Role of Transport Proteins for the Renal Handling of L-Arginine and Related Derivatives
Abstract
1. Introduction
2. Cardioactive Arginine Derivatives
2.1. L-Arginine
2.2. L-Homoarginine
2.3. ADMA
| L-Arginine | L-Homoarginine | ADMA | SDMA | |
|---|---|---|---|---|
| Structure | 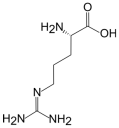 | 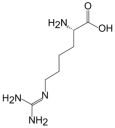 | 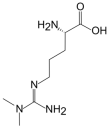 | 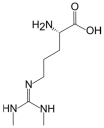 |
| Source or synthesis | Endogenous: via biosynthesis between 9.2 and 16 µmol × kg−1 × h−1 equals ~2.8–5 g/day in male adults [58,59] | Endogenous: synthesis by the enzyme AGAT [49] | Endogenous: hydrolysis of proteins after asymmetric methylation (~60 mg/day) [23,50,51] | Endogenous: hydrolysis of proteins after asymmetric methylation [23,50,51] |
| Diet: approx. 5 g/day [60] | Diet: unknown proportion | Diet: unknown proportion | Diet: unknown proportion | |
| Metabolism and elimination | Major enzymes: AGAT, NOS (3 isozymes), arginases (2 isozymes), and L-arginine decarboxylase [21,34] | Major enzymes: AGXT2 [61]; arginases and NO-Synthases [42,62] | Major enzymes: DDAH1 accounts for >80% of the metabolic elimination [9,52]; AGXT2 [53] Elimination: renal excretion ~20% [25,55] | Major enzyme: AGXT2 (mildly elevated plasma concentration in genetic AGXT2 deficiency) [53] Elimination: primarily by renal excretion [25,56] |
| Protein binding | <4% [63] | No data found | 8% [23] | 9% [23] |
| Plasma concentration mean values [µmol/L] | 83–153 [4,20,64,65,66,67,68] | 1.19–2.5 [65,69,70] | 0.23–0.67 [4,26,65,66,67,71,72] | 0.15–0.53 [4,26,65,66,72,73] |
| Renal clearance [mL/min] | 0.12–0.27 [17,18,19] | 1.06–1.50 [17,18] | 77.50–85.74 [17,20] | 80.10–81.73 [17,20] |
| Effect of impaired renal clearance on plasma concentration | Unchanged [4,37,38,39] | Lowered ~30% [13] | Elevated ~10–100% [4,5,26,66,74] | Highly elevated ~50–1000% [4,26,66,74] |
2.4. SDMA
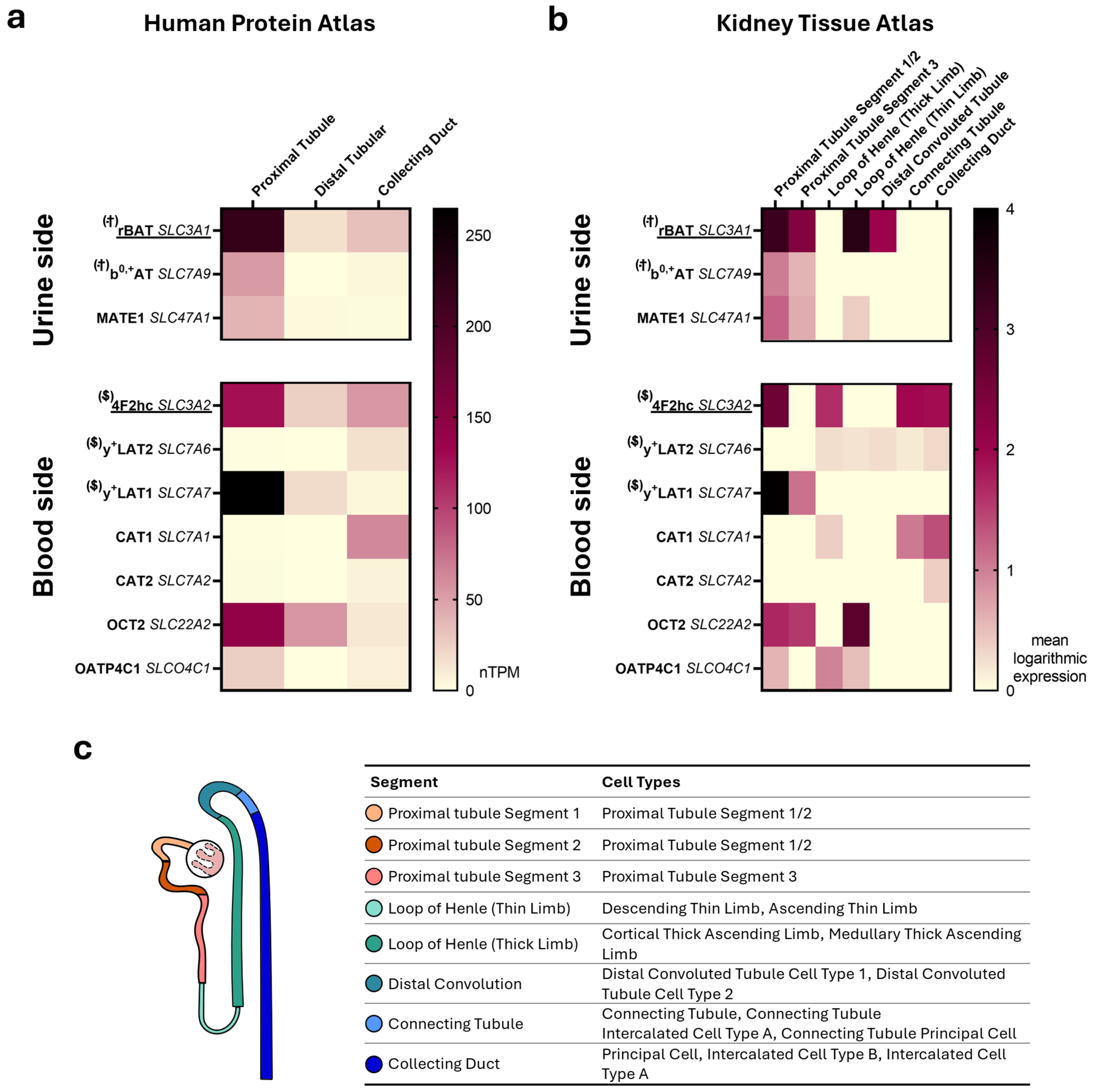
3. Renally-Expressed Transport Proteins Related to L-Arginine Transport
3.1. y+LAT1 (SLC7A7) and 4F2hc (SLC3A2)
3.2. b0,+AT (SLC7A9) and rBAT (SLC3A1)
3.3. OATP4C1 (SLCO4C1)
3.4. OCT2 (SLC22A2)
3.5. MATE1 (SLC47A1)
4. Conclusions
4.1. The Role of Overlapping Substrate Specificities of Tubular Transport Proteins
4.2. Key Candidates for Differential Transport of L-Arginine Derivatives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMA | Asymmetric dimethylarginine |
| AGAT | Arginine: glycine amidinotransferase |
| AGXT2 | Alanine-glyoxylate aminotransferase 2 |
| b0,+AT | b(0,+)-type amino acid transporter 1 |
| CAT1 | Cationic amino acid transporter 1 |
| CAT2 | Cationic amino acid transporter 2 |
| CKD | Chronic kidney disease |
| CVD | Cardiovascular disease |
| DDAH1 | Dimethylaminohydrolase 1 |
| eGFR | Estimated glomerular filtration rate |
| HPA | Human Protein Atlas |
| KTA | Kidney Tissue Atlas |
| MATE1 | Multidrug and toxin extrusion protein 1 |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| OATP4C1 | Organic anion transporting polypeptide 4C1 |
| OCT2 | Organic cation transporter 2 |
| scRNA | Single-cell RNA |
| SDMA | Symmetric dimethylarginine |
| SNP | Single nucleotide polymorphisms |
| y+LAT1 | y+L amino acid transporter 1 |
| y+LAT2 | y+L amino acid transporter 2 |
References
- Zoccali, C.; Bode-Böger, S.M.; Mallamaci, F.; Benedetto, F.A.; Tripepi, G.; Malatino, L.S.; Cataliotti, A.; Bellanuova, I.; Fermo, I.; Frölich, J.C.; et al. Plasma Concentration of Asymmetrical Dimethylarginine and Mortality in Patients with End-Stage Renal Disease: A Prospective Study. Lancet 2001, 358, 2113–2117. [Google Scholar] [CrossRef]
- Ravani, P.; Maas, R.; Malberti, F.; Pecchini, P.; Mieth, M.; Quinn, R.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Homoarginine and Mortality in Pre-Dialysis Chronic Kidney Disease (CKD) Patients. PLoS ONE 2013, 8, e72694. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and Symmetric Dimethylarginine as Risk Markers for Total Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef] [PubMed]
- Au, A.Y.M.; Mantik, K.; Bahadory, F.; Stathakis, P.; Guiney, H.; Erlich, J.; Walker, R.; Poulton, R.; Horvath, A.R.; Endre, Z.H. Plasma Arginine Metabolites in Health and Chronic Kidney Disease. Nephrol. Dial. Transplant. 2023, 38, 2767–2775. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leone, A.; Moncada, S.; Calver, A.; Collier, J. Accumulation of an Endogenous Inhibitor of Nitric Oxide Synthesis in Chronic Renal Failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- Tsikas, D.; Bollenbach, A.; Hanff, E.; Kayacelebi, A.A. Asymmetric Dimethylarginine (ADMA), Symmetric Dimethylarginine (SDMA) and Homoarginine (hArg): The ADMA, SDMA and hArg Paradoxes. Cardiovasc. Diabetol. 2018, 17, 1. [Google Scholar] [CrossRef]
- März, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Böhm, B.O.; Ritz, E.; et al. Homoarginine, Cardiovascular Risk, and Mortality. Circulation 2010, 122, 967–975. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ashton, D.; Moncada, S. Vascular Endothelial Cells Synthesize Nitric Oxide from L-Arginine. Nature 1988, 333, 664–666. [Google Scholar] [CrossRef]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric Dimethylarginine Causes Hypertension and Cardiac Dysfunction in Humans and Is Actively Metabolized by Dimethylarginine Dimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef]
