Integrative Approaches to Myopathies and Muscular Dystrophies: Molecular Mechanisms, Diagnostics, and Future Therapies
Abstract
1. Introduction
2. Myopathies
2.1. Cardiomyopathies
2.2. Metabolic Myopathies
2.3. Myofibrilal Myopathies
2.4. Congenital Myopathies
2.5. Distal Myopathies
2.6. Idiopathic Inflammatory Myopathies
2.7. Dystrophies
3. Discussion and Conclusions
| Subtype | Gene(s) Involved | Pathways | Clinical Phenotype | Current Therapies | Experimental Gene Therapy Approaches | Ref. |
|---|---|---|---|---|---|---|
| Dilated (DCM) | TTN, PLN, MYH7, BAG3, (frequent variants shown) | PI3K/Akt, NADPH/ROS, MAPK, PLC/IP3/Ca/NFATFAS-dependent apoptosis pathway | Arrhythmias, dyspnea, edema, thromboembolis, cardiac dilation, fibrosis, muscle wasting | ACE inhibitors, ARBs, β-blockers, MRAs, ivabradine; CRT; aerobic and resistance training; cardiac rehab | RNAi, ASO, CRISPR, TALEN, AAV vectors | [8,62,69,84,298,299,300] |
| Pompe disease (GSD II) | GAA | Lysosomal glycogen degradation; autophagy (mTORC1 regulation) | Infantile cardiomyopathy, hypotonia, respiratory failure; late-onset: proximal muscle weakness, fatigue; elevated CK | Enzyme replacement therapy (alglucosidase alfa); exercise restriction; nutritional support; emerging small-molecule chaperones (e.g., miglustat) | AAV-mediated GAA gene therapy in development | [87,99,101,104,105,310] |
| Cori disease (GSD III) | AGL | Glycogen debranching enzyme activity | Hepatomegaly, hypoglycemia (juvenile forms), myopathy; muscle weakness and fatigue; hepatic cirrhosis in severe cases | High-protein, frequent meals (uncooked cornstarch); monitoring of liver function; experimental liver-targeted gene therapy not yet available (no ERT) | AAV Vector Encoding a Bacterial Glycogen Debranching Enzyme | [95,97,106,302,310] |
| McArdle disease (GSD V) | PYGM | Muscle glycogen phosphorylase (glycogenolysis) | Exercise-induced cramps, myalgia, fatigue; “second wind” phenomenon (improved tolerance after brief rest); normal life expectancy with management | Pre-exercise simple sugars (glucose) or sucrose; regular moderate exercise; pain management; creatine supplementation (investigational) | rAAV8-Pygm improving glycogenolysis and muscle performance; AAV delivery of PYGM cDNA to ovine muscles; systemic rAAV8-Pygm delivery in murine model | [107,108,110,111,303,304] |
| CPT II deficiency | CPT2 | Mitochondrial fatty acid β-oxidation (entry into mitochondria) | Episodic myalgia and rhabdomyolysis triggered by prolonged exercise/fasting; mild fixed myopathy (adult form); episodes of myoglobinuria; normal CK between attacks | Prevent fasting/excess exercise; high-carbohydrate diet, medium-chain triglycerides; L-carnitine supplement; riboflavin in some cases | – | [31,112,113,115,116] |
| MELAS (m.3243A>G) | MT-TL1 (mitochondrial tRNA Leu(UUR)) | Oxidative phosphorylation (respiratory chain complexes); mitochondrial energy metabolism | Stroke-like episodes (neurologic deficits), seizures, migraines; lactic acidosis; progressive myopathy and exercise intolerance; cardiomyopathy; short survival (median ~17 yr post-onset) | Symptomatic: arginine for stroke-like episodes; coenzyme Q10, L-carnitine, vitamins (B complexes); exercise training; diet modifications | mitoARCUS nuclease for elimination of MELAS-associated m.3243G mtDNA | [86,120,121,122,123,311] |
| MTM1-associated myopathy | MTM1 | PI3P metabolism, Dysregulated autophagy | Severe muscle weakness, atrophy of fibers, disrupted membrane trafficking, accumulation of cellular debris, reduced lifespan | AT132 (AAV-MTM1 gene therapy), Non-invasive ventilation, tracheostomy/mechanical ventilation (if needed), physical therapy, nutritional support, multidisciplinary care | AT132 (AAV8-MTM1) | [169,170,171,172] |
| Central core disease (CCD) | RYR1 | Calcium dysregulation (SR Ca2+ release) | Single central core lesions, hypotonia, muscle weakness, increased risk of malignant hyperthermia | Avoidance of malignant hyperthermia-triggering agents (e.g., volatile anesthetics, succinylcholine), physical therapy, monitoring for cardiac or orthopedic issues | – | [158,159,167,168] |
| Multiminicore disease (MmD) | SEPN1 | Oxidative stress, Calcium homeostasis | Multiple small cores in fibers, progressive muscle weakness, respiratory involvement | Early and often intensive respiratory support, physical therapy, management of scoliosis and thoracic deformities, pulmonary care | – | [89,159,160,161] |
| Nemaline myopathy | NEB, TPM3, ACTA1, KLHL40, KLHL41, KBTBD13 | thin filament assembly), Ubiquitin–proteasome degradation | Rod-like (nemaline) inclusions in muscle, muscle weakness, dysmorphic facial features | Physical therapy, respiratory support, nutritional support | – | [157,160,162,163] |
| Centronuclear myopathy (CNM) | Autosomal: DNM2, BIN1, RYR1, X-linked: MTM1 | Membrane trafficking, PI3P metabolism, T-tubule defects | Centralized/internalized nuclei, muscle weakness, t-tubule disorganization | Physical therapy, respiratory support, nutritional support, monitoring for orthopedic and pulmonary complications | rAAV-shDnm2 (preclinical), AAV8-MTM1 for X-linked MTM | [158,159,170,175] |
| Dermatomyositis (DM) | – And autoantigens: Mi-2, NXP-2, TIF1γ | Complement-mediated microangiopathy, Type I IFN signaling | Proximal symmetrical weakness, skin rash (Gottron’s papules, heliotrope rash), dysphagia, ILD, elevated CK | Immunosuppression (steroids, methotrexate, azathioprine), IVIG (especially for refractory cases), antimalarials for skin rash, exercise therapy (aerobic, resistance); rituximab (RTX) for refractory cases | – | [203,204,205,213,214] |
| Polymyositis (PM) | – | CD8+ T-cell–mediated muscle fiber damage, MHC class I upregulation | Symmetrical proximal muscle weakness, no rash, dysphagia, ILD, elevated CK | Corticosteroids, steroid-sparing immunosuppressants (azathioprine, mycophenolate, methotrexate), exercise therapy (aerobic and resistance), rituximab for refractory cases | – | [204,205,218,219] |
| Inclusion-body myositis (IBM) | LDB3/Z And autoantigens ASP, | Mixed inflammatory (CD8+ T-cell–mediated) and degenerative processes involving β-amyloid and tau accumulation, MHC class I upregulation, granzyme B cleaves FHL1, and resulting fragments become autoantigens | Insidious onset quadriceps and finger flexor weakness, muscle biopsy shows rimmed vacuoles, β-amyloid inclusions, poor response to immunotherapy | No proven therapy, poor response to immunosuppressants, IVIG may help some, exercise improves function | AAV1-Follistatin (clinical trial) | [136,147,205,216,226] |
| Immune-mediated necrotizing myopathy (IMNM) | HMGC, SRP | Necrotizing (myocytolytic) muscle pathology; complement activation; anti-HMGCR or anti-SRP autoantibody–mediated injury | Rapidly progressive severe weakness, very high CK, necrosis on biopsy, can be statin-associated (HMGCR) | Corticosteroids; steroid-sparing agents (e.g., azathioprine), IVIG (effective in anti-HMGCR cases), rituximab (RTX) for refractory cases | – | [204,205,220] |
| MFM1 (Desmin myopathy) | DES | Intermediate filament network (Z-disk scaffold); protein quality control | Progressive distal and proximal weakness; cardiomyopathy; cores and inclusions on biopsy | Supportive (physical therapy, cardiac care) | Single high-dose AAV9 carrying human DES under a cardiac promoter | [129,130,131,132,144,312] |
| MFM2 (αB-crystallin myopathy) | CRYAB (HSPB5) | Small heat-shock protein (chaperone); aggregates clearance | Progressive myopathy with early cataracts; weakness often starts distal; cardiac involvement possible | Supportive; cataract management | – | [133,313] |
| MFM3 (Myotilin myopathy) | MYOT | Z-disk assembly (myotilin-actinin-filamin C complex) | Late-onset proximal and distal weakness; myalgia; cardiomyopathy in some; protein aggregates on biopsy | Supportive; manage cardiomyopathy | AAV6 vectors expressing microRNAs targeting the mutant MYOT transcript | [135,314] |
| MFM4 (LDB3/ZASP myopathy) | LDB3 (ZASP) | Z-disk integrity (actin-binding); autophagy (CASA complex) | Distal and proximal muscle weakness; cardiomyopathy; protein aggregates (ZASP, filamin, Hsp70) | Supportive; exercise, cardiac care | AAV vectors carrying shRNA/miRNA against mutant LDB3 | [136,315] |
| MFM5 (Filamin C myopathy) | FLNC | Filamin C cross-linking (actin-binding); autophagy (CASA) | Adult-onset distal/proximal weakness; cardiomyopathy common; protein aggregates (FLNC, CASA proteins) | Supportive; monitor heart; no cure yet (future: modulate autophagy) | CRISPR/Cas9 strategy for the generation of a FLNC knockout hESC line | [316,317] |
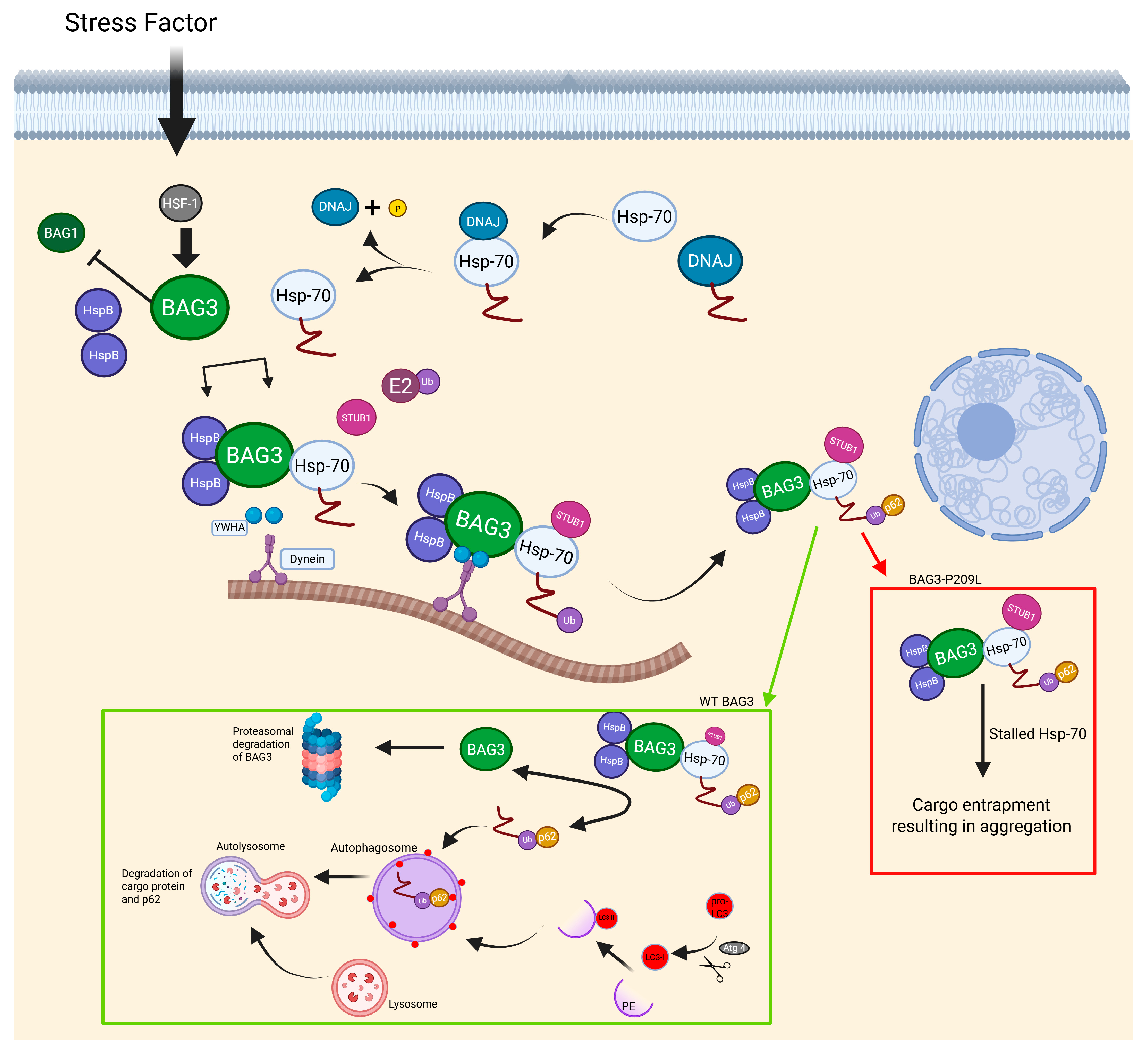
| MFM6 (BAG3 myopathy) | BAG3 | Chaperone-assisted selective autophagy (CASA complex) | Childhood-onset distal/proximal weakness, rigid spine, scoliosis; early cardiomyopathy; elevated CK | Supportive (ventilation, cardiac care) | Personalized allele-specific CRISPR-Cas9 (preclinical) | [11,138,139,140,142,149] |
| Welander distal myopathy | TIA1 | Impaired stress granule assembly (TIA1), disrupted RNA metabolism, mRNP accumulation and translation inhibition | Early weakness of thumb/index finger, difficulty extending fingers; later, tibialis anterior weakness, foot drop, steppage gait | Supportive (orthoses for hand/foot drop) | – | [178] |
| Tibial (Udd) myopathy | TTN | Disrupted sarcomeric structure, impaired muscle elasticity, altered protein-protein interactions (e.g., calpain-3, α-actinin, myosin), Progressive muscle fiber degeneration | Adult-onset foot dorsiflexor weakness, slow progression, often spares upper limbs | Supportive (ankle-foot orthoses); physical therapy, cardiac surveillance (titin variants) | – | [179,180] |
| GNE myopathy (Nonaka) | GNE | Impaired membrane repair (dysferlin deficiency), dysregulated Ca2+ signaling, enhanced phagocytosis in monocytes, accumulation of necrotic/regenerating fibers and fibrotic tissue | foot drop, sparing of quadriceps, hand/finger flexor weakness, respiratory function preserved | Exercise, L-carnitine, sialic acid precursor supplements (Ace-ER—mixed results) | GNE lipoplex (preclinical) | [180,181,183,184,185,194,196] |
| Miyoshi Myopathy Type 2 (MMD2) | DYSF | Membrane repair dysfunction, Vesicle fusion defects, T-tubule development and Ca2+ signaling disruption | Calf/thigh weakness and atrophy, progression to upper limbs, very high CK levels, necrosis, regeneration, fat/connective tissue replacement | Supportive care (physical therapy, orthoses) | SRP-6004 (clinical trial) NCT05906251 | [181,183,196,197,198] |
| Duchenne muscular dystrophy (DMD) | DMD | Sarcolemmal stability (dystrophin-glycoprotein complex) | Childhood-onset progressive proximal weakness, loss of ambulation by teens; dilated cardiomyopathy; scoliosis; elevated CK (~10–100× normal) | Corticosteroids; cardiac care (ACEI/β-blockers); | AAV-microdystrophin and exon-skipping (eteplirsen, golodirsen) in clinical use/trials | [243,244,245,318] |
| Myotonic dystrophy type 1 (DM1) | DMPK | RNA toxicity (toxic CUG-expanded RNA sequesters splicing factors) | Adult-onset distal and facial weakness, myotonia, cataracts, cardiac conduction defects; multisystem (insulin resistance); progressive | Symptomatic: mexiletine for myotonia; pacemakers for heart block; modafinil for fatigue; cognitive therapies; experimental antisense oligonucleotides (ATX-01) | siRNA (siRNA-CAG)—mutant DMPK mRNA degradation (preclinical); AOC-1001 (antibody-oligo conjugate)—DMPK mRNA knockdown (Phase I/II recruiting) (NCT05027269); SaCas9- or eSpCas9-paired sgRNAs (rAAV9), dSaCas9-KRAB (rAAV6/9) for CTG repeat excision or transcriptional repression (preclinical) | [246,269,306,307,319,320,321] |
