Carbon Monoxide as a Molecular Modulator of Ischemia–Reperfusion Injury: New Insights for Translational Application in Organ Transplantation
Abstract
1. Introduction
2. Toxicity of CO
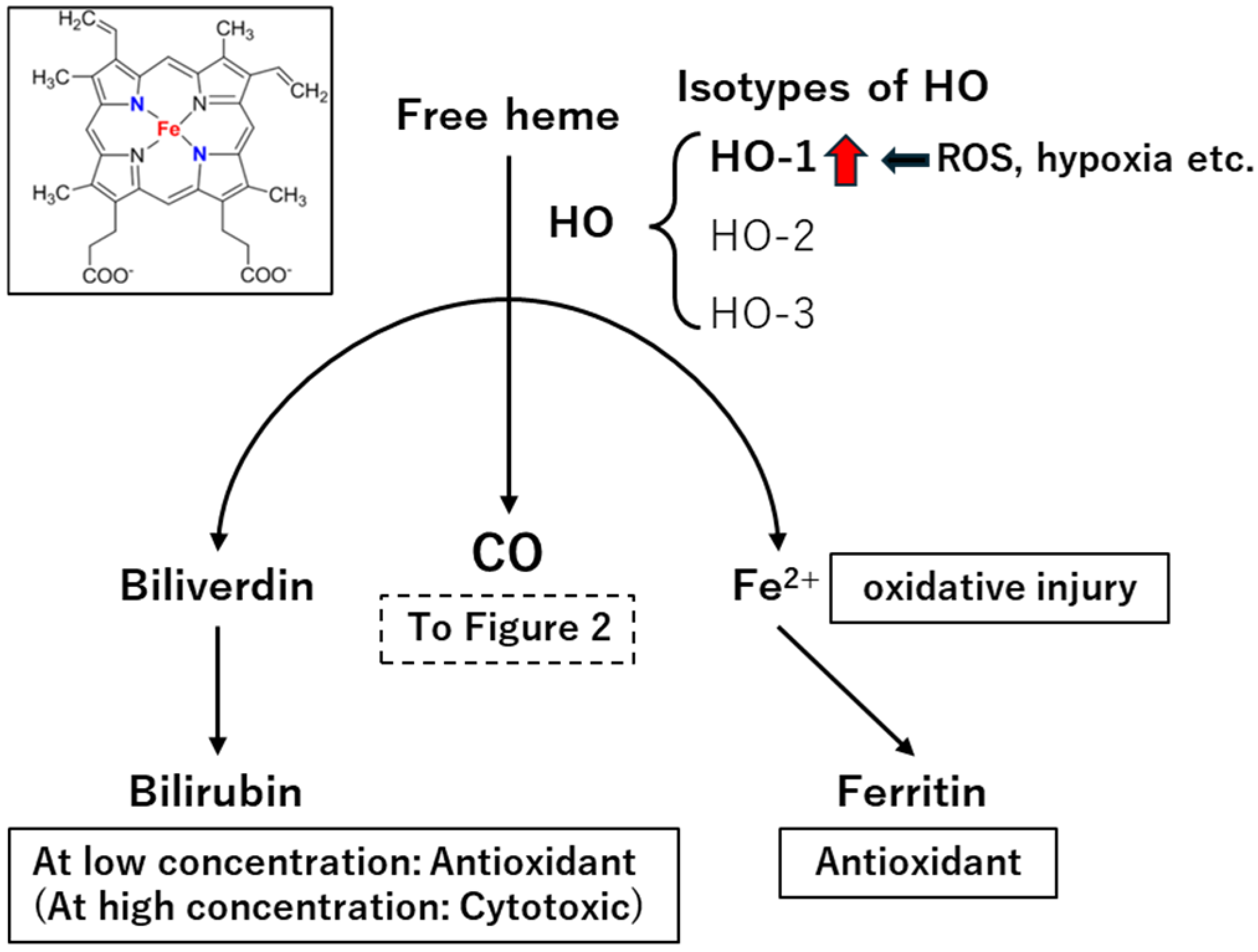
3. Endogenous CO Production Mechanism
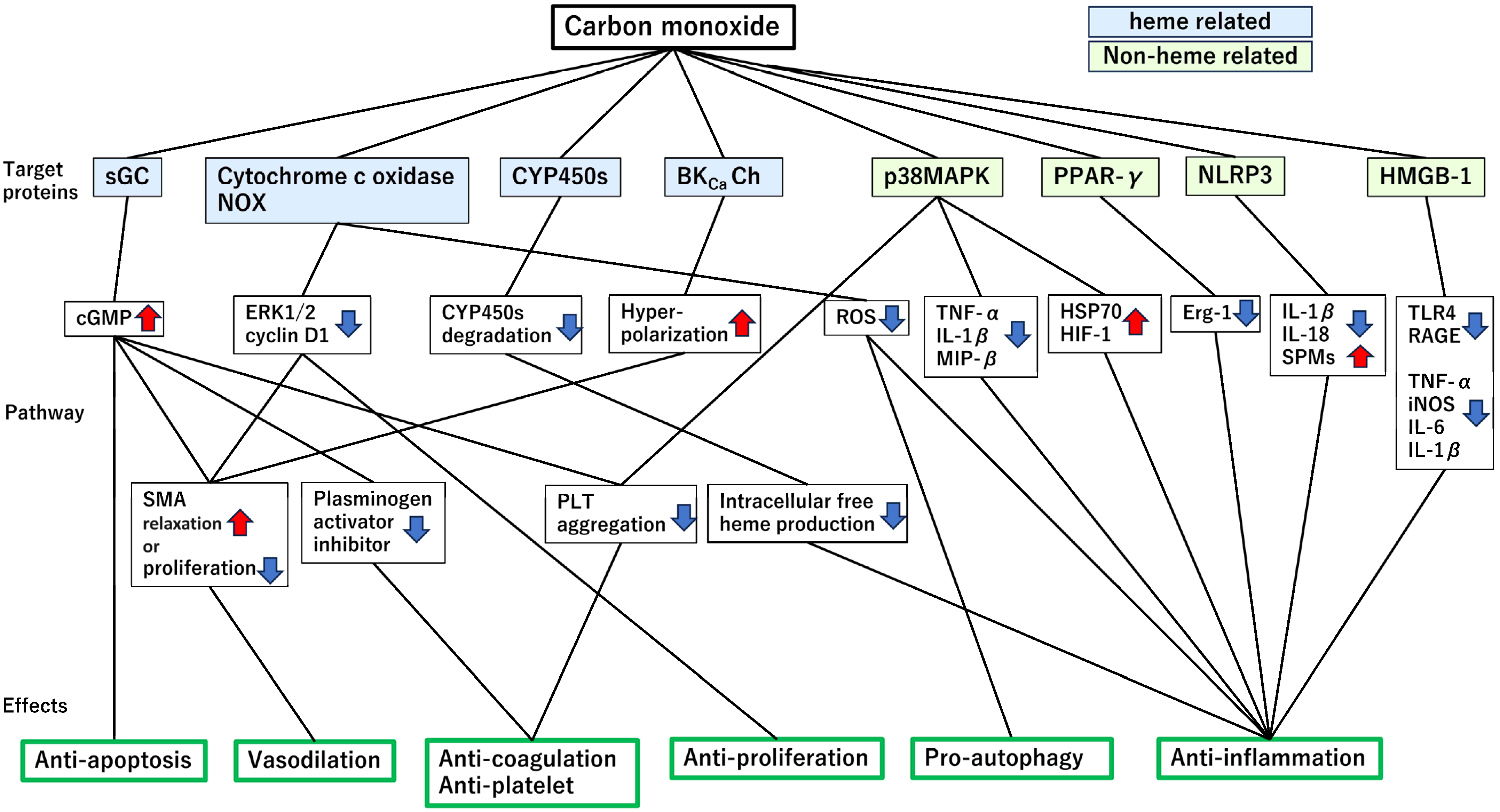
4. Target Proteins Mediating the Cytoprotective Effects of CO
4.1. Heme-Containing Proteins
4.1.1. Soluble Guanylate Cyclase (sGC)
4.1.2. Cytochrome c Oxidase (CytOx) and Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Reduced Form Oxidase
4.1.3. Cytochrome P450 Enzymes (CYP450s)
4.1.4. Large-Conductance Ca2+-Activated Potassium (BKCa) Channels
4.2. Non-Heme-Containing Proteins
4.2.1. Mitogen-Activated Protein Kinases (MAPKs)
4.2.2. Peroxisome Proliferator-Activated Receptor γ (PPARγ)
4.2.3. Nucleotide-Binding Domain, Leucine-Rich Repeat-Containing Family, Pyrin Domain-Containing 3 (NLRP3) Inflammasome
4.2.4. High-Mobility Group Box 1 (HMGB1)
4.2.5. Glycogen Synthase Kinase-3β (GSK3β)
5. Delivery Methods for Therapeutic CO
5.1. Inhalation of Gaseous CO
5.2. CO-Enriched Organ Preservation Solutions
5.3. CO-Releasing Molecules (CORMs)
5.4. Gas-Entrapping Materials (GEMs)
6. Effectiveness of CO as a Therapeutic Agent for Transplant-Related and IRI, Organized by Organs
6.1. CO Application in Transplantation: Findings from Rodent Models
6.1.1. Heart
6.1.2. Lung/Trachea
6.1.3. Kidney
6.1.4. Liver
6.2. Applications of CO in Experimental Evaluations Based on Non-Transplant Porcine Models
6.2.1. Heart
6.2.2. Lung
6.2.3. Intestine
6.3. Application of CO in the Transplantation Field Based on Porcine Model
6.3.1. Heart
6.3.2. Lung
6.3.3. Kidney
6.3.4. Liver
7. Application of CO in Clinical Research
8. Comparison Between CO and Other Gaseous Signaling Molecules
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Adora1 | Adenosine A1 receptor |
| A2A/A2B | Adenosine A2A/A2B receptors |
| Apaf-1 | Apoptotic protease activating factor 1 |
| ATP | Adenosine triphosphate |
| BAK | BCL2 antagonist/killer |
| BAX | BCL2-associated X protein |
| BCL2 | B-cell CLL/lymphoma 2 |
| CD39 | Cluster of differentiation 39 |
| cGMP | Cyclic guanosine monophosphate |
| CO | Carbon monoxide |
| COHb | Carboxyhemoglobin |
| COX | Cyclooxygenase |
| CORM | Carbon monoxide-releasing molecule |
| CytOx | Cytochrome c oxidase |
| DAMP | Damage-associated molecular pattern |
| eNOS | Endothelial nitric oxide synthase |
| ERK | Extracellular signal-regulated kinase |
| FasL | Fas ligand |