- Kittel, A.; Maas, R. Pharmacology and Clinical Pharmacology of Methylarginines Used as Inhibitors of Nitric Oxide Synthases. Curr. Pharm. Des. 2014, 20, 3530–3547. [Google Scholar] [CrossRef]
- Closs, E.I.; Basha, F.Z.; Habermeier, A.; Förstermann, U. Interference of L-Arginine Analogues with L-Arginine Transport Mediated by the y+carrier hCAT-2B. Nitric Oxide 1997, 1, 65–73. [Google Scholar] [CrossRef]
- Strobel, J.; Mieth, M.; Endreß, B.; Auge, D.; König, J.; Fromm, M.F.; Maas, R. Interaction of the Cardiovascular Risk Marker Asymmetric Dimethylarginine (ADMA) with the Human Cationic Amino Acid Transporter 1 (CAT1). J. Mol. Cell. Cardiol. 2012, 53, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Kollerits, B.; Meinitzer, A.; März, W.; Ritz, E.; König, P.; Neyer, U.; Pilz, S.; Wanner, C.; Kronenberg, F.; et al. Homoarginine and Progression of Chronic Kidney Disease: Results from the Mild to Moderate Kidney Disease Study. PLoS ONE 2013, 8, e63560. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Teerlink, T.; Scheffer, P.G.; Meinitzer, A.; Rutters, F.; Tomaschitz, A.; Drechsler, C.; Kienreich, K.; Nijpels, G.; Stehouwer, C.D.A.; et al. Homoarginine and Mortality in an Older Population: The Hoorn Study. Eur. J. Clin. Investig. 2014, 44, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Tomaschitz, A.; Meinitzer, A.; Pilz, S.; Rus-Machan, J.; Genser, B.; Drechsler, C.; Grammer, T.; Krane, V.; Ritz, E.; Kleber, M.E.; et al. Homoarginine, Kidney Function and Cardiovascular Mortality Risk. Nephrol. Dial. Transplant. 2014, 29, 663–671. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic Kidney Disease and Cardiovascular Risk: Epidemiology, Mechanisms, and Prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Gessner, A.; Gemeinhardt, A.; Bosch, A.; Kannenkeril, D.; Staerk, C.; Mayr, A.; Fromm, M.F.; Schmieder, R.E.; Maas, R. Effects of Treatment with SGLT-2 Inhibitors on Arginine-Related Cardiovascular and Renal Biomarkers. Cardiovasc. Diabetol. 2022, 21, 4. [Google Scholar] [CrossRef]
- Cox, B.; Cameron, J. Homoarginine in Cystinuria. Clin. Sci. Mol. Med. 1974, 46, 173–182. [Google Scholar] [CrossRef]
- Torremans, A.; Marescau, B.; Vanholder, R.; De Smet, R.; Billiouw, J.-M.; De Deyn, P. The Low Nanomolar Levels of NG-Monomethylarginine in Serum and Urine of Patients with Chronic Renal Insufficiency Are Not Significantly Different from Control Levels. Amino Acids 2003, 24, 375–381. [Google Scholar] [CrossRef]
- Kurko, J.; Tringham, M.; Tanner, L.; Näntö-Salonen, K.; Vähä-Mäkilä, M.; Nygren, H.; Pöhö, P.; Lietzen, N.; Mattila, I.; Olkku, A.; et al. Imbalance of Plasma Amino Acids, Metabolites and Lipids in Patients with Lysinuric Protein Intolerance (LPI). Metabolism 2016, 65, 1361–1375. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. Renal Arginine Metabolism. J. Nutr. 2004, 134, 2791S–2795S. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Meinitzer, A.; Gaksch, M.; Grübler, M.; Verheyen, N.; Drechsler, C.; ó Hartaigh, B.; Lang, F.; Alesutan, I.; Voelkl, J.; et al. Homoarginine in the Renal and Cardiovascular Systems. Amino Acids 2015, 47, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Speer, T.; Bode-Böger, S.M.; Fliser, D.; Kielstein, J.T. Dimethylarginines ADMA and SDMA: The Real Water-Soluble Small Toxins? Semin. Nephrol. 2014, 34, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef]
- Ronden, R.A.; Houben, A.J.; Teerlink, T.; Bakker, J.A.; Bierau, J.; Stehouwer, C.D.; De Leeuw, P.W.; Kroon, A.A. Reduced Renal Plasma Clearance Does Not Explain Increased Plasma Asymmetric Dimethylarginine in Hypertensive Subjects with Mild to Moderate Renal Insufficiency. Am. J. Physiol. Ren. Physiol. 2012, 303, F149–F156. [Google Scholar] [CrossRef]
- Al Banchaabouchi, M.; Marescau, B.; Possemiers, I.; D’Hooge, R.; Levillain, O.; De Deyn, P.P. NG,NG-Dimethylarginine and NG,N’G-Dimethylarginine in Renal Insufficiency. Pflügers Arch.—Eur. J. Physiol. 2000, 439, 524–531. [Google Scholar] [CrossRef]
- Banjarnahor, S.; Scherpinski, L.A.; Keller, M.; König, J.; Maas, R. Differential Uptake of Arginine Derivatives by the Human Heteromeric Amino Acid Transporter b0,+AT-rBAT (SLC7A9-SLC3A1). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 4419–4434. [Google Scholar] [CrossRef]
- Strobel, J.; Müller, F.; Zolk, O.; Endreß, B.; König, J.; Fromm, M.F.; Maas, R. Transport of Asymmetric Dimethylarginine (ADMA) by Cationic Amino Acid Transporter 2 (CAT2), Organic Cation Transporter 2 (OCT2) and Multidrug and Toxin Extrusion Protein 1 (MATE1). Amino Acids 2013, 45, 989–1002. [Google Scholar] [CrossRef]
- Taghikhani, E.; Maas, R.; Fromm, M.F.; König, J. The Renal Transport Protein OATP4C1 Mediates Uptake of the Uremic Toxin Asymmetric Dimethylarginine (ADMA) and Efflux of Cardioprotective L-Homoarginine. PLoS ONE 2019, 14, e0213747. [Google Scholar] [CrossRef]
- Banjarnahor, S.; Rodionov, R.N.; König, J.; Maas, R. Transport of L-Arginine Related Cardiovascular Risk Markers. J. Clin. Med. 2020, 9, 3975. [Google Scholar] [CrossRef]
