Human Glucose Transporters in Health and Selected Neurodegenerative Diseases
Abstract
1. Introduction
2. Characteristics of Human Glucose Transporters
2.1. The Human GLUT Transporters
Structure of GLUT Proteins
Pseudogenes
- SLC2A3P1 (alias GLUT6 or GLUT3 pseudogene) located on chromosome 5q35. It is a retroposon of SLC2A3.
- SLC2A3P2 (alias GLUT3 pseudogene 2) located on chromosome 1p31.3. It is a retroposon of SLC2A3.
- SLC2A3P4 (alias GLUT3 pseudogene 4) located on chromosome 8q21.3. It is a retroposon of SLC2A3.
- SLC2AXP1 is located on chromosome 2q11.2. It contains internal stop sequences.
2.2. The Human Sodium-Dependent Glucose Cotransporters
Structure of Sodium-Dependent Glucose Cotransporters
2.3. The Human SWEET Transporter
3. Association of Human Glucose Transporters with Neurodegenerative Diseases
3.1. Expression of Glucose Transporters in Alzheimer’s Disease
3.2. Expression of Glucose Transporters in Parkinson’s Disease
3.3. Expression of Glucose Transporters in Huntington’s Disease
3.4. Expression of Glucose Transporter in GLUT1 Deficiency Syndrome
3.5. Expression of Glucose Transporters in Stroke
3.6. Expression of Glucose Transporters in Traumatic Brain Injury
4. Therapeutic Strategies in Neurodegenerative Diseases
4.1. Incretins
4.2. Dipeptidyl Peptidase-4 Inhibitors
4.3. Thiazolidinediones
4.4. Biguanides
5. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klipp, A.; De Bock, K.; Bilan, P.J.; Richter, E.A. Transcellular barriers to glucose delivery in the body. Annu. Rev. Physiol. 2024, 86, 149–173. [Google Scholar] [CrossRef]
- Wright, E.M. Glucose transport families SLC5 and SLC50. Mol. Asp. Med. 2013, 34, 183–196. [Google Scholar] [CrossRef]
- Szablewski, L. Glucose transporters in brain: In health and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 55, 1307–1320. [Google Scholar] [CrossRef]
- Głuchowska, K.; Pliszka, M.; Szablewski, L. Expression of glucose transporters in human neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2021, 540, 8–15. [Google Scholar] [CrossRef]
- Kaepsel, H. Glucose transporters in brain in health and disease. Pflügers Archiv. Eur. J. Physiol. 2020, 472, 1299–1343. [Google Scholar] [CrossRef]
- Morea, V.; Bidollari, E.; Colotti, G.; Fiorillo, A.; Rosati, J.; De Filippis, L.; Squitieri, F.; Ilan, A. Glucose transportation in the brain and its impairment in Huntington disease: One more shade of the energetic metabolism failure. Amino Acids 2017, 49, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Human Glucose Transporters in Health and Diseases; Cambridge Scholar Publishing: Newcastle upon Tyne, UK, 2019; ISBN 978-1-5275-3558-9. [Google Scholar]
- Szablewski, L. Expression of glucose transporters in cancers. Biochim. Biophys. Acta Rev. Canc. 2013, 1835, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Distribution of glucose transporters in renal diseases. J. Biomed. Sci. 2017, 24, 64. [Google Scholar] [CrossRef]
- Szablewski, L. Glucose transporters in healthy heart and in cardiac disease. Int. J. Cardiol. 2017, 230, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Pao, S.S.; Paulsen, I.T.; Saier, M.H., Jr. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34. [Google Scholar] [CrossRef]
- Saier, M.H., Jr. Families of transmembrane sugar transport protein. Mol. Microbiol. 2000, 35, 699–710. [Google Scholar] [CrossRef]
- LeFevre, P.G. Evidence of active transfer of certain non-electrolytes across the human red cell membrane. J. Gen. Physiol. 1948, 31, 505–527. [Google Scholar] [CrossRef][Green Version]
- Kasahara, M.; Hinkle, P.C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J. Biol. Chem. 1997, 252, 7384–7390. [Google Scholar] [CrossRef]
- Mueckler, M.; Caruso, C.; Baldwin, S.A.; Panico, I.; Blench, I.; Morris, H.R. Sequence and structure of a human glucose transporter. Science 1985, 229, 941–945. [Google Scholar] [CrossRef]
- Joost, H.G.; Bell, G.I.; Best, J.D.; Birnbaum, M.J.; Charron, M.J.; Chen, Y.T.; Doege, H.; James, D.E.; Lodish, H.F.; Moley, K.H.; et al. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E974–E976. [Google Scholar] [CrossRef] [PubMed]
- Joost, H.-G.; Thorens, B. The extend GLUT-family of sugar-polyol transport facilitators: Nomenclature, sequence characteristics, and potential function of its novel members. Mol. Membr. Biol. 2001, 18, 247–256. [Google Scholar] [CrossRef]
- Uldry, M.; Thorens, B. The SLC2 family of facilitative hexose and polyol transporters. Pflügers Arch. 2004, 447, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Zhao, F.-Q.; Keating, A.F. Functional properties and genomic of glucose transporters. Curr. Genom. 2007, 8, 113–128. [Google Scholar] [CrossRef]
- Long, W.; Cheeseman, C.I. Structure and functional insight into the GLUT family of membrane transporters. Cell Health Cytosk. 2015, 7, 167–183. [Google Scholar]
- Thorens, B.; Mueckler, M. Glucose transporters in the 21st Century. AJP Endocrinol. Metab. 2010, 298, E141–E145. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, A.; Joost, H.G.; Schürmann, A. The glucose transporter families SGLT and GLUT: Molecular basis of normal and aberrant function. J. Parenter. Enter. Nutr. 2004, 28, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Manolescu, A.R.; Witkowska, K.; Kinnaird, A.; Cessford, T.; Cheeseman, C. Facilitated hexose transporters: New perspectives on form and function. Physiology 2007, 22, 234–240. [Google Scholar] [CrossRef]
- Mueckler, M.; Weng, W.; Kruse, M. Glutamine 161 of GLUT1 glucose transporter is critical for transport activity and exofacial ligand binding. J. Biol. Chem. 1994, 269, 20533–20538. [Google Scholar] [CrossRef]
- Doege, H.; Schürmann, A.; Ohnimus, H.; Monser, V.; Holman, G.D.; Joost, H.G. Serine 294 and threonine 259 in the exofacial loop domain between helices 7 and 8 of glucose transporters (GLUT) are involved in the conformational alterations during the transport process. Biochem. J. 1998, 329, 289–297. [Google Scholar] [CrossRef]
- Garcia, J.C.; Strube, M.; Leingang, K.; Keller, K.; Mueckler, M.M. Amino acids substitutions at tryptophan 388 and tryptophan 412 of the Hep62 (Glut1) glucose transporter inhibit transport activity and targeting to the plasma membrane in Xenopus oocytes. J. Biol. Chem. 1992, 267, 7770–7776. [Google Scholar] [CrossRef]
- Seater, M.J.; De La Ru, S.A.; Porter, L.M.; Gould, G.W. QLS motif in transmembrane helix VII of the glucose transporter family interacts with C-1 position of D-glucose and is involved in substrate selection at the exofacial binding site. Biochemistry 1998, 37, 1322–1326. [Google Scholar] [CrossRef]
- Uldry, M.; Ibberson, M.; Horsiberger, J.-D.; Rieder, B.M.; Thorens, B. Identification of a mammalian H+-myoinositol symporter expressed predominantly in the brain. EMBO J. 2001, 20, 4467–4477. [Google Scholar] [CrossRef]
- Longo, N.; Elsas, L.J. Human glucose transporters. Adv. Pediatr. 1998, 45, 293–313. [Google Scholar] [CrossRef]
- Hruz, P.W.; Mueckler, M.M. Structural analysis of the GLUT1 facilitative glucose transporter (Review). Mol. Membr. Biol. 2001, 18, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Maher, F.; Davies-Hill, T.M.; Lysko, P.G.; Henneberry, R.C.; Simpson, I.A. Expression of two glucose transporters GLUT1 and GLUT3 in cultured cerebral neurons: Evidence for neuron specific expression of GLUT3. Mol. Cell. Neurosci. 1991, 2, 351–360. [Google Scholar] [CrossRef]
- Maher, F.; Vannucci, S.J.; Simpson, I.A. Glucose transporter protein in brain. FASEB J. 1994, 8, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.L.; Pessin, J.E. Structure, function and regulation of the facilitative glucose transporter gene family. Annu. Rev. Nutr. 1996, 16, 235–256. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Boado, R.J.; Farrel, C.R. Brain-type glucose transporter (GLUT-1) is selectively located to the blood-brain barrier. Studies with the quantitative Western blotting and in situ hybridization. J. Biol. Chem. 1990, 265, 18035–18040. [Google Scholar] [CrossRef]
- Kumagai, A.K.; Dwyer, K.J.; Pardridge, W.M. Differential glycosylation of the GLUT1 glucose transporter in brain capillaries and choroid plexus. Biochim. Biophys. Acta 1994, 1193, 24–30. [Google Scholar] [CrossRef]
- Takata, K.; Kasahara, M.; Ezaki, O.; Hirano, H. Erythrocyte/HepG2-type glucose transporter is concentrated in cells of blood-tissue barriers. Biochem. Biophys. Res. Commun. 1990, 173, 67–73. [Google Scholar] [CrossRef]
- Gould, G.W.; Bell, G.J. Facilitative glucose transporters: An expanding family. Trends Biochem. Sci. 1990, 15, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.W.; Holman, G.D. The glucose transporter family: Structure, function and tissue-specific expression. Biochem. J. 1993, 295, 329–341. [Google Scholar] [CrossRef]
- Korgun, E.T.; Demir, R.; Sedlmayer, P.; Desoue, G.; Arikan, G.; Puerstner, P.; Haeusler, M.; Dohr, G.; Skofitsch, G.; Hahn, T. Physiological leukocytosis during pregnancy with changes in glucose transporter expression of maternal peripheral blood granulocytes and monocytes. Am. J. Reprod. Immunol. 2002, 48, 110–116. [Google Scholar] [CrossRef]
- Sagun, K.C.; Cárcano, J.M.; Golde, D.W. Vitamin C enter mitochondria via facilitative glucose transporter 1 (Glut 1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005, 19, 1657–1667. [Google Scholar]
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci. 2016, 25, 546–558. [Google Scholar] [CrossRef]
- Jin, Q.; Agrawal, L.; VanHorn-Ali, Z.; Alkhatib, G. Infection of CD4+ lymphocytes by the glucose transporter GLUT1: Evidence using antibodies specific to the receptor’s large extracellular domain. Virology 2006, 349, 184–196. [Google Scholar] [CrossRef]
- Jones, K.S.; Fugo, K.; Petrow-Sadowski, C.; Huang, Y.; Bertolette, D.C.; Lisinski, I.; Cushman, S.W.; Jacobson, S.; Ruscetti, F.W. Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 use different receptor complex to enter T cells. J. Virol. 2006, 80, 8291–8302. [Google Scholar] [CrossRef]
- Manel, N.; Kint, S.; Kim, F.J.; Taylor, N.; Sitbon, M.; Battini, J.J. GLUT-1 est le recepteur des retrovirus humains HTL. Méd. Sci. 2004, 20, 277–279. [Google Scholar]
- Wieczorke, R.; Dlugai, S.; Krampe, S.; Boles, E. Characterization of mammalian GLUT glucose transporters in a heterozygous yeast expression system. Cell Physiol. Biochem. 2003, 13, 123–134. [Google Scholar] [CrossRef]
- Uldry, M.; Ibberson, M.; Hosokawa, M.; Thorens, B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002, 524, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Seino, H.; Seino, Y.; Eddy, R.L.; Fukushima, Y.; Byers, M.G.; Shows, T.B.; Bell, G.I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc. Natl. Acad. Sci. USA 1988, 85, 5435–5438. [Google Scholar] [CrossRef]
- Thorens, B.; Sarkar, H.K.; Lodish, H.F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell 1988, 55, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Kayano, T.; Fukumoto, H.; Eddy, R.I.; Fan, Y.S.; Byers, M.G.; Shows, T.B.; Bell, G.I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J. Biol. Chem. 1988, 263, 15245–15248. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B.; Cheng, Z.Q.; Brown, D.; Lodish, H.F. Liver glucose transporter: A basolateral protein in hepatocytes and intestine and kidney cells. Am. J. Physiol. 1990, 259, C279–C285. [Google Scholar] [CrossRef]