| Facioscapulohumeral dystrophy (FSHD) | DUX4 (permissive 4q allele with D4Z4 contraction) | Misexpression of embryonic DUX4 transcription factor; epigenetic de-repression | Variable adult-onset weakness (facial, scapular, humeral muscles); scapular winging; foot drop; CK normal; slow progression | Supportive: physical therapy, scapular fixation surgery | Epigenetic CRISPRi (dSaCas9-KRAB) targeting DUX4 promoter | [249,250,269,322] |
| Emery–Dreifuss muscular dystrophy (EDMD) | EMD or LMNA (X-linked or AD); others (SYNE1/2, FHL1) | Nuclear envelope integrity; mechanical stability of nucleus | Early contractures (elbows/Achilles); humeroperoneal muscle weakness; cardiomyopathy with conduction defects; long QT | Pacemaker/ICD for conduction/cardiomyopath; ACE inhibitors; physical therapy for contractures | AAV-Net39 gene replacement (preclinical) | [248,269,274] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DCM | dilated cardiomyopathy |
| HF | heart failure |
| ACEI | angiotensin-converting enzyme inhibitor |
| ARB | angiotensin II receptor blocker |
| MRA | mineralocorticoid receptor antagonist |
| GSD | glycogen storage diseases |
| FAO | fatty acid oxidation |
| ERT | enzyme replacement therapy |
| GSDs | glycogen storage diseases |
| GAA | acid alpha-glucosidase |
| GSD II | Pompe disease |
| GSD III | Cori disease |
| GSD IV | Andersen disease |
| GSD V | McArdle disease |
| rhGAA | recombinant human GAA |
| M6P | mannose-6-phosphate |
| AGL | glycogen debranching enzyme |
| G6P | glucose-6-phosphate |
| CK | creatine kinase |
| MADD | multiple acyl-CoA dehydrogenase deficiency |
| LCFA | long-chain fatty acids |
| VLCFA | very long-chain fatty acids |
| NGS | next-generation sequencing |
| mtDNA | mitochondrial DNA |
| PCD | primary carnitine deficiency |
| CPT I | carnitine palmitoyltransferase I |
| CPT II | carnitine palmitoyltransferase II |
| RRFs | ragged red fibers |
| MELAS | mitochondrial encephalopathy with lactic acidosis and stroke-like episodes |
| MERRF | myoclonic epilepsy with ragged red fibers |
| ROS | reactive oxygen species |
| glucose 1-P | glucose-1-phosphate |
| MFMs | myofibrillar myopathies |
| HspB5 | alpha-crystalin B chain |
| CASA | chaperone-assisted selective autophagy |
| CMs | congenital myopathies |
| CCD | central core disease |
| MmD | minicore disease |
| CFTD | congenital fiber type disproportion |
| ActRIIB | activin receptor type IIB |
| MMD2 | Miyoshi myopathy type 2 |
| MMD1 | Miyoshi myopathy type 1 |
| EMG | electromyography |
| IIMs | idiopathic inflammatory myopathies |
| LMOD3 | leimodin-3 |
| NRAP | nebulin-related anchoring protein |
| CUL3 | Cullin-3 |
| PI3P | phosphatidylinositol 3-phosphate |
| mRNPs | messenger ribonucleoprotein complexes |
| mTORC1 | mammalian target of rapamycin complex 1 |
| AAV | adeno-associated virus |
| TLRs | toll-like receptors |
| DAMPs | damage associated with molecular patterns |
| MFM4 | Markesbery-Griggs distal myopathy |
| CAGR | compound annual growth rate |
| NF-κB | nuclear factor kappa B |
| DMRVs | distal myopathy with rimmed vacuoles |
| GNE myopathy | Nonaka myopathy |
| MRI | magnetic resonance imaging |
| CT | computed tomography |
| AI | artificial intelligence |
| LVEF | left ventricular ejection fraction |
| GPCR | G-protein-coupled receptor |
| rAAAV8 | recombinant AAV serotype 8 |
| LAMP1 | lysosome membrane-associated protein 1 |
| LAMP2 | lysosome membrane-associated protein 2 |
| shRNA | small hairpin RNA |
| PM | polymyositis |
| IMNM | immune-mediated necrotizing myopathy |
| NAM | necrotizing autoimmune myopathy |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| SRP | signal recognition particle |
| OM | overlap myositis |
| ASS | anti-synthetase syndrome |
| IBM | inclusion body myositis |
| CTD | connective tissue disorder |
| MSAs | myositis-specific antibodies |
| MHC-1 | Major Histocompatibility Complex 1 |
| DM | dermatomyositis |
| IVIG | intravenous immunoglobulin |
| ADL | activities of daily living |
| ZASP | Z-band alternatively spliced PDZ-motif protein |
| FHL1 | four-and-a-half LIM domain 1 |
| ILD | interstitial lung disease |
| RTX | rituximab |
| rAAV1 | recombinant AAV serotype 1 |
| TEEs | thromboembolic events |
| MSCs | mesenchymal stem cells |
| iPSCs | induced pluripotent stem cells |
| DMD | Duchenne muscular dystrophy |
| BMD | Becker muscular dystrophy |
| FSHD | facioscapulohumeral dystrophy |
| EDMD | Emery-Dreifuss muscular dystrophy |
| AONs | antisense oligonucleotides |
| MABs | mesoangioblasts |
| DUX4 | double homebox 4 protein |
| NORD | National Organization for Rare Disorders |
| MMPs | matrix metalloproteinases |
| RCM | restrictive cardiomyopathy |
| NINDS | National Institute of Neurological Disorders and Stroke |
| RDCRN | Rare Diseases Clinical Research Network |
| ERN | European Reference Networks |
| PPMD | Parent Project Muscular Dystrophy |
| MDA | Muscular Dystrophy Association |
| Ace-ER | acenouramic acid |
| HSP | hereditary spastic paraplegia |
| BDSRA | The Batten Disease Support & Research Association |
| CMT | Charcot-Marie-Tooth |
| HDSA | Huntington’s Disease Society of America |
| SMA | spinal muscular atrophy |
| LDB3 | LIM domain binding 3 |
References
- Elliott, P.; Charron, P.; Blanes, J.R.G.; Tavazzi, L.; Tendera, M.; Konté, M.; Laroche, C.; Maggioni, A.P. European Cardiomyopathy Pilot Registry: EURObservational Research Programme of the European Society of Cardiology. Eur. Heart J. 2016, 37, 164–173. [Google Scholar] [CrossRef]
- Ferreira, A.; Ferreira, V.; Antunes, M.M.; Lousinha, A.; Pereira-da-Silva, T.; Antunes, D.; Cunha, P.S.; Oliveira, M.; Ferreira, R.C.; Rosa, S.A. Dilated Cardiomyopathy: A Comprehensive Approach to Diagnosis and Risk Stratification. Biomedicines 2023, 11, 834. [Google Scholar] [CrossRef] [PubMed]
- Ababio, Y.; Kelly, S.P.; Angeli, F.S.; Berghout, J.; Huang, K.; Liu, K.; Burns, S.; Senerchia, C.; Moccia, R.; Brooks, G.C. Prevalence and Clinical Burden of Idiopathic Dilated Cardiomyopathy in the United States. Am. J. Med. Open 2023, 10, 100038. [Google Scholar] [CrossRef]
- Dec, G.W. The natural history of acute dilated cardiomyopathy. Trans. Am. Clin. Climatol. Assoc. 2014, 125, 76–86, discussion 86–87. [Google Scholar]
- Latronico, N.; Rasulo, F.A.; Eikermann, M.; Piva, S. Critical Illness Weakness, Polyneuropathy and Myopathy: Diagnosis, treatment, and long-term outcomes. Crit. Care 2023, 27, 439. [Google Scholar] [CrossRef]
- Månsson, A. Hypothesis and theory: Mechanical instabilities and non-uniformities in hereditary sarcomere myopathies. Front. Physiol. 2014, 5, 350. [Google Scholar] [CrossRef]
- Marston, S.; Memo, M.; Messer, A.; Papadaki, M.; Nowak, K.; McNamara, E.; Ong, R.; El-Mezgueldi, M.; Li, X.; Lehman, W. Mutations in repeating structural motifs of tropomyosin cause gain of function in skeletal muscle myopathy patients. Hum. Mol. Genet. 2013, 22, 4978–4987. [Google Scholar] [CrossRef]
- Ciarambino, T.; Menna, G.; Sansone, G.; Giordano, M. Cardiomyopathies: An Overview. Int. J. Mol. Sci. 2021, 22, 7722. [Google Scholar] [CrossRef]
- Savarese, M.; Sarparanta, J.; Vihola, A.; Jonson, P.H.; Johari, M.; Rusanen, S.; Hackman, P.; Udd, B. Panorama of the distal myopathies. Acta Myol. 2020, 39, 245–265. [Google Scholar] [CrossRef]
- Henderson, C.A.; Gomez, C.G.; Novak, S.M.; Mi-Mi, L.; Gregorio, C.C. Overview of the Muscle Cytoskeleton. Compr. Physiol. 2017, 7, 891–944. [Google Scholar] [CrossRef] [PubMed]
- Schröder, R.; Schoser, B. Myofibrillar Myopathies: A Clinical and Myopathological Guide. Brain Pathol. 2009, 19, 483–492. [Google Scholar] [CrossRef]
- Arena, I.G.; Pugliese, A.; Volta, S.; Toscano, A.; Musumeci, O. Molecular Genetics Overview of Primary Mitochondrial Myopathies. J. Clin. Med. 2022, 11, 632. [Google Scholar] [CrossRef]
- McNally, E.M.; Pytel, P. Muscle Diseases: The Muscular Dystrophies. Annu. Rev. Pathol. Mech. Dis. 2007, 2, 87–109. [Google Scholar] [CrossRef]
- Papadimas, G.K.; Xirou, S.; Kararizou, E.; Papadopoulos, C. Update on Congenital Myopathies in Adulthood. Int. J. Mol. Sci. 2020, 21, 3694. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, C.; Malfatti, E.; Meola, G. Editorial: Myopathology of inherited myopathies. Front. Neurol. 2022, 13, 1004562. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.; Uppin, M. Evolving classification and role of muscle biopsy in diagnosis of inflammatory myopathies. Indian J. Pathol. Microbiol. 2022, 65, S241–S251. [Google Scholar] [CrossRef]
- Sarohi, V.; Srivastava, S.; Basak, T. A Comprehensive Outlook on Dilated Cardiomyopathy (DCM): State-Of-The-Art Developments with Special Emphasis on OMICS-Based Approaches. J. Cardiovasc. Dev. Dis. 2022, 9, 174. [Google Scholar] [CrossRef]
- Punga, A.R.; Maddison, P.; Heckmann, J.M.; Guptill, J.T.; Evoli, A. Epidemiology, diagnostics, and biomarkers of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022, 21, 176–188. [Google Scholar] [CrossRef]
- Passos-Bueno, M.R.; Bakker, E.; Kneppers, A.L.; Takata, R.I.; Rapaport, D.; den Dunnen, J.T.; Zatz, M.; van Ommen, G.J. Different mosaicism frequencies for proximal and distal Duchenne muscular dystrophy (DMD) mutations indicate difference in etiology and recurrence risk. Am. J. Hum. Genet. 1992, 51, 1150–1155. [Google Scholar] [PubMed]
- Gomez Limia, C.; Baird, M.; Schwartz, M.; Saxena, S.; Meyer, K.; Wein, N. Emerging Perspectives on Gene Therapy Delivery for Neurodegenerative and Neuromuscular Disorders. J. Pers. Med. 2022, 12, 1979. [Google Scholar] [CrossRef]
- Ganassi, M.; Muntoni, F.; Zammit, P.S. Defining and identifying satellite cell-opathies within muscular dystrophies and myopathies. Exp. Cell Res. 2022, 411, 112906. [Google Scholar] [CrossRef]
- Ulm, J.W.; Barthélémy, F.; Nelson, S.F. Elucidation of bioinformatic-guided high-prospect drug repositioning candidates for DMD via Swanson linking of target-focused latent knowledge from text-mined categorical metadata. Front. Cell Dev. Biol. 2023, 11, 1226707. [Google Scholar] [CrossRef]
- Dowling, J.J.; North, K.N.; Goebel, H.H.; Beggs, A.H. Congenital and Other Structural Myopathies. In Neuromuscular Disorders of Infancy, Childhood, and Adolescence; Elsevier: Amsterdam, The Netherlands, 2015; pp. 499–537. [Google Scholar] [CrossRef]
- Maggi, L.; Mavroidis, M.; Psarras, S.; Capetanaki, Y.; Lattanzi, G. Skeletal and Cardiac Muscle Disorders Caused by Mutations in Genes Encoding Intermediate Filament Proteins. Int. J. Mol. Sci. 2021, 22, 4256. [Google Scholar] [CrossRef]
- Ignatieva, E.; Smolina, N.; Kostareva, A.; Dmitrieva, R. Skeletal Muscle Mitochondria Dysfunction in Genetic Neuromuscular Disorders with Cardiac Phenotype. Int. J. Mol. Sci. 2021, 22, 7349. [Google Scholar] [CrossRef]
- Bortolani, S.; Savarese, M.; Vattemi, G.; Bonanno, S.; Falzone, Y.M.; Pugliese, A.; Primiano, G.; Sancricca, C.; Lopergolo, D.; Greco, G.; et al. Clinical, Histopathologic, and Genetic Features of Patients with Myofibrillar and Distal Myopathies: Experience From the Italian Network. Neurology 2024, 103, e209697. [Google Scholar] [CrossRef]
- Vicino, A.; Veltsista, D.; Van Alfen, N. Muscle ultrasound in myopathies. Curr. Opin. Neurol. 2024, 37, 549–557. [Google Scholar] [CrossRef]
- Paoletti, M.; Pichiecchio, A.; Cotti Piccinelli, S.; Tasca, G.; Berardinelli, A.L.; Padovani, A.; Filosto, M. Advances in Quantitative Imaging of Genetic and Acquired Myopathies: Clinical Applications and Perspectives. Front. Neurol. 2019, 10, 78. [Google Scholar] [CrossRef]
- Marakhonov, A.V.; Tabakov, V.Y.; Zernov, N.V.; Dadali, E.L.; Sharkova, I.V.; Skoblov, M.Y. Two novel COL6A3 mutations disrupt extracellular matrix formation and lead to myopathy from Ullrich congenital muscular dystrophy and Bethlem myopathy spectrum. Gene 2018, 672, 165–171. [Google Scholar] [CrossRef]