- Chafai, A.; Fromm, M.F.; König, J.; Maas, R. The Prognostic Biomarker L-Homoarginine Is a Substrate of the Cationic Amino Acid Transporters CAT1, CAT2A and CAT2B. Sci. Rep. 2017, 7, 4767. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F.; Meier, C.; Rossier, G.; Kühn, L.C. Glycoprotein-Associated Amino Acid Exchangers: Broadening the Range of Transport Specificity. Pflügers Arch. 2000, 440, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Closs, E.I.; Simon, A.; Vékony, N.; Rotmann, A. Plasma Membrane Transporters for Arginine. J. Nutr. 2004, 134, 2752S–2759S. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M., Jr. Arginine Metabolism Revisited. J. Nutr. 2016, 146, 2579S–2586S. [Google Scholar] [CrossRef] [PubMed]
- Windmueller, H.G.; Spaeth, A.E. Source and Fate of Circulating Citrulline. Am. J. Physiol.-Endocrinol. Metab. 1981, 241, E473–E480. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Böger, R.H.; Bode-Böger, S.M.; Frölich, J.C.; Haller, H.; Ritz, E.; Fliser, D. Marked Increase of Asymmetric Dimethylarginine in Patients with Incipient Primary Chronic Renal Disease. J. Am. Soc. Nephrol. 2002, 13, 170–176. [Google Scholar] [CrossRef]
- Bouby, N.; Hassler, C.; Parvy, P.; Bankir, L. Renal Synthesis of Arginine in Chronic Renal Failure: In Vivo and in Vitro Studies in Rats with 5/6 Nephrectomy. Kidney Int. 1993, 44, 676–683. [Google Scholar] [CrossRef]
- Baylis, C. Nitric Oxide Deficiency in Chronic Kidney Disease. Am. J. Physiol.-Ren. Physiol. 2008, 294, F1–F9. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Baylis, C. Total Nitric Oxide Production Is Low in Patients with Chronic Renal Disease. Kidney Int. 2000, 58, 1261–1266. [Google Scholar] [CrossRef]
- Lau, T.; Owen, W.; Yu, Y.M.; Noviski, N.; Lyons, J.; Zurakowski, D.; Tsay, R.; Ajami, A.; Young, V.R.; Castillo, L. Arginine, Citrulline, and Nitric Oxide Metabolism in End-Stage Renal Disease Patients. J. Clin. Investig. 2000, 105, 1217–1225. [Google Scholar] [CrossRef]
- Bredt, D.S.; Snyder, S.H. Isolation of Nitric Oxide Synthetase, a Calmodulin-Requiring Enzyme. Proc. Natl. Acad. Sci. USA 1990, 87, 682–685. [Google Scholar] [CrossRef]
- Moali, C.; Boucher, J.-L.; Sari, M.-A.; Stuehr, D.J.; Mansuy, D. Substrate Specificity of NO Synthases: Detailed Comparison of L-Arginine, Homo-L-Arginine, Their Nω-Hydroxy Derivatives, and Nω-Hydroxynor-L-Arginine. Biochemistry 1998, 37, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.W.; Fernstrom, J.D.; Thompson, J.; Morris, S.M., Jr.; Kuller, L.H. Biochemical Responses of Healthy Subjects during Dietary Supplementation with L-Arginine. J. Nutr. Biochem. 2004, 15, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-Y.; Qin, L.-Q.; Zhang, Z.; Zhao, Y.; Wang, J.; Arigoni, F.; Zhang, W. Effect of Oral L-Arginine Supplementation on Blood Pressure: A Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled Trials. Am. Heart J. 2011, 162, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Teunissen-Beekman, K.F.; van Baak, M.A. The Role of Dietary Protein in Blood Pressure Regulation. Curr. Opin. Lipidol. 2013, 24, 65–70. [Google Scholar] [CrossRef]
- Appel, L.J. The Effects of Protein Intake on Blood Pressure and Cardiovascular Disease. Curr. Opin. Lipidol. 2003, 14, 55–59. [Google Scholar] [CrossRef]
- Schulman, S.P.; Becker, L.C.; Kass, D.A.; Champion, H.C.; Terrin, M.L.; Forman, S.; Ernst, K.V.; Kelemen, M.D.; Townsend, S.N.; Capriotti, A.; et al. L-Arginine Therapy in Acute Myocardial Infarction: The Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) Randomized Clinical Trial. JAMA 2006, 295, 58–64. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and Pharmacodynamic Properties of Oral L-Citrulline and L-Arginine: Impact on Nitric Oxide Metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Choe, C.; Atzler, D.; Wild, P.S.; Carter, A.M.; Böger, R.H.; Ojeda, F.; Simova, O.; Stockebrand, M.; Lackner, K.; Nabuurs, C.; et al. Homoarginine Levels Are Regulated by L-Arginine: Glycine Amidinotransferase and Affect Stroke Outcome: Results from Human and Murine Studies. Circulation 2013, 128, 1451–1461. [Google Scholar] [CrossRef]
- Maas, R.; Tan-Andreesen, J.; Schwedhelm, E.; Schulze, F.; Böger, R.H. A Stable-Isotope Based Technique for the Determination of Dimethylarginine Dimethylaminohydrolase (DDAH) Activity in Mouse Tissue. J. Chromatogr. B 2007, 851, 220–228. [Google Scholar] [CrossRef]
- Shirakawa, T.; Kako, K.; Shimada, T.; Nagashima, Y.; Nakamura, A.; Ishida, J.; Fukamizu, A. Production of Free Methylarginines via the Proteasome and Autophagy Pathways in Cultured Cells. Mol. Med. Rep. 2011, 4, 615–620. [Google Scholar] [CrossRef]
- Kimoto, M.; Whitley, G.S.J.; Tsuji, H.; Ogawa, T. Detection of NG, NG-Dimethylarginine Dimethylaminohydrolase in Human Tissues Using a Monoclonal Antibody. J. Biochem. 1995, 117, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Caplin, B.; Wang, Z.; Slaviero, A.; Tomlinson, J.; Dowsett, L.; Delahaye, M.; Salama, A.; The International Consortium for Blood Pressure Genome-Wide Association Studies; Wheeler, D.C.; Leiper, J. Alanine-Glyoxylate Aminotransferase-2 Metabolizes Endogenous Methylarginines, Regulates NO, and Controls Blood Pressure. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Jarzebska, N.; Mangoni, A.A.; Martens-Lobenhoffer, J.; Bode-Böger, S.M.; Rodionov, R.N. The Second Life of Methylarginines as Cardiovascular Targets. Int. J. Mol. Sci. 2019, 20, 4592. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, J.C. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef]