- Thorens, B.; Lodish, H.F.; Brown, D. Differential localization of two glucose transporter isoforms in rat kidney. Am. J. Physiol. 1990, 259, C286–C294. [Google Scholar] [CrossRef]
- Dwyer, D.S.; Vannucci, S.J.; Simpson, I.A. Expression, regulation, and functional role of glucose transporters (GLUTs) in brain. Int. Rev. Neurobiol. 2002, 51, 159–188. [Google Scholar]
- Garcia, M.L.; Millan, C.; Balmaceda-Aguilera, C.; Castro, T.; Pastor, P.; Montecinos, H. Hypothalamic ependymal-glial cells express the glucose transporter 2 a protein involved in glucose sensing. J. Neurochem. 2003, 86, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Young, J.K.; McKenzie, J.C. GLUT2 immunoreactivity in Gomori-positive astrocytes of hypothalamus. J. Histochem. Cytochem. 2004, 52, 1519–1524. [Google Scholar] [CrossRef]
- Leloup, C.; Arluison, M.; Lepetit, N.; Cartier, N.; Marfaing-Jallat, P.; Ferré, P.; Pénicaud, L. Glucose transporter 2 (GLUT2) expression in specific brain nuclei. Brain Res. 1994, 638, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Piroli, G.G.; Grillo, C.A.; Reznikov, L.R.; Reagan, L.P. Expression and functional activities of glucose transporters in central nervous system. In Handbook of Neurochemistry and Molecular Neurobiology, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 388–404. [Google Scholar]
- Arluison, M.; Quinon, M.; Nguyen, P.; Thorens, B.; Leloup, C.; Penicaud, L. Distribution and anatomical localization of the glucose transporter 2 (GLUT2) in the adult brain—An immunohistochemical study. J. Chem. Neuroanat. 2004, 28, 117–136. [Google Scholar] [CrossRef]
- Marty, N.; Dallaporta, M.; Thorens, B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology 2007, 22, 241–251. [Google Scholar] [CrossRef]
- Conville, C.A.; Seater, M.J.; Jess, T.J.; Gould, G.W.; Thomas, H.M. Kinetic analysis of the liver-type (GLUT2) and brain type (GLUT3) glucose transporters in Xenopus oocytes; substrate specificities and effect of transport inhibitors. Biochem. J. 1993, 290, 701–706. [Google Scholar] [CrossRef]
- Jihnson, J.H.; Newgard, C.B.; Milburn, J.L.; Lodish, H.F.; Thorens, B. The high Km glucose transporter of islet Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J. Biol. Chem. 1990, 265, 6548–6551. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Kornhauser, J.M.; Burant, C.F.; Seino, S.; Mayo, K.E.; Bell, G.J. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in-situ hybridization. J. Biol. Chem. 1992, 267, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Leino, R.L.; Gerhart, D.Z.; van Bueren, A.M.; McCall, A.L.; Drewes, L.R. Ultrastructural localization of GLUT1 and GLUT3 glucose transporters in rat brain. J. Neurosci. Res. 1997, 49, 617–626. [Google Scholar] [CrossRef]
- Burant, C.F.; Davidson, N.O. GLUT3 glucose transporter isoform in rat testis: Localization, effect of diabetes mellitus, and comparison to human testis. Am. J. Physiol. 1994, 267, R1488–R1495. [Google Scholar] [CrossRef]
- Illsley, N.P. Glucose transporters in the human placenta. Placenta 2000, 21, 14–22. [Google Scholar] [CrossRef]
- Pantaleon, M.; Harvey, M.B.; Pascoe, W.S.; James, D.E.; Kaye, P.L. Glucose transporter GLUT3: Ontogeny, targeting and role in the mouse blastocyst. Proc. Natl. Acad. Sci. USA 1997, 94, 3795–3800. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.F.G.; Oorschot, V.; Sixma, J.L.; Alat, J.W.; James, D.F. Trombin stimulates glucose transport in human platelets via the translocation of the glucose GLUT-3 from α-granules to the cell surface. J. Cell Biol. 1997, 138, 323–330. [Google Scholar] [CrossRef]
- Knott, R.M.; Robertson, M.; Muckensie, E.; Forrester, J.V. Regulation of glucose transporters (GLUT1 and GLUT3) in human endothelial cells. Biochem. J. 1996, 318, 313–317. [Google Scholar] [CrossRef]
- Leloup, C.; Arluison, M.; Kassis, N.; Lepetit, N.; Cartier, N.; Ferré, P.; Pénicaud, L. Discrete brain areas express the insulin-responsive glucose transporter GLUT4. Mol. Brain Res. 1996, 38, 45–53. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Clark, R.R.; Koehler-Stec, E.; Li, K.; Smith, C.B.; Davies, P.; Maher, F.; Simpson, I.A. Glucose transporters expression in brain: Relationship to cerebral glucose utilization. Dev. Neurosci. 1998, 20, 369–379. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Reagan, L.P. Glucose transporter expression in the central nervous system: Relationship to the synaptic function. Eur. J. Pharmacol. 2004, 490, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.A.; Owen, G.I. Glucose transporters: Expression, regulation and cancer. Biol. Res. 2002, 35, 9–26. [Google Scholar] [CrossRef]
- Pascoe, W.; Inuaki, K.; Oka, Y.; Slot, J.; James, D.E. Differential targeting of facilitative glucose transporter in polarized epithelial cells. Am. J. Physiol. 1996, 271, C547–C554. [Google Scholar] [CrossRef]
- Sweeney, G.; Garg, R.R.; Ceddia, R.B.; Li, D.; Ishiki, M.; Somwar, R.; Foster, L.J.; Neilsen, P.O.; Prestwich, G.D.; Rudich, G.D.; et al. Intracellular delivery of phosphatidylionositol(3, 4, 5)-triphosphate causes incorporation of GLUT4 into the plasma membrane of muscle and fat cells without increasing glucose uptake. J. Biol. Chem. 2004, 279, 32233–32242. [Google Scholar] [CrossRef]
- Thong, F.S.L.; Dugani, C.B.; Klip, A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology 2005, 20, 271–284. [Google Scholar] [CrossRef]
- Kasahara, T.; Kasahara, M. Characterization of the rat Glut4 glucose transporter expressed in the yeast Saccharomyces cerevisiae: Comparison with the Glut1 glucose transporter. Biochim. Biophys. Acta 1997, 1324, 111–119. [Google Scholar] [CrossRef]
- Drozdowski, L.A.; Thomas, A.B.R. Intestinal sugar transport. World J. Gastroenterol. 2006, 12, 1657–1670. [Google Scholar] [CrossRef]
- Davidson, N.O.; Hausman, A.M.; Ifkovits, C.A.; Buse, J.B.; Gould, G.W.; Burant, C.F.; Bell, G.I. Human intestinal glucose transporter expression and localization of GLUT5. Am. J. Physiol. 1992, 292, C795–C800. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, C. Fructose the odd man out. Why is the genomic control of intestinal GLUT5 expression different? J. Physiol. 2008, 586, 3563. [Google Scholar] [CrossRef]
- Burant, C.F.; Takeda, J.; Brat-Laroche, F.; Bell, G.J.; Davidson, N.O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem. 1992, 267, 14523–14526. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.A.; Yin, D.; Howell, M.E.A.; Dykes, R.J.; Laffan, J.L.; Ferrando, A.A. Hexose transporter mRNA for GLUT4, GLUT5 and GLUT12 predominate in human muscle. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1067–E1073. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.; Maher, F.; Simpson, I.; Mattice, L.; Davies, P. Glucose transporter Glut5 expression in microglial cells. Glia 1997, 21, 327–331. [Google Scholar] [CrossRef]
- Sasaki, A.; Horikoski, Y.; Yokoo, H.; Nakazano, Y.; Yamaguchi, H. Antiserum against human glucose transporter 5 is highly specific for microglia among cells of the mononuclear phagocyte system. Neurosci. Lett. 2003, 338, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef]
- Doege, H.; Bocianski, A.; Joost, H.G.; Schürmann, A. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar transport facilitators predominantly expressed in brain and leukocytes. Biochem. J. 2000, 350, 771–776. [Google Scholar] [CrossRef]
- Godoy, A.; Ulba, V.; Rodriquez, F.; Reinicke, K.; Yaňez, A.J.; Garcia Mde, L.; de los Angeles Garcia, M.; Medina, R.A.; Carrasco, M.; Barberis, S.; et al. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: Ultrastructural localization of GLUT1 and GLUT3 in breast tumor tissue. J. Cell. Physiol. 2006, 207, 614–627. [Google Scholar] [CrossRef]
- Li, Q.; Manolescu, A.; Ritzel, M.; Yao, S.; Slugoski, M.; Young, J.D.; Chen, X.-Z.; Cheeseman, C.I. Cloning and functional characterization of the human GLUT7 isoform SLC2A7 from the small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G236–G242. [Google Scholar] [CrossRef]
- Cheeseman, C. GLUT7: A new intestinal hexose transporter. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E238–E241. [Google Scholar] [CrossRef]
- Schürmann, A. Insight into the “odd” hexose transporters GLUT3, GLUT5, and GLUT7. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E225–E226. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Joost, H.-G.; Schürmann, A. Glut8, the enigmatic intracellular hexose transporter. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E614–E618. [Google Scholar] [CrossRef] [PubMed]
- Lisinski, Y.; Schürmann, A.; Joost, H.-G.; Cushman, S.W.; Al-Hasani, H. Targeting of GLUT6 (formerly GLUT9) and GLUT8 in rat adipose cells. Biochem. J. 2001, 358, 517–522. [Google Scholar] [CrossRef]
- Ibbersen, B.M.; Riederer, B.M.; Uldry, M.; Guhl, B.; Roth, J.; Thorens, B. Immunolocalization of GLUTX1 in the testis and to specific brain areas and vasopressin-containing neurons. Endocrinology 2002, 143, 276–284. [Google Scholar] [CrossRef]
- Henry, D.N.; Busik, J.V.; Botolin, D.; Grant, M.B.; Gorovits, N.; Charron, M. Glut8 expression in the blood retinal barrier. Investig. Ophtalmal. Vis. Sci. 2002, 43, 904. [Google Scholar]
- Goldman, N.A.; Katz, E.B.; Glen, A.S.; Weldon, R.H.; Jones, J.G.; Lynch, U.; Fezzari, M.J.; Runowicz, C.D.; Goldberg, G.L.; Charron, M.J. GLUT1 and GLUT8 in endometrium and endometrial adenocarcinoma. Modern Pathol. 2006, 19, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.B.; Carayannopoulos, M.O.; Hoehn, A.; Dowd, L.; Moley, K.H. Glucose transporter 8 expression and translocation are critical for murine blastocyst survival. Biol. Reprod. 2002, 66, 1729–1733. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Weng, X. Nucleic Acid Molecules Encoding GLUTX and Uses Thereof. USPTO Patent Application No. 09/031,392, 24 August 1999. [Google Scholar]
- Phay, J.E.; Hussein, H.B.; Moley, J.F. Cloning and expression analyses of a novel member of the facilitative glucose transporter SLC2A9 (GLUT9). Genomics 2000, 66, 217–220. [Google Scholar] [CrossRef]
- Augustin, R.; Carayannopoulos, M.O.; Dowd, L.O.; Phay, J.E.; Moley, J.F.; Moley, K.H. Identification and characterization of human glucose transporter-like protein 9 (GLUT9): Alternative splicing alters trafficking. J. Biol. Chem. 2004, 279, 16229–16236. [Google Scholar] [CrossRef] [PubMed]
- Keembiyehetty, C.; Augustin, R.; Carayannopoulos, M.O.; Steer, S.; Manolescu, A.; Cheeseman, C.I.; Moley, K.H. Mouse glucose transporter 9 splice variants are expressed in adult liver and kidney and are up-regulated in diabetes. Mol. Endocrinol. 2006, 20, 686–697. [Google Scholar] [CrossRef]
- Mobasheri, A.; Dobson, H.; Mason, S.L.; Moley, J.F.; Moley, K.H. Expression of GLUT1 and GLUT9 facilitative glucose transporters in embryonic chondroblasts and mature chondrocytes in ovine articular cartilage. Cell Biol. Int. 2005, 29, 249–260. [Google Scholar] [CrossRef]
- Bibert, S.; Hess, S.K.; Firsov, D.; Thorens, B.; Geering, K.; Horisberger, J.D.; Bonny, O. Mouse GLUT9: Evidences for a urate uniporter. Am. J. Physiol. Renal. Physiol. 2009, 297, F612–F619. [Google Scholar] [CrossRef]
- Caulfield, M.J.; Monroe, P.B.; O’Neil, D.; Witkowska, K.; Charchar, F.J.; Doblado, M.; Evans, S.; Eyheramendy, S.; Onipinla, A.; Howard, P.; et al. SLC2A9 is a high capacity urate transporter in humans. PLoS Med. 2008, 5, e197. [Google Scholar] [CrossRef]