- D’Avila, F.; Meregalli, M.; Lupoli, S.; Barcella, M.; Orro, A.; De Santis, F.; Sitzia, C.; Farini, A.; D’Ursi, P.; Erratico, S.; et al. Exome sequencing identifies variants in two genes encoding the LIM-proteins NRAP and FHL1 in an Italian patient with BAG3 myofibrillar myopathy. J. Muscle Res. Cell Motil. 2016, 37, 101–115. [Google Scholar] [CrossRef]
- Urtizberea, J.A.; Severa, G.; Malfatti, E. Metabolic Myopathies in the Era of Next-Generation Sequencing. Genes 2023, 14, 954. [Google Scholar] [CrossRef]
- Bentick, G.; Fairley, J.; Nadesapillai, S.; Wicks, I.; Day, J. Defining the clinical utility of PET or PET-CT in idiopathic inflammatory myopathies: A systematic literature review. Semin. Arthritis Rheum. 2022, 57, 152107. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, G.; Schirinzi, E.; Simoncini, C.; Ricci, G. Exercise therapy in muscle diseases: Open issues and future perspectives. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2019, 38, 233–238. [Google Scholar]
- Ansved, T. Muscle training in muscular dystrophies. Acta Physiol. Scand. 2001, 171, 359–366. [Google Scholar] [CrossRef]
- Shen, L.; Liao, T.; Chen, J.; Ma, J.; Wang, J.; Chen, L.; Zhang, S.; Zhao, Y.; Niu, L.; Zeng, C.; et al. Genistein Promotes Skeletal Muscle Regeneration by Regulating miR-221/222. Int. J. Mol. Sci. 2022, 23, 13482. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhou, S.; Li, P.; Liu, C.; Yuan, B.; Zhang, S.; Liu, A. Natural products and skeletal muscle health. J. Nutr. Biochem. 2021, 93, 108619. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Podlacha, M.; Gaffke, L.; Majkutewicz, I.; Mantej, J.; Węgrzyn, A.; Osiadły, M.; Myślińska, D.; Węgrzyn, G. Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer’s disease. Neuropharmacology 2019, 148, 332–346. [Google Scholar] [CrossRef]
- Li, M.-H.; Zhang, Y.-J.; Yu, Y.-H.; Yang, S.-H.; Iqbal, J.; Mi, Q.-Y.; Li, B.; Wang, Z.-M.; Mao, W.-X.; Xie, H.-G.; et al. Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur. J. Pharmacol. 2014, 728, 67–76. [Google Scholar] [CrossRef]
- Zeng, C.; Li, H.; Fan, Z.; Zhong, L.; Guo, Z.; Guo, Y.; Xi, Y. Crocin-Elicited Autophagy Rescues Myocardial Ischemia/Reperfusion Injury via Paradoxical Mechanisms. Am. J. Chin. Med. 2016, 44, 515–530. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Voumvouraki, A.; Augoustaki, A.; Samonakis, D.N. Autophagy in liver diseases. World J. Hepatol. 2021, 13, 6–65. [Google Scholar] [CrossRef]
- Ichimiya, T.; Yamakawa, T.; Hirano, T.; Yokoyama, Y.; Hayashi, Y.; Hirayama, D.; Wagatsuma, K.; Itoi, T.; Nakase, H. Autophagy and Autophagy-Related Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 8974. [Google Scholar] [CrossRef]
- Prakash, T.P.; Mullick, A.E.; Lee, R.G.; Yu, J.; Yeh, S.T.; Low, A.; Chappell, A.E.; Østergaard, M.E.; Murray, S.; Gaus, H.J.; et al. Fatty acid conjugation enhances potency of antisense oligonucleotides in muscle. Nucleic Acids Res. 2019, 47, 6029–6044. [Google Scholar] [CrossRef]
- Thibonnier, M.; Ghosh, S. Strategy for Pre-Clinical Development of Active Targeting MicroRNA Oligonucleotide Therapeutics for Unmet Medical Needs. Int. J. Mol. Sci. 2023, 24, 7126. [Google Scholar] [CrossRef]
- Tran, P.; Weldemichael, T.; Liu, Z.; Li, H. Delivery of Oligonucleotides: Efficiency with Lipid Conjugation and Clinical Outcome. Pharmaceutics 2022, 14, 342. [Google Scholar] [CrossRef] [PubMed]
- Kenjo, E.; Hozumi, H.; Makita, Y.; Iwabuchi, K.A.; Fujimoto, N.; Matsumoto, S.; Kimura, M.; Amano, Y.; Ifuku, M.; Naoe, Y.; et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat. Commun. 2021, 12, 7101. [Google Scholar] [CrossRef]
- Lohchania, B.; Christopher, A.C.; Arjunan, P.; Mahalingam, G.; Kathirvelu, D.; Prasannan, A.; Venkatesan, V.; Taneja, P.; Km, M.K.; Thangavel, S.; et al. Diosgenin enhances liposome-enabled nucleic acid delivery and CRISPR/Cas9-mediated gene editing by modulating endocytic pathways. Front. Bioeng. Biotechnol. 2023, 10, 1031049. [Google Scholar] [CrossRef] [PubMed]
- Johansen, A.K.; Molenaar, B.; Versteeg, D.; Leitoguinho, A.R.; Demkes, C.; Spanjaard, B.; De Ruiter, H.; Akbari Moqadam, F.; Kooijman, L.; Zentilin, L.; et al. Postnatal Cardiac Gene Editing Using CRISPR/Cas9 with AAV9-Mediated Delivery of Short Guide RNAs Results in Mosaic Gene Disruption. Circ. Res. 2017, 121, 1168–1181. [Google Scholar] [CrossRef]
- Weinmann, J.; Weis, S.; Sippel, J.; Tulalamba, W.; Remes, A.; El Andari, J.; Herrmann, A.-K.; Pham, Q.H.; Borowski, C.; Hille, S.; et al. Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nat. Commun. 2020, 11, 5432. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.L.; Poulard, K.; Goddard, M.A.; Latournerie, V.; Snyder, J.M.; Grange, R.W.; Elverman, M.R.; Denard, J.; Veron, P.; Buscara, L.; et al. Systemic AAV8-Mediated Gene Therapy Drives Whole-Body Correction of Myotubular Myopathy in Dogs. Mol. Ther. 2017, 25, 839–854. [Google Scholar] [CrossRef]
- Canonico, F.; Chirivi, M.; Maiullari, F.; Milan, M.; Rizzi, R.; Arcudi, A.; Galli, M.; Pane, M.; Gowran, A.; Pompilio, G.; et al. Focus on the road to modelling cardiomyopathy in muscular dystrophy. Cardiovasc. Res. 2022, 118, 1872–1884. [Google Scholar] [CrossRef]
- Vivekanandam, V.; Ellmers, R.; Jayaseelan, D.; Houlden, H.; Männikkö, R.; Hanna, M.G. In silico versus functional characterization of genetic variants: Lessons from muscle channelopathies. Brain 2023, 146, 1316–1321. [Google Scholar] [CrossRef]
- Margeta, M. Autophagy Defects in Skeletal Myopathies. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Holmes, S.; Fialho, D. Skeletal muscle channelopathies: A guide to diagnosis and management. Pract. Neurol. 2021, 21, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Heydemann, A.; Siemionow, M. A Brief Review of Duchenne Muscular Dystrophy Treatment Options, with an Emphasis on Two Novel Strategies. Biomedicines 2023, 11, 830. [Google Scholar] [CrossRef]
- Cortial, L.; Montero, V.; Tourlet, S.; Del Bano, J.; Blin, O. Artificial intelligence in drug repurposing for rare diseases: A mini-review. Front. Med. 2024, 11, 1404338. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Lee, S.-S.; Wen, Z.-H.; Lo, Y.-H. The changing scenario of drug discovery using AI to deep learning: Recent advancement, success stories, collaborations, and challenges. Mol. Ther.—Nucleic Acids 2024, 35, 102295. [Google Scholar] [CrossRef]
- Lovering, R.M.; Porter, N.C.; Bloch, R.J. The Muscular Dystrophies: From Genes to Therapies. Phys. Ther. 2005, 85, 1372–1388. [Google Scholar] [CrossRef] [PubMed]
- Hajahmadi, M.; Shemshadi, S.; Khalilipur, E.; Amin, A.; Taghavi, S.; Maleki, M.; Malek, H.; Naderi, N. Muscle wasting in young patients with dilated cardiomyopathy. J. Cachexia Sarcopenia Muscle 2017, 8, 542–548. [Google Scholar] [CrossRef]
- Harvey, P.A.; Leinwand, L.A. Cellular mechanisms of cardiomyopathy. J. Cell Biol. 2011, 194, 355–365. [Google Scholar] [CrossRef]
- Oudit, G.; Kassiri, Z.; Patel, M.; Chappell, M.; Butany, J.; Backx, P.; Tsushima, R.; Scholey, J.; Khokha, R.; Penninger, J. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc. Res. 2007, 75, 29–39. [Google Scholar] [CrossRef]
- Hong, B.K.; Kwon, H.M.; Byun, K.H.; Kim, D.S.; Choi, E.Y.; Kang, T.S.; Kang, S.M.; Chun, K.J.; Jang, Y.S.; Kim, H.S. Apoptosis in Dilated Cardiomyopathy. Korean J. Intern. Med. 2000, 15, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Phan, D.; Van Rooij, E.; Wang, D.-Z.; McAnally, J.; Qi, X.; Richardson, J.A.; Hill, J.A.; Bassel-Duby, R.; Olson, E.N. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J. Clin. Investig. 2008, 118, 124–132. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Russ. J. Cardiol. 2017, 1, 7–81. [Google Scholar] [CrossRef]
- Seo, Y.G.; Jang, M.J.; Lee, G.Y.; Jeon, E.S.; Park, W.H.; Sung, J.D. What Is the Optimal Exercise Prescription for Patients with Dilated Cardiomyopathy in Cardiac Rehabilitation? A SYSTEMATIC REVIEW. J. Cardiopulm. Rehabil. Prev. 2019, 39, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, M.; Pane, L.S.; Zhou, Q.; Chen, Z.; Murgia, M.; Schötterl, S.; Goedel, A.; Metzger, K.; Brade, T.; Parrotta, E.; et al. Antisense-mediated exon skipping: A therapeutic strategy for titin-based dilated cardiomyopathy. EMBO Mol. Med. 2015, 7, 562–576. [Google Scholar] [CrossRef]
- Lee, J.M.; Nobumori, C.; Tu, Y.; Choi, C.; Yang, S.H.; Jung, H.-J.; Vickers, T.A.; Rigo, F.; Bennett, C.F.; Young, S.G.; et al. Modulation of LMNA splicing as a strategy to treat prelamin A diseases. J. Clin. Investig. 2016, 126, 1592–1602. [Google Scholar] [CrossRef]
- Migliore, L.; Galvagni, F.; Pierantozzi, E.; Sorrentino, V.; Rossi, D. Allele-specific silencing by RNAi of R92Q and R173W mutations in cardiac troponin T. Exp. Biol. Med. 2022, 247, 805–814. [Google Scholar] [CrossRef]
- Grote Beverborg, N.; Später, D.; Knöll, R.; Hidalgo, A.; Yeh, S.T.; Elbeck, Z.; Silljé, H.H.W.; Eijgenraam, T.R.; Siga, H.; Zurek, M.; et al. Phospholamban antisense oligonucleotides improve cardiac function in murine cardiomyopathy. Nat. Commun. 2021, 12, 5180. [Google Scholar] [CrossRef]
- Stillitano, F.; Turnbull, I.C.; Karakikes, I.; Nonnenmacher, M.; Backeris, P.; Hulot, J.-S.; Kranias, E.G.; Hajjar, R.J.; Costa, K.D. Genomic correction of familial cardiomyopathy in human engineered cardiac tissues. Eur. Heart J. 2016, 37, 3282–3284. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Ghahremani, S.; Zimmerman, T.; Legere, N.; Thakar, K.; Ladha, F.A.; Pettinato, A.M.; Hinson, J.T. Reading Frame Repair of TTN Truncation Variants Restores Titin Quantity and Functions. Circulation 2022, 145, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Hoshijima, M.; Ikeda, Y.; Iwanaga, Y.; Minamisawa, S.; Date, M.; Gu, Y.; Iwatate, M.; Li, M.; Wang, L.; Wilson, J.M.; et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat. Med. 2002, 8, 864–871. [Google Scholar] [CrossRef]
- Woitek, F.; Zentilin, L.; Hoffman, N.E.; Powers, J.C.; Ottiger, I.; Parikh, S.; Kulczycki, A.M.; Hurst, M.; Ring, N.; Wang, T.; et al. Intracoronary Cytoprotective Gene Therapy. J. Am. Coll. Cardiol. 2015, 66, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, Y.; Zhan, Y.; Zhou, X.; Li, Y.; Zhao, C.; Sun, N.; Xu, C.; Liang, Q. Cardiac Overexpression of XIN Prevents Dilated Cardiomyopathy Caused by TNNT2 ΔK210 Mutation. Front. Cell Dev. Biol. 2021, 9, 691749. [Google Scholar] [CrossRef]
- Huang, S.; Li, J.; Li, Q.; Wang, Q.; Zhou, X.; Chen, J.; Chen, X.; Bellou, A.; Zhuang, J.; Lei, L. Cardiomyopathy: Pathogenesis and therapeutic interventions. MedComm 2024, 5, e772. [Google Scholar] [CrossRef]
- Lee, Y.; Lau, Y.; Cai, Z.; Lai, W.; Wong, L.; Tse, H.; Ng, K.; Siu, C. Modeling Treatment Response for Lamin A/C Related Dilated Cardiomyopathy in Human Induced Pluripotent Stem Cells. J. Am. Heart Assoc. 2017, 6, e005677. [Google Scholar] [CrossRef] [PubMed]
- Caragnano, A.; Aleksova, A.; Bulfoni, M.; Cervellin, C.; Rolle, I.G.; Veneziano, C.; Barchiesi, A.; Mimmi, M.C.; Vascotto, C.; Finato, N.; et al. Autophagy and Inflammasome Activation in Dilated Cardiomyopathy. J. Clin. Med. 2019, 8, 1519. [Google Scholar] [CrossRef]
- Kanamori, H.; Yoshida, A.; Naruse, G.; Endo, S.; Minatoguchi, S.; Watanabe, T.; Kawaguchi, T.; Tanaka, T.; Yamada, Y.; Takasugi, N.; et al. Impact of Autophagy on Prognosis of Patients With Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2022, 79, 789–801. [Google Scholar] [CrossRef]
- Wei, H.; Qu, H.; Wang, H.; Ji, B.; Ding, Y.; Liu, D.; Duan, Y.; Liang, H.; Peng, C.; Xiao, X.; et al. 1,25-Dihydroxyvitamin-D3 prevents the development of diabetic cardiomyopathy in type 1 diabetic rats by enhancing autophagy via inhibiting the β-catenin/TCF4/GSK-3β/mTOR pathway. J. Steroid Biochem. Mol. Biol. 2017, 168, 71–90. [Google Scholar] [CrossRef]