- Carello, K.A.; Whitesall, S.E.; Lloyd, M.C.; Billecke, S.S.; D’Alecy, L.G. Asymmetrical Dimethylarginine Plasma Clearance Persists after Acute Total Nephrectomy in Rats. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H209–H216. [Google Scholar] [CrossRef]
- Lluch, P.; Segarra, G.; Medina, P. Asymmetric Dimethylarginine as a Mediator of Vascular Dysfunction in Cirrhosis. World J. Gastroenterol. WJG 2015, 21, 9466–9475. [Google Scholar] [CrossRef]
- Castillo, L.; Ajami, A.; Branch, S.; Chapman, T.; Yu, Y.-M.; Burke, J.; Young, V. Plasma Arginine Kinetics in Adult Man: Response to an Arginine-Free Diet. Metabolism 1994, 43, 114–122. [Google Scholar] [CrossRef]
- Castillo, L.; Beaumier, L.; Ajami, A.M.; Young, V.R. Whole Body Nitric Oxide Synthesis in Healthy Men Determined from [15N] Arginine-to-[15N] Citrulline Labeling. Proc. Natl. Acad. Sci. USA 1996, 93, 11460–11465. [Google Scholar] [CrossRef]
- Böger, R.H. The Pharmacodynamics of L-Arginine. J. Nutr. 2007, 137, 1650S–1655S. [Google Scholar] [CrossRef]
- Rodionov, R.N.; Oppici, E.; Martens-Lobenhoffer, J.; Jarzebska, N.; Brilloff, S.; Burdin, D.; Demyanov, A.; Kolouschek, A.; Leiper, J.; Maas, R.; et al. A Novel Pathway for Metabolism of the Cardiovascular Risk Factor Homoarginine by Alanine: Glyoxylate Aminotransferase 2. Sci. Rep. 2016, 6, 35277. [Google Scholar] [CrossRef] [PubMed]
- Bollenbach, A.; Cordts, K.; Hanff, E.; Atzler, D.; Choe, C.; Schwedhelm, E.; Tsikas, D. Evidence by GC-MS That Lysine Is an Arginase-Catalyzed Metabolite of Homoarginine in Vitro and in Vivo in Humans. Anal. Biochem. 2019, 577, 59–66. [Google Scholar] [CrossRef] [PubMed]
- McMenamy, R.H.; Lund, C.C.; Oncley, J.L. Unbound Amino Acid Concentrations in Human Blood Plasmas. J. Clin. Investig. 1957, 36, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Said, M.Y.; Douwes, R.M.; van Londen, M.; Minović, I.; Frenay, A.-R.; de Borst, M.H.; van den Berg, E.; Heiner-Fokkema, M.R.; Kayacelebi, A.A.; Bollenbach, A.; et al. Effect of Renal Function on Homeostasis of Asymmetric Dimethylarginine (ADMA): Studies in Donors and Recipients of Renal Transplants. Amino Acids 2019, 51, 565–575. [Google Scholar] [CrossRef]
- Servillo, L.; Giovane, A.; D’Onofrio, N.; Casale, R.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Determination of Homoarginine, Arginine, NMMA, ADMA, and SDMA in Biological Samples by HPLC-ESI-Mass Spectrometry. Int. J. Mol. Sci. 2013, 14, 20131–20138. [Google Scholar] [CrossRef]
- Akyurek, F.; Celik, G.; Ozturk, B. Predictive Role of Methylarginines in Renal Failure. Ann. Med. Res. 2020, 27, 2129–2133. [Google Scholar] [CrossRef]
- Lluch, P.; Torondel, B.; Medina, P.; Segarra, G.; Del Olmo, J.A.; Serra, M.A.; Rodrigo, J.M. Plasma Concentrations of Nitric Oxide and Asymmetric Dimethylarginine in Human Alcoholic Cirrhosis. J. Hepatol. 2004, 41, 55–59. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Wallaschofski, H.; Atzler, D.; Dörr, M.; Nauck, M.; Völker, U.; Kroemer, H.K.; Völzke, H.; Böger, R.H.; Friedrich, N. Incidence of All-Cause and Cardiovascular Mortality Predicted by Symmetric Dimethylarginine in the Population-Based Study of Health in Pomerania. PLoS ONE 2014, 9, e96875. [Google Scholar] [CrossRef]
- Kato, T.; Sano, M.; Mizutani, N. Homocitrullinuria and Homoargininuria in Lysinuric Protein Intolerance. J. Inherit. Metab. Dis. 1989, 12, 157–161. [Google Scholar] [CrossRef]
- Atzler, D.; Mieth, M.; Maas, R.; Böger, R.H.; Schwedhelm, E. Stable Isotope Dilution Assay for Liquid Chromatography–Tandem Mass Spectrometric Determination of L-Homoarginine in Human Plasma. J. Chromatogr. B 2011, 879, 2294–2298. [Google Scholar] [CrossRef]
- May, M.; Batkai, S.; Zörner, A.A.; Tsikas, D.; Jordan, J.; Engeli, S. Clinical Evaluation of Extracellular ADMA Concentrations in Human Blood and Adipose Tissue. Int. J. Mol. Sci. 2014, 15, 1189–1200. [Google Scholar] [CrossRef]
- Nijveldt, R.; Teerlink, T.; Van Der Hoven, B.; Siroen, M.; Kuik, D.; Rauwerda, J.; Van Leeuwen, P. Asymmetrical Dimethylarginine (ADMA) in Critically Ill Patients: High Plasma ADMA Concentration Is an Independent Risk Factor of ICU Mortality. Crit. Care 2003, 7, 23–30. [Google Scholar] [CrossRef]
- Teerlink, T. HPLC Analysis of ADMA and Other Methylated L-Arginine Analogs in Biological Fluids. J. Chromatogr. B 2007, 851, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Emrich, I.E.; Zawada, A.M.; Martens-Lobenhoffer, J.; Fliser, D.; Wagenpfeil, S.; Heine, G.H.; Bode-Böger, S.M. Symmetric Dimethylarginine (SDMA) Outperforms Asymmetric Dimethylarginine (ADMA) and Other Methylarginines as Predictor of Renal and Cardiovascular Outcome in Non-Dialysis Chronic Kidney Disease. Clin. Res. Cardiol. 2018, 107, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Glorieux, G.; Dhondt, A.; Leybaert, L.; Vanholder, R. Role of Symmetric Dimethylarginine in Vascular Damage by Increasing ROS via Store-Operated Calcium Influx in Monocytes. Nephrol. Dial. Transplant. 2009, 24, 1429–1435. [Google Scholar] [CrossRef]