- Mc Vie-Wylie, A.J.; Lamson, D.R.; Chen, Y.T. Molecular cloning of a novel member of the GLUT family of Transporters, SLC2A10 (GLUT10), localized on chromosome 20q13.1: A candidate gene for NIDDM susceptibility. Genomics 2001, 72, 113–117. [Google Scholar] [CrossRef]
- Woods, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expressed families of sugar transport protein. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef]
- Dawson, P.A.; Mychaleckyj, J.C.; Fossey, S.C.; Mihic, S.J.; Bowden, B.W. Sequence and functional analysis of GLUT10: A glucose transporter in Type 2 diabetes-linked region of chromosome 20q12-13.1. Mol. Genet. Metab. 2001, 74, 186–199. [Google Scholar] [CrossRef]
- Doege, H.; Bocianski, A.; Scheepers, A.; Axer, H.; Eckel, J.; Joost, H.G.; Schürmann, A. Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar-transport facilitator specifically expressed in heart and skeletal muscle. Biochem. J. 2001, 359, 443–449. [Google Scholar] [CrossRef]
- Scheepers, A.; Schmidt, S.; Manolescu, A.; Cheeseman, C.I.; Bell, A.; Zahn, C.; Joost, H.-G.; Schürmann, A. Characterization of the human SLC2A11 (GLUT11) gene: Alternative promoter, usage, function, expression, and subcellular distribution of three isoforms, and lack of mouse orthologue. Mol. Membr. Biol. 2005, 22, 339–351. [Google Scholar] [CrossRef]
- Rogers, S.; Macheda, M.L.; Docherty, S.E.; Carty, M.D.; Henderson, M.A.; Soeller, W.C.; Gibbs, E.M.; James, D.E.; Best, J.D. Identification of a novel transporter-like protein-GLUT-12. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E733–E738. [Google Scholar] [CrossRef]
- Stuart, C.A.; Howel, M.E.A.; Zhang, Y.; Yin, D. Insulin-stimulated translocation of GLUT12 parallels that of GLUT4 in normal muscle. J. Clin. Endocrinol. Metab. 2009, 94, 3535–3542. [Google Scholar] [CrossRef]
- Gude, N.M.; Stevenson, J.L.; Rogers, S.; Best, J.D.; Kalionis, B.; Huisman, M.A.; Erwich, J.J.H.M.; Timmer, A.; King, R.G. GLUT12 expression in human placenta in first trimester and term. Placenta 2003, 24, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R. The protein family of glucose transporters: It’s not only about glucose after all. IUBMB Life 2010, 62, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Uldry, M.; Steiner, P.; Zurich, M.G.; Begun, P.; Hirling, H.; Dolci, W.; Thorens, B. Regulated exocytosis of an H+/myo-inositol symporter at synapses and growth cones. EMBO J. 2004, 23, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Di Daniel, E.; Mok, M.H.; Mead, E.; Mutinelli, C.; Zambello, E.; Caberlotto, L.L.; Pell, T.J.; Langmead, C.J.; Shah, A.J.; Duddy, G.; et al. Evaluation of expression and function of the H+/myo-inositol transporter HMIT. BMC Cell Biol. 2009, 10, 54. [Google Scholar] [CrossRef]
- Wu, X.; Freeze, H.H. GLUT14, a duplication of GLUT3, is especially expressed in testis as alternative splice forms. Genomics 2002, 80, 553–557. [Google Scholar] [CrossRef]
- Riklis, E.; Quastel, J.H. Effects of cation on sugar absorption by isolated surviving guinea pig intestine. Can. J. Biochem. Physiol. 1958, 36, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.K. Hypothesis for mechanism of intestinal active transport of sugars. Fed. Proc. 1962, 21, 891–895. [Google Scholar] [PubMed]
- Crane, R.K. Na+-dependent transporter in the intestine and other animal sugars. Fed. Proc. 1965, 24, 1000–1006. [Google Scholar]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A.; Turk, E. Surprising versatility of Na+/glucose cotransporters: SLC5. Physiology 2004, 19, 371–376. [Google Scholar] [CrossRef]
- Wright, E.M. Renal Na+/glucose cotransporters. Am. J. Physiol. 2001, 280, F10–F18. [Google Scholar] [CrossRef]
- Wright, E.M.; Turk, E. The sodium/glucose cotransporter family SLC5. Pflügers Arch. Eur. J. Physiol. 2004, 447, 510–518. [Google Scholar] [CrossRef]
- Turk, E.; Wright, E.M. Membrane topology motifs in the SGLT cotransporter family. J. Membr. Biol. 1997, 159, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bianche, L.; Diez-Sampedro, A. A single amino acid change converts the sugar sensor SGLT3 into a sugar transporter. PLoS ONE 2010, 5, e10241. [Google Scholar] [CrossRef]
- Turk, E.; Martin, M.G.; Wright, E.M. Structure of the human Na+/glucose cotransporter gene SGLT1. J. Biol. Chem. 1994, 269, 15204–15209. [Google Scholar] [CrossRef] [PubMed]
- Panayotova-Heiermann, M.; Loo, D.D.F.; Kong, C.T.; Lever, J.E.; Wright, E.M. Sugar binding to Na+/glucose cotransporters is determined by the C-terminal half of the protein. J. Biol. Chem. 1996, 271, 10029–10034. [Google Scholar] [CrossRef]
- Zhou, L.; Cryan, E.V.; D’Andrea, M.R.; Belkowski, S.; Conway, B.R.; Demarest, K.T. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J. Cell. Biochem. 2003, 90, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-Q.; Zheng, Y.C.; Wall, E.H.; McFadden, T.B. Cloning and expression of bovine sodium/glucose cotransporters. J. Diary Sci. 2005, 88, 182–194. [Google Scholar] [CrossRef]
- Elfeber, K.; Koehler, A.; Lutzenburg, M.; Osswald, C.; Galla, H.J.; Witte, O.W.; Koepsell, H. Localization of the Na+-D-glucose cotransporter SGLT1 in the blood-brain barrier. Histochem. Cell Biol. 2004, 121, 201–207. [Google Scholar] [CrossRef]
- Shah, K.; DeSilva, S.; Abbruscato, T. The role of glucose transporters in brain disease: Diabetes and Alzheimer’s disease. Int. J. Mol. Sci. 2012, 13, 12629–12655. [Google Scholar] [CrossRef]
- Venula, S.; Roderer, K.; Yang, T.; Bhat, G.J.; Thekkumkara, T.J.; Abbruscato, T.J. A functional role of sodium-dependent glucose transporter across the blood-brain barrier during oxygen glucose deprivation. J. Pharmacol. Exp. Therapeut. 2009, 328, 487–495. [Google Scholar] [CrossRef]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.O.; Guo, W.-J.; Kim, J.-G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intracellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Zeuthen, T.; Meinild, A.K.; Lao, D.D.; Wright, E.M.; Klaerke, D.A. Isotonic transport by the Na+-glucose cotransporter SGLT1 from humans and rabbit. J. Physiol. 2001, 531, 631–644. [Google Scholar] [CrossRef]
- Loo, D.D.F.; Wright, E.M.; Zeuthen, T. Water pumps. J. Physiol. 2002, 42, 53–60. [Google Scholar] [CrossRef]
- Leung, D.W.; Loo, D.D.F.; Hirayama, B.A.; Zeuthen, T.; Wright, E.M. Urea transport by cotransporters. J. Physiol. 2000, 528, 251–257. [Google Scholar] [CrossRef]
- Panayotova-Heiermann, M.; Wright, E.M. Mapping the urea channel through the rabbit Na+/glucose cotransporter SGLT1. J. Physiol. 2001, 535, 419–425. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.F.; Turk, E.; Hirayama, B.A. Sodium cotransporters. Curr. Opin. Cell Biol. 1996, 8, 468–473. [Google Scholar] [CrossRef]
- Wells, R.G.; Kamai, Y.; Pajor, A.M.; Turk, E.; Wright, E.M.; Hediger, M.A. The cloning of a human kidney cDNA with similarity to the sodium/glucose cotransporter. Am. J. Physiol. 1992, 263, F459–F465. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G.; Mohandas, T.K.; Hediger, M.A. Localization of the Na+/glucose cotransporter gene SGLT2 to human chromosome 16 close to the centromere. Genomics 1993, 17, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.T.; Yet, S.F.; Lever, J.E. Cloning and expression of a mammalian Na+/amino acid cotransporter with sequence similarity to Na+/glucose cotransporters. J. Biol. Chem. 1993, 268, 1509–1512. [Google Scholar] [CrossRef]
- Mackenzie, B.; Panayotova-Heiermann, M.; Loo, D.D.; Lever, J.E.; Wright, E.M. SAA1 is a low affinity Na+/glucose cotransporter and not an amino acid transporter. A reinterpretation. J. Biol. Chem. 1994, 269, 22488–22491. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Lee, W.S.; You, G.; Brown, D.; Hediger, M.A. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorption mechanism for D-glucose. J. Clin. Investig. 1994, 93, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Diez-Sampedro, A.; Eskanderi, S.; Wright, E.M.; Hiryama, B.A. Na+-to-sugar stoichiometry of SGLT3. Am. J. Physiol. Renal. Physiol. 2001, 280, F278–F282. [Google Scholar] [CrossRef]
- Kothinti, R.K.; Blodgett, A.B.; North, P.E.; Roman, R.J.; Tabatabai, N.M. A novel SGLT is expressed in the human kidney. Eur. J. Pharmacol. 2012, 690, 77–83. [Google Scholar] [CrossRef]
- Tazawa, S.; Yamamoto, T.; Fujikura, H.; Hiratochi, M.; Itoh, F.; Tomae, M.; Takemura, Y.; Maruyama, H.; Sugiyama, T.; Wakamatsu, A.; et al. SLC5A9/SGLT4, a new Na+-dependent glucose transporter, is an essential transporter for mannose and fructose. Life Sci. 2005, 76, 1039–1050. [Google Scholar] [CrossRef]
- Grempler, R.; Augustin, R.; Froehner, S.; Hildebrandt, T.; Simon, E.; Mark, M.; Eickelmann, P. Functional characterization of SGLT-5 as a novel kidney-specific sodium-dependent sugar transporter. FEBS Lett. 2012, 586, 248–253. [Google Scholar] [CrossRef]
- Augustin, R.; Mayoux, E. Mammalian sugar transporters. In Glucose Homeostasis, 1st ed.; Szablewski, L., Ed.; InTech: Rijeka, Croatia, 2014; pp. 3–36. [Google Scholar]
- Coady, M.J.; Wallendorf, B.; Cagan, D.G.; Lapointe, J.Y. Identification of a novel Na+/myo-inositol cotransporter. J. Biol. Chem. 2002, 299, 35219–35224. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, T.; Helms, G.; Merboldt, K.D.; Hanicka, W.; Bruhn, H.; Frahm, J. Identification of Syllo-inositol in proton NMR spectra in human brain in vivo. NMR Biomed. 1993, 6, 105–109. [Google Scholar] [CrossRef]
- Tsai, L.J.; Hsiao, S.H.; Tsai, L.M.; Lin, C.Y.; Tsai, J.J.; Liou, D.M.; Lan, J.L. The sodium-dependent glucose cotransporter SLC5A11 as an autoimmune modifier gene in SLE. Tissue Antigens 2008, 71, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.T.B.; Dahlin, A.; Wang, J. Expression profiling of solute carrier gene families at the blood-CSF barrier. Front. Pharmacol. 2012, 3, 154. [Google Scholar] [CrossRef]
- De La Vieja, A.; Dohan, O.; Levy, O.; Carrasco, N. Molecular analysis of the sodium/iodide symporter: Impact on thyroid and extrathyroid pathophysiology. Physiol. Rev. 2000, 80, 1083–1105. [Google Scholar] [CrossRef] [PubMed]
- Van Sande, J.; Massart, C.; Beauwens, R.; Schouters, A.; Costaglia, S.; Dumont, J.E.; Wolff, J. Anion selectivity by the sodium iodide symporter. Endocrinology 2003, 144, 247–252. [Google Scholar] [CrossRef]
- Appansundaram, S.; Fergusson, S.M.; Blakely, R.D. Molecular cloning and characterization of a murine hemi-cholinum-3-sensitive choline transporter. Biochem. Soc. Trans. 2001, 29, 711–716. [Google Scholar] [CrossRef]
- Coady, M.J.; Wallendorf, B.; Bourgeois, F.; Charron, F.; Lapointe, J.Y. Establishing a definitive stoichiometry for the Na+/monocarboxylate cotransporter SMCT. Biophys. J. 2007, 93, 2325–2331. [Google Scholar] [CrossRef]
- Gopal, E.; Umapathy, N.S.; Martin, P.M.; Ananth, S.; Gnoma-Prakasom, J.P.; Becker, H.; Wagner, C.A.; Ganapathy, V.; Prasad, P.D. Cloning and functional characterization of human SMCT2 (SLC5A12) and expression pattern in kidney. Biochim. Biophys. Acta 2012, 1768, 2690–2697. [Google Scholar] [CrossRef]
- Expression of SLC50A1 in Cancer–Summary the Human Protein Atlas. Available online: www.proteinatlas.org/ENSG00000169241-SLC50A1/pathology (accessed on 1 June 2025).