| Fe | Iron |
| GSK3β | Glycogen synthase kinase 3 beta |
| H2O2 | Hydrogen peroxide |
| H2S | Hydrogen sulfide |
| HIF-1 | Hypoxia-inducible factor 1 |
| HMGB1 | High-mobility group box 1 |
| Hsp70 | Heat shock protein 70 |
| HO | Heme oxygenase |
| IL | Interleukin |
| IFN-γ | Interferon gamma |
| IRI | Ischemia–reperfusion injury |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| KIM-1 | Kidney injury molecule-1 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MIP-1β | Macrophage inflammatory protein-1 beta |
| MMP | Matrix metalloproteinase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NO | Nitric oxide |
| NOX | NADPH oxidase |
| nNOS | Neuronal nitric oxide synthase |
| PPAR | Peroxisome proliferator-activated receptor |
| PI3K/Akt | Phosphoinositide 3-kinase/protein kinase B |
| PolyPHb | Polymerized human placenta hemoglobin |
| RBC | Red blood cell |
| ROS | Reactive oxygen species |
| SMA | Smooth muscle actin |
| STAT | Signal transducer and activator of transcription |
| SIRT1 | Sirtuin 1 |
| TGF-β1 | Transforming growth factor beta 1 |
| TNF-α | Tumor necrosis factor alpha |
| Tx | Transplantation |
| UW | University of Wisconsin |
| Xeno | Xenotransplantation |
| sGC | Soluble guanylate cyclase |
References
- Terrault, N.A.; Francoz, C.; Berenguer, M.; Charlton, M.; Heimbach, J. Liver Transplantation 2023: Status Report, Current and Future Challenges. Clin. Gastroenterol. Hepatol. 2023, 21, 2150–2166. [Google Scholar] [CrossRef]
- Al-Tawil, M.; Wang, W.; Chandiramani, A.; Zaqout, F.; Diab, A.H.; Sicouri, S.; Ramlawi, B.; Haneya, A. Survival after heart transplants from circulatory-dead versus brain-dead donors: Meta-analysis of reconstructed time-to-event data. Transplant. Rev. 2025, 39, 100917. [Google Scholar] [CrossRef]
- Siracusa, R.; Schaufler, A.; Calabrese, V.; Fuller, P.M.; Otterbein, L.E. Carbon Monoxide: From Poison to Clinical Trials. Trends Pharmacol. Sci. 2021, 42, 329–339. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M.K. Carbon monoxide: Present and future indications for a medical gas. Korean J. Intern. Med. 2013, 28, 123–140. [Google Scholar] [CrossRef]
- Weaver, L.K. Clinical practice. Carbon monoxide poisoning. N. Engl. J. Med. 2009, 360, 1217–1225. [Google Scholar] [CrossRef]
- Burg, R.V. Toxicology update. Bis (2-ethylhexyl) phthalate. J. Appl. Toxicol. JAT 1988, 8, 75–78. [Google Scholar] [CrossRef]
- Wegiel, B.; Hanto, D.W.; Otterbein, L.E. The social network of carbon monoxide in medicine. Trends Mol. Med. 2013, 19, 3–11. [Google Scholar] [CrossRef]