- Schepers, E.; Barreto, D.V.; Liabeuf, S.; Glorieux, G.; Eloot, S.; Barreto, F.C.; Massy, Z.; Vanholder, R. Symmetric Dimethylarginine as a Proinflammatory Agent in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 2374–2383. [Google Scholar] [CrossRef]
- Riddell, A.; Flynn, A.; Bergugnat, H.; Dowsett, L.B.; Miller, A.A. SDMA as a Marker and Mediator in Cerebrovascular Disease. Clin. Sci. 2024, 138, 1305–1323. [Google Scholar] [CrossRef]
- Surrer, D.B.; Fromm, M.F.; Maas, R.; König, J. L-Arginine and Cardioactive Arginine Derivatives as Substrates and Inhibitors of Human and Mouse NaCT/Nact. Metabolites 2022, 12, 273. [Google Scholar] [CrossRef]
- Lüneburg, N.; Lieb, W.; Zeller, T.; Chen, M.-H.; Maas, R.; Carter, A.M.; Xanthakis, V.; Glazer, N.L.; Schwedhelm, E.; Seshadri, S.; et al. Genome-Wide Association Study of L-Arginine and Dimethylarginines Reveals Novel Metabolic Pathway for Symmetric Dimethylarginine. Circ. Cardiovasc. Genet. 2014, 7, 864–872. [Google Scholar] [CrossRef]
- Marescau, B.; Nagels, G.; Possemiers, I.; De Broe, M.E.; Becaus, I.; Billiouw, J.-M.; Lornoy, W.; De Deyn, P.P. Guanidino Compounds in Serum and Urine of Nondialyzed Patients with Chronic Renal Insufficiency. Metabolism 1997, 46, 1024–1031. [Google Scholar] [CrossRef]
- Veldink, H.; Faulhaber-Walter, R.; Park, J.-K.; Martens-Lobenhoffer, J.; Bode-Böger, S.; Schuett, H.; Haghikia, A.; Hilfiker-Kleiner, D.; Kielstein, J.T. Effects of Chronic SDMA Infusion on Glomerular Filtration Rate, Blood Pressure, Myocardial Function and Renal Histology in C57BL6/J Mice. Nephrol. Dial. Transplant. 2013, 28, 1434–1439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bröer, S.; Fairweather, S.J. Amino Acid Transport across the Mammalian Intestine. Compr. Physiol. 2018, 9, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Matsumoto, T.; Morimoto, R.; Arioka, S.; Omote, H.; Moriyama, Y. A Human Transporter Protein That Mediates the Final Excretion Step for Toxic Organic Cations. Proc. Natl. Acad. Sci. USA 2005, 102, 17923–17928. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Yoshimoto, E.; Meruelo, D. Molecular Cloning and Characterization of a Novel Human Gene Homologous to the Murine Ecotropic Retroviral Receptor. Virology 1991, 185, 10–17. [Google Scholar] [CrossRef]
- Wu, F.; Cholewa, B.; Mattson, D.L. Characterization of L-Arginine Transporters in Rat Renal Inner Medullary Collecting Duct. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1506–R1512. [Google Scholar] [CrossRef]
- Burger-Kentischer, A.; Müller, E.; Klein, H.G.; Schober, A.; Neuhofer, W.; Beck, F.X. Cationic Amino Acid Transporter mRNA Expression in Rat Kidney and Liver. Kidney Int. Suppl. 1998, 67, S136–S138. [Google Scholar] [CrossRef]
- Bröer, A.; Wagner, C.A.; Lang, F.; Bröer, S. The Heterodimeric Amino Acid Transporter 4F2hc/y+LAT2 Mediates Arginine Efflux in Exchange with Glutamine. Biochem. J. 2000, 349, 787–795. [Google Scholar] [CrossRef]
- Torrents, D.; Mykkänen, J.; Pineda, M.; Feliubadaló, L.; Estévez, R.; de Cid, R.; Sanjurjo, P.; Zorzano, A.; Nunes, V.; Huoponen, K.; et al. Identification of SLC7A7, Encoding y+LAT-1, as the Lysinuric Protein Intolerance Gene. Nat. Genet. 1999, 21, 293–296. [Google Scholar] [CrossRef]
- Mikkaichi, T.; Suzuki, T.; Onogawa, T.; Tanemoto, M.; Mizutamari, H.; Okada, M.; Chaki, T.; Masuda, S.; Tokui, T.; Eto, N.; et al. Isolation and Characterization of a Digoxin Transporter and Its Rat Homologue Expressed in the Kidney. Proc. Natl. Acad. Sci. USA 2004, 101, 3569–3574. [Google Scholar] [CrossRef]
- Urakami, Y.; Akazawa, M.; Saito, H.; Okuda, M.; Inui, K. cDNA Cloning, Functional Characterization, and Tissue Distribution of an Alternatively Spliced Variant of Organic Cation Transporter hOCT2 Predominantly Expressed in the Human Kidney. J. Am. Soc. Nephrol. 2002, 13, 1703–1710. [Google Scholar] [CrossRef]
- Gorboulev, V.; Ulzheimer, J.C.; Akhoundova, A.; Ulzheimer-Teuber, I.; Karbach, U.; Quester, S.; Baumann, C.; Lang, F.; Busch, A.E.; Koepsell, H. Cloning and Characterization of Two Human Polyspecific Organic Cation Transporters. DNA Cell Biol. 1997, 16, 871–881. [Google Scholar] [CrossRef]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A Single–Cell Type Transcriptomics Map of Human Tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef]
- Kidney Precision Medicine Project (KPMP). Available online: https://www.kpmp.org (accessed on 4 March 2025).
- Human Protein Atlas V24.0-Single Cell Type—SLC7A9. Available online: https://www.proteinatlas.org/ENSG00000021488-SLC7A9/single+cell#single_cell_type_summary (accessed on 4 March 2025).
- Human Protein Atlas V24.0-Single Cell Type—SLC47A1. Available online: https://www.proteinatlas.org/ENSG00000142494-SLC47A1/single+cell#single_cell_type_summary (accessed on 4 March 2025).
- Human Protein Atlas V24.0-Single Cell Type—SLC3A2. Available online: https://www.proteinatlas.org/ENSG00000168003-SLC3A2/single+cell#single_cell_type_summary (accessed on 4 March 2025).
- Human Protein Atlas V24.0-Single Cell Type—SLC7A1. Available online: https://www.proteinatlas.org/ENSG00000139514-SLC7A1/single+cell#single_cell_type_summary (accessed on 4 March 2025).
- Human Protein Atlas V24.0-Single Cell Type—SLC7A2. Available online: https://www.proteinatlas.org/ENSG00000003989-SLC7A2/single+cell#single_cell_type_summary (accessed on 4 March 2025).