- Santer, R.; Hillebrand, G.; Steinmann, B.; Schaub, J. Intestinal glucose transport: Evidence for a membrane traffic-based pathway in humans. Gastroenterology 2003, 124, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Larrabel, M.G. Lactate metabolism and its effect on glucose metabolism in an exercised tissue. J. Neurochem. 1995, 64, 1734–1741. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Pellerin, L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos. Trans. R. Soc. Lond. 1999, 254, 1155–1163. [Google Scholar] [CrossRef]
- Goldstein, G.W. Relation of potassium transport to oxidative metabolism in isolated brain capillaries. J. Physiol. 1979, 286, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Blazquez, C. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol. Metab. 2001, 12, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.T.K.; Ha, H.T.T.; Nguyen, T.H.; Nguyen, L.N. The role of SLC transporters for brain health and disease. Cell. Mol. Life. Sci. 2022, 79, 20. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Lopez, O.L.; McDade, E.; Riverol, N.; Becker, J.T. Evaluation of the diagnostic criteria for degenerative and cognitive disorders. Curr. Opin. Neurol. 2011, 24, 532–541. [Google Scholar] [CrossRef]
- Marcelina de Naraneth, A. Type 2 diabetes mellitus in the pathophysiology of Alzheimer’s disease. Dement. Neuropsychol. 2017, 11, 105–113. [Google Scholar]
- Sun, Y.; Ma, C.; Sun, H.; Wang, H.; Peng, W.; Zhou, Z.; Wang, H.; Pi, C.; Shi, Y.; He, X. Metabolism: A novel shared link between diabetes mellitus and Alzheimer’s disease. J. Diabetes Res. 2020, 2020, 4981814. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Association between diabetes mellitus and neurodegenerative diseases. Int. J. Mol. Sci. 2025, 26, 542. [Google Scholar] [CrossRef]
- Rorbach-Dolata, A.; Piwowar, A. Neurometabolic evidence supporting the hypothesis of increased incidence of Type 3 diabetes mellitus in the 21st century. Biomed. Res. Int. 2019, 2019, 1435276. [Google Scholar] [CrossRef] [PubMed]
- Hamzé, R.; Delangre, E.; Tolu, S.; Moreau, M.; Janel, N.; Bailbé, D.; Movassat, J. Type 2 diabetes mellitus and Alzheimer’s disease: Shared molecular mechanisms and potential common therapeutic targets. Int. J. Mol. Sci. 2022, 23, 15287. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhanan, J.; Suresh, G.; Devanathan, V. Neurodegeneration in type 2 diabetes: Alzheimer’s as a case study. Brain Behav. 2020, 10, e01577. [Google Scholar] [CrossRef]
- Diniz Pereira, J.; Gomes Fraga, V.; Morais Santos, A.L.; das Graças Carvalho, M.; Caramelli, P.; Braga Gomes, K. Alzheimer’s disease and type 2 diabetes mellitus, a systematic review of proteomic studies. J. Neurochem. 2021, 156, 753–776. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mudher, A. Alzheimer’s disease and type 2 diabetes: A critical assessment of the shared pathological traits. Front. Neurosci. 2018, 12, 383. [Google Scholar] [CrossRef]
- Hoyer, S. The brain insulin signal transduction system and sporadic (type II) Alzheimer disease: An update. J. Neural Transm. 2002, 109, 341–360. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Zhang, B.; Gong, C.-X. Deregulation of brain insulin signaling in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 282–294. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Nugent, S.; Roy, M.; Courchesne-Loyer, A.; Croteau, E.; Tremblay, S.; Castellano, A.; Pifferi, F.; Bocti, C.; Paquet, N.; et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 2011, 27, 3–20. [Google Scholar] [CrossRef]
- De Strooper, B. Loss-of-function presenilin mutations in Alzheimer’s disease, talking point on the role of presenilin mutations in Alzheimer’s disease. EMBO Rep. 2007, 8, 141–146. [Google Scholar] [CrossRef]
- Doroszewska, J.; Prendecki, M.; Oczkowska, A.; Dezor, M.; Kozubski, W. Molecular basis of familial and sporadic Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 952–963. [Google Scholar] [CrossRef]
- Bertram, L.; Tanzi, R.E. Genome-wide association studies in Alzheimer’s disease. Hum. Mol. Genet. 2009, 18, R137–R145. [Google Scholar] [CrossRef]
- Li, X.; Song, D.; Leng, S.X. Link between type 2 diabetes and Alzheimer’s disease from epidemiology to mechanism and treatment. Clin. Intern. Aging 2015, 10, 549–560. [Google Scholar] [CrossRef]
- Kisilevsky, R.; Raimondi, S.; Bellotti, V. Historical and current concepts of fibrillogenesis and in vivo amyloidogenesis: Implications of amyloid tissue targeting. Front. Mol. Biosci. 2016, 3, 17. [Google Scholar] [CrossRef]
- Ancarcona, M.; Winblad, B.; Monteiro, C.; Feans, C.; Powers, J.; Johansson, J.; Westmark, T.; Presto, J.; Kelly, J.W.; Institutet, K.; et al. Current and future treatment of amyloid diseases. J. Intern. Med. 2016, 280, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Widespread τ and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012, 22, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef] [PubMed]
- Sędzikowska, A.; Szablewski, L. Insulin and insulin resistance in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416, 535–539. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Guillén-Nieto, G.; Rodriguez-Rodriguez, N.; Bringas-Vegá, M.L.; Garcia-del-Barcó-Herrera, D.; Berlanga-Saez, J.O.; Garcia-Ojálvo, A.; Valdés-Sosa, M.J.; Valdés-Sosa, P.A. Insulin resistance at the crossroad of Alzheimer disease pathology: A review. Front. Endocrinol. 2020, 11, 560375. [Google Scholar] [CrossRef]
- Zhao, W.Q.; Lacor, P.N.; Chen, H.; Lambert, M.P.; Buon, M.J.; Krafft, G.A.; Klein, W.L. Insulin receptor dysfunction of neurotic oligomeric Aβ. J. Biol. Chem. 2009, 289, 18742–18753. [Google Scholar] [CrossRef] [PubMed]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Frittitla, L. Type 2 diabetes mellitus and Alzheimer’s disease: Role of insulin signaling and therapeutic implications. Int. J. Mol. Sci. 2018, 19, 3306. [Google Scholar] [CrossRef]
- Caillet-Boudin, M.L.; Buee, L.; Sergrant, N.; Lefebvre, B. Regulation of human MAPT gene expression. Mol. Neurodegener. 2015, 10, 28. [Google Scholar] [CrossRef]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef]
- Stoothoff, W.H.; Johnson, G.V. Tau phosphorylation, physiological and pathological consequences. Biochim. Biophys. Acta 2005, 1739, 280–297. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sang, T.K.; Lawless, G.M.; Jackson, G.R. Dissociation of tau toxicity and phosphorylation: Role of GSK-3beta, MARK, and Cdk5 in Drosophila model. Hum. Mol. Genet. 2009, 18, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Burillo, J.; Margués, P.; Jiménez, B.; González-Blanco, C.; Benito, M.; Guillén, C. Insulin resistance and diabetes mellitus in Alzheimer’s disease. Cells 2021, 10, 1236. [Google Scholar] [CrossRef]
- Van Der Velpen, V.; Teav, T.; Gallart-Ayala, H.; Mehl, F.; Konz, I.; Clark, C.; Oikonomidi, A.; Peratout, G.; Henry, H.; Delorenzi, M.; et al. Systemic and central nervous system metabolic alterations in Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 93. [Google Scholar] [CrossRef]
- Mark, R.J.; Pang, Z.; Geddes, J.W.; Uchida, K.; Mattson, M.P. Amyloid β-peptide impairs glucose transport in hippocampal and cortical neurons: Involvement of membrane lipid peroxidation. J. Neurosci. 1997, 17, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, T.; Cabecinha, M.; Tillman, A.; Kerr, F.; Wong, C.T.; Cardenes, D.; Vincent, A.J.; Bettedi, L.; Li, L.; Gronke, S.; et al. Increased glucose transport into neurons rescues Aβ toxicity in Drosophila. Curr. Biol. 2016, 26, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, S. Oxidative energy metabolism in Alzheimer brain. Studies in early-onset and late-onset cases. Mol. Chem. Neuropathol. 1992, 16, 207–224. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Scheff, S.W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann. Neurol. 1990, 27, 457–464. [Google Scholar] [CrossRef]
- Jia, Y.J.; Wang, N.; Zhang, Y.; Xue, D.; Lou, H.; Liu, X. Alteration in the function and expression of SLC and ABC transporters in the neurovascular unit in Alzheimer’s disease and the clinical significance. Aging Dis. 2020, 11, 390–404. [Google Scholar] [CrossRef]
- Jagust, W.J.; Seab, J.P.; Huesman, R.H.; Valk, P.E.; Mathis, C.A.; Reed, B.R.; Coxon, P.G.; Budinger, T.F. Diminished glucose transport in Alzheimer’s disease: Dynamic PET studies. J. Cereb. Blood Flow Metab. 1991, 11, 323–330. [Google Scholar] [CrossRef]
- Piert, M.; Koeppe, R.A.; Giordani, B.; Berent, S.; Kuhl, D.E. Diminished glucose transport and phosphorylation in Alzheimer’s disease determined by FDG-PET. J. Nucl. Med. 1996, 37, 201–208. [Google Scholar] [PubMed]
- Friedland, R.P.; Jagust, W.J.; Huesman, R.H.; Koss, E.; Knittel, B.; Mathis, C.A.; Ober, B.A.; Mazoyer, B.M.; Budinger, T.F. Regional cerebral glucose transport and utilization in Alzheimer’s disease. Neurology 1989, 39, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.A.; Davies, P. Reduced glucose transporter concentrations in brain of patients with Alzheimer’s disease. Ann. Neurol. 1994, 36, 800–801. [Google Scholar] [CrossRef]
- Harik, S.I. Changes in the glucose transporter of brain capillaries. Can. J. Physiol. Pharmacol. 1992, 70 (Suppl. l), S113–S117. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Harik, S.I. Reduced glucose transporter at the blood-brain barrier in cerebral cortex in Alzheimer’s disease. J. Neurochem. 1989, 53, 1083–1088. [Google Scholar] [CrossRef]
- Kyrtata, N.; Emsley, H.C.A.; Sparasci, O.; Parkes, L.M.; Dickie, B.R. A systemic review of glucose transport alterations in Alzheimer’s disease. Front. Neurosci. 2021, 15, 626636. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Gang, C.-X. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer’s disease. FEBS Lett. 2008, 582, 359–364. [Google Scholar] [CrossRef]
- An, Y.; Varma, V.R.; Varma, S.; Casanova, R.; Dammer, E.; Pletnikova, O.; Chia, C.W.; Egan, J.M.; Ferrucci, L.; Troncoso, J.; et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 318–329. [Google Scholar] [CrossRef]
- Hendrix, R.D.; Ou, Y.; Davis, J.E.; Odle, A.K.; Groves, T.R.; Allen, A.R.; Childs, G.V.; Barger, S.W. Alzheimer amyloid-β-peptide disrupts membrane localization of glucose transporter 1 in astrocytes: Implications for glucose levels in brain and blood. Neurobiol. Aging 2021, 97, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, B.; Liu, Y.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.-X. Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain. Am. J. Pathol. 2009, 175, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.-X. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer’s disease. J. Neurochem. 2009, 111, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lei, L.; Liu, D.; Jovin, I.; Russel, R.; Johnson, R.S.; Di Lorenzo, A.; Giordano, F.J. Normal glucose uptake in the brain and heart requires an endothelial cell-specific HIF-1α-dependent function. Proc. Natl. Acad. Sci. USA 2012, 109, 17478–17483. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shi, J.; Tanimukai, H.; Gu, J.; Gu, J.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain 2009, 132, 1820–1832. [Google Scholar] [CrossRef]
- Jin, N.; Qian, W.; Yin, X.; Zhang, L.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.-X.; Liu, F. CREB regulates the expression of neuronal glucose transporter 3: A possible mechanism related to impaired brain glucose uptake in Alzheimer’s disease. Nucl. Acid. Res. 2013, 41, 3240–3256. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Graven, C.; Dederen, P.J.; Tanila, H.; van Groen, T.; Kiliaan, A.J. Amyloid beta deposition is related to decreased glucose transporter-1 levels and hippocampal atrophy in brain of aged APP/PS1 mice. Brain Res. 2007, 1181, 93–103. [Google Scholar] [CrossRef]