- Miró, O.; Casademont, J.; Barrientos, A.; Urbano-Márquez, A.; Cardellach, F. Mitochondrial cytochrome c oxidase inhibition during acute carbon monoxide poisoning. Pharmacol. Toxicol. 1998, 82, 199–202. [Google Scholar] [CrossRef]
- Alonso, J.-R.; Cardellach, F.; López, S.; Casademont, J.; Miró, O. Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol. Toxicol. 2003, 93, 142–146. [Google Scholar] [CrossRef]
- Leemann, T.; Bonnabry, P.; Dayer, P. Selective inhibition of major drug metabolizing cytochrome P450 isozymes in human liver microsomes by carbon monoxide. Life Sci. 1994, 54, 951–956. [Google Scholar] [CrossRef]
- Zuckerbraun, B.S.; Chin, B.Y.; Bilban, M.; d’Avila, J.d.C.; Rao, J.; Billiar, T.R.; Otterbein, L.E. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007, 21, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, R. Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005, 57, 585–630. [Google Scholar] [CrossRef]
- Keyse, S.M.; Tyrrell, R.M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc. Natl. Acad. Sci. USA 1989, 86, 99–103. [Google Scholar] [CrossRef]
- Maines, M.D. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988, 2, 2557–2568. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fierro, A.; Funes, S.C.; Rios, M.; Covián, C.; González, J.; Kalergis, A.M. Immune Modulation by Inhibitors of the HO System. Int. J. Mol. Sci. 2020, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Ollinger, R.; Wang, H.; Yamashita, K.; Wegiel, B.; Thomas, M.; Margreiter, R.; Bach, F.H. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid. Redox Signal. 2007, 9, 2175–2185. [Google Scholar] [CrossRef]
- Khan, Z.A.; Barbin, Y.P.; Cukiernik, M.; Adams, P.C.; Chakrabarti, S. Heme-oxygenase-mediated iron accumulation in the liver. Can. J. Physiol. Pharmacol. 2004, 82, 448–456. [Google Scholar] [CrossRef]
- Yuan, Z.; De La Cruz, L.K.; Yang, X.; Wang, B. Carbon Monoxide Signaling: Examining Its Engagement with Various Molecular Targets in the Context of Binding Affinity, Concentration, and Biologic Response. Pharmacol. Rev. 2022, 74, 823–873. [Google Scholar] [CrossRef]
- Durante, W.; Johnson, F.K.; Johnson, R.A. Role of carbon monoxide in cardiovascular function. J. Cell. Mol. Med. 2006, 10, 672–686. [Google Scholar] [CrossRef]
- Suematsu, M.; Goda, N.; Sano, T.; Kashiwagi, S.; Egawa, T.; Shinoda, Y.; Ishimura, Y. Carbon monoxide: An endogenous modulator of sinusoidal tone in the perfused rat liver. J. Clin. Investig. 1995, 96, 2431–2437. [Google Scholar] [CrossRef]
