi on the Growth of Cyclocarya Paliurus Cutting Seedlings. Turk. J. Agric. For. 2024, 48, 102–115. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, B.; Zhang, M.; Chen, S.; Deng, B. Mycorrhizal Fungi Synergistically Promote the Growth and Secondary Metabolism of Cyclocarya paliurus. Forests 2022, 13, 2188. [Google Scholar] [CrossRef]
- Babuin, M.F.; Autonoma, C.; Aires, D.B.; Menendez, A.B. Arbuscular Mycorrhizal Pecan Water Supply. HortScience 2016, 51, 212–215. [Google Scholar] [CrossRef]
- Janowski, D.; Wilgan, R.; Leski, T.; Karliński, L.; Rudawska, M. Effective Molecular Identification of Ectomycorrhizal Fungi: Revisiting DNA Isolation Methods. Forests 2019, 10, 218. [Google Scholar] [CrossRef]
- Rudawska; Wilgan, R.; Janowski, D.; Iwański, M.; Leski, T. Shifts in Taxonomical and Functional Structure of Ectomycorrhizal Fungal Community of Scots Pine (Pinus sylvestris L.) Underpinned by Partner Tree Ageing. Pedobiologia 2018, 71, 20–30. [Google Scholar] [CrossRef]
- Diez-Hermano, S.; Poveda, J.; Benito, Á.; Peix, Á.; Martín-Pinto, P.; Diez, J.J. Soil Mycobiome and Forest Endophytic Fungi: Is There a Relationship between Them? For. Ecol. Manag. 2024, 562, 121924. [Google Scholar] [CrossRef]
- Corrales, A.; Henkel, T.W.; Smith, M.E. Ectomycorrhizal Associations in the Tropics—Biogeography, Diversity Patterns and Ecosystem Roles. New Phytol. 2018, 220, 1076–1091. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.; Xu, H.; Garibay-Orijel, R.; Alfonso-Corrado, C.; Williams-Linera, G.; Chu, C.; Truong, C.; Jusino, M.A.; Clark-Tapia, R.; Dalling, J.W.; et al. Fungal Communities Associated with Roots of Two Closely Related Juglandaceae Species with a Disjunct Distribution in the Tropics. Fungal Ecol. 2021, 50, 101023. [Google Scholar] [CrossRef]
- Edwards, J.D.; Krichels, A.H.; Seyfried, G.S.; Dalling, J.; Kent, A.D.; Yang, W.H. Soil Microbial Community Response to Ectomycorrhizal Dominance in Diverse Neotropical Montane Forests. Mycorrhiza 2024, 34, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Maunoury, L.; Deveau, A.; Moreno, M.; Todesco, F.; Belmondo, S.; Murat, C.; Courty, P.E.; Jąkalski, M.; Selosse, M.A. Two Ectomycorrhizal Truffles, Tuber melanosporum and T. aestivum, Endophytically Colonise Roots of Non-Ectomycorrhizal Plants in Natural Environments. New Phytol. 2019, 225, 2542–2556. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, M.A.; Horton, T.R.; Simberloff, D. Lack of Belowground Mutualisms Hinders Pinaceae Invasions. Ecology 2009, 90, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Policelli, N.; Bruns, T.D.; Vilgalys, R.; Nuñez, M.A. Suilloid Fungi as Global Drivers of Pine Invasions. New Phytol. 2018, 222, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, M.A.; Chiuffo, M.C.; Torres, A.; Paul, T.; Dimarco, R.D.; Raal, P.; Policelli, N.; Moyano, J.; García, R.A.; van Wilgen, B.W.; et al. Ecology and Management of Invasive Pinaceae around the World: Progress and Challenges. Biol. Invasions 2017, 19, 3099–3120. [Google Scholar] [CrossRef]
- Nuñez, M.A.; Dickie, I.A. Invasive Belowground Mutualists of Woody Plants. Biol. Invasions 2014, 16, 645–661. [Google Scholar] [CrossRef]
- Dickie, I.A.; Nu??ez, M.A.; Pringle, A.; Lebel, T.; Tourtellot, S.G.; Johnston, P.R. Towards Management of Invasive Ectomycorrhizal Fungi. Biol. Invasions 2016, 18, 3383–3395. [Google Scholar] [CrossRef]
- Dickie, I.A.; Bufford, J.L.; Cobb, R.C.; Desprez-Loustau, M.L.; Grelet, G.; Hulme, P.E.; Klironomos, J.; Makiola, A.; Nuñez, M.A.; Pringle, A.; et al. The Emerging Science of Linked Plant–Fungal Invasions. New Phytol. 2017, 215, 1314–1332. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.M.; Allsopp, N.; D’antonio, C.M.; Milton, S.J.; Rejmánek, M. Plant Invasions—The Role of Mutualisms. Biol. Rev. 2000, 75, 65–93. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, M.K.; Paź-Dyderska, S.; Jagodziński, A.M.; Puchałka, R. Shifts in Native Tree Species Distributions in Europe under Climate Change. J. Environ. Manag. 2025, 373, 123504. [Google Scholar] [CrossRef] [PubMed]