- Pujol-Gimenez, J.; Martisova, E.; Perez-Mediavilla, A.; Lostao, M.P.; Ramirez, M.J. Expression of glucose transporter GLUT12 in Alzheimer’s disease patients. J. Alzheimer’s Dis. 2014, 42, 97–101. [Google Scholar] [CrossRef]
- Gil-Iturbe, E.; Solas, M.; Cuadrado-Tejedo, M.; Garcia-Osta, A.; Escote, X.; Ramirez, M.J.; Lostao, M.P. GLUT12 expression in brain of mouse models of Alzheimer’s disease. Mol. Neurobiol. 2020, 57, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet Lond. Engl. 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Chase, T.N.; Oh, J.D.; Blanchet, P.J. Neostriatal mechanisms in Parkinson’s disease. Neurology 1998, 51 (Suppl. 2), S30–S35. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Qin, L.; Zhang, H. Parkinson’s disease and glucose metabolism impairment. Translat. Neurodegener. 2025, 14, 10. [Google Scholar] [CrossRef]
- Jia, F.; Fellner, A.; Kumar, K.R. Monogenic Parkinson’s disease: Genotype, phenotype, pathophysiology, and genetic testing. Genes 2022, 13, 471. [Google Scholar] [CrossRef]
- Inamdar, N.N.; Arulmozhi, D.K.; Tandon, A.; Bodhanker, S.L. Parkinson’s disease: Genetics and beyond. Curr. Neuropharmacol. 2007, 5, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, X.; Liu, X.; Liao, J.; Lin, J.; Zhu, C.; Meng, X.; Xie, D.; Chao, D.; Fenoy, A.J.; et al. Low cerebral glucose metabolism: A potential predictor for severity of vascular Parkinsonism and Parkinson’s disease. Aging Dis. 2015, 6, 426–436. [Google Scholar] [CrossRef]
- Dai, C.; Tai, C.; Zhao, L.; Liang, Y.; Liu, G.; Liu, H.; Zhong, Y.; Liu, Z.; Mo, L.; Liu, X.; et al. Glucose metabolism impairment in Parkinson’s disease. Brain Res. Bull. 2023, 199, 110672. [Google Scholar] [CrossRef]
- Sian-Hulsmann, J.; Rieder, P.; Michel, T.M. Metabolic dysfunction in Parkinson’s disease: Unraveling the glucose-lipid connection. Biomedicines 2024, 12, 2841. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, A.; Jo, J.; Park, S.M. Targeting glucose metabolism: A novel therapeutic approach for Parkinson’s diseases. Cells 2024, 13, 1876. [Google Scholar] [CrossRef]
- Schmidt, S.; Stautner, C.; Vu, D.T.; Heinz, A.; Regensburger, M.; Karayel, O.; Trümbach, D.; Artati, A.; Kaltenhäuser, S.; Nassef, M.Z.; et al. A reversible state of hypometabolism in a human cellular model of sporadic Parkinson’s disease. Nat. Commun. 2023, 14, 7674. [Google Scholar] [CrossRef]
- Puchades, M.; Sogn, C.J.; Maehlen, J.; Bergsen, L.H.; Gundersen, V. Unaltered lactate and glucose transport levels in the MPTP mouse model of Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Chigurupati, S.; Raymick, J.; Mann, D.; Bowyer, J.F.; Schmitt, T.; Beger, R.D.; Hanig, J.P.; Schmued, L.C.; Paule, M.G. Neuroprotective effect of the chemical chaperone, trehalose in a chronic MPTP-induced Parkinson’s disease mouse model. Neurotoxicology 2014, 44, 250–262. [Google Scholar] [CrossRef]
- Burks, S.; Raymick, J.; Robinson, B.; Hanig, J.; Sarkar, S. Neuroprotective effects of acetyl-1-carmitine (ALC) in a chronic MPTP-induced Parkinson’s disease mouse model: Endothelial and microglial effects. Neurosci. Lett. 2019, 703, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, L.; Zhang, C.; Liu, C.; Li, Y.; Cheng, L.; Ouyang, Y.; Rutledge, C.; Anderson, J.; Wei, Z.; et al. Glymphatic influx and clearance are perturbed in Huntington’s disease. JCI Insight 2024, 9, 172286. [Google Scholar] [CrossRef]
- Andrew, S.E.; Goldberg, Y.P.; Kremer, B.; Telenius, H.; Theilmann, J.; Adam, S.; Starr, E.; Squitieri, F.; Lin, B.; Kalchman, M.A.; et al. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat. Genet. 1993, 4, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Jucker, M.; Walker, L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013, 501, 45–51. [Google Scholar] [CrossRef]
- Vittori, A.; Breda, C.; Repici, M.; Orth, M.; Roos, R.A.C.; Outeiro, T.F.; Giorgini, F.; Hollox, E.J.; The Registry Investigators of the European Huntington’s Disease Network. Copy- number variation of the neuronal glucose transporter gene SLC2A3 and age of onset in Huntington’s disease. Hum. Mol. Genet. 2014, 23, 3129–3137. [Google Scholar] [CrossRef]
- Chang, C.-P.; Wu, C.-W.; Chern, Y. Metabolic dysregulation in Huntington’s disease. Neuronal and glial perspectives. Neurobiol. Dis. 2024, 201, 106672. [Google Scholar] [CrossRef]
- Chaves, G.; Özel, R.E.; Rao, N.; Hadiprodjo, H.; Da Costa, Y.; Tokuno, Z.; Pourmand, N. Metabolic and transcriptomic analysis of Huntington’s disease model reveal changes in intracellular glucose levels and related genes. Heliyon 2017, 3, e00381. [Google Scholar] [CrossRef]
- Martin, W.R.; Clark, C.; Ammann, W.; Stossl, A.J.; Shtybel, W.; Hayden, M.R. Cortical glucose metabolism in Huntington disease. Neurology 1992, 42, 223–229. [Google Scholar] [CrossRef]
- Berent, S.; Giordari, B.; Lehtinen, S.; Markel, D.; Penney, J.B.; Buchtel, H.A.; Starosta-Rubinstein, S.; Hichwa, R.; Young, A.B. Positron emission tomography scan investigations of Huntington’s disease: Cerebral metabolic correlates of cognitive function. Ann. Neurol. 1988, 23, 541–546. [Google Scholar] [CrossRef]
- Antonini, A.; Leenders, K.L.; Eidelberg, D. [11C] Raclopride-PET studies of the Huntington’s disease rate of progression: Relevance of the trinucleotide repeat length. Ann. Neurol. 1998, 43, 253–255. [Google Scholar] [CrossRef]
- Gambino, W.C.; Brennan, W.A., Jr. Glucose transporter isoform expression in Huntington’s disease brain. J. Neurochem. 1994, 63, 1392–1397. [Google Scholar] [CrossRef]
- McDonald, T.S.; Lerskiatiphanich, T.; Woodruff, T.M.; McCombe, P.A.; Lee, J.D. Potential mechanisms to modify impaired glucose metabolism in neurodegenerative disorders. J. Cereb. Blood Flow Metab. 2023, 43, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, O.A.; Dedeoglu, A.; Stanojevic, V.; Hughes, D.B.; Browne, S.E.; Leech, C.A.; Ferrante, R.J.; Habener, J.F.; Beal, J.F.; Thomas, M.K. Huntington’s disease of the endocrine pancreas: Insulin deficiency and diabetes mellitus due to impaired insulin gene expression. Neurobiol. Dis. 2002, 11, 410–424. [Google Scholar] [CrossRef]
- Mochel, F.; Haller, R.G. Energy deficit in Huntington disease: Why it matters. J. Clin. Investig. 2011, 121, 493–499. [Google Scholar] [CrossRef]
- Guyot, M.C.; Hantraye, P.; Dolan, R.; Palfi, S.; Maziére, M.; Brouillet, E. Quantifiable bradykinesia, gait abnormalities and Huntington’s disease-like striatal lesions in rats chronically treated with 3-nitropropionic acid. Neuroscience 1997, 79, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, J.M. Towards an understanding of energy impairment in Huntington’s disease brain. J. Huntingt. Dis. 2017, 6, 267–302. [Google Scholar] [CrossRef] [PubMed]
- Bossy-Wetzel, E.; Petrilli, A.; Knott, A.B. Mutant huntingtin and mitochondrial dysfunction. Trend. Neurosci. 2008, 31, 609–616. [Google Scholar] [CrossRef]
- Covarrubias-Pint, A.; Moll, P.; Solis-Maldonado, A.; Acuña, A.I.; Riveros, A.; Paz Miró, M.; Papic, E.; Beltrán, F.A.; Cepeda, C.; Concha, I.I.; et al. Beyond the redox imbalance: Oxidative stress contributes to an impaired GLUT3 modulation in Huntington’s disease. Free Rad. Biol. Med. 2015, 89, 1085–1096. [Google Scholar] [CrossRef]
- McClory, H.; Williams, D.; Sapp, E.; Gatune, L.W.; Wang, P.; DiFiglia, M.; Li, X. Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons CAG140 Huntington’s mice. Acta Neuropathol. Commun. 2014, 2, 179. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Trifiletti, R.R.; Jacobson, R.I.; Ronen, G.M.; Behmand, R.A.; Harik, S.I. Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N. Engl. J. Med. 1991, 325, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, K. The expanding phenotype of GLUT1-deficiency syndrome. Brain Develop. 2009, 31, 545–552. [Google Scholar] [CrossRef] [PubMed]
- De Giorgis, V.; Veggiotti, P. GLUT1 deficiency syndrome 2013: Current state of the art. Seizure 2013, 22, 803–811. [Google Scholar] [CrossRef]
- Arsov, T.; Mullen, S.A.; Damiano, J.A.; Lawrence, K.M.; Huh, L.L.; Nolan, M.; Young, H.; Touin, A.; Dahl, H.-H.M.; Bergovic, S.F.; et al. Early onset absence epilepsy: 1 in 10 cases is caused by GLUT1 deficiency. Epilepsia 2012, 53, e204–e207. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Brain glucose transporters. Implications for neurologic disease. Clin. Implic. Neurosci. Res. 2014, 85, 1374–1379. [Google Scholar]
- Patching, S.G. Glucose transporters at the blood-brain barrier: Function, regulation and gateways for drug delivery. Mol. Neurobiol. 2017, 54, 1046–1077. [Google Scholar] [CrossRef] [PubMed]
- Seider, G.; Alvarez, M.G.; Yeh, J.-J.; O’Driscoll, K.R.; Klepper, J.; Stump, T.S.; Wang, D.; Spiner, N.B.; Birnbaum, M.J.; De Vivo, D.C. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat. Genet. 1998, 18, 188–191. [Google Scholar] [CrossRef]
- Hashimoto, N.; Kagitani-Shimonok, K.; Sakai, N.; Otomo, T.; Tominaga, K.; Nabatame, S.; Mogami, Y.; Takahashi, Y.; Imai, K.; Yanagihara, K.; et al. SLC2A1 gene analysis of Japanese patients with glucose transporter 1 deficiency syndrome. J. Hum. Genet. 2011, 56, 846–851. [Google Scholar] [CrossRef]
- Leen, W.G.; Klepper, J.; Verbeek, M.M.; Leferink, M.; Hofste, T.; van Engelen, B.G.; Wevers, R.A.; Artur, T.; Bahi-Buisson, N.; Ballhausen, D.; et al. Glucose transporter-1 deficiency syndrome: The expanding clinical and genetic spectrum of a treatable disorder. Brain 2010, 133, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Lee, J.W.; Bellows, S.T.; Damiano, J.A.; Mullen, S.A.; Berkovic, S.F.; Bahlo, M.; Scheffer, I.E.; Hildebrand, M.S.; Clinical, G. Evaluation of non-coding variation in GLUT1 deficiency. Dev. Med. Child Neurol. 2016, 58, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, M.A.; Vissers, L.E.; Verbeek, M.M.; Van Bon, B.W.; Geuer, S.; Gilissen, C.; Klepper, J.; Kwint, M.P.; Leen, W.G.; Pennings, M.; et al. Upstream SLC2A1 translocation initiation causes GLUT1 deficiency syndrome. Eur. J. Hum. Genet. 2017, 25, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Klepper, J.; Scheffer, H.; Elsaid, M.F.; Kamsteeg, E.J.; Leferink, M.; Ben-Omran, T. Autosomal recessive inheritance of GLUT1 deficiency syndrome. Neuropediatrics 2009, 40, 207–210. [Google Scholar] [CrossRef]
- Rotstein, M.; Engelstad, K.; Yang, H.; Wang, D.; Levy, B.; Chung, W.K.; De Vivo, D.C. Glut1 deficiency: Inheritance pattern determined by haploinsufficiency. Am. Neurol. 2010, 68, 955–958. [Google Scholar] [CrossRef]
- Wang, D.; Pascual, J.M.; Yang, H.; Engelstad, K.; Ihung, S.; Sun, R.P.; De Vivo, D.C. Glut-1 deficiency syndrome: Clinical, genetic and therapeutic aspects. Ann. Neurol. 2005, 57, 111–118. [Google Scholar] [CrossRef]
- Klepper, J.; Akman, C.; Armeno, M.; Auvin, S.; Cervenko, M.; Cross, H.J.; De Giorgis, V.; Marina, M.; Engelstad, K.; Heussinger, N.; et al. Glut1 Deficiency Syndrome (Glut1DS): State of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open 2020, 5, 354–365. [Google Scholar] [CrossRef]
- Ohtsuki, S.; Kikkawa, T.; Hori, S.; Terasaki, T. Modulation and compensation of the mRNA expression of energy related transporters in the brain of glucose transporter 1-deficient mice. Biol. Pharm. Bull. 2006, 29, 1587–1591. [Google Scholar] [CrossRef]
- Wang, D.; Pascual, J.M.; Yang, H.; Engelstad, K.; Mao, X.; Chen, J.; Yoo, J.; Noebels, J.L.; De Vivo, D.C. A mouse model for Glut-1 haploinsufficiency. Hum. Mol. Genet. 2006, 15, 1169–1179. [Google Scholar] [CrossRef]
