Urinary Metabolites Variation After High-Intensity Rowing Training and Potential Biomarker Screening for Exercise-Induced Muscle Damage
Abstract
1. Introduction
2. Results and Discussion
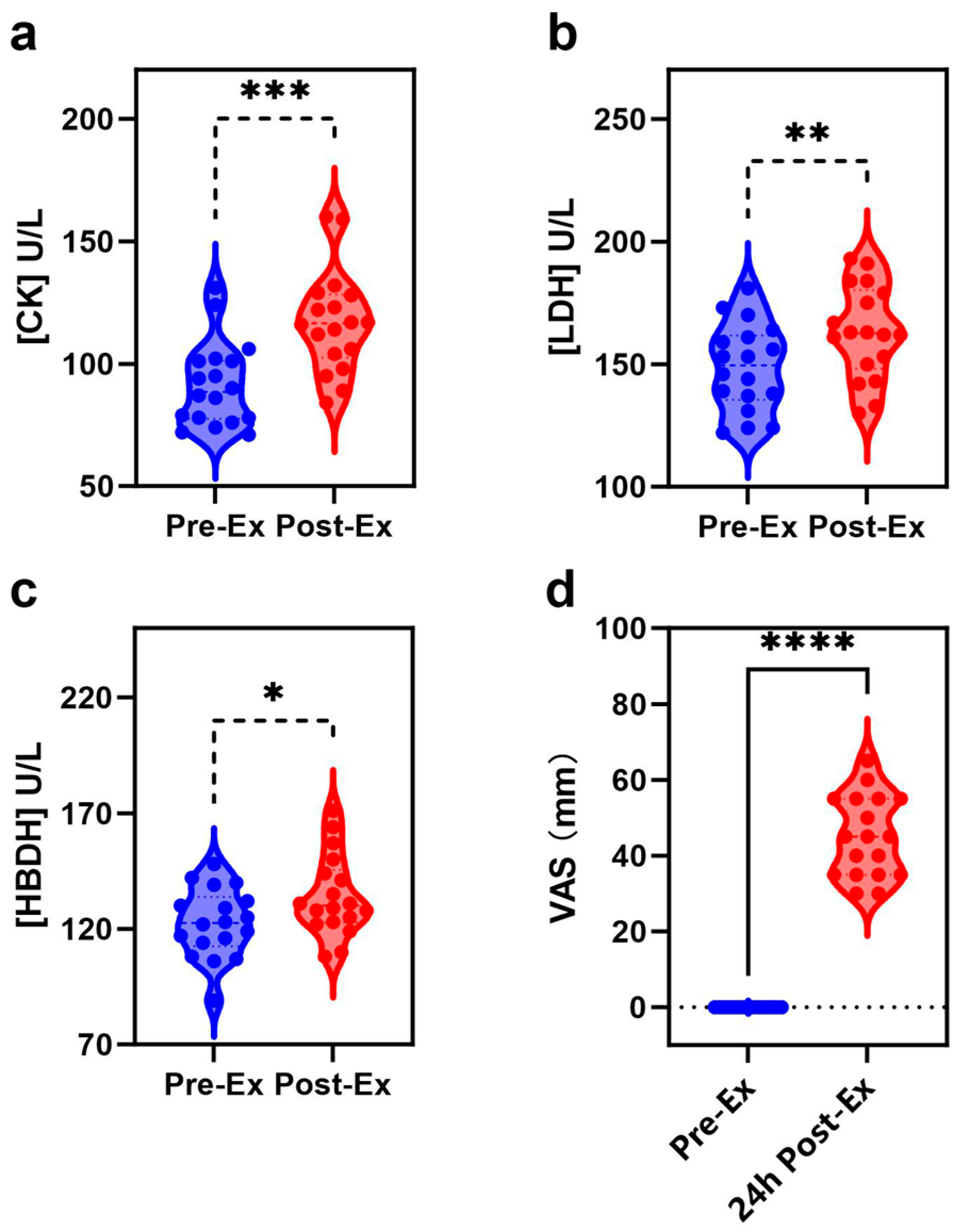
2.1. Biochemical Index and EIMD Analyses
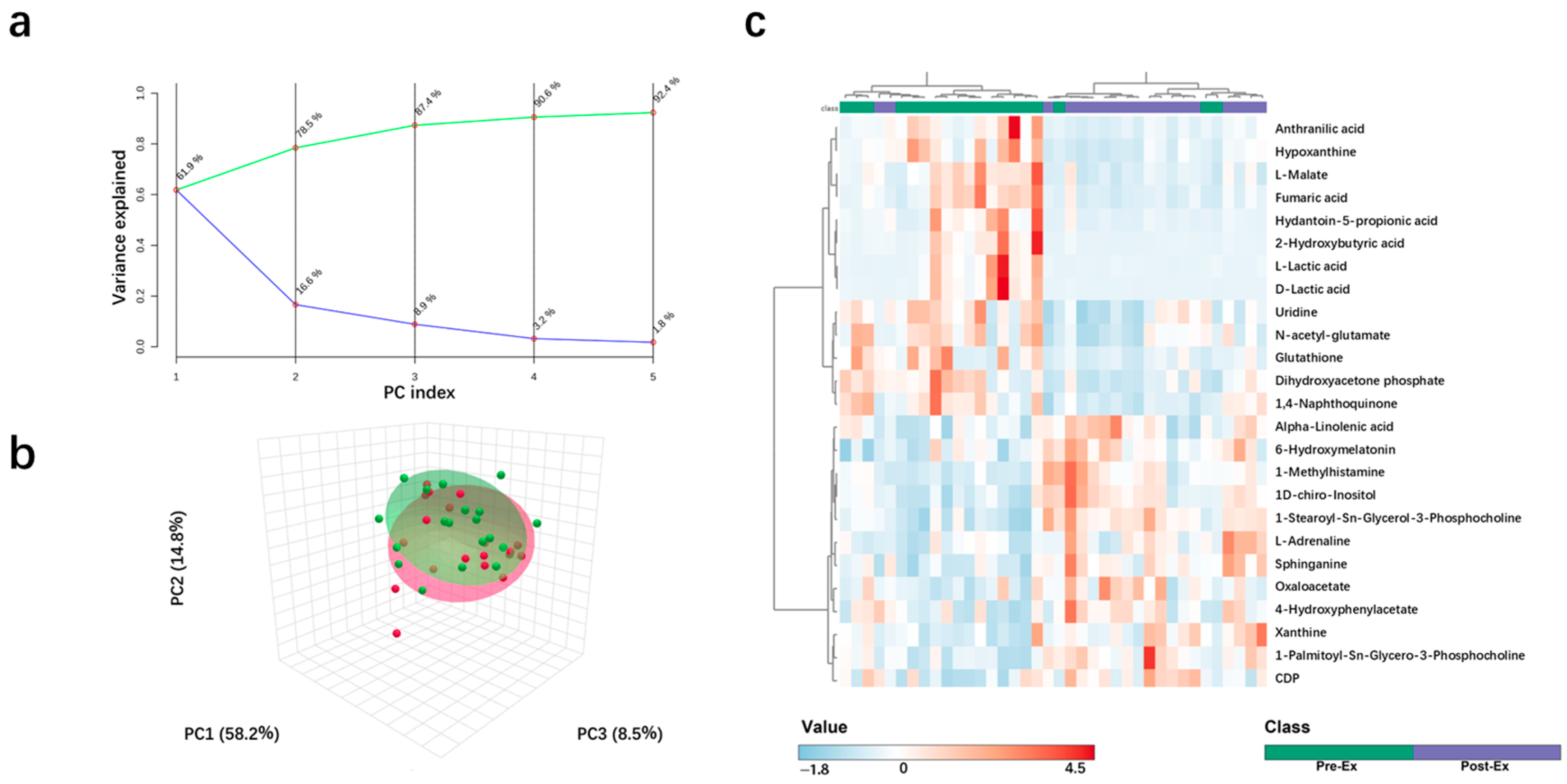
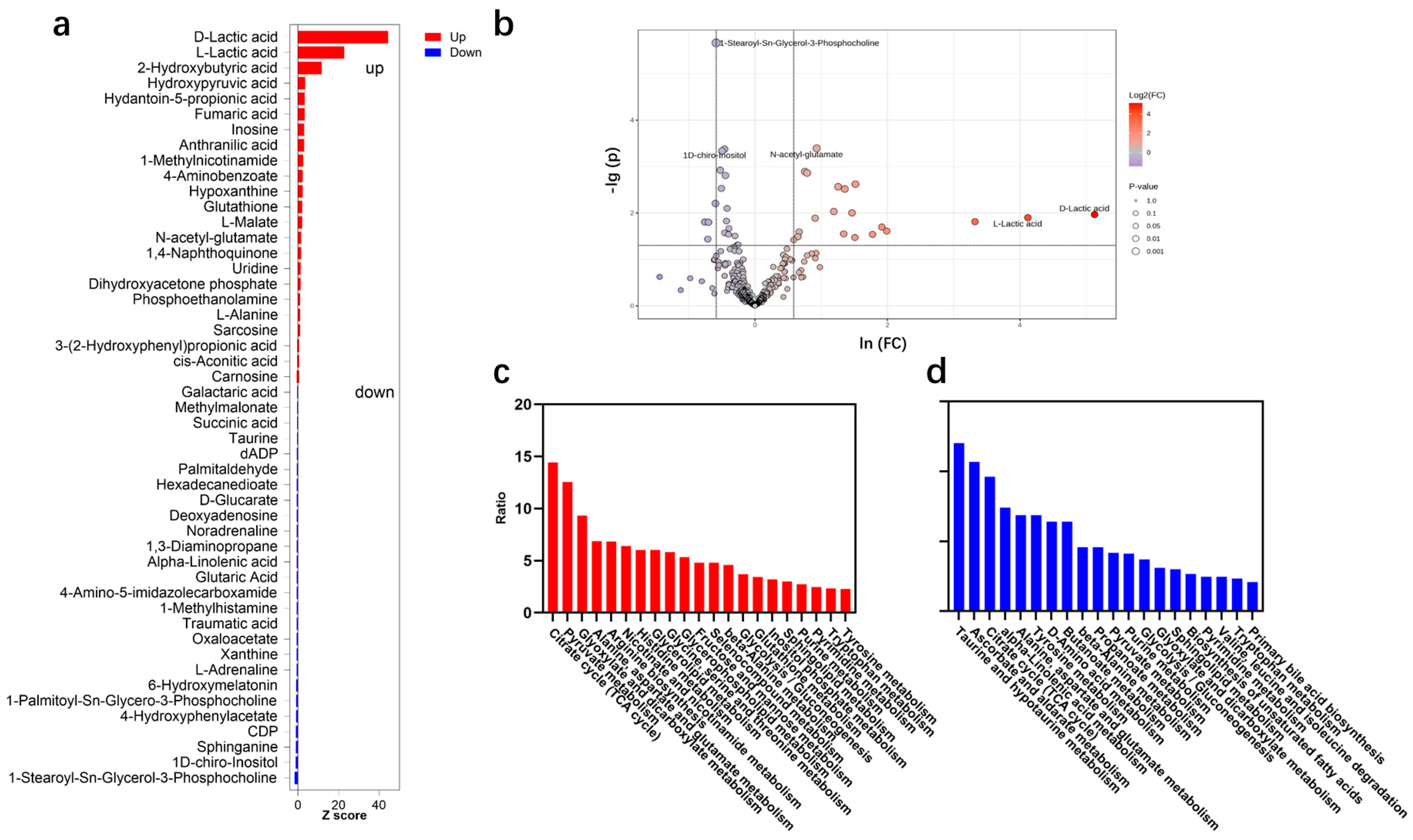
2.2. Metabolite Change Characteristics After High-Intensity Rowing Training
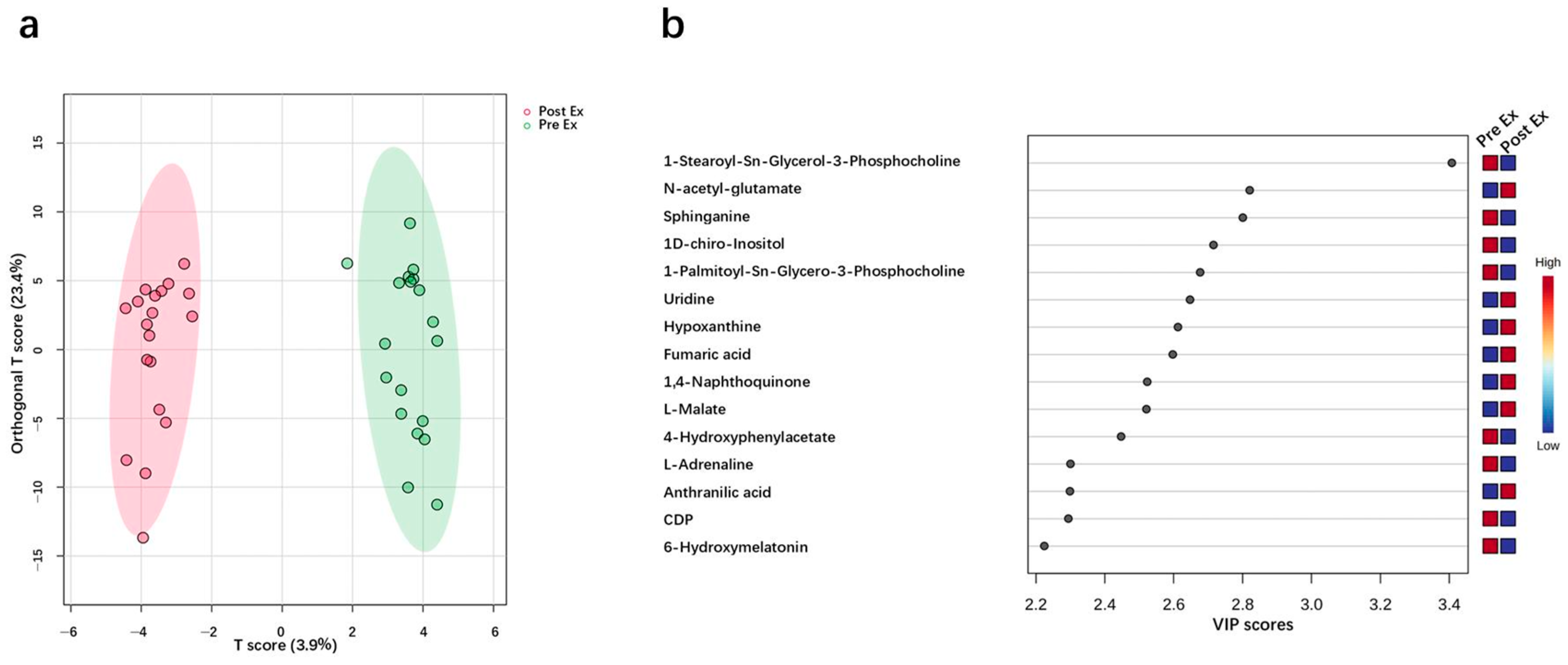
2.3. Potential Urinary Biomarker for EIMD Prediction
3. Materials and Methods
3.1. Subjects
3.2. Exercise Protocol and Sample Collection
3.3. Biochemical Index and Urine Metabolite Analyses
3.4. Bioinformatics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EIMD | Exercise-induced muscle damage |
| DOMS | Delayed onset muscle soreness |
| CK | Creatine kinase |
| VAS | Visual analog scale |
| LDH | Lactate dehydrogenase |
| HBDH | Hydroxybutyrate dehydrogenase |
| PCA | Principal component analysis |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| ROC | Receiver operating characteristic |
| TCA | Tricarboxylic acid |
| ALA | alpha-Linolenic acid |
| VIP | Variable importance in the projection |
| AUC | Area under the curve |
References
- MoTrPAC Study Group; Analysts, L.; Amar, D.; Gay, N.R.; Jean-Beltran, P.M.; Generators, L.D.; Bae, D.; Dasari, S.; Dennis, C.; Evans, C.R.; et al. Temporal dynamics of the multi-omic response to endurance exercise training. Nature 2024, 629, 174–183. [Google Scholar] [CrossRef]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef]
- Stozer, A.; Vodopivc, P.; Bombek, L.K. Pathophysiology of Exercise-Induced Muscle Damage and Its Structural, Functional, Metabolic, and Clinical Consequences. Physiol. Res. 2020, 69, 565–598. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, S.; Ryu, H.Y.; Cha, K.S.; Sung, D.J. Exercise-induced rhabdomyolysis mechanisms and prevention: A literature review. J. Sport Health Sci. 2016, 5, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Cleak, M.J.; Eston, R.G. Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. Br. J. Sports Med. 1992, 26, 267–272. [Google Scholar] [CrossRef]