- Kanamori, H.; Naruse, G.; Yoshida, A.; Minatoguchi, S.; Watanabe, T.; Kawaguchi, T.; Yamada, Y.; Mikami, A.; Kawasaki, M.; Takemura, G.; et al. Metformin Enhances Autophagy and Provides Cardioprotection in δ-Sarcoglycan Deficiency-Induced Dilated Cardiomyopathy. Circ. Heart Fail. 2019, 12, e005418. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, H.; Takemura, G.; Goto, K.; Tsujimoto, A.; Ogino, A.; Takeyama, T.; Kawaguchi, T.; Watanabe, T.; Morishita, K.; Kawasaki, M.; et al. Resveratrol Reverses Remodeling in Hearts with Large, Old Myocardial Infarctions through Enhanced Autophagy-Activating AMP Kinase Pathway. Am. J. Pathol. 2013, 182, 701–713. [Google Scholar] [CrossRef]
- Watanabe, T.; Takemura, G.; Kanamori, H.; Goto, K.; Tsujimoto, A.; Okada, H.; Kawamura, I.; Ogino, A.; Takeyama, T.; Kawaguchi, T.; et al. Restriction of Food Intake Prevents Postinfarction Heart Failure by Enhancing Autophagy in the Surviving Cardiomyocytes. Am. J. Pathol. 2014, 184, 1384–1394. [Google Scholar] [CrossRef]
- Fatkin, D.; Huttner, I.G.; Kovacic, J.C.; Seidman, J.G.; Seidman, C.E. Precision Medicine in the Management of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2019, 74, 2921–2938. [Google Scholar] [CrossRef]
- Sinagra, G.; Merlo, M.; Pinamonti, B. (Eds.) Dilated Cardiomyopathy: From Genetics to Clinical Management; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Liang, H.; Liu, F.; Li, Y.; Zhang, H.; Wang, C.; Wang, Q. Identification of Signature Genes of Dilated Cardiomyopathy Using Integrated Bioinformatics Analysis. Int. J. Mol. Sci. 2023, 24, 7339. [Google Scholar] [CrossRef] [PubMed]
- Berardo, A.; DiMauro, S.; Hirano, M. A Diagnostic Algorithm for Metabolic Myopathies. Curr. Neurol. Neurosci. Rep. 2010, 10, 118–126. [Google Scholar] [CrossRef]
- Angelini, C.; Marozzo, R.; Pegoraro, V.; Sacconi, S. Diagnostic challenges in metabolic myopathies. Expert Rev. Neurother. 2020, 20, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- De Barcelos, I.P.; Emmanuele, V.; Hirano, M. Advances in primary mitochondrial myopathies. Curr. Opin. Neurol. 2019, 32, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Moraczewska, J. Thin filament dysfunctions caused by mutations in tropomyosin Tpm3.12 and Tpm1.1. J. Muscle Res. Cell Motil. 2020, 41, 39–53. [Google Scholar] [CrossRef]
- Nilipour, Y.; Fatehi, F.; Sanatinia, S.; Bradshaw, A.; Duff, J.; Lochmüller, H.; Horvath, R.; Nafissi, S. Multiple acyl-coenzyme A dehydrogenase deficiency shows a possible founder effect and is the most frequent cause of lipid storage myopathy in Iran. J. Neurol. Sci. 2020, 411, 116707. [Google Scholar] [CrossRef]
- Lefèvre, C.R.; Labarthe, F.; Dufour, D.; Moreau, C.; Faoucher, M.; Rollier, P.; Arnoux, J.-B.; Tardieu, M.; Damaj, L.; Bendavid, C.; et al. Newborn Screening of Primary Carnitine Deficiency: An Overview of Worldwide Practices and Pitfalls to Define an Algorithm before Expansion of Newborn Screening in France. Int. J. Neonatal Screen. 2023, 9, 6. [Google Scholar] [CrossRef]
- Fan, H.-C.; Lee, H.-F.; Yue, C.-T.; Chi, C.-S. Clinical Characteristics of Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes. Life 2021, 11, 1111. [Google Scholar] [CrossRef]
- Godfrey, R.; Quinlivan, R. Skeletal muscle disorders of glycogenolysis and glycolysis. Nat. Rev. Neurol. 2016, 12, 393–402. [Google Scholar] [CrossRef]
- Finsterer, J. An update on diagnosis and therapy of metabolic myopathies. Expert Rev. Neurother. 2018, 18, 933–943. [Google Scholar] [CrossRef]
- Gümüş, E.; Özen, H. Glycogen storage diseases: An update. World J. Gastroenterol. 2023, 29, 3932–3963. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Kishnani, P.S.; Koeberl, D. Glycogen Storage Diseases. In The Online Metabolic and Molecular Bases of Inherited Disease; Valle, D.L., Antonarakis, S., Ballabio, A., Beaudet, A.L., Mitchell, G.A., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Lilleker, J.B.; Keh, Y.S.; Roncaroli, F.; Sharma, R.; Roberts, M. Metabolic myopathies: A practical approach. Pract. Neurol. 2018, 18, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.; Cammarata, G.; Colomba, P.; Sciarrino, S.; Zizzo, C.; Francofonte, D.; Zora, M.; Scalia, S.; Brando, C.; Curto, A.L.; et al. Pompe disease: Pathogenesis, molecular genetics and diagnosis. Aging 2020, 12, 15856–15874. [Google Scholar] [CrossRef]
- Roig-Zamboni, V.; Cobucci-Ponzano, B.; Iacono, R.; Ferrara, M.C.; Germany, S.; Bourne, Y.; Parenti, G.; Moracci, M.; Sulzenbacher, G. Structure of human lysosomal acid α-glucosidase–a guide for the treatment of Pompe disease. Nat. Commun. 2017, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- Kohler, L.; Puertollano, R.; Raben, N. Pompe Disease: From Basic Science to Therapy. Neurotherapeutics 2018, 15, 928–942. [Google Scholar] [CrossRef]
- Meena, N.K.; Raben, N. Pompe Disease: New Developments in an Old Lysosomal Storage Disorder. Biomolecules 2020, 10, 1339. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Xu, T.; Guan, K.-L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003, 17, 1829–1834. [Google Scholar] [CrossRef]
- Lim, J.-A.; Sun, B.; Puertollano, R.; Raben, N. Therapeutic Benefit of Autophagy Modulation in Pompe Disease. Mol. Ther. 2018, 26, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Do, H.V.; Khanna, R.; Gotschall, R. Challenges in treating Pompe disease: An industry perspective. Ann. Transl. Med. 2019, 7, 291. [Google Scholar] [CrossRef]
- Remes, A.; Franz, M.; Mohr, F.; Weber, A.; Rapti, K.; Jungmann, A.; Karck, M.; Hecker, M.; Kallenbach, K.; Müller, O.J.; et al. AAV-Mediated Expression of AP-1-Neutralizing RNA Decoy Oligonucleotides Attenuates Transplant Vasculopathy in Mouse Aortic Allografts. Mol. Ther.—Methods Clin. Dev. 2019, 15, 246–256. [Google Scholar] [CrossRef]
- Decostre, V.; Laforêt, P.; De Antonio, M.; Kachetel, K.; Canal, A.; Ollivier, G.; Nadaj-Pakleza, A.; Petit, F.M.; Wahbi, K.; Fayssoil, A.; et al. Long term longitudinal study of muscle function in patients with glycogen storage disease type IIIa. Mol. Genet. Metab. 2017, 122, 108–116. [Google Scholar] [CrossRef]
- Deschauer, M.; Morgenroth, A.; Joshi, P.R.; Gläser, D.; Chinnery, P.F.; Aasly, J.; Schreiber, H.; Knape, M.; Zierz, S.; Vorgerd, M. Analysis of spectrum and frequencies of mutations in McArdle disease: Identification of 13 novel mutations. J. Neurol. 2007, 254, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Voth, A.; Sayas Catalan, J.; Corral Blanco, M.; Castaño Mendez, A.; Martin, M.A.; De Fuenmayor Fernandez De La Hoz, C.; Villena Garrido, V.; Dominguez-Gonzalez, C. Deoxynucleoside therapy for respiratory involvement in adult patients with thymidine kinase 2-deficient myopathy. BMJ Open Respir. Res. 2020, 7, e000774. [Google Scholar] [CrossRef] [PubMed]
- Pizzamiglio, C.; Mahroo, O.A.; Khan, K.N.; Patasin, M.; Quinlivan, R. Phenotype and genotype of 197 British patients with McArdle disease: An observational single-centre study. J. Inherit. Metab. Dis. 2021, 44, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Salazar, M.; Brull, A.; Nogales-Gadea, G.; Andreu, A.L.; Martín, M.A.; Arenas, J.; Santalla, A.; Lucia, A.; Vissing, J.; Krag, T.O.; et al. Preclinical Research in McArdle Disease: A Review of Research Models and Therapeutic Strategies. Genes 2021, 13, 74. [Google Scholar] [CrossRef]
- Llavero, F.; Arrazola Sastre, A.; Luque Montoro, M.; Gálvez, P.; Lacerda, H.M.; Parada, L.A.; Zugaza, J.L. McArdle Disease: New Insights into Its Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2019, 20, 5919. [Google Scholar] [CrossRef]
- Sproule, D.M.; Kaufmann, P. Mitochondrial Encephalopathy, Lactic Acidosis, and Strokelike Episodes: Basic Concepts, Clinical Phenotype, and Therapeutic Management of MELAS Syndrome. Ann. N. Y. Acad. Sci. 2008, 1142, 133–158. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Metabolic Myopathies. Continuum 2016, 22, 1829–1851. [Google Scholar] [CrossRef]
- Merritt Ii, J.L.; Norris, M.; Kanungo, S. Fatty acid oxidation disorders. Ann. Transl. Med. 2018, 6, 473. [Google Scholar] [CrossRef]
- Horvath, R.; Schneiderat, P.; Schoser, B.G.H.; Gempel, K.; Neuen-Jacob, E.; Plöger, H.; Müller-Höcker, J.; Pongratz, D.E.; Naini, A.; DiMauro, S.; et al. Coenzyme Q10 deficiency and isolated myopathy. Neurology 2006, 66, 253–255. [Google Scholar] [CrossRef]
- Toscano, A.; Barca, E.; Musumeci, O. Update on diagnostics of metabolic myopathies. Curr. Opin. Neurol. 2017, 30, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Schuier, A.M.; Wood, P.A. Mouse Models for Disorders of Mitochondrial Fatty Acid β-Oxidation. ILAR J. 2002, 43, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Van Adel, B.A.; Tarnopolsky, M.A. Metabolic Myopathies: Update 2009. J. Clin. Neuromuscul. Dis. 2009, 10, 97–121. [Google Scholar] [CrossRef]
- Calvo, S.E.; Clauser, K.R.; Mootha, V.K. MitoCarta2.0: An updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016, 44, D1251–D1257. [Google Scholar] [CrossRef] [PubMed]
- Di Donfrancesco, A.; Massaro, G.; Di Meo, I.; Tiranti, V.; Bottani, E.; Brunetti, D. Gene Therapy for Mitochondrial Diseases: Current Status and Future Perspective. Pharmaceutics 2022, 14, 1287. [Google Scholar] [CrossRef]
- Quinlivan, R.; Jungbluth, H. Myopathic causes of exercise intolerance with rhabdomyolysis. Dev. Med. Child Neurol. 2012, 54, 886–891. [Google Scholar] [CrossRef]
- Elston, T.; Wang, H.; Oster, G. Energy transduction in ATP synthase. Nature 1998, 391, 510–513. [Google Scholar] [CrossRef]
- Sharp, L.J.; Haller, R.G. Metabolic and Mitochondrial Myopathies. Neurol. Clin. 2014, 32, 777–799. [Google Scholar] [CrossRef]
- Shemesh, A.; Margolin, E. Kearns-Sayre Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK482341/ (accessed on 14 July 2025).
- Angelini, C.; Nascimbeni, A.C.; Semplicini, C. Therapeutic advances in the management of Pompe disease and other metabolic myopathies. Ther. Adv. Neurol. Disord. 2013, 6, 311–321. [Google Scholar] [CrossRef]
- Laforêt, P.; Vianey-Saban, C. Disorders of muscle lipid metabolism: Diagnostic and therapeutic challenges. Neuromuscul. Disord. 2010, 20, 693–700. [Google Scholar] [CrossRef]
- Fichna, J.P.; Maruszak, A.; Żekanowski, C. Myofibrillar myopathy in the genomic context. J. Appl. Genet. 2018, 59, 431–439. [Google Scholar] [CrossRef]
- Selcen, D. Myofibrillar myopathies. Neuromuscul. Disord. 2011, 21, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.G.; Waddell, L.B.; Ghaoui, R.; Evesson, F.J.; Cummings, B.B.; Bryen, S.J.; Joshi, H.; Wang, M.-X.; Brammah, S.; Kritharides, L.; et al. Recessive DES cardio/myopathy without myofibrillar aggregates: Intronic splice variant silences one allele leaving only missense L190P-desmin. Eur. J. Hum. Genet. 2019, 27, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Wittig, I.; Peeva, V.; Eggers, B.; Heidler, J.; Chevessier, F.; Kley, R.A.; Barkovits, K.; Strecker, V.; Berwanger, C.; et al. Mutant desmin substantially perturbs mitochondrial morphology, function and maintenance in skeletal muscle tissue. Acta Neuropathol. 2016, 132, 453–473. [Google Scholar] [CrossRef]
- Brodehl, A.; Gaertner-Rommel, A.; Milting, H. Molecular insights into cardiomyopathies associated with desmin (DES) mutations. Biophys. Rev. 2018, 10, 983–1006. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Huang, W.; Horak, K.M.; Zheng, H.; Mestril, R.; Wang, X. Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J. 2006, 20, 362–364. [Google Scholar] [CrossRef]
- Fichna, J.P.; Potulska-Chromik, A.; Miszta, P.; Redowicz, M.J.; Kaminska, A.M.; Zekanowski, C.; Filipek, S. A novel dominant D109A CRYAB mutation in a family with myofibrillar myopathy affects αB-crystallin structure. BBA Clin. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Smyth, C.; Špakulová, I.; Cotton-Barratt, O.; Rafiq, S.; Tapper, W.; Upstill-Goddard, R.; Hopper, J.L.; Makalic, E.; Schmidt, D.F.; Kapuscinski, M.; et al. Quantifying the cumulative effect of low-penetrance genetic variants on breast cancer risk. Mol. Genet. Genom. Med. 2015, 3, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Olivé, M.; Goldfarb, L.G.; Shatunov, A.; Fischer, D.; Ferrer, I. Myotilinopathy: Refining the clinical and myopathological phenotype. Brain 2005, 128, 2315–2326. [Google Scholar] [CrossRef]