- Amolou, S.; Gras, D.; Ilea, A.; Greneche, M.D.; Francois, L.; Bellavoine, V.; Delanoe, C.; Auvin, S. Use of modified Atkins diet in glucose transporter type 1 deficiency syndrome. Des. Med. Child Neurol. 2016, 58, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Bondy, C.A. Ischemic injury induces brain glucose transporter gene expression. Endocrinology 1993, 133, 2540–2544. [Google Scholar] [CrossRef] [PubMed]
- Urabe, T.; Hattori, N.; Nagamatsu, S.; Sawa, H.; Mizuno, Y. Expression of glucose transporters in rat brain following transient focal ischemic injury. J. Neurochem. 1996, 67, 265–271. [Google Scholar] [CrossRef]
- Gerhart, D.Z.; Leino, R.L.; Taylor, W.E.; Borson, N.D.; Drewes, L.R. GLUT1 and GLUT3 gene expression in gerbil brain following brief ischemia: On in situ hybridization study. Brain Res. Mol. Brain Res. 1994, 25, 313–322. [Google Scholar] [CrossRef]
- Zhang, W.W.; Zhang, L.; Hou, W.K.; Xu, Y.X.; Hu, H.; Lou, F.C.; Zhang, Y.; Wang, Q. Dynamic expression of glucose transporters 1 and 3 in the brain of diabetic rats with cerebral ischemia reperfusion. Chin. Med. J. 2009, 122, 1996–2001. [Google Scholar] [PubMed]
- Chavez, J.C.; LaManna, J.C. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: Potential role of insulin-like growth factor-1. J. Neurosci. 2002, 22, 8922–8932. [Google Scholar] [CrossRef]
- McCall, A.L.; Van Bueren, A.M.; Nipper, V.; Moholt-Siebert, M.; Downes, H.; Lessov, N. Forebrain ischemia increases GLUT1 protein in brain microvessels and parenchyma. J. Cereb. Blood Flow Metab. 1996, 16, 69–76. [Google Scholar] [CrossRef]
- Weisova, P.; Concannon, C.G.; Devocelle, M.; Prehn, J.H.; Ward, M.W. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J. Neurosci. 2009, 29, 2997–3008. [Google Scholar] [CrossRef]
- Cruz, J. An additional therapeutic effect of adequate hyperventilation in severe acute brain trauma: Normalization of cerebral glucose uptake. J. Neurosurg. 1995, 82, 379–385. [Google Scholar] [CrossRef]
- Bergsneider, M.; Hovda, D.A.; Shalmon, E.; Kelly, D.F.; Vespa, P.M.; Martin, N.A.; Phelps, M.E.; McArthur, D.L.; Caron, M.J.; Kraus, J.F. Cerebral hyperglycolysis following severe traumatic brain injury in humans: A positron emission tomography study. J. Neurosurg. 1997, 86, 241–251. [Google Scholar] [CrossRef]
- Cornford, E.M.; Hyman, S.; Comford, M.E.; Caron, M.J. Glut1 glucose transporter activity in human brain injury. J. Neurotrauma 1996, 13, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Umschweif, G.; Alexandrovich, A.G.; Trembovler, V.; Horowitz, M.; Shohami, E. Hypoxia-induces factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection. J. Cereb. Blood Flow Metab. 2013, 33, 524–531. [Google Scholar] [CrossRef]
- Ding, J.Y.; Kreipke, C.W.; Speirs, S.L.; Schafer, P.; Schafer, S.; Rafols, J.A. Hypoxia-inducible factor-1 alpha signaling in aquaporin upregulation after traumatic brain injury. Neurosci. Lett. 2009, 453, 68–72. [Google Scholar] [CrossRef]
- Hamlin, G.P.; Cernak, I.; Wixey, J.A.; Vink, R. Increased expression of neuronal glucose transporter 3 but not glial glucose transporter 1 following severe diffuse traumatic brain injury in rats. J. Neurotrauma 2001, 18, 1011–1018. [Google Scholar] [CrossRef]
- Hu, L.; Wang, W.; Chen, X.; Bai, G.; Ma, L.; Yang, X.; Shu, Q.; Li, X. Prospects of antidiabetic drugs in the treatment of neurodegenerative disease. Brain-X 2024, 2, e52. [Google Scholar] [CrossRef]
- Cereda, E.; Barichella, M.; Pedrolli, C.; Klersy, C.; Cassani, E.; Caccialanza, R.; Pezzoli, G. Diabetes and risk of Parkinson’s disease: A systematic review and meta-analysis. Diabetes Care 2011, 34, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Fu, D.L.; Li, H.Q.; Liu, A.J.; Li, J.H.; Zheng, G.Q. Diabetes and risk of Parkinson’s disease: An updated meta-analysis of case-control studies. PLoS ONE 2014, 9, e85781. [Google Scholar] [CrossRef]
- Noyce, A.J.; Bestwick, J.P.; Silveira-Moriyama, L.; Hawkes, C.H.; Giovannoni, G.; Lees, A.J.; Schrag, A. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann. Neurol. 2012, 72, 893–901. [Google Scholar] [CrossRef]
- Yue, X.; Li, H.; Yan, H.; Zhang, P.; Chang, L.; Li, T. Risk of Parkinson disease in diabetes mellitus: An updated meta-analysis of population-based cohort studies. Medicine 2016, 95, e3549. [Google Scholar] [CrossRef]
- Labandeira, C.M.; Fraga-Bau, A.; Ron, D.A.; Alvarez-Rodriguez, E.; Vincente-Alba, P.; Lago-Garma, J.; Rodriguez-Perez, A.I. Parkinson’s disease and diabetes mellitus: Common mechanisms and treatment repurposing. Neural Regen. Res. 2022, 17, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, K.; Siuda, J. Alzheimer’s disease and type 2 diabetes mellitus: Similarities in pathomechanisms lead to therapeutic opportunities. Neurol. Neurochir. Pol. 2021, 55, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.N.; Chorawala, M.R.; Shah, M.B.; Shah, K.C.; Dave, B.P.; Shah, M.P.; Patel, T.M. Emerging pathophysiological mechanisms linking diabetes mellitus and Alzheimer’s disease: An old wine in a new bottle. J. Alzheimer’s Dis. Rep. 2022, 6, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C. Protective properties of GLP-1 and associated hormones in neurodegenerative disorders. Br. J. Pharmacol. 2022, 179, 695–714. [Google Scholar] [CrossRef]
- Li, Y.; Perry, T.; Kindy, M.S.; Harvey, B.K.; Tweedie, D.; Holloway, H.W.; Powers, K.; Shen, H.; Egan, J.M.; Sambamurti, K.; et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci. USA 2009, 106, 1285–1290. [Google Scholar] [CrossRef]
- Bertilsson, G.; Patrone, C.; Zachrison, O.; Andersson, A.; Dannaeus, K.; Heidrich, J.; Kortesmaa, J.; Mercer, A.; Nielsen, E.; Rönnholm, H.; et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J. Neurosci. Res. 2008, 86, 326–338. [Google Scholar] [CrossRef]
- Harkavyi, A.; Abuirmeileh, A.; Lever, R.; Kingsbury, A.E.; Biggs, C.S.; Whitton, P.S. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J. Neuroinflamm. 2008, 5, 19. [Google Scholar] [CrossRef]
- Qi, L.; Ke, L.; Liu, X.; Liao, L.; Ke, S.; Liu, X.; Wang, Y.; Lin, X.; Zhou, Y.; Wu, L.; et al. Subcutaneous administration of liraglutide ameliorates learning and memory impairment by modulation tau hyperphosphorylation via the glycogen synthase kinase-3β pathway in an amyloid β protein induced Alzheimer disease mouse model. Eur. J. Pharmacol. 2016, 783, 23–32. [Google Scholar] [CrossRef]
- McClean, P.L.; Parthsarathy, V.; Faivre, E.; Hölscher, C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 6587–6594. [Google Scholar] [CrossRef] [PubMed]
- Han, W.N.; Hölscher, C.; Yuan, L.; Yang, W.; Wang, X.-H.; Wu, M.-N.; Qi, J.-S. Liraglutide protects against amyloid-β protein-induced impairment of spatial learning and memory in rats. Neurobiol. Aging 2013, 34, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Lin, T.C.; Ho, H.L.; Kuo, C.-Y.; Li, H.-H.; Korolenko, T.A.; Chen, W.-J.; Lai, T.-J.; Ho, Y.-J.; Lin, C.-L. GLP-1 analogue liraglutide attenuates mutant huntingtin-induced neurotoxicity by restoration of neuronal insulin signaling. Int. J. Mol. Sci. 2018, 19, 2505. [Google Scholar] [CrossRef]
- Nizari, S.; Basalay, M.; Chapman, P.; Korte, N.; Korsak, A.; Christie, I.N.; Theparambil, S.M.; Davidson, S.M.; Reimann, F.; Trapp, S.; et al. Glucagon-like peptide-1 (GLP-1) receptor activation dilates cerebral arterioles, increases cerebral blood flow, and mediates remote (pre)conditioning neuroprotection against ischemic stroke. Basic Res. Cardiol. 2021, 116, 32. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhou, Y.; Zhang, M.; Xie, Z.; Ke, S.; Liu, L.; Pan, X.; Chen, Z. Exenatide alleviates mitochondrial dysfunction and cognitive impairment in 5xFAD, mouse model of Alzheimer’s disease. Behav. Brain Res. 2019, 370, 111932. [Google Scholar] [CrossRef]
- Garabadu, D.; Verma, J. Extendin-4 attenuates brain mitochondrial toxicity through PI3K/Akt-independent pathway in amyloid (1–42)-induced cognitive deficit rats. Neurochem. Int. 2019, 128, 39–49. [Google Scholar] [CrossRef]
- Yu, H.Y.; Sun, T.; Wang, Z.; Li, H.; Xu, D.; An, J.; Wen, L.L.; Li, J.Y.; Li, W.; Feng, J. Exendin-4 and lingliptin attenuate neuroinflammation in a mouse model of Parkinson’s disease. Neural. Regen. 2023, 18, 1818–1826. [Google Scholar]
- Nowell, J.; Blunt, E.; Edison, P. Incretin and insulin targets for Alzheimer’s and Parkinson’s disease. Mol. Psychiatr. 2023, 28, 217–229. [Google Scholar] [CrossRef]
- Hölscher, C. Brain insulin resistance: Role in neurodegenerative disease and potential for targeting. Expert Opin. Investig. Drugs 2020, 29, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.K.; Goel, R.; Nandakumar, K.; Nemmani, K.V.S. Effect of D-Ala2GIP, a stable GIP receptor agonist on MPTP-induced neuronal impairments in mice. Eur. J. Pharmacol. 2017, 84, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.M.; Hölscher, C. The incretin analogue D-Ala261P reduces plague load, astrogliosis and oxidative stress in an APP/PS1 mouse model of Alzheimer’s disease. Neuroscience 2013, 228, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, W.; Li, L.; Hölscher, C. D-Ala2-GIP-glu-PAL is neuroprotective in a chronic Parkinson’s disease mouse model and increases BNDF expression while reducing neuroinflammation and lipid peroxidation. Eur. J. Pharm. 2017, 797, 162–172. [Google Scholar] [CrossRef]
- Panagaki, T.; Gengler, S.; Hölscher, C. The novel DA-CH3 dual incretin restores endoplasmic reticulum stress and autophagy impairments to attenuate Alzheimer-like pathology and cognitive decrements in the APPSWE/PS1ΔE9 mouse model. J. Alzheimer’s Dis. 2018, 66, 195–218. [Google Scholar] [CrossRef]
- Maskery, M.; Goulding, E.M.; Gengler, S.; Melchiorsen, J.U.; Rosenkilde, M.M.; Holscher, C. The dual GLP/GIP receptor agonist DA4-JC shows superior protective properties compared to GLP-1 analog Liraglutide in APP/PS1 mouse model of Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Demen. 2020, 35, 1533317520953041. [Google Scholar] [CrossRef]
- Cao, Y.; Hölscher, C.; Hu, M.-M.; Wang, T.; Zhao, F.; Bai, Y.; Zhang, J.; Wu, M.-N.; Qi, J.-S. DAS-CH, a novel GLP-GIP dual agonist, effectively ameliorates the cognitive impairments and pathology in the APP/PS1 mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 2018, 827, 215–226. [Google Scholar] [CrossRef]
- Tai, J.; Liu, W.; Li, Y.; Hölscher, C. Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Brain Res. 2018, 1678, 64–74. [Google Scholar] [CrossRef]
- Li, T.; Jiao, J.J.; Hölscher, C.; Wu, M.N.; Zhang, J.; Tong, J.Q.; Dong, X.-F.; Qu, X.-S.; Cao, Y.; Cai, H.-Y.; et al. A novel GLP-1/GIP/Gcg triagonist reduces cognitive deficits and pathology in the 3xTg mouse model of Alzheimer’s disease. Hippocampus 2018, 28, 358–372. [Google Scholar] [CrossRef]
- Li, T.; Jiao, J.J.; Su, Q.; Hölscher, C.; Zhang, J.; Yan, X.D.; Zhao, H.-M.; Cai, H.-Y.; Qi, J.-S. A GLP-1/GIP/Gcg receptor trigonist improves memory behavior, as well as synaptic transmission, neuronal excitability and Ca2+ homeostasis in 3xTg-AD mice. Neuropharmacology 2020, 170, 108042. [Google Scholar] [CrossRef]
- Choi, I.Y.; Lee, J.S.; Kim, J.K.; Park, Y.J.; Lee, S.H.; Kim, Y.H.; Kim, S.J.; Kwon, S.C. Potent body weight loss and efficacy in a NASH animal model by a novel long-lasting GLP-1/Glucagon/GIP triple-agonist (HM15211). In Proceedings of the American Diabetes Association’s 77th Scientific Session, San Diego, CA, USA, 9–13 June 2017. [Google Scholar]