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, physical activity, exercise and health: A review of the current evidence. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165936. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Clarkson, P.M. Exercise-induced muscle damage and adaptation. Sports Med. 1989, 7, 207–234. [Google Scholar] [CrossRef]
- De Rosa, A.; Verrengia, E.P.; Merlo, I.; Rea, F.; Siciliano, G.; Corrao, G.; Prelle, A. Muscle manifestations and CK levels in COVID infection: Results of a large cohort of patients inside a Pandemic COVID-19 Area. Acta Myol 2021, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Neto, G.R.; Novaes, J.S.; Salerno, V.P.; Gonçalves, M.M.; Batista, G.R.; Cirilo-Sousa, M.S. Does a resistance exercise session with continuous or intermittent blood flow restriction promote muscle damage and increase oxidative stress? J. Sports Sci. 2018, 36, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, R.; Sunderland, C. Muscle damage, endocrine, and immune marker response to a soccer match. J. Strength Cond. Res. 2012, 26, 2783–2790. [Google Scholar] [CrossRef]
- Lindsay, A.; Costello, J.T. Realising the potential of urine and saliva as diagnostic tools in sport and exercise medicine. Sports Med. 2017, 47, 11–31. [Google Scholar] [CrossRef]
- Fulford, J.; Eston, R.G.; Rowlands, A.V.; Davies, R.C. Assessment of magnetic resonance techniques to measure muscle damage 24 h after eccentric exercise. Scand. J. Med. Sci. Sports 2015, 25, E28–E39. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fuentes, I.; Oliva-Lozano, J.M.; Muyor, J.M. Electromyographic activity in deadlift exercise and its variants. A systematic review. PLoS ONE 2020, 15, e0229507. [Google Scholar] [CrossRef]