- Human Protein Atlas V24.0-Single Cell Type—SLC7A6. Available online: https://www.proteinatlas.org/ENSG00000103064-SLC7A6/single+cell#single_cell_type_summary (accessed on 4 March 2025).
- Human Protein Atlas V24.0-Single Cell Type—SLC7A7. Available online: https://www.proteinatlas.org/ENSG00000155465-SLC7A7/single+cell#single_cell_type_summary (accessed on 4 March 2025).
- Human Protein Atlas V24.0-Single Cell Type—SLC22A2. Available online: https://www.proteinatlas.org/ENSG00000112499-SLC22A2/single+cell#single_cell_type_summary (accessed on 4 March 2025).
- Human Protein Atlas V24.0-Single Cell Type—SLCO4C1. Available online: https://www.proteinatlas.org/ENSG00000173930-SLCO4C1/single+cell#single_cell_type_summary (accessed on 4 March 2025).
- Lake, B.B.; Menon, R.; Winfree, S.; Hu, Q.; Melo Ferreira, R.; Kalhor, K.; Barwinska, D.; Otto, E.A.; Ferkowicz, M.; Diep, D.; et al. An Atlas of Healthy and Injured Cell States and Niches in the Human Kidney. Nature 2023, 619, 585–594. [Google Scholar] [CrossRef]
- Makrides, V.; Camargo, S.M.R.; Verrey, F. Transport of Amino Acids in the Kidney. Compr. Physiol. 2014, 4, 367–403. [Google Scholar] [CrossRef]
- Kakoki, M.; Kim, H.-S.; Arendshorst, W.J.; Mattson, D.L. L-Arginine Uptake Affects Nitric Oxide Production and Blood Flow in the Renal Medulla. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R1478–R1485. [Google Scholar] [CrossRef]
- Kakoki, M.; Wang, W.; Mattson, D.L. Cationic Amino Acid Transport in the Renal Medulla and Blood Pressure Regulation. Hypertension 2002, 39, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Estévez, R.; Camps, M.; Rojas, A.M.; Testar, X.; Devîs, R.; Hediger, M.A.; Zorzano, A.; Palacín, M. The Amino Acid Transport System y+L/4F2hc Is a Heteromultimeric Complex. FASEB J. 1998, 12, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Grahammer, F.; Ramakrishnan, S.K.; Rinschen, M.M.; Larionov, A.A.; Syed, M.; Khatib, H.; Roerden, M.; Sass, J.O.; Helmstaedter, M.; Osenberg, D.; et al. mTOR Regulates Endocytosis and Nutrient Transport in Proximal Tubular Cells. J. Am. Soc. Nephrol. 2017, 28, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Fukasawa, Y.; Cha, S.H.; Segawa, H.; Chairoungdua, A.; Kim, D.K.; Matsuo, H.; Kim, J.Y.; Miyamoto, K.; Takeda, E.; et al. Transport Properties of a System y+L Neutral and Basic Amino Acid Transporter. J. Biol. Chem. 2000, 275, 20787–20793. [Google Scholar] [CrossRef]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 Families of Amino Acid Transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef]
- Rotoli, B.M.; Barilli, A.; Visigalli, R.; Ferrari, F.; Dall’Asta, V. y+LAT1 and y+LAT2 Contribution to Arginine Uptake in Different Human Cell Models: Implications in the Pathophysiology of Lysinuric Protein Intolerance. J. Cell. Mol. Med. 2020, 24, 921–929. [Google Scholar] [CrossRef]
- Closs, E.I.; Ostad, M.A.; Simon, A.; Warnholtz, A.; Jabs, A.; Habermeier, A.; Daiber, A.; Förstermann, U.; Münzel, T. Impairment of the Extrusion Transporter for Asymmetric Dimethyl-L-Arginine: A Novel Mechanism Underlying Vasospastic Angina. Biochem. Biophys. Res. Commun. 2012, 423, 218–223. [Google Scholar] [CrossRef]
- Bauch, C.; Verrey, F. Apical Heterodimeric Cystine and Cationic Amino Acid Transporter Expressed in MDCK Cells. Am. J. Physiol.-Ren. Physiol. 2002, 283, F181–F189. [Google Scholar] [CrossRef]
- Toyohara, T.; Suzuki, T.; Morimoto, R.; Akiyama, Y.; Souma, T.; Shiwaku, H.O.; Takeuchi, Y.; Mishima, E.; Abe, M.; Tanemoto, M.; et al. SLCO4C1 Transporter Eliminates Uremic Toxins and Attenuates Hypertension and Renal Inflammation. J. Am. Soc. Nephrol. 2009, 20, 2546–2555. [Google Scholar] [CrossRef] [PubMed]
- Scherpinski, L.A.; Fromm, M.F.; Maas, R.; König, J. Transport of the Uremic Toxin Symmetric Dimethylarginine (SDMA) by Renal Transport Proteins. Amino Acids 2025, 57, 34. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Nagaretani, H.; Tamura, S.; Ohama, T.; Maruyama, T.; Hiraoka, H.; Yamashita, S.; Yamada, A.; Kiso, S.; Inui, Y.; et al. Vascular Endothelial Dysfunction Resulting from L-Arginine Deficiency in a Patient with Lysinuric Protein Intolerance. J. Clin. Investig. 2001, 108, 717–724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kayanoki, Y.; Kawata, S.; Yamasaki, E.; Kiso, S.; Inoue, S.; Tamura, S.; Taniguchi, N.; Matsuzawa, Y. Reduced Nitric Oxide Production by L-Arginine Deficiency in Lysinuric Protein Intolerance Exacerbates Intravascular Coagulation. Metabolism 1999, 48, 1136–1140. [Google Scholar] [CrossRef]
- Fernández, E.; Jiménez-Vidal, M.; Calvo, M.; Zorzano, A.; Tebar, F.; Palacín, M.; Chillarón, J. The Structural and Functional Units of Heteromeric Amino Acid Transporters: The Heavy Subunit rBAT Dictates Oligomerization of the Heteromeric Amino Acid Transporters. J. Biol. Chem. 2006, 281, 26552–26561. [Google Scholar] [CrossRef]
- Wagner, C.A.; Lang, F.; Bröer, S. Function and Structure of Heterodimeric Amino Acid Transporters. Am. J. Physiol.-Cell Physiol. 2001, 281, C1077–C1093. [Google Scholar] [CrossRef][Green Version]
- Nagamori, S.; Wiriyasermkul, P.; Guarch, M.E.; Okuyama, H.; Nakagomi, S.; Tadagaki, K.; Nishinaka, Y.; Bodoy, S.; Takafuji, K.; Okuda, S.; et al. Novel Cystine Transporter in Renal Proximal Tubule Identified as a Missing Partner of Cystinuria-Related Plasma Membrane Protein rBAT/SLC3A1. Proc. Natl. Acad. Sci. USA 2016, 113, 775–780. [Google Scholar] [CrossRef]