- Brüne, B.; Ullrich, V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol. Pharmacol. 1987, 32, 497–504. [Google Scholar] [CrossRef]
- Fujita, T.; Toda, K.; Karimova, A.; Yan, S.F.; Naka, Y.; Yet, S.F.; Pinsky, D.J. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat. Med. 2001, 7, 598–604. [Google Scholar] [CrossRef]
- Morita, T.; Kourembanas, S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J. Clin. Investig. 1995, 96, 2676–2682. [Google Scholar] [CrossRef]
- Günther, L.; Berberat, P.O.; Haga, M.; Brouard, S.; Smith, R.N.; Soares, M.P.; Bach, F.H.; Tobiasch, E. Carbon monoxide protects pancreatic beta-cells from apoptosis and improves islet function/survival after transplantation. Diabetes 2002, 51, 994–999. [Google Scholar] [CrossRef]
- Lu, W.; Yang, X.; Wang, B. Carbon monoxide signaling and soluble guanylyl cyclase: Facts, myths, and intriguing possibilities. Biochem. Pharmacol. 2022, 200, 115041. [Google Scholar] [CrossRef]
- D’Amico, G.; Lam, F.; Hagen, T.; Moncada, S. Inhibition of cellular respiration by endogenously produced carbon monoxide. J. Cell Sci. 2006, 119, 2291–2298. [Google Scholar] [CrossRef]
- Taillé, C.; El-Benna, J.; Lanone, S.; Boczkowski, J.; Motterlini, R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the anti-proliferative effect of carbon monoxide in human airway smooth muscle. J. Biol. Chem. 2005, 280, 25350–25360. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, L.; Chen, S.; Zhao, G.; Fu, C.; Xu, B.; Tan, X.; Xiang, Y.; Chen, G. Carbon Monoxide Inhibits T Cell Proliferation by Suppressing Reactive Oxygen Species Signaling. Antioxid. Redox Signal. 2020, 32, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Ryter, S.W.; Xu, J.-F.; Nakahira, K.; Kim, H.P.; Choi, A.M.K.; Kim, Y.S. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am. J. Respir. Cell Mol. Biol. 2011, 45, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef]
- Nakao, A.; Faleo, G.; Shimizu, H.; Nakahira, K.; Kohmoto, J.; Sugimoto, R.; Choi, A.M.K.; McCurry, K.R.; Takahashi, T.; Murase, N. Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int. 2008, 74, 1009–1016. [Google Scholar] [CrossRef]
- Kawahara, B.; Faull, K.F.; Janzen, C.; Mascharak, P.K. Carbon Monoxide Inhibits Cytochrome P450 Enzymes CYP3A4/2C8 in Human Breast Cancer Cells, Increasing Sensitivity to Paclitaxel. J. Med. Chem. 2021, 64, 8437–8446. [Google Scholar] [CrossRef]