- Zhao, J.J.; Wang, Y.; Zhao, D.; Zhang, L.Z.; Chen, P.J.; Xu, X. Integration of metabolomics and proteomics to reveal the metabolic characteristics of high-intensity interval training. Analyst 2020, 145, 6500–6510. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wu, Y.; Wu, X.P.; Ma, H.F. Metabolomic profiles investigation on athletes’ urine 35 minutes after an 800-meter race. J. Sports Med. Phys. Fit. 2017, 57, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Mougios, V.; Gika, H.G.; Mikros, E.; Theodoridis, G.A. 1H NMR-based metabonomic investigation of the effect of two different exercise sessions on the metabolic fingerprint of human urine. J. Proteome Res. 2010, 9, 6405–6416. [Google Scholar] [CrossRef]
- Prado, E.; Souza, G.; Pegurier, M.; Vieira, C.; Lima-Neto, A.B.M.; Assis, M.; Guedes, M.I.F.; Koblitz, M.G.B.; Ferreira, M.S.L.; Macedo, A.F.; et al. Non-targeted sportomics analyses by mass spectrometry to understand exercise-induced metabolic stress in soccer players. Int. J. Mass Spectrom. 2017, 418, 1–5. [Google Scholar] [CrossRef]
- Ali, A.M.; Burleigh, M.; Daskalaki, E.; Zhang, T.; Easton, C.; Watson, D.G. Metabolomic Profiling of Submaximal Exercise at a Standardised Relative Intensity in Healthy Adults. Metabolites 2016, 6, 9. [Google Scholar] [CrossRef]
- Mukherjee, K.; Edgett, B.A.; Burrows, H.W.; Castro, C.; Griffin, J.L.; Schwertani, A.G.; Gurd, B.J.; Funk, C.D. Whole blood transcriptomics and urinary metabolomics to define adaptive biochemical pathways of high-intensity exercise in 50–60 year old masters athletes. PLoS ONE 2014, 9, e0092031. [Google Scholar] [CrossRef]