- Fernández, E.; Carrascal, M.; Rousaud, F.; Abián, J.; Zorzano, A.; Palacín, M.; Chillarón, J. rBAT-b0,+AT Heterodimer Is the Main Apical Reabsorption System for Cystine in the Kidney. Am. J. Physiol.-Ren. Physiol. 2002, 283, F540–F548. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.; Wagner, C.A.; Bröer, A.; Stehberger, P.A.; Kaltenbach, S.; Gelpí, J.L.; Martin del Rio, R.; Zorzano, A.; Palacín, M.; Lang, F.; et al. Cystinuria-Specific rBAT (R365W) Mutation Reveals Two Translocation Pathways in the Amino Acid Transporter rBAT-b0,+AT. Biochem. J. 2004, 377, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Li, Y.; Shi, Y.; Zhou, J.; Lei, J.; Huang, J.; Zhou, Q. Cryo-EM Structure of the Human Heteromeric Amino Acid Transporter b0,+AT-rBAT. Sci. Adv. 2020, 6, eaay6379. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhao, X.; Lei, J.; Zhou, Q. Structure of the Human LAT1–4F2hc Heteromeric Amino Acid Transporter Complex. Nature 2019, 568, 127–130. [Google Scholar] [CrossRef]
- Lee, Y.; Wiriyasermkul, P.; Jin, C.; Quan, L.; Ohgaki, R.; Okuda, S.; Kusakizako, T.; Nishizawa, T.; Oda, K.; Ishitani, R.; et al. Cryo-EM Structure of the Human L-Type Amino Acid Transporter 1 in Complex with Glycoprotein CD98hc. Nat. Struct. Mol. Biol. 2019, 26, 510–517. [Google Scholar] [CrossRef]
- Mizoguchi, K.; Cha, S.H.; Chairoungdua, A.; Kim, D.K.; Shigeta, Y.; Matsuo, H.; Fukushima, J.-I.; Awa, Y.; Akakura, K.; Goya, T.; et al. Human Cystinuria-Related Transporter: Localization and Functional Characterization. Kidney Int. 2001, 59, 1821–1833. [Google Scholar] [CrossRef][Green Version]
- Reig, N.; Chillarón, J.; Bartoccioni, P.; Fernández, E.; Bendahan, A.; Zorzano, A.; Kanner, B.; Palacín, M.; Bertran, J. The Light Subunit of System bo,+ Is Fully Functional in the Absence of the Heavy Subunit. EMBO J. 2002, 21, 4906–4914. [Google Scholar] [CrossRef]
- Torras-Llort, M.; Torrents, D.; Soriano-Garcia, J.; Gelpi, J.; Estévez, R.; Ferrer, R.; Palacin, M.; Moreto, M. Sequential Amino Acid Exchange across b0,+-like System in Chicken Brush Border Jejunum. J. Membr. Biol. 2001, 180, 213–220. [Google Scholar] [CrossRef]
- Iuliano, I.; Iervolino, A.; Suzumoto, Y.; Shams, A.; Longobardi, C.; Capasso, G.; Perna, A.F.; Capolongo, G. Cystinuria: A Genetic and Molecular View. What Is Known about Animal Models and Cells. Kidney Blood Press. Res. 2025. [Google Scholar] [CrossRef]
- Strologo, L.D.; Pras, E.; Pontesilli, C.; Beccia, E.; Ricci-Barbini, V.; de Sanctis, L.; Ponzone, A.; Gallucci, M.; Bisceglia, L.; Zelante, L.; et al. Comparison between SLC3A1 and SLC7A9 Cystinuria Patients and Carriers: A Need for a New Classification. J. Am. Soc. Nephrol. 2002, 13, 2547–2553. [Google Scholar] [CrossRef]
- Brodehl, J. Isolated Cystinuria (without Lysin-, Ornithinand Argininuria) in a Family with Hypocalcemic Tetany. Monatsschr. Kinderheilkd. 1967, 115, 317–320. [Google Scholar]
- Hagenbuch, B.; Meier, P.J. Organic Anion Transporting Polypeptides of the OATP/SLC21 Family: Phylogenetic Classification as OATP/SLCO Superfamily, New Nomenclature and Molecular/Functional Properties. Pflügers Arch. Eur. J. Physiol. 2004, 447, 653–665. [Google Scholar] [CrossRef]
- Chu, X.-Y.; Bleasby, K.; Yabut, J.; Cai, X.; Chan, G.H.; Hafey, M.J.; Xu, S.; Bergman, A.J.; Braun, M.P.; Dean, D.C.; et al. Transport of the Dipeptidyl Peptidase-4 Inhibitor Sitagliptin by Human Organic Anion Transporter 3, Organic Anion Transporting Polypeptide 4C1, and Multidrug Resistance P-Glycoprotein. J. Pharmacol. Exp. Ther. 2007, 321, 673–683. [Google Scholar] [CrossRef]
- Suzuki, T.; Toyohara, T.; Akiyama, Y.; Takeuchi, Y.; Mishima, E.; Suzuki, C.; Ito, S.; Soga, T.; Abe, T. Transcriptional Regulation of Organic Anion Transporting Polypeptide SLCO4C1 as a New Therapeutic Modality to Prevent Chronic Kidney Disease. J. Pharm. Sci. 2011, 100, 3696–3707. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Holzwarth, J.A.; Smith, B.; Karaz, S.; Membrez, M.; Sorrentino, V.; Summers, S.; Spears, J.; Migliavacca, E. Impaired Renal Transporter Gene Expression and Uremic Toxin Excretion as Aging Hallmarks in Cats with Naturally Occurring Chronic Kidney Disease. Aging 2024, 16, 13588–13607. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Kikuchi, K.; Saigusa, D.; Suzuki, T.; Takeuchi, Y.; Mishima, E.; Yamamoto, Y.; Ishida, A.; Sugawara, D.; Jinno, D.; et al. Indoxyl Sulfate Down-Regulates SLCO4C1 Transporter through Up-Regulation of GATA3. PLoS ONE 2013, 8, e66518. [Google Scholar] [CrossRef]
- Ailabouni, A.; Prasad, B. Organic Cation Transporters 2: Structure, Regulation, Functions, and Clinical Implications. Drug Metab. Dispos. 2025, 53, 100044. [Google Scholar] [CrossRef]
- Motohashi, H.; Nakao, Y.; Masuda, S.; Katsura, T.; Kamba, T.; Ogawa, O.; Inui, K.-I. Precise Comparison of Protein Localization among OCT, OAT, and MATE in Human Kidney. J. Pharm. Sci. 2013, 102, 3302–3308. [Google Scholar] [CrossRef]
- Jonker, J.W.; Wagenaar, E.; Van Eijl, S.; Schinkel, A.H. Deficiency in the Organic Cation Transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in Mice Abolishes Renal Secretion of Organic Cations. Mol. Cell. Biol. 2003, 23, 7902–7908. [Google Scholar] [CrossRef]
- Giacomini, K.M.; Huang, S.-M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane Transporters in Drug Development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef]
- Schophuizen, C.M.S.; Wilmer, M.J.; Jansen, J.; Gustavsson, L.; Hilgendorf, C.; Hoenderop, J.G.J.; van den Heuvel, L.P.; Masereeuw, R. Cationic Uremic Toxins Affect Human Renal Proximal Tubule Cell Functioning through Interaction with the Organic Cation Transporter. Pflugers Arch. 2013, 465, 1701–1714. [Google Scholar] [CrossRef]
- Severance, A.C.; Sandoval, P.J.; Wright, S.H. Correlation between Apparent Substrate Affinity and OCT2 Transport Turnover. J. Pharmacol. Exp. Ther. 2017, 362, 405–412. [Google Scholar] [CrossRef]