- Jaggar, J.H.; Li, A.; Parfenova, H.; Liu, J.; Umstot, E.S.; Dopico, A.M.; Leffler, C.W. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ. Res. 2005, 97, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Jaggar, J.H.; Leffler, C.W.; Cheranov, S.Y.; Tcheranova, D.; E, S.; Cheng, X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ. Res. 2002, 91, 610–617. [Google Scholar] [CrossRef]
- Naik, J.S.; Walker, B.R. Heme oxygenase-mediated vasodilation involves vascular smooth muscle cell hyperpolarization. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H220–H228. [Google Scholar] [CrossRef]
- Bae, H.; Kim, T.; Lim, I. Carbon monoxide activates large-conductance calcium-activated potassium channels of human cardiac fibroblasts through various mechanisms. Korean J. Physiol. Pharmacol. 2021, 25, 227–237. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Wang, X.; Zhang, J.; Suh, G.Y.; Benjamin, I.J.; Ryter, S.W.; Choi, A.M.K. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: Involvement of p38 beta MAPK and heat shock factor-1. J. Immunol. 2005, 175, 2622–2629. [Google Scholar] [CrossRef] [PubMed]
- Hoetzel, A.; Dolinay, T.; Vallbracht, S.; Zhang, Y.; Kim, H.P.; Ifedigbo, E.; Alber, S.; Kaynar, A.M.; Schmidt, R.; Ryter, S.W.; et al. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am. J. Respir. Crit. Care Med. 2008, 177, 1223–1232. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme oxygenase-1/carbon monoxide as modulators of autophagy and inflammation. Arch. Biochem. Biophys. 2019, 678, 108186. [Google Scholar] [CrossRef]
- Jung, S.-S.; Moon, J.-S.; Xu, J.-F.; Ifedigbo, E.; Ryter, S.W.; Choi, A.M.K.; Nakahira, K. Carbon monoxide negatively regulates NLRP3 inflammasome activation in macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L1058–L1067. [Google Scholar] [CrossRef]
- Ruan, Y.; Wang, L.; Zhao, Y.; Yao, Y.; Chen, S.; Li, J.; Guo, H.; Ming, C.; Chen, S.; Gong, F.; et al. Carbon monoxide potently prevents ischemia-induced high-mobility group box 1 translocation and release and protects against lethal renal ischemia-reperfusion injury. Kidney Int. 2014, 86, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, E.; Yang, J.; Yang, Y.; Liu, S.; Hu, J.; Jiang, X.; Dirsch, O.; Dahmen, U.; Dong, W.; et al. Carbon monoxide ameliorates hepatic ischemia/reperfusion injury via sirtuin 1-mediated deacetylation of high-mobility group box 1 in rats. Liver Transplant. 2017, 23, 510–526. [Google Scholar] [CrossRef]