- Pathak, P.; Blech-Hermoni, Y.; Subedi, K.; Mpamugo, J.; Obeng-Nyarko, C.; Ohman, R.; Molloy, I.; Kates, M.; Hale, J.; Stauffer, S.; et al. Myopathy associated LDB3 mutation causes Z-disc disassembly and protein aggregation through PKCα and TSC2-mTOR downregulation. Commun. Biol. 2021, 4, 355. [Google Scholar] [CrossRef]
- Kley, R.A.; Leber, Y.; Schrank, B.; Zhuge, H.; Orfanos, Z.; Kostan, J.; Onipe, A.; Sellung, D.; Güttsches, A.K.; Eggers, B.; et al. FLNC-Associated Myofibrillar Myopathy: New Clinical, Functional, and Proteomic Data. Neurol. Genet. 2021, 7, e590. [Google Scholar] [CrossRef]
- Tedesco, B.; Vendredy, L.; Timmerman, V.; Poletti, A. The chaperone-assisted selective autophagy complex dynamics and dysfunctions. Autophagy 2023, 19, 1619–1641. [Google Scholar] [CrossRef]
- Semmler, A.-L.; Sacconi, S.; Bach, J.E.; Liebe, C.; Bürmann, J.; Kley, R.A.; Ferbert, A.; Anderheiden, R.; Van Den Bergh, P.; Martin, J.-J.; et al. Unusual multisystemic involvement and a novel BAG3 mutation revealed by NGS screening in a large cohort of myofibrillar myopathies. Orphanet J. Rare Dis. 2014, 9, 121. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kokubun, N.; Inoue, M.; Komagamine, T.; Aoki, R.; Nishino, I.; Hirata, K. A family with adult-onset myofibrillar myopathy with BAG3 mutation (P470S) presenting with axonal polyneuropathy. Neuromuscul. Disord. 2020, 30, 727–731. [Google Scholar] [CrossRef]
- Meister-Broekema, M.; Freilich, R.; Jagadeesan, C.; Rauch, J.N.; Bengoechea, R.; Motley, W.W.; Kuiper, E.F.E.; Minoia, M.; Furtado, G.V.; Van Waarde, M.A.W.H.; et al. Myopathy associated BAG3 mutations lead to protein aggregation by stalling Hsp70 networks. Nat. Commun. 2018, 9, 5342. [Google Scholar] [CrossRef]
- Ruparelia, A.A.; Oorschot, V.; Vaz, R.; Ramm, G.; Bryson-Richardson, R.J. Zebrafish models of BAG3 myofibrillar myopathy suggest a toxic gain of function leading to BAG3 insufficiency. Acta Neuropathol. 2014, 128, 821–833. [Google Scholar] [CrossRef]
- Adriaenssens, E.; Tedesco, B.; Mediani, L.; Asselbergh, B.; Crippa, V.; Antoniani, F.; Carra, S.; Poletti, A.; Timmerman, V. BAG3 Pro209 mutants associated with myopathy and neuropathy relocate chaperones of the CASA-complex to aggresomes. Sci. Rep. 2020, 10, 8755. [Google Scholar] [CrossRef]
- Selcen, D.; Muntoni, F.; Burton, B.K.; Pegoraro, E.; Sewry, C.; Bite, A.V.; Engel, A.G. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann. Neurol. 2009, 65, 83–89. [Google Scholar] [CrossRef]
- Konersman, C.G.; Bordini, B.J.; Scharer, G.; Lawlor, M.W.; Zangwill, S.; Southern, J.F.; Amos, L.; Geddes, G.C.; Kliegman, R.; Collins, M.P. BAG3 myofibrillar myopathy presenting with cardiomyopathy. Neuromuscul. Disord. 2015, 25, 418–422. [Google Scholar] [CrossRef]
- Robertson, R.; Conte, T.; Dicaire, M.; Bryson-Richardson, R.; Lavoie, J.; O’Ferrall, E.; Young, J.; Brais, B. Mouse model of BAG3 myofibrillar myopathy. Neuromuscul. Disord. 2017, 27, S121. [Google Scholar] [CrossRef]
- Piga, D.; Zanotti, S.; Ripolone, M.; Napoli, L.; Ciscato, P.; Gibertini, S.; Maggi, L.; Fortunato, F.; Rigamonti, A.; Ronchi, D.; et al. Association between ZASP/LDB3 Pro26Ser and Inclusion Body Myopathy. Int. J. Mol. Sci. 2024, 25, 6547. [Google Scholar] [CrossRef]
- Ruparelia, A.A.; McKaige, E.A.; Williams, C.; Schulze, K.E.; Fuchs, M.; Oorschot, V.; Lacene, E.; Meregalli, M.; Lee, C.; Serrano, R.J.; et al. Metformin rescues muscle function in BAG3 myofibrillar myopathy models. Autophagy 2021, 17, 2494–2510. [Google Scholar] [CrossRef]
- Shin, J.W.; Kim, K.-H.; Lee, Y.; Choi, D.E.; Lee, J.-M. Personalized allele-specific CRISPR-Cas9 strategies for myofibrillar myopathy 6. medRxiv 2024. [Google Scholar] [CrossRef]
- Mout, R.; Ray, M.; Lee, Y.-W.; Scaletti, F.; Rotello, V.M. In Vivo Delivery of CRISPR/Cas9 for Therapeutic Gene Editing: Progress and Challenges. Bioconjug. Chem. 2017, 28, 880–884. [Google Scholar] [CrossRef]
- Rabaan, A.A.; AlSaihati, H.; Bukhamsin, R.; Bakhrebah, M.A.; Nassar, M.S.; Alsaleh, A.A.; Alhashem, Y.N.; Bukhamseen, A.Y.; Al-Ruhimy, K.; Alotaibi, M.; et al. Application of CRISPR/Cas9 Technology in Cancer Treatment: A Future Direction. Curr. Oncol. 2023, 30, 1954–1976. [Google Scholar] [CrossRef]
- Stewart, M.D.; Ritterhoff, T.; Klevit, R.E.; Brzovic, P.S. E2 enzymes: More than just middle men. Cell Res. 2016, 26, 423–440. [Google Scholar] [CrossRef]
- Franceschelli, S.; Rosati, A.; Lerose, R.; De Nicola, S.; Turco, M.C.; Pascale, M. bag3 gene expression is regulated by heat shock factor 1. J. Cell. Physiol. 2008, 215, 575–577. [Google Scholar] [CrossRef]
- Stürner, E.; Behl, C. The Role of the Multifunctional BAG3 Protein in Cellular Protein Quality Control and in Disease. Front. Mol. Neurosci. 2017, 10, 177. [Google Scholar] [CrossRef]
- Brenner, C.M.; Choudhary, M.; McCormick, M.G.; Cheung, D.; Landesberg, G.P.; Wang, J.-F.; Song, J.; Martin, T.G.; Cheung, J.Y.; Qu, H.-Q.; et al. BAG3: Nature’s Quintessential Multi-Functional Protein Functions as a Ubiquitous Intra-Cellular Glue. Cells 2023, 12, 937. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.-A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 Binds Directly to Atg8/LC3 to Facilitate Degradation of Ubiquitinated Protein Aggregates by Autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef]
- the Italian Network on Congenital Myopathies; Cassandrini, D.; Trovato, R.; Rubegni, A.; Lenzi, S.; Fiorillo, C.; Baldacci, J.; Minetti, C.; Astrea, G.; Bruno, C.; et al. Congenital myopathies: Clinical phenotypes and new diagnostic tools. Ital. J. Pediatr. 2017, 43, 101. [Google Scholar] [CrossRef]
- Huang, K.; Bi, F.-F.; Yang, H. Corrigendum: A Systematic Review and Meta-Analysis of the Prevalence of Congenital Myopathy. Front. Neurol. 2022, 13, 857959, Erratum in Front. Neurol. 2022, 13, 857959. [Google Scholar] [CrossRef]
- North, K.N.; Wang, C.H.; Clarke, N.; Jungbluth, H.; Vainzof, M.; Dowling, J.J.; Amburgey, K.; Quijano-Roy, S.; Beggs, A.H.; Sewry, C.; et al. Approach to the diagnosis of congenital myopathies. Neuromuscul. Disord. 2014, 24, 97–116. [Google Scholar] [CrossRef]
- Sewry, C.A.; Brown, S.C.; Pelin, K.; Jungbluth, H.; Wallgren-Pettersson, C.; Labeit, S.; Manzur, A.; Muntoni, F. Abnormalities in the expression of nebulin in chromosome-2 linked nemaline myopathy. Neuromuscul. Disord. 2001, 11, 146–153. [Google Scholar] [CrossRef]
- Karpicheva, O.E.; Simonyan, A.O.; Rysev, N.A.; Redwood, C.S.; Borovikov, Y.S. Looking for Targets to Restore the Contractile Function in Congenital Myopathy Caused by Gln147Pro Tropomyosin. Int. J. Mol. Sci. 2020, 21, 7590. [Google Scholar] [CrossRef]
- Sambuughin, N.; Swietnicki, W.; Techtmann, S.; Matrosova, V.; Wallace, T.; Goldfarb, L.; Maynard, E. KBTBD13 interacts with Cullin 3 to form a functional ubiquitin ligase. Biochem. Biophys. Res. Commun. 2012, 421, 743–749. [Google Scholar] [CrossRef]
- Garg, A.; O’Rourke, J.; Long, C.; Doering, J.; Ravenscroft, G.; Bezprozvannaya, S.; Nelson, B.R.; Beetz, N.; Li, L.; Chen, S.; et al. KLHL40 deficiency destabilizes thin filament proteins and promotes nemaline myopathy. J. Clin. Investig. 2014, 124, 3529–3539. [Google Scholar] [CrossRef]
- De Winter, J.M.; Molenaar, J.P.; Yuen, M.; Van Der Pijl, R.; Shen, S.; Conijn, S.; Van De Locht, M.; Willigenburg, M.; Bogaards, S.J.P.; Van Kleef, E.S.B.; et al. KBTBD13 is an actin-binding protein that modulates muscle kinetics. J. Clin. Investig. 2020, 130, 754–767. [Google Scholar] [CrossRef]
- Gómez-Oca, R.; Edelweiss, E.; Djeddi, S.; Gerbier, M.; Massana-Muñoz, X.; Oulad-Abdelghani, M.; Crucifix, C.; Spiegelhalter, C.; Messaddeq, N.; Poussin-Courmontagne, P.; et al. Differential impact of ubiquitous and muscle dynamin 2 isoforms in muscle physiology and centronuclear myopathy. Nat. Commun. 2022, 13, 6849. [Google Scholar] [CrossRef]
- Lionello, V.M.; Kretz, C.; Edelweiss, E.; Crucifix, C.; Gómez-Oca, R.; Messaddeq, N.; Buono, S.; Koebel, P.; Massana Muñoz, X.; Diedhiou, N.; et al. BIN1 modulation in vivo rescues dynamin-related myopathy. Proc. Natl. Acad. Sci. USA 2022, 119, e2109576119. [Google Scholar] [CrossRef]
- Arbogast, S.; Beuvin, M.; Fraysse, B.; Zhou, H.; Muntoni, F.; Ferreiro, A. Oxidative stress in SEPN1-related myopathy: From pathophysiology to treatment. Ann. Neurol. 2009, 65, 677–686. [Google Scholar] [CrossRef]
- Endo, Y.; Groom, L.; Wang, S.M.; Pannia, E.; Griffiths, N.W.; Van Gennip, J.L.M.; Ciruna, B.; Laporte, J.; Dirksen, R.T.; Dowling, J.J. Two zebrafish cacna1s loss-of-function variants provide models of mild and severe CACNA1S-related myopathy. Hum. Mol. Genet. 2024, 33, 254–269. [Google Scholar] [CrossRef]
- Laporte, J.; Hu, L.J.; Kretz, C.; Mandel, J.-L.; Kioschis, P.; Coy, J.F.; Klauck, S.M.; Poustka, A.; Dahl, N. A gene mutated in X–linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat. Genet. 1996, 13, 175–182. [Google Scholar] [CrossRef]
- Dowling, J.J.; Vreede, A.P.; Low, S.E.; Gibbs, E.M.; Kuwada, J.Y.; Bonnemann, C.G.; Feldman, E.L. Loss of Myotubularin Function Results in T-Tubule Disorganization in Zebrafish and Human Myotubular Myopathy. PLoS Genet. 2009, 5, e1000372. [Google Scholar] [CrossRef]
- Sabha, N.; Volpatti, J.R.; Gonorazky, H.; Reifler, A.; Davidson, A.E.; Li, X.; Eltayeb, N.M.; Dall’Armi, C.; Di Paolo, G.; Brooks, S.V.; et al. PIK3C2B inhibition improves function and prolongs survival in myotubular myopathy animal models. J. Clin. Investig. 2016, 126, 3613–3625. [Google Scholar] [CrossRef]
- Giraud, Q.; Spiegelhalter, C.; Messaddeq, N.; Laporte, J. MTM1 overexpression prevents and reverts BIN1-related centronuclear myopathy. Brain 2023, 146, 4158–4173. [Google Scholar] [CrossRef]
- Lawlor, M.W.; Viola, M.G.; Meng, H.; Edelstein, R.V.; Liu, F.; Yan, K.; Luna, E.J.; Lerch-Gaggl, A.; Hoffmann, R.G.; Pierson, C.R.; et al. Differential Muscle Hypertrophy Is Associated with Satellite Cell Numbers and Akt Pathway Activation Following Activin Type IIB Receptor Inhibition in Mtm1 p.R69C Mice. Am. J. Pathol. 2014, 184, 1831–1842. [Google Scholar] [CrossRef]
- Lawlor, M.W.; Read, B.P.; Edelstein, R.; Yang, N.; Pierson, C.R.; Stein, M.J.; Wermer-Colan, A.; Buj-Bello, A.; Lachey, J.L.; Seehra, J.S.; et al. Inhibition of Activin Receptor Type IIB Increases Strength and Lifespan in Myotubularin-Deficient Mice. Am. J. Pathol. 2011, 178, 784–793. [Google Scholar] [CrossRef]
- Trochet, D.; Prudhon, B.; Beuvin, M.; Peccate, C.; Lorain, S.; Julien, L.; Benkhelifa-Ziyyat, S.; Rabai, A.; Mamchaoui, K.; Ferry, A.; et al. Allele-specific silencing therapy for Dynamin 2-related dominant centronuclear myopathy. EMBO Mol. Med. 2018, 10, 239–253. [Google Scholar] [CrossRef]
- Rabai, A.; Reisser, L.; Reina-San-Martin, B.; Mamchaoui, K.; Cowling, B.S.; Nicot, A.-S.; Laporte, J. Allele-Specific CRISPR/Cas9 Correction of a Heterozygous DNM2 Mutation Rescues Centronuclear Myopathy Cell Phenotypes. Mol. Ther.—Nucleic Acids 2019, 16, 246–256. [Google Scholar] [CrossRef]
- Savarese, M.; Jokela, M.; Udd, B. Chapter 20—Distal myopathy. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 497–519. [Google Scholar] [CrossRef]
- Klar, J.; Sobol, M.; Melberg, A.; Mäbert, K.; Ameur, A.; Johansson, A.C.V.; Feuk, L.; Entesarian, M.; Örlén, H.; Casar-Borota, O.; et al. Welander Distal Myopathy Caused by an Ancient Founder Mutation in TIA1 Associated with Perturbed Splicing. Hum. Mutat. 2013, 34, 572–577. [Google Scholar] [CrossRef]