- Gessner, A.; König, J.; Fromm, M.F. Clinical Aspects of Transporter-Mediated Drug-Drug Interactions. Clin. Pharmacol. Ther. 2019, 105, 1386–1394. [Google Scholar] [CrossRef]
- Galetin, A.; Brouwer, K.L.R.; Tweedie, D.; Yoshida, K.; Sjöstedt, N.; Aleksunes, L.; Chu, X.; Evers, R.; Hafey, M.J.; Lai, Y.; et al. Membrane Transporters in Drug Development and as Determinants of Precision Medicine. Nat. Rev. Drug Discov. 2024, 23, 255–280. [Google Scholar] [CrossRef] [PubMed]
- Budiman, T.; Bamberg, E.; Koepsell, H.; Nagel, G. Mechanism of Electrogenic Cation Transport by the Cloned Organic Cation Transporter 2 from Rat. J. Biol. Chem. 2000, 275, 29413–29420. [Google Scholar] [CrossRef] [PubMed]
- Gessner, A.; König, J.; Fromm, M.F. Contribution of Multidrug and Toxin Extrusion Protein 1 (MATE1) to Renal Secretion of Trimethylamine-N-Oxide (TMAO). Sci. Rep. 2018, 8, 6659. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.; Galetin, A.; Tomita, Y.; Giacomini, K.M.; Chu, X.; Yang, X.; Nakamura, T.; Kusuhara, H.; Sugiyama, Y. Predicting OCT2/MATEs-Mediated Drug Interactions in Healthy Volunteers and Patients with Chronic Kidney Disease: Insights from Extended Clearance Concept, Endogenous Biomarkers, and In Vitro Inhibition Studies (Perspectives from the International Transporter Consortium). Clin. Pharmacol. Ther. 2025. [Google Scholar] [CrossRef]
- Cheung, K.W.K.; Hsueh, C.-H.; Zhao, P.; Meyer, T.W.; Zhang, L.; Huang, S.-M.; Giacomini, K.M. The Effect of Uremic Solutes on the Organic Cation Transporter 2. J. Pharm. Sci. 2017, 106, 2551–2557. [Google Scholar] [CrossRef]
- Tanihara, Y.; Masuda, S.; Sato, T.; Katsura, T.; Ogawa, O.; Inui, K.-I. Substrate Specificity of MATE1 and MATE2-K, Human Multidrug and Toxin Extrusions/H+-Organic Cation Antiporters. Biochem. Pharmacol. 2007, 74, 359–371. [Google Scholar] [CrossRef]
- Yin, O.Q.; Tomlinson, B.; Chow, M.S. Variability in Renal Clearance of Substrates for Renal Transporters in Chinese Subjects. J. Clin. Pharmacol. 2006, 46, 157–163. [Google Scholar] [CrossRef]
- Kajiwara, M.; Terada, T.; Ogasawara, K.; Iwano, J.; Katsura, T.; Fukatsu, A.; Doi, T.; Inui, K. Identification of Multidrug and Toxin Extrusion (MATE1 and MATE2-K) Variants with Complete Loss of Transport Activity. J. Hum. Genet. 2009, 54, 40–46. [Google Scholar] [CrossRef]
- Masereeuw, R.; Mutsaers, H.A.M.; Toyohara, T.; Abe, T.; Jhawar, S.; Sweet, D.H.; Lowenstein, J. The Kidney and Uremic Toxin Removal: Glomerulus or Tubule? Semin. Nephrol. 2014, 34, 191–208. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing Kidney Function—Measured and Estimated Glomerular Filtration Rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Chen, L.; Lederer, E.D.; Yang, C.-R.; Knepper, M.A. Transcriptomes of Major Proximal Tubule Cell Culture Models. J. Am. Soc. Nephrol. 2021, 32, 86–97. [Google Scholar] [CrossRef]

| Transport Protein | Direction | Counter ion(s) | L-Arginine | L-Homoarginine | ADMA | SDMA |
|---|---|---|---|---|---|---|
| y+LAT1-4F2hc | Efflux | Na+, neutral amino acids [32,109,110] | Km: 182 ± 35 µM [111] Vmax: 3.822 ± 0.24 nmol × mg protein−1 × min−1 [111] Elevated urine and lowered plasma concentrations in LPI patients [69] | No in vitro data Elevated urine concentrations in LPI patients [69] | No in vitro data Case report of elevated intracellular ADMA concentrations [112] | No in vitro data No clinical data |
| b0,+AT-rBAT | Uptake | Neutral amino acids [82,110] | Km: 179.0 µM [113] Km: 512.6 ± 109.3 µM [27] Vmax: 1.9 ± 0.1 nmol × mg protein−1 × min−1 [27] Elevated urine concentrations in cystinuria patients [18] | Km: 197.0 ± 31 µM [27] Vmax: 0.7 ± 0.02 nmol × mg protein−1 × min−1 [27] Elevated urine concentrations in cystinuria patients [18] | Km: not detected Vmax: >5 ± 0.5 nmol × mg protein−1 × min−1 [27] No clinical data | No in vitro data No clinical data |
| OATP4C1 | Uptake and efflux | / | Km: 48.1 ± 5.7 µM [29] Vmax: 500.0 ± 19.9 pmol × mg protein−1 × min−1 [29] No clinical data | Km: 49.9 ± 9.6 µM Vmax: 355.7 ± 23.0 pmol × mg protein−1 × min−1 [29] No clinical data | Km: 232.1 ± 78.9 µM [29] Vmax: 351.6 ± 55.0 pmol × mg protein−1 × min−1 [29] No clinical data but increased SLCO4C1 mRNA expression was associated with elevated ADMA elimination in rats [114] | Km: 70 µM [115] No clinical data |
| OCT2 | Uptake | / | Km: >10,000 µM [28] Vmax: >50.0 nmol × mg protein−1 × min−1 [28] No clinical data | No in vitro data No clinical data | Km: 967 ± 143 µM [28] Vmax: 6.3 ± 0.3 nmol × mg protein−1 × min−1 [28] No clinical data | Km: no saturation [115] No clinical data |
| MATE1 | Efflux | H+ | Substrate [28] No clinical data | No in vitro data No clinical data | Transported [28] No clinical data | Km: 1973 µM [115] No clinical data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherpinski, L.A.; König, J.; Maas, R. Role of Transport Proteins for the Renal Handling of L-Arginine and Related Derivatives. Int. J. Mol. Sci. 2025, 26, 7899. https://doi.org/10.3390/ijms26167899
Scherpinski LA, König J, Maas R. Role of Transport Proteins for the Renal Handling of L-Arginine and Related Derivatives. International Journal of Molecular Sciences. 2025; 26(16):7899. https://doi.org/10.3390/ijms26167899
Chicago/Turabian StyleScherpinski, Lorenz A., Jörg König, and Renke Maas. 2025. "Role of Transport Proteins for the Renal Handling of L-Arginine and Related Derivatives" International Journal of Molecular Sciences 26, no. 16: 7899. https://doi.org/10.3390/ijms26167899
APA StyleScherpinski, L. A., König, J., & Maas, R. (2025). Role of Transport Proteins for the Renal Handling of L-Arginine and Related Derivatives. International Journal of Molecular Sciences, 26(16), 7899. https://doi.org/10.3390/ijms26167899