- Schader, J.F.; Haid, M.; Cecil, A.; Schoenfeld, J.; Halle, M.; Pfeufer, A.; Prehn, C.; Adamski, J.; Nieman, D.C.; Scherr, J. Metabolite shifts induced by marathon race competition differ between athletes based on level of fitness and performance: A substudy of the Enzy-MagIC Study. Metabolites 2020, 10, 87. [Google Scholar] [CrossRef]
- Wang, F.Q.; Han, J.; He, Q.; Geng, Z.F.; Deng, Z.W.; Qiao, D.C. Applying 1H NMR spectroscopy to detect changes in the urinary metabolite levels of Chinese half-pipe snowboarders after different exercises. J. Anal. Methods Chem. 2015, 2015, 315217. [Google Scholar] [CrossRef]
- Jang, H.-J.; Lee, J.D.; Jeon, H.-S.; Kim, A.-R.; Kim, S.; Lee, H.-S.; Kim, K.-B. Metabolic profiling of eccentric exercise-induced muscle damage in human urine. Toxicol. Res. 2018, 34, 199–210. [Google Scholar] [CrossRef]
- Hammouda, O.; Chtourou, H.; Chahed, H.; Ferchichi, S.; Kallel, C.; Miled, A.; Chamari, K.; Souissi, N. Diurnal variations of plasma homocysteine, total antioxidant status, and biological markers of muscle injury during repeated sprint: Effect on performance and muscle fatigue—A pilot study. Chronobiol. Int. 2011, 28, 958–967. [Google Scholar] [CrossRef]
- Pokora, I.; Kempa, K.; Chrapusta, S.J.; Langfort, J. Effects of downhill and uphill exercises of equivalent submaximal intensities on selected blood cytokine levels and blood creatine kinase activity. Biol. Sport 2014, 31, 173–178. [Google Scholar] [CrossRef]
- Gomes, J.H.; Mendes, R.R.; Franca, C.S.; Da Silva-Grigoletto, M.E.; Pereira da Silva, D.R.; Antoniolli, A.R.; de Oliveira, E.S.A.M.; Quintans-Júnior, L.J. Acute leucocyte, muscle damage, and stress marker responses to high-intensity functional training. PLoS ONE 2020, 15, e0243276. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.J.; Jude, B.; Lanner, J.T. Intramuscular mechanisms of overtraining. Redox Biol. 2020, 35, 101480. [Google Scholar] [CrossRef] [PubMed]