- Labeit, S.; Gautel, M.; Lakey, A.; Trinick, J. Towards a molecular understanding of titin. EMBO J. 1992, 11, 1711–1716. [Google Scholar] [CrossRef]
- Dimachkie, M.M.; Barohn, R.J. Distal Myopathies. Neurol. Clin. 2014, 32, 817–842. [Google Scholar] [CrossRef]
- Eisenberg, I.; Avidan, N.; Potikha, T.; Hochner, H.; Chen, M.; Olender, T.; Barash, M.; Shemesh, M.; Sadeh, M.; Grabov-Nardini, G.; et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat. Genet. 2001, 29, 83–87. [Google Scholar] [CrossRef]
- Mullen, J.; Alrasheed, K.; Mozaffar, T. GNE myopathy: History, etiology, and treatment trials. Front. Neurol. 2022, 13, e2109576119. [Google Scholar] [CrossRef]
- Singh, R.; Chaudhary, P.; Arya, R. Role of IGF-1R in ameliorating apoptosis of GNE deficient cells. Sci. Rep. 2018, 8, 7323. [Google Scholar] [CrossRef]
- Singh, R.; Arya, R. GNE Myopathy and Cell Apoptosis: A Comparative Mutation Analysis. Mol. Neurobiol. 2016, 53, 3088–3101. [Google Scholar] [CrossRef]
- Malicdan, M.C.V.; Noguchi, S.; Nishino, I. Autophagy in a Mouse Model of Distal Myopathy with Rimmed Vacuoles or Hereditary Inclusion Body Myopathy. Autophagy 2007, 3, 396–398. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Liu, F.; Zhang, X.; Li, W.; Liu, S.; Zhao, Y.; Gong, Y.; Yan, C. Unfolded Protein Response and Activated Degradative Pathways Regulation in GNE Myopathy. PLoS ONE 2013, 8, e58116. [Google Scholar] [CrossRef]
- Khadilkar, S.V.; Nallamilli, B.R.R.; Bhutada, A.; Hegde, M.; Gandhi, K.; Faldu, H.D.; Patil, S.B. A report on GNE myopathy: Individuals of Rajasthan ancestry share the Roma gene. J. Neurol. Sci. 2017, 375, 239–240. [Google Scholar] [CrossRef]
- Bosch-Morató, M.; Iriondo, C.; Guivernau, B.; Valls-Comamala, V.; Vidal, N.; Olivé, M.; Querfurth, H.; Muñoz, F.J. Increased amyloid β-peptide uptake in skeletal muscle is induced by hyposialylation and may account for apoptosis in GNE myopathy. Oncotarget 2016, 7, 13354–13371. [Google Scholar] [CrossRef]
- Strodel, B.; Lee, J.W.L.; Whittleston, C.S.; Wales, D.J. Transmembrane Structures for Alzheimer’s Aβ1−42 Oligomers. J. Am. Chem. Soc. 2010, 132, 13300–13312. [Google Scholar] [CrossRef]
- Carrillo, N.; Malicdan, M.C.; Huizing, M. GNE Myopathy: Etiology, Diagnosis, and Therapeutic Challenges. Neurotherapeutics 2018, 15, 900–914. [Google Scholar] [CrossRef]
- Oswalia, J.; Singh, S.; Gautam, V.; Arya, R. Altered autophagic flux in GNE mutant cells of Indian origin: Potential drug target for GNE myopathy. Exp. Cell Res. 2024, 440, 114118. [Google Scholar] [CrossRef]
- Yadav, R.; Devi, S.S.; Oswalia, J.; Ramalingam, S.; Arya, R. Role of HSP70 chaperone in protein aggregate phenomenon of GNE mutant cells: Therapeutic lead for GNE Myopathy. Int. J. Biochem. Cell Biol. 2022, 149, 106258. [Google Scholar] [CrossRef]
- Devi, S.S.; Yadav, R.; Arya, R. Altered Actin Dynamics in Cell Migration of GNE Mutant Cells. Front. Cell Dev. Biol. 2021, 9, 603742. [Google Scholar] [CrossRef]
- Lochmüller, H.; Behin, A.; Caraco, Y.; Lau, H.; Mirabella, M.; Tournev, I.; Tarnopolsky, M.; Pogoryelova, O.; Woods, C.; Lai, A.; et al. A phase 3 randomized study evaluating sialic acid extended-release for GNE myopathy. Neurology 2019, 92, e2109-17. [Google Scholar] [CrossRef]
- Zhang, T.; Yin, X.; Yu, X.; Shang, R.; Lu, L.; Miao, J. Metformin protects fibroblasts from patients with GNE myopathy by restoring autophagic flux via an AMPK/mTOR-independent pathway. Biomed. Pharmacother. 2023, 164, 114958. [Google Scholar] [CrossRef]
- Nemunaitis, G.; Jay, C.M.; Maples, P.B.; Gahl, W.A.; Huizing, M.; Yardeni, T.; Tong, A.W.; Phadke, A.P.; Pappen, B.O.; Bedell, C.; et al. Hereditary Inclusion Body Myopathy: Single Patient Response to Intravenous Dosing of GNE Gene Lipoplex. Hum. Gene Ther. 2011, 22, 1331–1341. [Google Scholar] [CrossRef]
- Hofhuis, J.; Bersch, K.; Büssenschütt, R.; Drzymalski, M.; Liebetanz, D.; Nikolaev, V.O.; Wagner, S.; Maier, L.S.; Gärtner, J.; Klinge, L.; et al. Dysferlin mediates membrane tubulation and links T-tubule biogenesis to muscular dystrophy. J. Cell Sci. 2017, 130, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, A.M.; González-Jamett, A.M.; Cea, L.A.; Bevilacqua, J.A.; Caviedes, P. Dysferlin function in skeletal muscle: Possible pathological mechanisms and therapeutical targets in dysferlinopathies. Exp. Neurol. 2016, 283, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Reyngoudt, H.; Smith, F.E.; Caldas De Almeida Araújo, E.; Wilson, I.; Fernández-Torrón, R.; James, M.K.; Moore, U.R.; Díaz-Manera, J.; Marty, B.; Azzabou, N.; et al. Three-year quantitative magnetic resonance imaging and phosphorus magnetic resonance spectroscopy study in lower limb muscle in dysferlinopathy. J. Cachexia Sarcopenia Muscle 2022, 13, 1850–1863. [Google Scholar] [CrossRef]
- Woudt, L.; Di Capua, G.A.; Krahn, M.; Castiglioni, C.; Hughes, R.; Campero, M.; Trangulao, A.; González-Hormazábal, P.; Godoy-Herrera, R.; Lévy, N.; et al. Toward an objective measure of functional disability in dysferlinopathy. Muscle Nerve 2016, 53, 49–57. [Google Scholar] [CrossRef]
- Harris, E.; Bladen, C.L.; Mayhew, A.; James, M.; Bettinson, K.; Moore, U.; Smith, F.E.; Rufibach, L.; Cnaan, A.; Bharucha-Goebel, D.X.; et al. The Clinical Outcome Study for dysferlinopathy: An international multicenter study. Neurol. Genet. 2016, 2, e89. [Google Scholar] [CrossRef]
- Ranta-aho, J.; Johari, M.; Udd, B. Current advance on distal myopathy genetics. Curr. Opin. Neurol. 2024, 37, 515–522. [Google Scholar] [CrossRef]
- Ashton, C.; Paramalingam, S.; Stevenson, B.; Brusch, A.; Needham, M. Idiopathic inflammatory myopathies: A review. Intern. Med. J. 2021, 51, 845–852. [Google Scholar] [CrossRef]
- Triplett, J.; Kassardjian, C.D.; Liewluck, T.; Tahir, A.; Lennon, V.; Kopecky, S.; Milone, M. Cardiac and Respiratory Complications of Necrotizing Autoimmune Myopathy. Mayo Clin. Proc. 2020, 95, 2144–2149. [Google Scholar] [CrossRef]
- Lu, Z.; Guo-chun, W.; Li, M.; Ning, Z. Cardiac Involvement in Adult Polymyositis or Dermatomyositis: A Systematic Review. Clin. Cardiol. 2012, 35, 685–691. [Google Scholar] [CrossRef]
- Cobos, G.A.; Femia, A.; Vleugels, R.A. Dermatomyositis: An Update on Diagnosis and Treatment. Am. J. Clin. Dermatol. 2020, 21, 339–353. [Google Scholar] [CrossRef]
- Basharat, P.; Christopher-Stine, L. Immune-Mediated Necrotizing Myopathy: Update on Diagnosis and Management. Curr. Rheumatol. Rep. 2015, 17, 72. [Google Scholar] [CrossRef]
- Khoo, T.; Lilleker, J.B.; Thong, B.Y.-H.; Leclair, V.; Lamb, J.A.; Chinoy, H. Epidemiology of the idiopathic inflammatory myopathies. Nat. Rev. Rheumatol. 2023, 19, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Onchan, T.; Foocharoen, C.; Pongkulkiat, P.; Suwannaroj, S.; Mahakkanukrauh, A. Incidence and prevalence of idiopathic inflammatory myopathies in Thailand from the Ministry of Public Health data analysis. Sci. Rep. 2024, 14, 20646. [Google Scholar] [CrossRef]
- Al Adhoubi, N.K.; Liyanage, P.; Al Salmi, I.; Abdul Hameed, Z.; Al Abrawi, S.; Al Lawati, T.; Almouslem, A.; Al Ghafri, A.; Al Shamsi, A.; Alismaeili, Z.; et al. The prevalence, epidemiological characteristics and mortality trends of inflammatory myopathies patients in Oman: The Prevision study. Clin. Exp. Rheumatol. 2024, 42, 1333–1342. [Google Scholar] [CrossRef]
- Dobloug, G.C.; Svensson, J.; Lunmdberg, I.E.; Holmqvist, M. Correction: Mortality in idiopathic inflammatory myopathy: Results from a Swedish nationwide population-based cohort study. Ann. Rheum. Dis. 2018, 77, 786. [Google Scholar] [CrossRef] [PubMed]
- Che, W.I.; Lundberg, I.E.; Holmqvist, M. Environmental Risks for Inflammatory Myopathies. Rheum. Dis. Clin. N. Am. 2022, 48, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Hayat, G.; Kalia, J.S.; Guzman, M.A. Idiopathic Inflammatory Myopathies: Clinical Approach and Management. Front. Neurol. 2016, 7, 64. [Google Scholar] [CrossRef]
- Dalakas, M.C. Inflammatory Muscle Diseases. N. Engl. J. Med. 2015, 372, 1734–1747. [Google Scholar] [CrossRef]
- Albrecht, I.; Wick, C.; Hallgren, Å.; Tjärnlund, A.; Nagaraju, K.; Andrade, F.; Thompson, K.; Coley, W.; Phadke, A.; Diaz-Gallo, L.-M.; et al. Development of autoantibodies against muscle-specific FHL1 in severe inflammatory myopathies. J. Clin. Investig. 2015, 125, 4612–4624. [Google Scholar] [CrossRef]
- Cai, H.; Yabe, I.; Sato, K.; Kano, T.; Nakamura, M.; Hozen, H.; Sasaki, H. Clinical, pathological, and genetic mutation analysis of sporadic inclusion body myositis in Japanese people. J. Neurol. 2012, 259, 1913–1922. [Google Scholar] [CrossRef]
- Van Thillo, A.; Vulsteke, J.-B.; Van Assche, D.; Verschueren, P.; De Langhe, E. Physical therapy in adult inflammatory myopathy patients: A systematic review. Clin. Rheumatol. 2019, 38, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Alexanderson, H.; Boström, C. Exercise therapy in patients with idiopathic inflammatory myopathies and systemic lupus erythematosus—A systematic literature review. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101547. [Google Scholar] [CrossRef]
- Špiritović, M.; Heřmánková, B.; Oreská, S.; Štorkánová, H.; Růžičková, O.; Vernerová, L.; Klein, M.; Kubínová, K.; Šmucrová, H.; Rathouská, A.; et al. The effect of a 24-week training focused on activities of daily living, muscle strengthening, and stability in idiopathic inflammatory myopathies: A monocentric controlled study with follow-up. Arthritis Res. Ther. 2021, 23, 173. [Google Scholar] [CrossRef]
- Kassardjian, C.D.; Lennon, V.A.; Alfugham, N.B.; Mahler, M.; Milone, M. Clinical Features and Treatment Outcomes of Necrotizing Autoimmune Myopathy. JAMA Neurol. 2015, 72, 996. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Illa, I.; Dambrosia, J.M.; Soueidan, S.A.; Stein, D.P.; Otero, C.; Dinsmore, S.T.; McCrosky, S. A Controlled Trial of High-Dose Intravenous Immune Globulin Infusions as Treatment for Dermatomyositis. N. Engl. J. Med. 1993, 329, 1993–2000. [Google Scholar] [CrossRef]
- Aggarwal, R.; Schessl, J.; Charles-Schoeman, C.; Bata-Csörgő, Z.; Dimachkie, M.M.; Griger, Z.; Moiseev, S.; Oddis, C.V.; Schiopu, E.; Vencovský, J.; et al. Safety and tolerability of intravenous immunoglobulin in patients with active dermatomyositis: Results from the randomised, placebo-controlled ProDERM study. Arthritis Res. Ther. 2024, 26, 27. [Google Scholar] [CrossRef]
- Barsotti, S.; Cioffi, E.; Tripoli, A.; Tavoni, A.; D’Ascanio, A.; Mosca, M.; Neri, R. The use of rituximab in idiopathic inflammatory myopathies: Description of a monocentric cohort and review of the literature. Reumatismo 2018, 70, 78–84. [Google Scholar] [CrossRef]
- Kyriakidou, Y.; Wood, C.; Ferrier, C.; Dolci, A.; Elliott, B. The effect of Omega-3 polyunsaturated fatty acid supplementation on exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2021, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Oddis, C.V.; Rockette, H.E.; Zhu, L.; Koontz, D.C.; Lacomis, D.; Venturupalli, S.; Moghadam-Kia, S.; Ascherman, D.P.; Crofford, L.; Dimachkie, M.M.; et al. Randomized Trial of Tocilizumab in the Treatment of Refractory Adult Polymyositis and Dermatomyositis. ACR Open Rheumatol. 2022, 4, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Sahenk, Z.; Al-Zaidy, S.; Rodino-Klapac, L.R.; Lowes, L.P.; Alfano, L.N.; Berry, K.; Miller, N.; Yalvac, M.; Dvorchik, I.; et al. Follistatin Gene Therapy for Sporadic Inclusion Body Myositis Improves Functional Outcomes. Mol. Ther. 2017, 25, 870–879. [Google Scholar] [CrossRef]
- Dohi, K.; Manabe, Y.; Fujii, N.L.; Furuichi, Y. Achieving myoblast engraftment into intact skeletal muscle via extracellular matrix. Front. Cell Dev. Biol. 2025, 12, 1502332. [Google Scholar] [CrossRef]
- Qu, X.; Liu, X.; Cheng, K.; Yang, R.; Zhao, R.C.H. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Exp. Hematol. 2012, 40, 761–770. [Google Scholar] [CrossRef]