- Wonki, K.; Kim, J.A.; Lee, S.H.; Bae, S.; Choi, I.Y.; Kim, Y.H. 1810-P: Effect of HM15211, a novel long-acting GLP-1/GIP/Glucagon triple agonist in the neurodegenerative disease models. Diabetes 2019, 68, 1810-P. [Google Scholar] [CrossRef]
- Egan, M.F.; Kost, J.; Tariot, P.N.; Tariot, P.N.; Aisen, P.S.; Cummings, J.L.; Vellas, B.; Sur, C.; Michelson, D. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2018, 378, 1691–1703. [Google Scholar] [CrossRef]
- Kosaraju, J.; Holsinger, R.M.; Guo, L.; Tam, K.Y. Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3xTg-AD mouse model of Alzheimer’s disease. Mol. Neurobiol. 2017, 54, 6074–6084. [Google Scholar] [CrossRef]
- Brauer, R.; Wei, L.; Ma, T.; Athuda, D.; Girges, C.; Vijiaratnam, N.; Auld, G.; Whittlesea, C.; Wong, I.; Foltynie, T. Diabetes medications and risk of Parkinson’s disease: A cohort study of patients with diabetes. Brain 2020, 143, 3067–3076. [Google Scholar] [CrossRef]
- Svennigsson, P.; Wirdefeldt, K.; Yin, L.; Fang, F.; Markaki, I.; Efendic, S.; Ludvigsson, J.F. Reduced incidence of Parkinson’s disease after dipeptidyl peptidase-4 inhibitors–A nationwide case-control study. Mov. Disord. 2016, 31, 1422–1423. [Google Scholar] [CrossRef]
- Justin, A.; Ananda Kumar, T.D.; Chinaswamy, M.; Kumar, B.R.P. Glitazones activate PGC-1α signaling via PPAR-γ a promising strategy for antiparkinsonism therapeutics. ACS Chem. Neurosci. 2021, 12, 2261–2272. [Google Scholar]
- Agarwal, S.; Yada, A.; Chaturvedi, R.K. Peroxisome proliferator activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem. Biophys. Res. Commun. 2017, 483, 1166–1177. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.N.; Jia, J.P. Peroxisome proliferator-activated receptor-gamma agonists for Alzheimer’s disease and amnestic mild cognitive impairment: A systematic review and meta-analysis. Drugs Aging 2015, 32, 57–65. [Google Scholar] [CrossRef]
- Risner, M.E.; Saunders, A.M.; Altman, J.F.B.; Ormandy, G.C.; Craft, S.; Foley, I.M.; Zvartau-Hind, M.E.; Hosford, D.A.; Roses, A.D.; Rosiglitazone in Alzheimer’s Disease Study Group. Efficacy of rosiglitazone in a genetically defined population with mild-to moderate Alzheimer’s disease. Pharmacogenomics 2006, 6, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.; Alderton, C.; Zvartau-Hind, M.; Egginton, S.; Saunders, A.M.; Irizarry, M.; Craft, S.; Landreth, G.; Linnamägi, U.; Sawchak, S. Rosiglitazone, monotherapy in mild-to-moderate Alzheimer’s disease: Results from a randomized, double-blind, placebo-controlled phase III study. Dement. Geriatr. Cogn. Disord. 2010, 30, 131–146. [Google Scholar] [CrossRef]
- Sluggett, J.K.; Koponen, M.; Bell, J.S.; Taipale, H.; Tnaskanen, A.; Tiihonen, J.; Uusitupa, M.; Tolppanen, A.; Hartikainen, S. Metformin and risk of Alzheimer’s disease among community-dwelling people with diabetes: A randomized case-control study. J. Clin. Endocrinol. Metab. 2020, 105, e963–e972. [Google Scholar] [CrossRef]
- Ping, F.; Jiang, N.; Li, Y. Association between metformin and neurodegenerative diseases of observational studies: Systematic review and meta-analysis. BMJ Open Diab. Res. Care 2020, 8, e001370. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- McLean, B.A.; Wong, C.K.; Campbell, J.E.; Hodson, D.J.; Trapp, S.; Drucker, D.J. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor reaction. Endocr. Rev. 2021, 42, 101–132. [Google Scholar] [CrossRef]
- Heppner, K.M.; Kirigiti, M.; Secher, A.; Paulsen, S.J.; Buckingham, R.; Pyke, C.; Knudsen, L.B.; Vrang, N.; Grove, K.L. Expression and distribution of glucose-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015, 156, 255–267. [Google Scholar] [CrossRef]
- Farr, O.M.; Sofopoulos, M.; Tsoukas, M.A.; Dincer, F.; Thakkar, B.; Sahin-Efe, A.; Filippaios, A.; Bowers, J.; Srnka, A.; Gavrieli, A.; et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: A crossover randomized, placebo-controlled trial. Diabetologia 2016, 59, 954–965. [Google Scholar]
- Kim, D.S.; Choi, H.I.; Wang, Y.; Luo, Y.; Hoffer, B.J.; Greig, N.H. A new treatment strategy for Parkinson’s disease through the gut-brain axis: The glucagon-like peptide-1 receptor pathway. Cell Transplant. 2017, 26, 1560–1571. [Google Scholar] [CrossRef]
- Joint Formulary Committee. British National Formulary on Line; BMJ Group and Pharmaceutical Press: London, UK, 2020. [Google Scholar]
- Nyberg, J.; Anderson, M.F.; Alborn, A.; Stro, A.; Brederlau, A.; Illerskog, A.; Nilsson, O.; Kieffer, T.J.; Hietala, M.A.; Rickstein, A.; et al. Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J. Neurosci. 2005, 25, 1816–1825. [Google Scholar] [CrossRef]
- Nyberg, J.; Jacobsson, C.; Andersen, M.F.; Eriksson, P.S. Immunohistochemical distribution of glucose-dependent insulinotropic polypeptide in the adult rat brain. J. Neurosci. 2007, 85, 2099–2119. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, X.; Li, D.; Ji, C.; Yuan, Z.; Wang, R.; Xue, G.; Li, G.; Hölscher, C. Two dual GLP1/GIP receptor agonists are neuroprotective in the MPTP mouse model of Parkinson’s disease. Neuropharmacology 2018, 133, 385–394. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Zhu, L.; Sha, L.; Yang, S.; Wei, J.; Ji, L.; Tang, X.; Mao, K.; Cao, L.; et al. Insulin degrading enzyme contributes to the pathology in a mixed model of Type 2 diabetes and Alzheimer’s disease: Possible mechanisms of IDE in T2D and AD. Biosci. Rep. 2018, 38, BSR20170862. [Google Scholar] [CrossRef]
- Ou, Z.; Kong, X.; Sun, X.; He, X.; Zhang, L.; Gong, Z.; Huang, J.; Xu, B.; Long, D.; Li, J.; et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav. Immun. 2018, 69, 351–363. [Google Scholar] [CrossRef]
- Farr, S.A.; Raesler, E.; Niehoff, M.L.; Roby, D.A.; Mckee, A.; Morley, J.E. Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 68, 1699–1710. [Google Scholar] [CrossRef]
- Biosa, A.; Outeiro, T.F.; Bubacco, L.; Bisaglia, M. Diabetes mellitus as a risk factor for Parkinson’s disease: A molecular point of view. Mol. Neurobiol. 2018, 55, 8754–8763. [Google Scholar] [CrossRef]
- Moore, E.M.; Mander, A.G.; Ames, D.; Kotowicz, M.A.; Carne, R.P.; Brodaty, H.; Woodward, M.; Boundy, K.; Ellis, K.A.; Bush, A.I.; et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 2013, 36, 2981–2987. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Ashrafizadeh, M.; Henney, N.C.; Sathyapalan, T.; Jamialahmadi, T.; Sabebkar, A. Neuromodulatory effects of anti-diabetes medications: A mechanistic review. Pharmacol. Res. 2020, 152, 104611. [Google Scholar] [CrossRef] [PubMed]
- Labandeira, C.M.; Fraga-Bau, A.; Ron, D.A.; Muñoz, A.; Alonso-Losada, G.; Koukoulis, A.; Romero-Lopez, J.; Rodriguez-Perez, A.I. Diabetes, insulin and new therapeutic strategies for Parkinson’s disease: Focus on glucagon-like peptide-1 receptor agonists. Front. Neuroendocrinol. 2021, 62, 100914. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.; Go, J.; Park, H.; Choi, Y.; Jeong, Y.; Hyeon, J.; Rhee, M.; Geal, T.; Lee, C.; Kim, K. Metformin regulates astrocyte reactivity in Parkinson’s disease and normal aging. Neuropharmacology 2020, 175, 108173. [Google Scholar] [CrossRef] [PubMed]
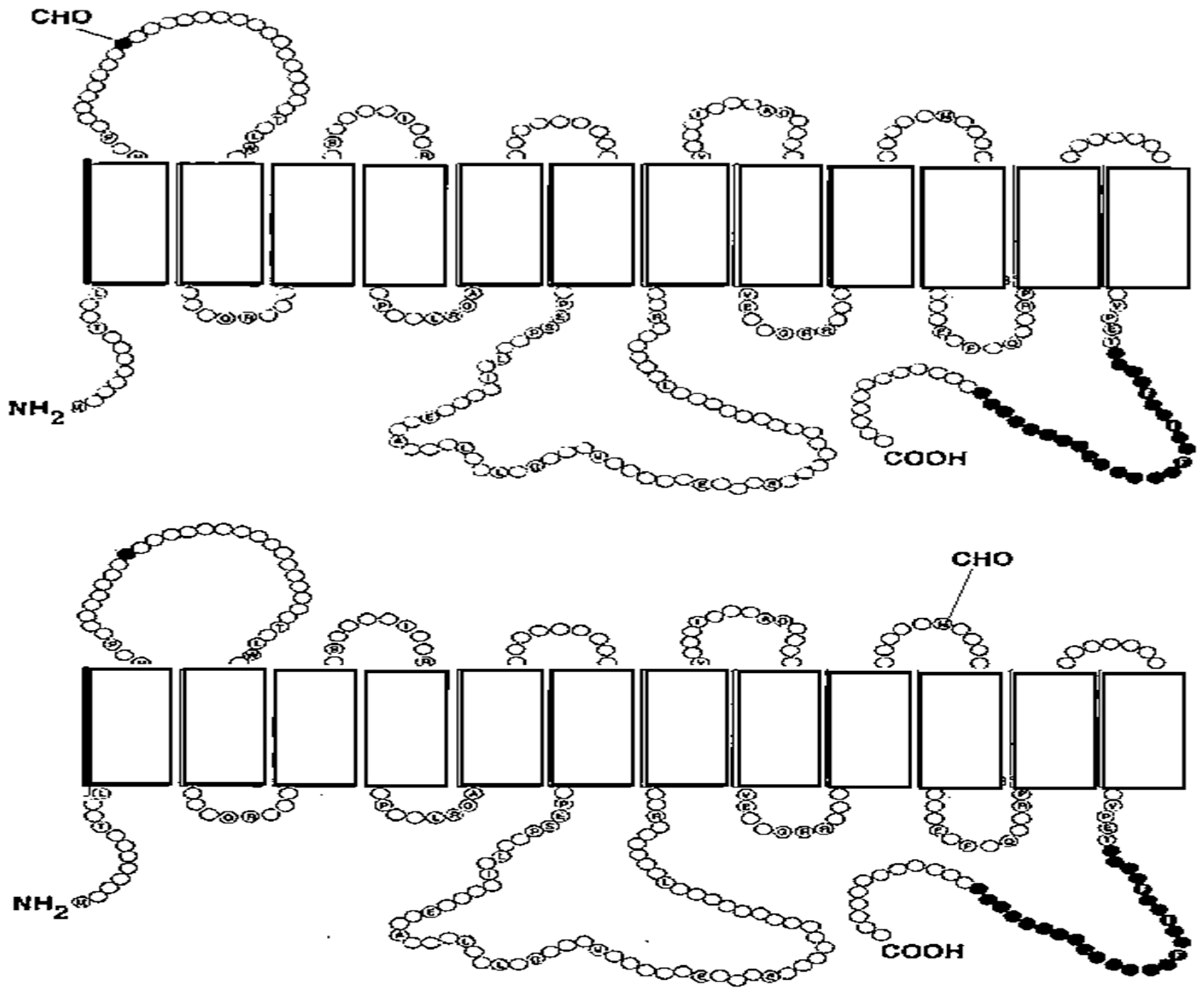
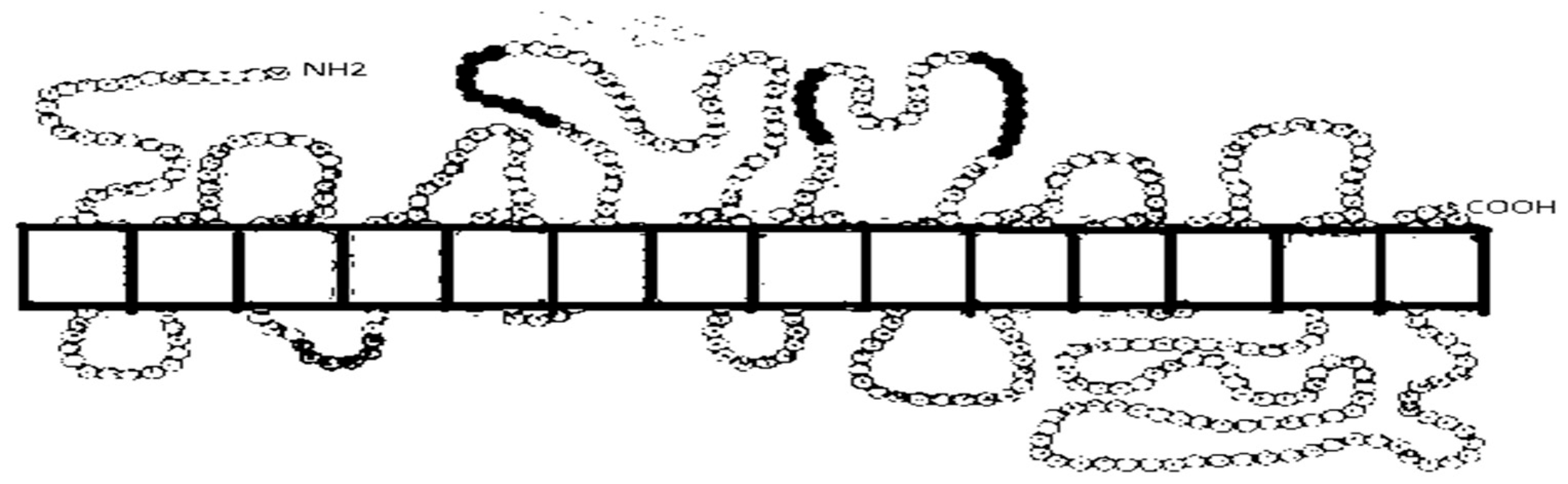
| Glucose Transporter Gene Localization | Organ/Tissue of Transporter Expression | Transported Substrates | References |
|---|---|---|---|
| GLUT1 SLC2A1 1p35-p31.3 | The large-molecular-weight GLUT1 (55 kDa) is detected in the cerebral cortex microvessels; the low-molecular-weight isoform (45 kDa) is present in the microvessel-depleted membrane of the brain (neuronal/glial membranes) and synaptosomes; an intermediate isoform of this transport protein is detected in the choroid plexus. It is expressed in fetal cells, from oocyte to the blastocyst. This transporter transports glucose through the epithelial or epithelial-like barrier cells of the brain, eye, peripheral nerve, and placenta. These barriers express high levels of GLUT1. Its expression is detected also in the blood cells, such as erythrocytes, granulocytes, and lymphocytes, as well as in adipocytes, muscle cells, kidney cells, and colon. Expression of GLUT1 was also detected in mitochondria of the human kidney cells 293T. | Glucose, galactose, mannose, glucosamine, the oxidized form of vitamin C. | [19,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] |
| GLUT2 SLC2A2 3q26.1-26.2. | It is expressed in the intestinal absorptive epithelial cells, hepatocytes, pancreatic β cells, and in the kidney cells. In the epithelial cells, GLUT2 is expressed in the basolateral membrane. In the brain, it is expressed in the brainstem, thalamus, cortex, hypothalamus, and hippocampus. Low levels of GLUT2 mRNA are detected in the nuclei of the brain, such as the nucleus tractus solitatius, the motor nucleus of the vagus, the paraventricular hypothalamic nucleus, the lateral hypothalamic area, the arcuate nucleus, and the olfactory bulbs, as well as in the neurons of limbic areas and related nuclei. | Glucose, galactose, mannose, fructose, glucosamine. | [17,20,30,42,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61] |
| GLUT3 SLC2A3 12p13.3 | Its high expression is detected in the brain, especially in the neurons, while not in the glia. In the brain, its expression is detected in the hippocampus, gyrus, and temporal neocortex. It is also found in tissues and cells with high demand for glucose, such as spermatozoa, placenta, preimplantation embryos, fibroblasts, platelets, white blood cells, and retinal endothelial cells. | Glucose, galactose. | [7,16,17,19,30,32,33,57,62,63,64,65,66,67,68] |
| GLUT4 SLC2A4 17p13 | Its preferential sites of expression are the skeletal muscle, cardiomyocytes, and adipose tissue. It is expressed also in the brain in the hypothalamus, cerebellum, cortex, and hippocampus. | Glucose, dehydroascorbic acid, glucosamine. | [20,57,69,70,71,72,73,74,75,76] |
| GLUT5 SLC2A5 1p36.2 | At high levels, it is expressed in the apical membrane of enterocytes. GLUT5 mRNA levels increase with age, and its highest levels are found in the small intestine of adults. Its expression is found also in the small intestine (proximal region). GLUT5 mRNA is detected in the plasma membrane of mature spermatozoa, and GLUT5 mRNA and/or GLUT5 protein have been detected in the kidneys, adipose tissue, skeletal muscle, and in the brain. Its expression is also detected in the microglial cells of the brain, but only in microglia among cells of the mononuclear phagocyte system. | Fructose. | [16,17,19,20,30,33,50,56,57,77,78,79,80,81,82,83,84] |
| GLUT6 Formerly designated as GLUT9 SLC2A6 9q34 | It is predominantly expressed in the brain, peripheral leukocytes, spleen, and germinal cells of the testis. | Glucose. | [16,17,20,85,86] |
| GLUT7 SLC2A7 1p36.22 | Its primary expression is detected in the apical membrane of the small intestine (distal region) and colon. Its mRNA is detected in the testis and prostate. | Glucose, fructose. | [17,20,87,88,89] |
| GLUT8 Formerly designated as GLUTX1 SLC2A8 9q33.3 | It is detected in endosomes, lysosomes, and membranes of the endoplasmic reticulum. GLUT8 mRNA is detected in the testis, cerebellum, adrenal gland, liver, spleen, and brown adipose tissue. In the brain, expression of GLUT8 is reported in the hippocampus, dente gyrus, amygdala, primary olfactory cortex, hypothalamic nuclei, and tractus solitaries in the blood–retinal barrier. It is also reported in the endometrium and endometrial adenocarcinoma and in blastocysts. It transports sugars or sugar derivatives through the intracellular membranes. | Glucose. | [16,19,20,57,90,91,92,93,94,95] |