- Burt, D.; Hayman, O.; Forsyth, J.; Doma, K.; Twist, C. Monitoring indices of exercise-induced muscle damage and recovery in male field hockey: Is it time to retire creatine kinase? Sci. Sports 2020, 35, 402–404. [Google Scholar] [CrossRef]
- Berton, R.; Conceiçao, M.S.; Libardi, C.A.; Canevarolo, R.R.; Gáspari, A.F.; Chacon-Mikahil, M.P.T.; Zeri, A.C.; Cavaglieri, C.R. Metabolic time-course response after resistance exercise: A metabolomics approach. J. Sports Sci. 2017, 35, 1211–1218. [Google Scholar] [CrossRef]
- Shi, R.F.; Zhang, J.; Fang, B.Q.; Tian, X.Y.; Feng, Y.; Cheng, Z.P.; Fu, Z.Y.; Zhang, J.J.; Wu, J.X. Runners’ metabolomic changes following marathon. Nutr. Metab. 2020, 17, 19. [Google Scholar] [CrossRef]
- Su, Y.B.; Peng, B.; Li, H.; Cheng, Z.X.; Zhang, T.T.; Zhu, J.X.; Li, D.; Li, M.Y.; Ye, J.Z.; Du, C.C.; et al. Pyruvate cycle increases aminoglycoside efficacy and provides respiratory energy in bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, E1578–E1587. [Google Scholar] [CrossRef]
- Pachnis, P.; Wu, Z.; Faubert, B.; Tasdogan, A.; Gu, W.; Shelton, S.; Solmonson, A.; Rao, A.D.; Kaushik, A.K.; Rogers, T.J.; et al. In vivo isotope tracing reveals a requirement for the electron transport chain in glucose and glutamine metabolism by tumors. Sci. Adv. 2022, 8, eabn9550. [Google Scholar] [CrossRef] [PubMed]
- Sklirou, E.; Alodaib, A.N.; Dobrowolski, S.F.; Mohsen, A.A.; Vockley, J. Physiological perspectives on the use of triheptanoin as anaplerotic therapy for long chain fatty acid oxidation disorders. Front. Genet. 2020, 11, 598760. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jung, I.-R.; Tu-Sekine, B.; Jin, S.; Anokye-Danso, F.; Ahima, R.S.; Kim, S.F. IPMK Deficiency Reduces Skeletal Muscle Oxidative Metabolism and Exercise Capacity. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Turgut, M.; Cinar, V.; Pala, R.; Tuzcu, M.; Orhan, C.; Telceken, H.; Sahin, N.; Deeh, P.B.D.; Komorowski, J.R.; Sahin, K. Biotin and chromium histidinate improve glucose metabolism and proteins expression levels of IRS-1, PPAR-γ, and NF-κB in exercise-trained rats. J. Int. Soc. Sports Nutr. 2018, 15, 45. [Google Scholar] [CrossRef]