- Selleri, S.; Dieng, M.M.; Nicoletti, S.; Louis, I.; Beausejour, C.; Le Deist, F.; Haddad, E. Cord-Blood-Derived Mesenchymal Stromal Cells Downmodulate CD4+ T-Cell Activation by Inducing IL-10-Producing Th1 Cells. Stem Cells Dev. 2013, 22, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Wirthlin, L.; Hess, D.; Zhou, P.; Nolta, J. Human Stem Cells for Tissue Repair. Biol. Blood Marrow Transplant. 2008, 14, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, J.; Wu, L.; Tao, X.; Yaqub, N.; Chang, J. Induced Pluripotent Stem Cells for Tissue-Engineered Skeletal Muscles. Int. J. Mol. Sci. 2023, 24, 11520. [Google Scholar] [CrossRef] [PubMed]
- Muravyeva, A.; Smirnikhina, S. Adenoviral Vectors for Gene Therapy of Hereditary Diseases. Biology 2024, 13, 1052. [Google Scholar] [CrossRef]
- Ahi, Y.S.; Bangari, D.S.; Mittal, S.K. Adenoviral Vector Immunity: Its Implications and Circumvention Strategies. Curr. Gene Ther. 2011, 11, 307–320. [Google Scholar] [CrossRef]
- Chen, H.-H.; Mack, L.M.; Kelly, R.; Ontell, M.; Kochanek, S.; Clemens, P.R. Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc. Natl. Acad. Sci. USA 1997, 94, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Bremer, J.; Weller, M. Gene therapy for myositis. In Gene Therapy for Autoimmune and Inflammatory Diseases; Springer: Basel, Switzerland, 2010; pp. 79–90. [Google Scholar] [CrossRef]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 141. [Google Scholar] [CrossRef]
- Mah, J.K.; Korngut, L.; Fiest, K.M.; Dykeman, J.; Day, L.J.; Pringsheim, T.; Jette, N. A Systematic Review and Meta-analysis on the Epidemiology of the Muscular Dystrophies. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2016, 43, 163–177. [Google Scholar] [CrossRef]
- Broomfield, J.; Hill, M.; Guglieri, M.; Crowther, M.; Abrams, K. Life Expectancy in Duchenne Muscular Dystrophy: Reproduced Individual Patient Data Meta-analysis. Neurology 2021, 97, e2304-14. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Fatahi, B.; Valipour, E.; Kazeminia, M.; Fatahian, R.; Kiaei, A.; Shohaimi, S.; Mohammadi, M. Global prevalence of Duchenne and Becker muscular dystrophy: A systematic review and meta-analysis. J. Orthop. Surg. 2022, 17, 96. [Google Scholar] [CrossRef]
- Meola, G. Myotonic dystrophy type 2: The 2020 update. Acta Myol. 2020, 39, 222–234. [Google Scholar] [CrossRef]
- Szwec, S.; Kapłucha, Z.; Chamberlain, J.S.; Konieczny, P. Dystrophin- and Utrophin-Based Therapeutic Approaches for Treatment of Duchenne Muscular Dystrophy: A Comparative Review. BioDrugs 2024, 38, 95–119, Erratum in BioDrugs 2025, 39, 167. [Google Scholar] [CrossRef]
- Bez Batti Angulski, A.; Hosny, N.; Cohen, H.; Martin, A.A.; Hahn, D.; Bauer, J.; Metzger, J.M. Duchenne muscular dystrophy: Disease mechanism and therapeutic strategies. Front. Physiol. 2023, 14, 1183101. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primer 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- Cohn, R.D.; Campbell, K.P. Molecular basis of muscular dystrophies. Muscle Nerve 2000, 23, 1456–1471. [Google Scholar] [CrossRef] [PubMed]
- Lanni, S.; Pearson, C.E. Molecular genetics of congenital myotonic dystrophy. Neurobiol. Dis. 2019, 132, 104533. [Google Scholar] [CrossRef]
- Heller, S.A.; Shih, R.; Kalra, R.; Kang, P.B. Emery-Dreifuss muscular dystrophy. Muscle Nerve 2020, 61, 436–448. [Google Scholar] [CrossRef]
- Zhang, Y.; Ramirez-Martinez, A.; Chen, K.; McAnally, J.R.; Cai, C.; Durbacz, M.Z.; Chemello, F.; Wang, Z.; Xu, L.; Bassel-Duby, R.; et al. Net39 protects muscle nuclei from mechanical stress during the pathogenesis of Emery-Dreifuss muscular dystrophy. J. Clin. Investig. 2023, 133, e163333. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; DeSimone, A.; Lek, M.; Lek, A. Therapeutic Approaches in Facioscapulohumeral Muscular Dystrophy. Trends Mol. Med. 2021, 27, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Schätzl, T.; Kaiser, L.; Deigner, H.-P. Facioscapulohumeral muscular dystrophy: Genetics, gene activation and downstream signalling with regard to recent therapeutic approaches: An update. Orphanet J. Rare Dis. 2021, 16, 129. [Google Scholar] [CrossRef]
- Alegre-Cortés, E.; Giménez-Bejarano, A.; Uribe-Carretero, E.; Paredes-Barquero, M.; Marques, A.R.A.; Lopes-da-Silva, M.; Vieira, O.V.; Canales-Cortés, S.; Camello, P.J.; Martínez-Chacón, G.; et al. Delay of EGF-Stimulated EGFR Degradation in Myotonic Dystrophy Type 1 (DM1). Cells 2022, 11, 3018. [Google Scholar] [CrossRef]
- Gopal Krishnan, P.D.; Lee, W.X.; Goh, K.Y.; Choy, S.M.; Turqueza, L.R.R.; Lim, Z.H.; Tang, H.-W. Transcriptional regulation of autophagy in skeletal muscle stem cells. Dis. Model. Mech. 2025, 18, DMM052007. [Google Scholar] [CrossRef]
- Call, J.A.; Nichenko, A.S. Autophagy: An essential but limited cellular process for timely skeletal muscle recovery from injury. Autophagy 2020, 16, 1344–1347. [Google Scholar] [CrossRef]
- You, J.-S.; Karaman, K.; Reyes-Ordoñez, A.; Lee, S.; Kim, Y.; Bashir, R.; Chen, J. Leucyl-tRNA Synthetase Contributes to Muscle Weakness through Mammalian Target of Rapamycin Complex 1 Activation and Autophagy Suppression in a Mouse Model of Duchenne Muscular Dystrophy. Am. J. Pathol. 2024, 194, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo, C.A.; Garibotti, M.C.; Perry, C.G.R. Mitochondrial stress responses in Duchenne muscular dystrophy: Metabolic dysfunction or adaptive reprogramming? Am. J. Physiol.-Cell Physiol. 2022, 323, C718–C730. [Google Scholar] [CrossRef]
- Yazid, M.D.; Hung-Chih, C. Perturbation of PI3K/Akt signaling affected autophagy modulation in dystrophin-deficient myoblasts. Cell Commun. Signal. 2021, 19, 105. [Google Scholar] [CrossRef]
- Mastrapasqua, M.; Rossi, R.; De Cosmo, L.; Resta, A.; Errede, M.; Bizzoca, A.; Zampatti, S.; Resta, N.; Giardina, E.; Ruggieri, M.; et al. Autophagy increase in Merosin-Deficient Congenital Muscular Dystrophy type 1A. Eur. J. Transl. Myol. 2023, 33, 11501. [Google Scholar] [CrossRef] [PubMed]
- Budzinska, M.; Zimna, A.; Kurpisz, M. The role of mitochondria in Duchenne muscular dystrophy. J. Physiol. Pharmacol. 2021, 72, 157–166. [Google Scholar] [CrossRef]
- Kleefeld, F.; Horvath, R.; Pinal-Fernandez, I.; Mammen, A.L.; Casal-Dominguez, M.; Hathazi, D.; Melchert, S.; Hahn, K.; Sickmann, A.; Muselmann-Genschow, C.; et al. Multi-level profiling unravels mitochondrial dysfunction in myotonic dystrophy type 2. Acta Neuropathol. 2024, 147, 19. [Google Scholar] [CrossRef]
- Gómez Armengol, E.; Merckx, C.; De Sutter, H.; De Bleecker, J.L.; De Paepe, B. Changes to the Autophagy-Related Muscle Proteome Following Short-Term Treatment with Ectoine in the Duchenne Muscular Dystrophy Mouse Model mdx. Int. J. Mol. Sci. 2025, 26, 439. [Google Scholar] [CrossRef]
- Baechler, B.L.; Bloemberg, D.; Quadrilatero, J. Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation. Autophagy 2019, 15, 1606–1619. [Google Scholar] [CrossRef]
- Rosati, A.; Graziano, V.; De Laurenzi, V.; Pascale, M.; Turco, M.C. BAG3: A multifaceted protein that regulates major cell pathways. Cell Death Dis. 2011, 2, e141. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; He, J.; Liu, J.; Guo, X.; Chu, H.; Xu, H.; Wang, Y. Comprehensive review on gene mutations contributing to dilated cardiomyopathy. Front. Cardiovasc. Med. 2023, 10, 1296389. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, R.E.; Morales, A.; Siegfried, J.D. Clinical and genetic issues in dilated cardiomyopathy: A review for genetics professionals. Genet. Med. 2010, 12, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Otway, R.; Chin, E.; Horvat, C.; Ohanian, M.; Wilcox, J.A.L.; Su, Z.; Prestes, P.; Smolnikov, A.; Soka, M.; et al. DMD-Associated Dilated Cardiomyopathy: Genotypes, Phenotypes, and Phenocopies. Circ. Genom. Precis. Med. 2023, 16, 421–430. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, W.; Shu, S.; Zhang, W. Duchenne muscular dystrophy involves the myocardium and causes arrhythmia: Case report. Front. Cardiovasc. Med. 2022, 9, 974843. [Google Scholar] [CrossRef]
- Sun, L.; Shen, D.; Xiong, T.; Zhou, Z.; Lu, X.; Cui, F. Limb-girdle muscular dystrophy due to GMPPB mutations: A case report and comprehensive literature review. Bosn. J. Basic Med. Sci. 2019, 20, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, Y.; Zhou, Y.; Zeng, J. Becker muscular dystrophy with dilated cardiomyopathy: A case report. Clin. Case Rep. 2021, 9, e04777. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.A.; Siciliano, G.; Angelini, C. Advances in Dystrophinopathy Diagnosis and Therapy. Biomolecules 2023, 13, 1319. [Google Scholar] [CrossRef] [PubMed]
- Voet, N.B.; Van Der Kooi, E.L.; Van Engelen, B.G.; Geurts, A.C. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst. Rev. 2019, 2019, CD003907. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Vissing, J. Exercise therapy for muscle and lower motor neuron diseases. Acta Myol. 2019, 38, 215–232. [Google Scholar]
- Gianola, S.; Castellini, G.; Pecoraro, V.; Monticone, M.; Banfi, G.; Moja, L. Effect of Muscular Exercise on Patients with Muscular Dystrophy: A Systematic Review and Meta-Analysis of the Literature. Front. Neurol. 2020, 11, 958. [Google Scholar] [CrossRef]
- Hammer, S.; Toussaint, M.; Vollsæter, M.; Nesbjørg Tvedt, M.; Drange Røksund, O.; Reychler, G.; Lund, H.; Andersen, T. Exercise Training in Duchenne Muscular Dystrophy: A Systematic Review and Meta-Analysis. J. Rehabil. Med. 2021, 54, jrm00250. [Google Scholar] [CrossRef]
- D’Ambrosio, E.S.; Mendell, J.R. Evolving Therapeutic Options for the Treatment of Duchenne Muscular Dystrophy. Neurotherapeutics 2023, 20, 1669–1681. [Google Scholar] [CrossRef]
- Happi Mbakam, C.; Lamothe, G.; Tremblay, J.P. Therapeutic Strategies for Dystrophin Replacement in Duchenne Muscular Dystrophy. Front. Med. 2022, 9, 859930. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.T.; Christie, K.A.; Whittaker, M.N.; Kleinstiver, B.P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 2020, 368, 290–296. [Google Scholar] [CrossRef]
- Findlay, A.R.; Weihl, C.C. Genetic-Based Treatment Strategies for Muscular Dystrophy and Congenital Myopathies. Continuum 2022, 28, 1800–1816. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N. Toward the correction of muscular dystrophy by gene editing. Proc. Natl. Acad. Sci. USA 2021, 118, e2004840117. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Serra, C.; Lee, G.; Wagner, K.R. Stem cell-based therapies for Duchenne muscular dystrophy. Exp. Neurol. 2020, 323, 113086. [Google Scholar] [CrossRef] [PubMed]
- Chulanova, Y.; Breier, D.; Peer, D. Delivery of genetic medicines for muscular dystrophies. Cell Rep. Med. 2025, 6, 101885. [Google Scholar] [CrossRef]
- McGrath, M.J.; Eramo, M.J.; Gurung, R.; Sriratana, A.; Gehrig, S.M.; Lynch, G.S.; Lourdes, S.R.; Koentgen, F.; Feeney, S.J.; Lazarou, M.; et al. Defective lysosome reformation during autophagy causes skeletal muscle disease. J. Clin. Investig. 2021, 131, e135124. [Google Scholar] [CrossRef]
- Allameen, N.A.; Salam, S.; Reddy, V.; Machado, P.M. Inclusion body myositis and immunosenescence: Current evidence and future perspectives. Rheumatology 2025, 64, 952–961. [Google Scholar] [CrossRef]
- Brady, S.; Poulton, J.; Muller, S. Inclusion body myositis: Correcting impaired mitochondrial and lysosomal autophagy as a potential therapeutic strategy. Autoimmun. Rev. 2024, 23, 103644. [Google Scholar] [CrossRef]