| GLUT9 Formerly designated as GLUTX SLC2A9 4p15.3-p16 | Due to the use of alternative promoters, the gene encodes two isoforms of GLUT9: the major isoform, i.e., GLUT9 or GLUT9a, or an alternative splice variant of GLUT9 mRNA that codes the GLUT9ΔN or GLUT9b isoform. GLUT9 is expressed in the liver, kidneys, intestine, leukocytes, and chondrocytes, whereas GLUT9b is detected only in the liver and kidneys. GLUT9a is expressed in the basolateral membrane of the epithelial cells, while GLUT9b is localized to the apical pole. | Urate, glucose, fructose. | [19,20,96,97,98,99,100,101,102] |
| GLUT10 SLC2A10 20q13.1 | It is detected in insulin-sensitive tissues such as the skeletal muscle and heart. GLUT10 mRNA is detected in the liver, pancreas, placenta, and the kidneys, as well as in human adipose tissue (omental and subcutaneous), in human preadipocyte cell strains (SGBS), and in 3T3-L1 adipocytes. | Deoxy-D-glucose, D-galactose. | [17,20,24,103,104,105] |
| GLUT11 Formerly designated as GLUT10 SLC2A11 22q11.2 | In humans, three isoforms of GLUT11 are expressed. These isoforms are due to the separate exons (exons 1A, 1B, and 1C) of the SLC2A11 gene, which generate three variants of GLUT11 (GLUT11-A, GLUT11-B, and GLUT11-C). GLUT11-A is detected in the heart, skeletal muscle, and in the kidneys; GLUT11-B is expressed in the kidneys, adipose tissue, and the placenta; and GLUT11-C is expressed in the adipose tissue, heart, skeletal muscle, and pancreas. This transporter has no rodent ortholog. | Fructose, glucose. | [19,20,106,107] |
| GLUT12 Formerly designated as GLUT8 SLC2A12 6q23.2 | It is detected in the heart, skeletal muscle, adipose tissue, prostate glands, kidneys, small intestine, and chondrocytes. In the human placenta, different expressions of GLUT12 have been detected. In the first trimester, this glucose transporter is detected predominantly in the syncytiotrophoblast, in the lesser extent in the villous cytotrophoblast, and in extra-villous trophoblast cells. At term, GLUT12 is detected predominantly in the villous vascular smooth muscle and stromal cells, whereas in the syncytiotrophoblast, it is not detected. | Glucose, galactose, fructose. | [16,20,21,24,108,109,110] |
| GLUT13 (HMIT) SLC2A13 22q12 | GLUT13 (HMIT) is an H+/myo-inositol cotransporter. Its high expression is detected predominantly in the brain in the hippocampus, hypothalamus, cerebellum, and brainstem regions. In the brain, this transporter is expressed in neurons and in the glial cells. Low levels of GLUT13 are detected in the white and brown adipose tissue and in the kidneys. It uses a proton gradient that is a source of energy for movement of substrate. | Myo-inositol, inositol-3-phosphate. | [17,19,20,111,112,113] |
| GLUT14 SLC2A14 12p13.3 | It is identified and has been cloned as a duplication of GLUT3. There are two alternatively spliced forms: the short form of GLUT14 (GLUT14-S) and the long form of GLUT14 (GLUT14-L). Both isoforms are detected in the human testis. The ortholog of GLUT14 is not detected in mice. | Glucose, fructose. | [114] |
| Glucose Cotransporter Gene Localization | Organ/Tissue of Cotransporter Expression | Transported Substrates | References |
|---|---|---|---|
| SGLT1 SLC5A1 22q12.3 | It is expressed in the BBM of the mature enterocytes in the small intestine, trachea, prostate gland, heart, kidneys, mammary glands, liver, and in the endothelial cells of the luminal membrane of intracerebral capillaries. Its expression is detected in the cortical, pyramidal, and Purkinje neuronal cells of the brain; in neurons (hippocampal) and the cortical granule (pyramidal); and the BBB. It is suggested that in the brain, SGLT1 plays a role as a glucose receptor. Its mRNA is also detected in the uterus (cervix). | Water molecules, urea, fluid across the intestine; D-glucose; D-galactose. | [2,20,77,119,120,121,122,124,125,126,127,128,129,130,131,132,133,134,135,136] |
| SGLT2 SLC5A2 16p11.2 | Its predominant expression is detected on the apical membrane of renal convoluted proximal tubules (S1 and S2 segments). SGLT mRNA is detected also in the mammary glands, liver, lungs, intestine, skeletal muscle, and spleen. It is suggested also to have a role in the heart and brain as the receptor of glucose. | Glucose. | [2,20,119,120,137,138,139,140,141] |
| SGLT3 Alias SAAT1 SLC5A4 22q12.3 | Its expression is detected in the kidneys, uterus, testis, intestinal autonomic nervous system, skeletal muscle, brain, the cholinergic neurons in the enteric nervous system, pancreas, lungs, and liver. | Sodium absorption in kidneys. | [20,119,121,131,142,143] |
| SGLT4 SLC5A9 1p32 | Its expression is detected in the small intestine, kidneys, brain, liver, heart, uterus, and lungs. SGLT4 mRNA is detected in the testis, pancreas, and skeletal muscle. | D-mannose, D-fructose, D-glucose. | [2,104,119,121,131,144] |
| SGLT5 SLC5A10 17p11.2 | SGLT5 is expressed in the human kidney cortex, and its mRNA is detected in the left atrium of the heart, ovary, skin (foreskin), testis, and vas deferens. Its precise location on the chromosome remains unknown. | Mannose, fructose, α-methyl-D-glucopyanoside. | [121,131,145,146] |
| SGLT6 SMIT2 SLC5A11 16p12.1 | SGLT6 protein and its mRNA are detected in the brain, heart, kidneys, skeletal muscle, spleen, liver, placenta, lungs, and leukocytes. The SLC5A11 gene reveals interaction with immune-related genes, and therefore, it is suggested that it may play a role as an autoimmune modifier gene. | Myo-inositol, D-chiro-inositol, D-glucose, D-xylose. | [121,131,146,147,148,149] |
| SMIT1 SLC5A3 21q22.11 | There are three transcripts, SMIT1-1, SMIT1-2, and SMIT1-3, caused by splicing within and distal to exon 2. Its expression is detected in the kidneys, choroid plexus, brain, placenta, pancreas, heart, skeletal muscle, and lungs. SMIT1 mRNA is found in the blood vessels, choroid plexus, and uterus (cervix). | Myo-inositol, L-fucose, L-xylose. | [2,131,146,150] |
| NIS SLC5A5 19p13.11 | It is a sodium/iodide cotransporter mainly expressed in the thyroid as well as in the lactating breast, colon, stomach, and ovaries. | I−, ClO4−, SCN−, NO3−, Br−. | [2,151,152] |
| SMVT SLC5A6 2q12 | It is detected in the brain, heart, kidneys, lungs, and placenta. SMVT is a multivitamin/sodium cotransporter. | Vitamins such as pantothenic acid, biotin, α-lipoic acid. | [2] |
| CHT1 SLC5A7 2q13 | It is expressed in the central nervous system, spinal cord, and medulla. | Choline. | [2,153] |
| SMCT1 SLC5A8 12q23.1 | Its expression is detected in the small intestine, kidneys, brain, retina, and muscle. | Lactate, pyruvate, nicotinate. | [2,154] |
| SMCT2 SLC5A12 11p14.2 | Its expression is detected in the small intestine, kidneys, brain, retina, and muscle. | Lactate, pyruvate, nicotinate. | [2,154,155] |
| Anti-Diabetic Drugs | Mechanism of Action and Effects [References] |
|---|---|
| Incretins | Glucagon-like peptide-1 (GLP-1) receptor agonists: Liraglutide reveals neuroprotective properties in ND patients and ND animal models [288,289,290,291]. It ameliorates learning and memory impairment in animal models of AD. Liraglutide attenuates mutant huntingtin-induced neurotoxicity in human neuronal cells [292,293,294,295]. These agonists promote neuroprotection against ischemic stroke [296]. Exenatide halts cognitive decline in animal models of AD [297,298] and improves degeneration of dopaminergic neurons and motor function in PD animal models [289,299]. Exendin-4 decreases mitochondrial toxicity, alleviates cognitive dysfunction, prevents Aβ accumulation, and protects mitochondrial functions [297,298]. Glucose-dependent insulinotropic polypeptide (GIP) receptor agonists: These agonists have neuroprotective properties in animal models of AD and PD [300,301,302] and reduce the activation of chronic inflammatory response in the brain, oxidative stress, synapse loss, amyloid plaque burden, and DNA damage. They reduce also lipid peroxidation and α-synuclein levels, protecting dopaminergic neurons in the substantia nigra [303,304]. Dual-receptor agonists of GLP-1 and GIP, which activate both GLP-1 and GIP receptors, show promising effects in animal models of AD and PD [300,301]. These novel agonists rescue or prevent spatial learning and memory dysfunction [305,306,307], reduce amyloid plaques and phosphorylated tau protein [305], and prevent overactivation of p-GSK3β. In animal models of PD, these agonists improve motor function and protect dopaminergic neurons. Triple-receptor agonists activate GLP-1, GIP, and glucagon (Gcg) receptors. Treatment of a mouse model of AD with these agonists revealed anti-apoptotic effects and reduced Aβ deposition and hyperphosphorylated tau protein. These agonists protect against loss of synapses and reduce inflammatory symptoms and oxidative stress [308,309,310]. In PD mice, the triple-receptor agonist protects against dopaminergic neuronal death, decreases α-synuclein levels, and prevents nigrostriatal neurodegeneration [311,312]. |
| Dipeptidyl Peptidase-4 Inhibitors | Linagliptin improves cognitive function and decreases tau phosphorylation and Aβ aggregation in animal models of AD [313,314]. It has neuroprotective effects on human nerve cells caused by the protection of neurons from the effect of Aβ on mitochondrial damage, oxidative stress, and impaired insulin signaling. In humans who receive DPP-4 inhibitors, the incidence of PD is significantly decreased; however, high doses of inhibitors must be used [315,316]. |
| Thiazolidinediones | This class of drugs reveals neuroprotective effects in animal models of AD and PD, significantly improving behavior and motor responses [317,318,319]. Rosiglitazone improves spatial memory tested in the Morris water maze. Treatment of AD patients with rosiglitazone revealed different results in the case of dementia. Some results indicate improved cognitive performance in these patients [320], while other results do not confirm this effect [321]. |
| Biguanides | Metformin decreases α-synuclein expression in animal models of PD, and in AD animal models, it has a beneficial effect on cognitive function [322]. This effect is due to the promotion of neurogenesis and prevention of amyloid deposition and tau phosphorylation [320,321]. Performed observations revealed that metformin may have a negative effect in patients. This drug may stimulate the production of Aβ, and in diabetic patients treated with metformin, a significantly higher incidence of PD was observed in comparison with non-metformin diabetic patients [323]. A meta-analysis that studied the effects of metformin on neurodegeneration revealed that this drug has no effect on NDs [323]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szablewski, L. Human Glucose Transporters in Health and Selected Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 7392. https://doi.org/10.3390/ijms26157392
Szablewski L. Human Glucose Transporters in Health and Selected Neurodegenerative Diseases. International Journal of Molecular Sciences. 2025; 26(15):7392. https://doi.org/10.3390/ijms26157392
Chicago/Turabian StyleSzablewski, Leszek. 2025. "Human Glucose Transporters in Health and Selected Neurodegenerative Diseases" International Journal of Molecular Sciences 26, no. 15: 7392. https://doi.org/10.3390/ijms26157392
APA StyleSzablewski, L. (2025). Human Glucose Transporters in Health and Selected Neurodegenerative Diseases. International Journal of Molecular Sciences, 26(15), 7392. https://doi.org/10.3390/ijms26157392