- Thurm, E.A.; Hernandez, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative Tree Species under Climate Warming in Managed European Forests. For. Ecol. Manag. 2018, 430, 485–497. [Google Scholar] [CrossRef]
- Andrew, C.; Heegaard, E.; Halvorsen, R.; Martinez-Peña, F.; Egli, S.; Kirk, P.M.; Bässler, C.; Büntgen, U.; Aldea, J.; Høiland, K.; et al. Climate Impacts on Fungal Community and Trait Dynamics. Fungal Ecol. 2016, 22, 17–25. [Google Scholar] [CrossRef]
- Wilgan, R.; Dyderski, M.K.; Pietras, M.; Walas, Ł.; Kolanowska, M.; Leski, T. Northward Shifting in the Distribution of Optimal Niches for Tuber aestivum, Tuber melanosporum, and Their Ectomycorrhizal Tree Partners in Europe. Acta Oecologica 2025, 126, 104057. [Google Scholar] [CrossRef]
- Steidinger, B.S.; Crowther, T.W.; Liang, J.; Van Nuland, M.E.; Werner, G.D.A.; Reich, P.B.; Nabuurs, G.; de-Miguel, S.; Zhou, M.; Picard, N.; et al. Climatic Controls of Decomposition Drive the Global Biogeography of Forest-Tree Symbioses. Nature 2019, 569, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, J.; Barbero-López, A.; Vestberg, M.; Heiskanen, J.; Lehto, T. Does Severe Soil Drought Have After-Effects on Arbuscular and Ectomycorrhizal Root Colonisation and Plant Nutrition? Plant Soil 2017, 418, 377–386. [Google Scholar] [CrossRef]
- Kilpeläinen, J.; Aphalo, P.J.; Lehto, T. Temperature Affected the Formation of Arbuscular Mycorrhizas and Ectomycorrhizas in Populus angustifolia Seedlings More than a Mild Drought. Soil Biol. Biochem. 2020, 146, 107798. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Egerton-Warburton, L.M.; Allen, M.F. Topographic Position Modulates the Mycorrhizal Response of Oak Trees to Interannual Rainfall Variability. Ecology 2009, 90, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Gehring, C.A.; Mueller, R.C.; Whitham, T.G. Environmental and Genetic Effects on the Formation of Ectomycorrhizal and Arbuscular Mycorrhizal Associations in Cottonwoods. Oecologia 2006, 149, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, H.; Wu, C.; Zhu, L. Flooding Tolerance and Recovery Capacity of Carya illinoinensis. Horticulturae 2025, 11, 590. [Google Scholar] [CrossRef]
- Tendersoo, L.; Brundrett, M. Evolution of Ectomycorrhizal Symbiosis in Plants. In Biogeography of Mycorrhizal Symbiosi; Springer: Cham, Switzerland, 2017; Volume 230, p. 563. ISBN 978-3-319-56362-6. [Google Scholar]
- Luciani, E.; Palliotti, A.; Tombesi, S.; Gardi, T.; Micheli, M.; Berrios, J.G.; Zadra, C.; Farinelli, D. Mitigation of Multiple Summer Stresses on Hazelnut (Corylus avellana L.): Effects of the New Arbuscular Mycorrhiza Glomus Iranicum Tenuihypharum Sp. Nova. Sci. Hortic. 2019, 257, 108659. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, Y.; Ebrahimi, A.; Liu, P.; Woeste, K.; Zhao, P.; Zhang, S. Whole Genome Based Insights into the Phylogeny and Evolution of the Juglandaceae. BMC Ecol. Evol. 2021, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- ul Haq, H.; Hauer, A.; Singavarapu, B.; Christel, H.; Cesarz, S.; Eisenhauer, N.; Ferlian, O.; Bruelheide, H.; Wubet, T. The Interactive Effect of Tree Mycorrhizal Type, Mycorrhizal Type Mixture and Tree Diversity Shapes Rooting Zone Soil Fungal Communities in Temperate Forest Ecosystems. Funct. Ecol. 2024, 39, 1441–1454. [Google Scholar] [CrossRef]