- Tan, Y.; Gong, Y.; Dong, M.; Pei, Z.; Ren, J. Role of autophagy in inherited metabolic and endocrine myopathies. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2019, 1865, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Antuña, E.; Cachán-Vega, C.; Bermejo-Millo, J.C.; Potes, Y.; Caballero, B.; Vega-Naredo, I.; Coto-Montes, A.; Garcia-Gonzalez, C. Inflammaging: Implications in Sarcopenia. Int. J. Mol. Sci. 2022, 23, 15039. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Zhang, L.; Han, R. Autophagy in striated muscle diseases. Front. Cardiovasc. Med. 2022, 9, 1000067. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.M.; Roncaroli, F.; Turton, N.; Hendriksz, C.J.; Roberts, M.; Heaton, R.A.; Hargreaves, I. Mechanisms of Mitochondrial Dysfunction in Lysosomal Storage Disorders: A Review. J. Clin. Med. 2020, 9, 2596. [Google Scholar] [CrossRef]
- Alves De Siqueira Carvalho, A.; Gomes Silva, V.; Silva, A.P.; Corazzini, R.; Lacene, E. New insights about myofibrillar myopathies: The role of metalloproteinases 2 and 9 in the pathogenesis. J. Hum. Growth Dev. 2022, 32, 101–107. [Google Scholar] [CrossRef]
- Wallgren-Pettersson, C.; Sewry, C.A.; Nowak, K.J.; Laing, N.G. Nemaline Myopathies. Semin. Pediatr. Neurol. 2011, 18, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Varghese, V.; Rajadurai, I.; Paul, M.; Shyamprasad, S.B.; Mathew, A.A. 209P Custom orthosis improves mobility and caregiver experience in an adolescent with congenital myasthenic syndrome and myofibrillar myopathy. Neuromuscul. Disord. 2024, 43, 104441. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.-M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Nagappa, M.; Narayanappa, G. Approach to the diagnosis of metabolic myopathies. Indian J. Pathol. Microbiol. 2022, 65, S277–S290. [Google Scholar] [CrossRef]
- González-Jamett, A.M.; Bevilacqua, J.A.; Díaz, A.M. Hereditary Myopathies. In Muscle Cell and Tissue—Current Status of Research Field; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Arbustini, E.; Di Toro, A.; Giuliani, L.; Favalli, V.; Narula, N.; Grasso, M. Cardiac Phenotypes in Hereditary Muscle Disorders. J. Am. Coll. Cardiol. 2018, 72, 2485–2506. [Google Scholar] [CrossRef]
- Cacciani, N.; Skärlén, Å.; Wen, Y.; Zhang, X.; Addinsall, A.B.; Llano-Diez, M.; Li, M.; Gransberg, L.; Hedström, Y.; Bellander, B.; et al. A prospective clinical study on the mechanisms underlying critical illness myopathy—A time-course approach. J. Cachexia Sarcopenia Muscle 2022, 13, 2669–2682. [Google Scholar] [CrossRef]
- Toscano, A.; Schoser, B. Enzyme replacement therapy in late-onset Pompe disease: A systematic literature review. J. Neurol. 2013, 260, 951–959. [Google Scholar] [CrossRef]
- Mendell, J.R.; Muntoni, F.; McDonald, C.M.; Mercuri, E.M.; Ciafaloni, E.; Komaki, H.; Leon-Astudillo, C.; Nascimento, A.; Proud, C.; Schara-Schmidt, U.; et al. AAV gene therapy for Duchenne muscular dystrophy: The EMBARK phase 3 randomized trial. Nat. Med. 2025, 31, 332–341. [Google Scholar] [CrossRef]
- Chamberlain, K.; Riyad, J.M.; Weber, T. Cardiac gene therapy with adeno-associated virus-based vectors. Curr. Opin. Cardiol. 2017, 32, 275–282. [Google Scholar] [CrossRef]
- Karakikes, I.; Termglinchan, V.; Cepeda, D.A.; Lee, J.; Diecke, S.; Hendel, A.; Itzhaki, I.; Ameen, M.; Shrestha, R.; Wu, H.; et al. A Comprehensive TALEN-Based Knockout Library for Generating Human-Induced Pluripotent Stem Cell–Based Models for Cardiovascular Diseases. Circ. Res. 2017, 120, 1561–1571. [Google Scholar] [CrossRef]
- FDA approves Alnylam’s anti-amyloid RNAi drug for cardiomyopathy. Nat. Biotechnol. 2025, 43, 457. [CrossRef]
- Salabarria, S.M.; Nair, J.; Clement, N.; Smith, B.K.; Raben, N.; Fuller, D.D.; Byrne, B.J.; Corti, M. Advancements in AAV-mediated Gene Therapy for Pompe Disease. J. Neuromuscul. Dis. 2020, 7, 15–31. [Google Scholar] [CrossRef]
- Lim, J.-A.; Choi, S.J.; Gao, F.; Kishnani, P.S.; Sun, B. A Novel Gene Therapy Approach for GSD III Using an AAV Vector Encoding a Bacterial Glycogen Debranching Enzyme. Mol. Ther.—Methods Clin. Dev. 2020, 18, 240–249. [Google Scholar] [CrossRef]
- McNamara, E.L.; Taylor, R.L.; Clayton, J.S.; Goullee, H.; Dilworth, K.L.; Pinós, T.; Brull, A.; Alexander, I.E.; Lisowski, L.; Ravenscroft, G.; et al. Systemic AAV8-mediated delivery of a functional copy of muscle glycogen phosphorylase (Pygm) ameliorates disease in a murine model of McArdle disease. Hum. Mol. Genet. 2020, 29, 20–30. [Google Scholar] [CrossRef]
- Howell, J.M.; Walker, K.R.; Davies, L.; Dunton, E.; Everaardt, A.; Laing, N.; Karpati, G. Adenovirus and adeno-associated virus-mediated delivery of human myophosphorylase cDNA and LacZ cDNA to muscle in the ovine model of McArdle’s disease: Expression and re-expression of glycogen phosphorylase. Neuromuscul. Disord. 2008, 18, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Trundle, J.; Boulinguiez, A.; Lu-Nguyen, N.; March, J.; Malerba, A.; Popplewell, L. Periostin exon 17 skipping enhances the efficacy of local AAV-microdystrophin administration in a fibrotic model of Duchenne muscular dystrophy. bioRXiv 2025. [Google Scholar] [CrossRef]
- Cardinali, B.; Provenzano, C.; Izzo, M.; Voellenkle, C.; Battistini, J.; Strimpakos, G.; Golini, E.; Mandillo, S.; Scavizzi, F.; Raspa, M.; et al. Time-controlled and muscle-specific CRISPR/Cas9-mediated deletion of CTG-repeat expansion in the DMPK gene. Mol. Ther. Nucleic Acids 2022, 27, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, K.; Wheeler, T.M.; Wang, W.; Thornton, C.A. RNA Interference Targeting CUG Repeats in a Mouse Model of Myotonic Dystrophy. Mol. Ther. 2013, 21, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaidy, S.A.; Sahenk, Z.; Rodino-Klapac, L.R.; Kaspar, B.; Mendell, J.R. Follistatin Gene Therapy Improves Ambulation in Becker Muscular Dystrophy. J. Neuromuscul. Dis. 2015, 2, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ganassi, M.; Zammit, P.S. Involvement of muscle satellite cell dysfunction in neuromuscular disorders: Expanding the portfolio of satellite cell-opathies. Eur. J. Transl. Myol. 2022, 32, 10064. [Google Scholar] [CrossRef]
- Beck, M. Treatment strategies for lysosomal storage disorders. Dev. Med. Child Neurol. 2018, 60, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Shoop, W.K.; Lape, J.; Trum, M.; Powell, A.; Sevigny, E.; Mischler, A.; Bacman, S.R.; Fontanesi, F.; Smith, J.; Jantz, D.; et al. Efficient elimination of MELAS-associated m.3243G mutant mitochondrial DNA by an engineered mitoARCUS nuclease. Nat. Metab. 2023, 5, 2169–2183. [Google Scholar] [CrossRef]
- Ruppert, T.; Heckmann, M.B.; Rapti, K.; Schultheis, D.; Jungmann, A.; Katus, H.A.; Winter, L.; Frey, N.; Clemen, C.S.; Schröder, R.; et al. AAV-mediated cardiac gene transfer of wild-type desmin in mouse models for recessive desminopathies. Gene Ther. 2020, 27, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Maksimiuk, M.; Sobiborowicz, A.; Tuzimek, A.; Deptała, A.; Czerw, A.; Badowska-Kozakiewicz, A. αB-crystallin as a promising target in pathological conditions—A review. Ann. Agric. Environ. Med. 2020, 27, 326–334. [Google Scholar] [CrossRef]
- Liu, J.; Wallace, L.M.; Garwick-Coppens, S.E.; Sloboda, D.D.; Davis, C.S.; Hakim, C.H.; Hauser, M.A.; Brooks, S.V.; Mendell, J.R.; Harper, S.Q. RNAi-mediated Gene Silencing of Mutant Myotilin Improves Myopathy in LGMD1A Mice. Mol. Ther.—Nucleic Acids 2014, 3, e160. [Google Scholar] [CrossRef]
- Abstracts of the 17th International Congress on Neuromuscular Diseases (ICNMD 2022). J. Neuromuscul. Dis. 2022, 9, S1–S331. [CrossRef]
- Fürst, D.O.; Goldfarb, L.G.; Kley, R.A.; Vorgerd, M.; Olivé, M.; Van Der Ven, P.F.M. Filamin C-related myopathies: Pathology and mechanisms. Acta Neuropathol. 2013, 125, 33–46. [Google Scholar] [CrossRef]
- Qin, Z.; Sun, L.; Sun, X.; Su, H.; Gao, X. A CRISPR/Cas9 strategy for the generation of a FLNC knockout hESC line (WAe009-A-70) to model dilated cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. Stem Cell Res. 2021, 56, 102562. [Google Scholar] [CrossRef]
- Grabich, S.; Santra, S.; Waldrop, M.; Mathews, K.; Abid, F.; Ramos-Platt, L.; Scharf, R.; Zaidman, C.; Sehinovych, I.; McDonald, C. P31 Interim analysis of EVOLVE: Evaluating Eteplirsen, Golodirsen, or Casimersen treatment in patients <7 years old in routine clinical practice. Neuromuscul. Disord. 2023, 33, S104–S105. [Google Scholar] [CrossRef]
- Nakamori, M.; Gourdon, G.; Thornton, C.A. Stabilization of Expanded (CTG)•(CAG) Repeats by Antisense Oligonucleotides. Mol. Ther. 2011, 19, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Lo Scrudato, M.; Poulard, K.; Sourd, C.; Tomé, S.; Klein, A.F.; Corre, G.; Huguet, A.; Furling, D.; Gourdon, G.; Buj-Bello, A. Genome Editing of Expanded CTG Repeats within the Human DMPK Gene Reduces Nuclear RNA Foci in the Muscle of DM1 Mice. Mol. Ther. 2019, 27, 1372–1388. [Google Scholar] [CrossRef]
- Pinto, B.S.; Saxena, T.; Oliveira, R.; Méndez-Gómez, H.R.; Cleary, J.D.; Denes, L.T.; McConnell, O.; Arboleda, J.; Xia, G.; Swanson, M.S.; et al. Impeding Transcription of Expanded Microsatellite Repeats by Deactivated Cas9. Mol. Cell 2017, 68, 479–490.e5. [Google Scholar] [CrossRef]
- Himeda, C.L.; Jones, T.I.; Jones, P.L. Targeted epigenetic repression by CRISPR/dSaCas9 suppresses pathogenic DUX4-fl expression in FSHD. Mol. Ther.—Methods Clin. Dev. 2021, 20, 298–311. [Google Scholar] [CrossRef] [PubMed]
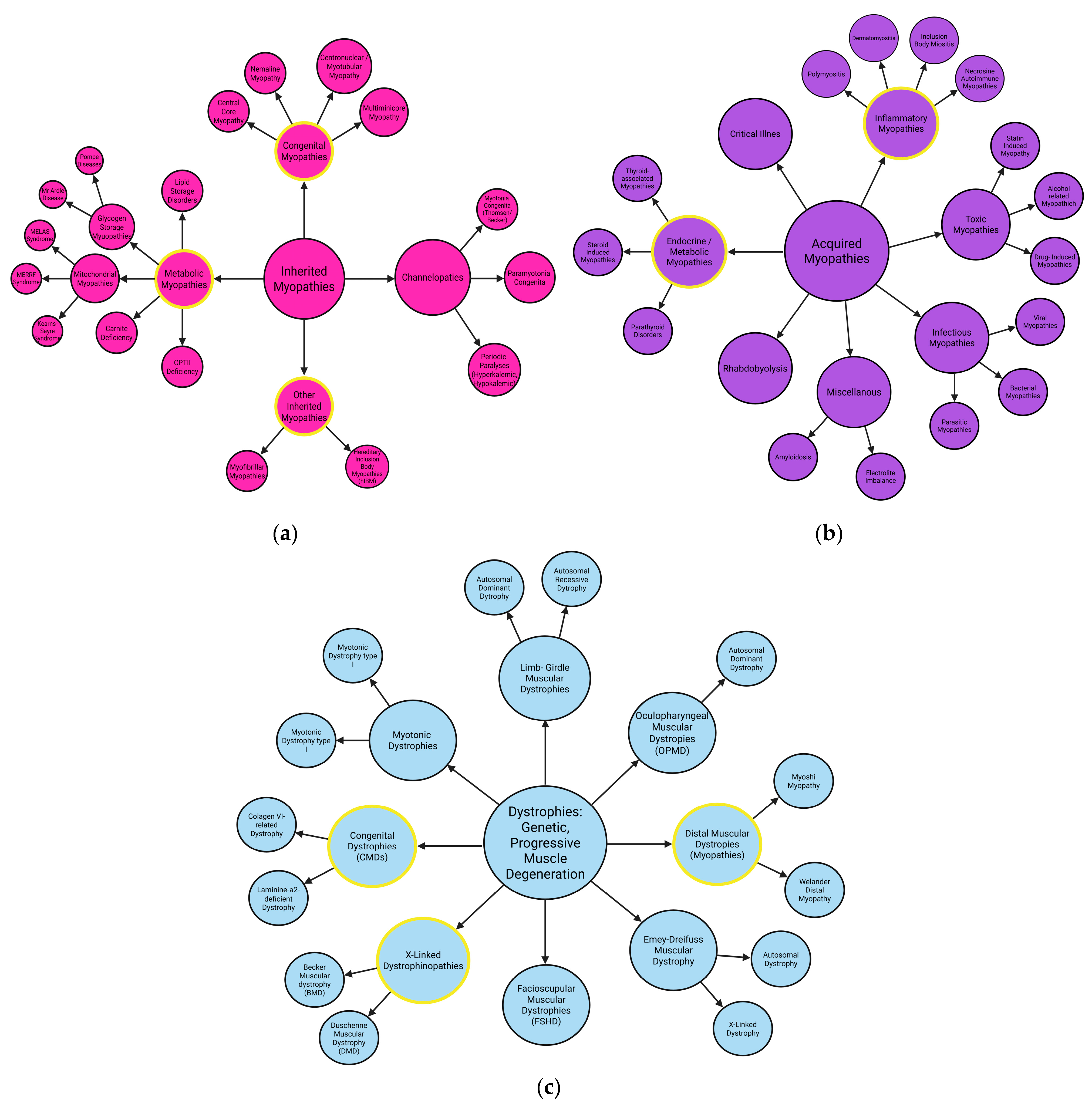

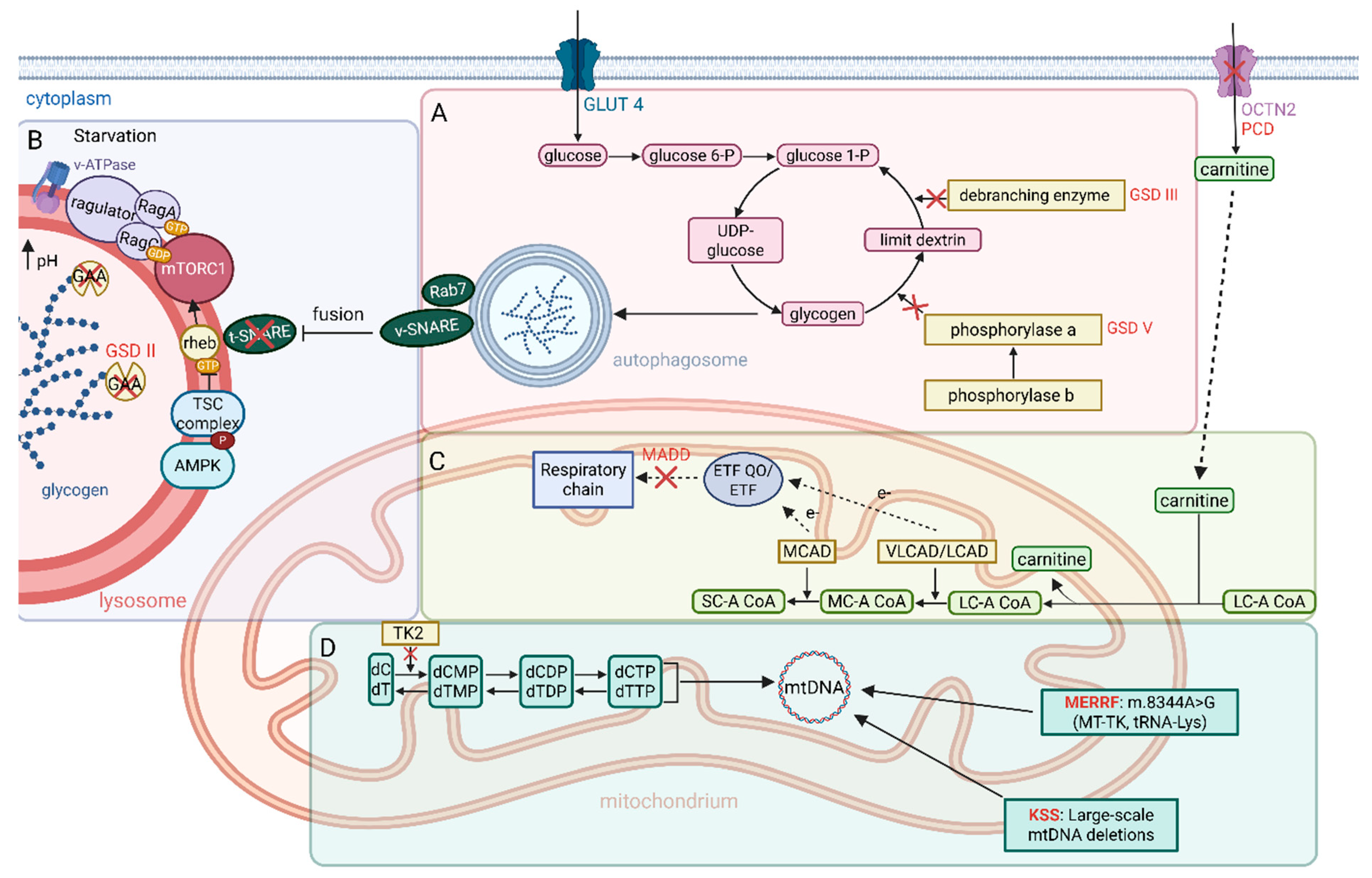
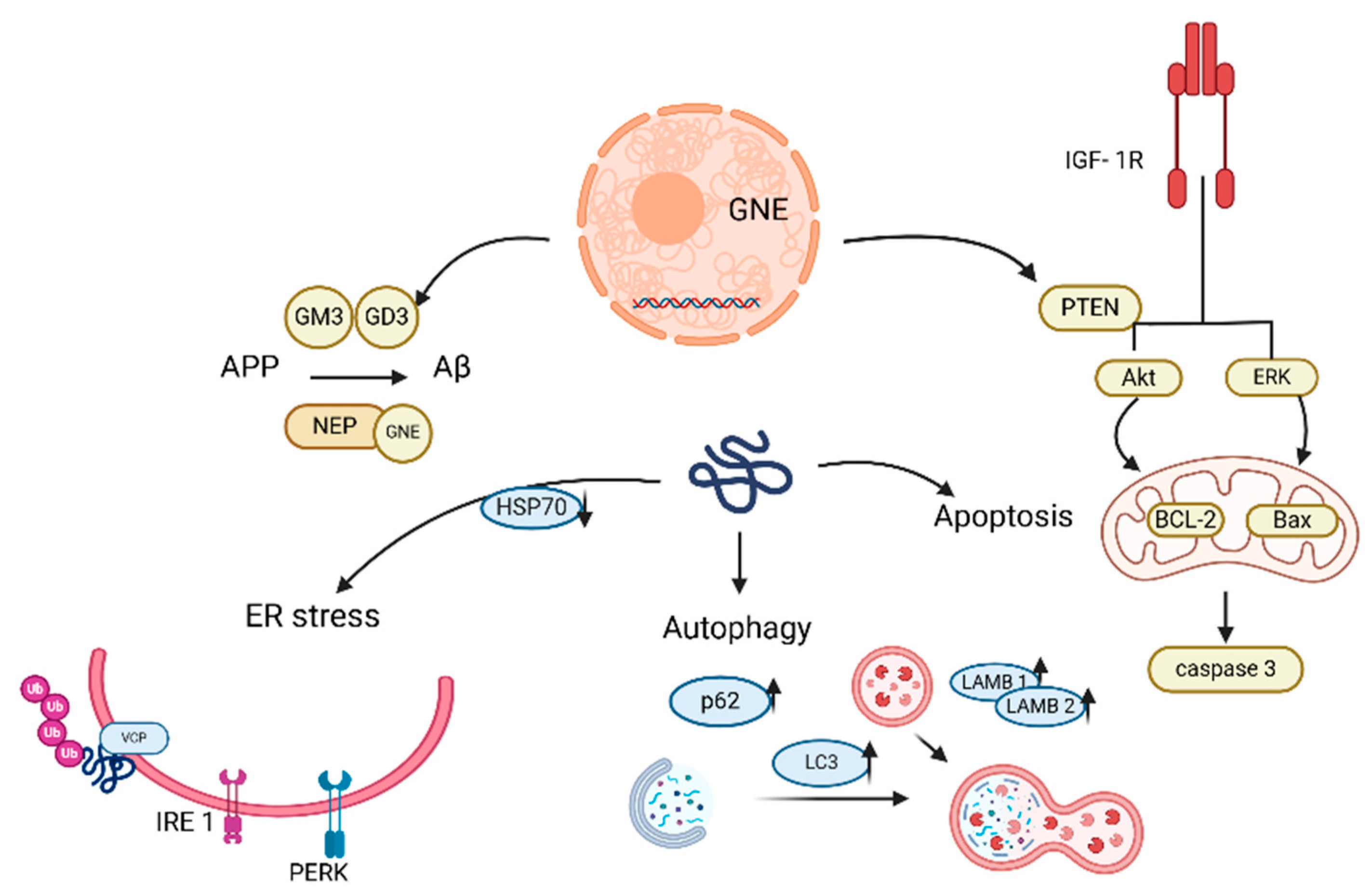
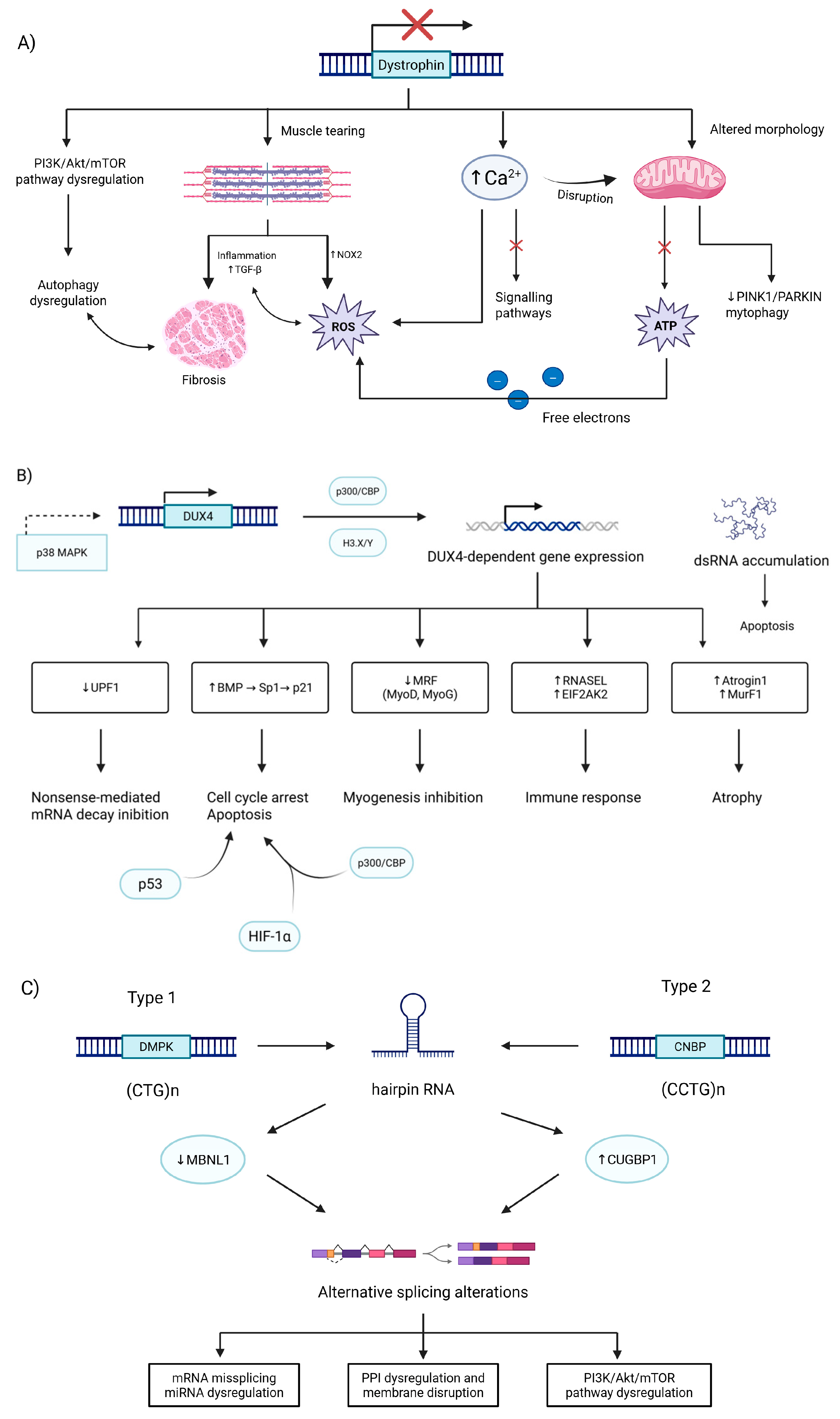
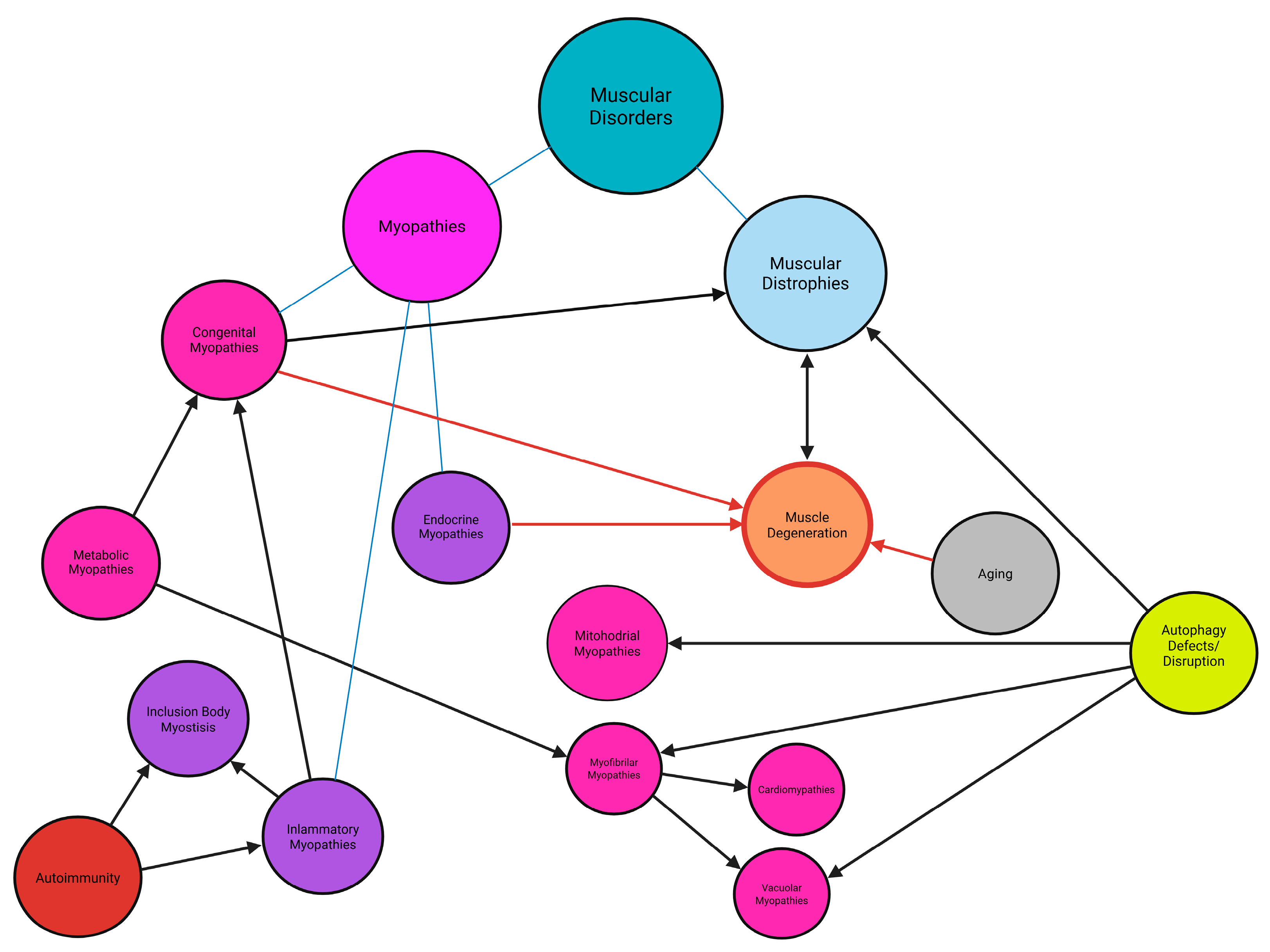
| Type | PM | DM | IMNM/NAM | OM | IBM |
|---|---|---|---|---|---|
| Subtypes | unknown | juvenile DM | statin-associated HMGCR antibody NAM | ASS | unknown |
| myopathic DM | statin-naïve HMGCR antibody NAM | ||||
| DM sine dermatitis | SRP antibody NAM | ||||
| Symptoms | - pathognomonic skin changes of Gottron’s papules - heliotrope rash - cutaneous changes | - atrophy in affected muscles - weight loss - necrosis on biopsy - cardiorespiratory complications | - systemic lupus erythematosus - rheumatoid arthritis - scleroderma - associated with underlying CTD | - loss of finger dexterity - forearm medial compartment flexors and quadriceps atrophy - muscle involvement more asymmetric than in other IIMs (usually the non-dominant arm more affected) | |
| Overall comparison | - proximal weakness - higher CK | - long finger flexors and quadriceps - normal/moderately higher CK | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziemian, M.; Szmydtka, J.; Snoch, W.; Milner, S.; Wojciechowski, S.; Dłuszczakowska, A.; Chojnowski, J.W.; Pallach, Z.; Żamojda, K.; Węgrzyn, G.; et al. Integrative Approaches to Myopathies and Muscular Dystrophies: Molecular Mechanisms, Diagnostics, and Future Therapies. Int. J. Mol. Sci. 2025, 26, 7972. https://doi.org/10.3390/ijms26167972
Ziemian M, Szmydtka J, Snoch W, Milner S, Wojciechowski S, Dłuszczakowska A, Chojnowski JW, Pallach Z, Żamojda K, Węgrzyn G, et al. Integrative Approaches to Myopathies and Muscular Dystrophies: Molecular Mechanisms, Diagnostics, and Future Therapies. International Journal of Molecular Sciences. 2025; 26(16):7972. https://doi.org/10.3390/ijms26167972
Chicago/Turabian StyleZiemian, Maja, Joanna Szmydtka, Wojciech Snoch, Sandra Milner, Szymon Wojciechowski, Aleksandra Dłuszczakowska, Jakub W. Chojnowski, Zofia Pallach, Katarzyna Żamojda, Grzegorz Węgrzyn, and et al. 2025. "Integrative Approaches to Myopathies and Muscular Dystrophies: Molecular Mechanisms, Diagnostics, and Future Therapies" International Journal of Molecular Sciences 26, no. 16: 7972. https://doi.org/10.3390/ijms26167972
APA StyleZiemian, M., Szmydtka, J., Snoch, W., Milner, S., Wojciechowski, S., Dłuszczakowska, A., Chojnowski, J. W., Pallach, Z., Żamojda, K., Węgrzyn, G., & Rintz, E. (2025). Integrative Approaches to Myopathies and Muscular Dystrophies: Molecular Mechanisms, Diagnostics, and Future Therapies. International Journal of Molecular Sciences, 26(16), 7972. https://doi.org/10.3390/ijms26167972