- Nassis, G.P.; Sporer, B.; Stathis, C.G. β-alanine efficacy for sports performance improvement: From science to practice. Br. J. Sports Med. 2017, 51, 626–627. [Google Scholar] [CrossRef]
- Monaco, C.M.F.; Proudfoot, R.; Miotto, P.M.; Herbst, E.A.F.; MacPherson, R.E.K.; Holloway, G.P. α-linolenic acid supplementation prevents exercise-induced improvements in white adipose tissue mitochondrial bioenergetics and whole-body glucose homeostasis in obese Zucker rats. Diabetologia 2018, 61, 433–444. [Google Scholar] [CrossRef]
- Seidel, U.; Huebbe, P.; Rimbach, G. Taurine: A Regulator of Cellular Redox Homeostasis and Skeletal Muscle Function. Mol. Nutr. Food Res. 2018, 63, e1800569. [Google Scholar] [CrossRef]
- Peng, B.; Su, Y.B.; Li, H.; Han, Y.; Guo, C.; Tian, Y.M.; Peng, X.X. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab. 2015, 21, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Chen, Z.G.; Yang, T.C.; Jiang, M.; Wang, J.; Cheng, Z.X.; Yang, M.J.; Zhu, J.X.; Zhang, T.T.; Li, H.; et al. Glutamine promotes antibiotic uptake to kill multidrug-resistant uropathogenic bacteria. Sci. Transl. Med. 2021, 13, eabj0716. [Google Scholar] [CrossRef]
- Krug, S.; Kastenmüller, G.; Stückler, F.; Rist, M.J.; Skurk, T.; Sailer, M.; Raffler, J.; Römisch-Margl, W.; Adamski, J.; Prehn, C.; et al. The dynamic range of the human metabolome revealed by challenges. Faseb J. 2012, 26, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.R.; Lieberman, S.; Turino, G.M.; Lin, Y.Y. The detection and quantitation of free desmosine and isodesmosine in human urine and their peptide-bound forms in sputum. Proc. Natl. Acad. Sci. USA 2003, 100, 12941–12943. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.; Janmale, T.; Draper, N.; Gieseg, S.P. Measurement of changes in urinary neopterin and total neopterin in body builders using SCX HPLC. Pteridines 2014, 25, 53–62. [Google Scholar] [CrossRef]


| Physical Indicators | Range | Average Value |
|---|---|---|
| Height/cm | 168–186 | 177.9 |
| Weight/kg | 66–86 | 72.1 |
| BMI | 20.3–27.1 | 22.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Ding, J.; Zhao, Z.; Wang, B.; Cheng, Y.; Li, Y.; Wang, L.; Bo, S.; Luo, A.; Zhang, C.; et al. Urinary Metabolites Variation After High-Intensity Rowing Training and Potential Biomarker Screening for Exercise-Induced Muscle Damage. Int. J. Mol. Sci. 2025, 26, 7897. https://doi.org/10.3390/ijms26167897
Wu J, Ding J, Zhao Z, Wang B, Cheng Y, Li Y, Wang L, Bo S, Luo A, Zhang C, et al. Urinary Metabolites Variation After High-Intensity Rowing Training and Potential Biomarker Screening for Exercise-Induced Muscle Damage. International Journal of Molecular Sciences. 2025; 26(16):7897. https://doi.org/10.3390/ijms26167897
Chicago/Turabian StyleWu, Jie, Junjie Ding, Ziyue Zhao, Baoguo Wang, Yang Cheng, Yuxian Li, Liming Wang, Shumin Bo, Aiqin Luo, Changyong Zhang, and et al. 2025. "Urinary Metabolites Variation After High-Intensity Rowing Training and Potential Biomarker Screening for Exercise-Induced Muscle Damage" International Journal of Molecular Sciences 26, no. 16: 7897. https://doi.org/10.3390/ijms26167897
APA StyleWu, J., Ding, J., Zhao, Z., Wang, B., Cheng, Y., Li, Y., Wang, L., Bo, S., Luo, A., Zhang, C., & Yi, Y. (2025). Urinary Metabolites Variation After High-Intensity Rowing Training and Potential Biomarker Screening for Exercise-Induced Muscle Damage. International Journal of Molecular Sciences, 26(16), 7897. https://doi.org/10.3390/ijms26167897