- Větrovský, T.; Kolaříková, Z.; Lepinay, C.; Awokunle Hollá, S.; Davison, J.; Fleyberková, A.; Gromyko, A.; Jelínková, B.; Kolařík, M.; Krüger, M.; et al. GlobalAMFungi: A Global Database of Arbuscular Mycorrhizal Fungal Occurrences from High-Throughput Sequencing Metabarcoding Studies. New Phytol. 2023, 240, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Shigaeva, J.; Darr, D. On the Socio-Economic Importance of Natural and Planted Walnut (Juglans regia L.) Forests in the Silk Road Countries: A Systematic Review. For. Policy Econ. 2020, 118, 102233. [Google Scholar] [CrossRef]
- Schroder, E.S.; Ekanayake, D.B.; Romano, S.P. Indiana Bat Maternity Roost Habitat Preference within Midwestern United States Upland Oak-Hickory (Quercus-Carya) Forests. For. Ecol. Manag. 2017, 404, 65–74. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Jensen, R.; Kabrick, J. Changes in Aboveground Biomass Following Alternative Harvesting in Oak-Hickory Forests in the Eastern USA. IForest 2015, 8, 652–660. [Google Scholar] [CrossRef]
- Westwood, M.; Cavender, N.; Meyer, A.; Smith, P. Botanic Garden Solutions to the Plant Extinction Crisis. Plants People Planet 2021, 3, 22–32. [Google Scholar] [CrossRef]
- Varga, T.; Krizsán, K.; Földi, C.; Dima, B.; Sánchez-García, M.; Sánchez-Ramírez, S.; Szöllősi, G.J.; Szarkándi, J.G.; Papp, V.; Albert, L.; et al. Megaphylogeny Resolves Global Patterns of Mushroom Evolution. Nat. Ecol. Evol. 2019, 3, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Strullu-Derrien, C.; Selosse, M.A.; Kenrick, P.; Martin, F.M. The Origin and Evolution of Mycorrhizal Symbioses: From Palaeomycology to Phylogenomics. New Phytol. 2018, 220, 1012–1030. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, S.; Neubert, K.; Wöllecke, J.; Münzenberger, B.; Hüttl, R.F. Ectomycorrhiza Communities of Red Oak (Quercus rubra L.) of Different Age in the Lusatian Lignite Mining District, East Germany. Mycorrhiza 2007, 17, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Wilgan, R. High Species Diversity but Low Specificity to Ectomycorrhizal Tree Partners Exhibited by Native Truffle Species (Tuber Spp., Pezizales) in Poland, Central Europe. Forests 2023, 14, 2407. [Google Scholar] [CrossRef]
- Rudawska, M.; Kujawska, M.; Leski, T.; Janowski, D.; Karliński, L.; Wilgan, R. Ectomycorrhizal Community Structure of the Admixture Tree Species Betula pendula, Carpinus betulus, and Tilia cordata Grown in Bare-Root Forest Nurseries. For. Ecol. Manag. 2019, 473, 113–125. [Google Scholar] [CrossRef]
- Arraiano-Castilho, R.; Bidartondo, M.I.; Niskanen, T.; Clarkson, J.J.; Brunner, I.; Zimmermann, S.; Senn-Irlet, B.; Frey, B.; Peintner, U.; Mrak, T.; et al. Habitat Specialisation Controls Ectomycorrhizal Fungi above the Treeline in the European Alps. New Phytol. 2021, 229, 2901–2916. [Google Scholar] [CrossRef] [PubMed]
- Campagnaro, T.; Brundu, G.; Sitzia, T. Five Major Invasive Alien Tree Species in European Union Forest Habitat Types of the Alpine and Continental Biogeographical Regions. J. Nat. Conserv. 2018, 43, 227–238. [Google Scholar] [CrossRef]
- Ferrara, G.; Lombardini, L.; Mazzeo, A.; Bruno, G.L. Evaluation of Pecan [Carya illinoinensis (Wangenh.) K. Koch] Cultivars for Possible Cultivation for Both Fruit and Truffle Production in the Puglia Region, Southeastern Italy. Horticulturae 2023, 9, 261. [Google Scholar] [CrossRef]
- Marozzi, G.; Sánchez, S.; Benucci, G.M.N.; Bonito, G.; Falini, L.B.; Albertini, E.; Donnini, D. Mycorrhization of Pecan (Carya illinoinensis) with Black Truffles: Tuber Melanosporum and Tuber Brumale. Mycorrhiza 2017, 27, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Benucci, G.M.N.; Bonito, G.; Falini, L.B.; Bencivenga, M. Mycorrhization of Pecan Trees (Carya illinoinensis) with Commercial Truffle Species: Tuber aestivum Vittad. and Tuber borchii Vittad. Mycorrhiza 2012, 22, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Cramer, W. Climate Change: The 2015 Paris Agreement Thresholds and Mediterranean Basin Ecosystems. Science 2016, 354, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, C.R.; Glendon, S.; Duffy, P.B. RCP8.5 Tracks Cumulative CO2 Emissions. Proc. Natl. Acad. Sci. USA 2020, 117, 19656–19657. [Google Scholar] [CrossRef] [PubMed]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The Projected Timing of Abrupt Ecological Disruption from Climate Change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, A.; Aizpurua, O.; Gilbert, M.T.P.; Bohmann, K. Scrutinizing Key Steps for Reliable Metabarcoding of Environmental Samples. Methods Ecol. Evol. 2018, 9, 134–147. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of Roots and Mycorrhiza of Land Plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Wurzburger, N.; Brookshire, E.N.J.; McCormack, M.L.; Lankau, R.A. Mycorrhizal Fungi as Drivers and Modulators of Terrestrial Ecosystem Processes. New Phytol. 2017, 213, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.L.; Gilbert, M.T.P.; Araújo, M.B.; Matias, M.G. Fine-Tuning Biodiversity Assessments: A Framework to Pair EDNA Metabarcoding and Morphological Approaches. Methods Ecol. Evol. 2021, 12, 2397–2409. [Google Scholar] [CrossRef]
- Rudawska, M.; Leski, T.; Stasińska, M.; Karliński, L.; Wilgan, R.; Kujawska, M. The Contribution of Forest Reserves and Managed Forests to the Diversity of Macrofungi of Different Trophic Groups in European Mixed Coniferous Forest Ecosystem. For. Ecol. Manag. 2022, 518, 120274. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Published Online 2020. Suppl. Inf. Ref. S 2021, 1, 371–378. [Google Scholar]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize Implements and Enhances Circular Visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef] [PubMed]

| Juglandaceae | Number of Research Papers | |||
|---|---|---|---|---|
| Phylogenetic Lineage | Genus | Tree Ecology * | Root-Associated Symbionts | |
| AM Fungi | ECM Fungi | |||
| /juglandoide | Juglans | ~5000 | 29 studies (e.g., [35,36,37,38,39,40,41]) | 2 studies [47,49] |
| Carya | >2500 | 3 studies [24,25,52] | 10 studies [18,20,25,42,44,45,46,53,54,55] | |
| Pterocarya | ~300 | - | - | |
| Cyclocarya | ~500 | 2 study [50,51] | - | |
| Platycarya | ~100 | - | - | |
| /engelhardioidea | Alfaroa | ~20 | - | 1 study [56] |
| Engelhardtia (=Alfaropsis) | ~60 | 1 study [57] | 1 study [57] | |
| Oreomunnea | ~50 | 1 study [57] | 4 studies [32,43,57,58] | |
| outgroup | Rhoiptelea | ~30 | - | - |
| All Juglandaceae trees | ~8500 (100%) | 35 (0.4%) | 17 (0.2%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilgan, R. Phylogenetic Determinants Behind the Ecological Traits of Relic Tree Family Juglandaceae, Their Root-Associated Symbionts, and Response to Climate Change. Int. J. Mol. Sci. 2025, 26, 6866. https://doi.org/10.3390/ijms26146866
Wilgan R. Phylogenetic Determinants Behind the Ecological Traits of Relic Tree Family Juglandaceae, Their Root-Associated Symbionts, and Response to Climate Change. International Journal of Molecular Sciences. 2025; 26(14):6866. https://doi.org/10.3390/ijms26146866
Chicago/Turabian StyleWilgan, Robin. 2025. "Phylogenetic Determinants Behind the Ecological Traits of Relic Tree Family Juglandaceae, Their Root-Associated Symbionts, and Response to Climate Change" International Journal of Molecular Sciences 26, no. 14: 6866. https://doi.org/10.3390/ijms26146866
APA StyleWilgan, R. (2025). Phylogenetic Determinants Behind the Ecological Traits of Relic Tree Family Juglandaceae, Their Root-Associated Symbionts, and Response to Climate Change. International Journal of Molecular Sciences, 26(14), 6866. https://doi.org/10.3390/ijms26146866