- Kim, H.J.; Joe, Y.; Kong, J.S.; Jeong, S.-O.; Cho, G.J.; Ryter, S.W.; Chung, H.T. Carbon Monoxide Protects against Hepatic Ischemia/Reperfusion Injury via ROS-Dependent Akt Signaling and Inhibition of Glycogen Synthase Kinase 3β. Oxid. Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kohmoto, J.; Nakao, A.; Sugimoto, R.; Wang, Y.; Zhan, J.; Ueda, H.; McCurry, K.R. Carbon monoxide-saturated preservation solution protects lung grafts from ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2008, 136, 1067–1075. [Google Scholar] [CrossRef]
- Hu, H.-J.; Sun, Q.; Ye, Z.-H.; Sun, X.-J. Characteristics of exogenous carbon monoxide deliveries. Med. Gas Res. 2016, 6, 96–101. [Google Scholar] [PubMed]
- Motterlini, R.; Clark, J.E.; Foresti, R.; Sarathchandra, P.; Mann, B.E.; Green, C.J. Carbon monoxide-releasing molecules: Characterization of biochemical and vascular activities. Circ. Res. 2002, 90, E17–E24. [Google Scholar] [CrossRef]
- Romão, C.C.; Blättler, W.A.; Seixas, J.D.; Bernardes, G.J.L. Developing drug molecules for therapy with carbon monoxide. Chem. Soc. Rev. 2012, 41, 3571–3583. [Google Scholar] [CrossRef]
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef]
- García-Gallego, S.; Bernardes, G.J.L. Carbon-monoxide-releasing molecules for the delivery of therapeutic CO in vivo. Angew. Chem. 2014, 53, 9712–9721. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.; Yuan, Z.; Yang, X.; Wang, B. Plight of CORMs: The unreliability of four commercially available CO-releasing molecules, CORM-2, CORM-3, CORM-A1, and CORM-401, in studying CO biology. Biochem. Pharmacol. 2023, 214, 115642. [Google Scholar] [CrossRef]
- Byrne, J.D.; Gallo, D.; Boyce, H.; Becker, S.L.; Kezar, K.M.; Cotoia, A.T.; Feig, V.R.; Lopes, A.; Csizmadia, E.; Longhi, M.S.; et al. Delivery of therapeutic carbon monoxide by gas-entrapping materials. Sci. Transl. Med. 2022, 14, eabl4135. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.S.; Kimura, S.; Murase, N. Use of carbon monoxide in minimizing ischemia/reperfusion injury in transplantation. Transplant. Rev. 2012, 26, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nie, H.; Li, S.; Deng, Y.; Zhou, W.; Wu, W.; Xu, X.; Yu, H.; Li, T. Carbon Monoxide-Saturated Polymerized Placenta Hemoglobin Optimizes Mitochondrial Function and Protects Heart Against Ischemia-Reperfusion Injury. J. Cardiovasc. Pharmacol. 2021, 77, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Boovarahan, S.R.; Prem, P.N.; Ramanathan, M.; Chellappan, D.R.; Kurian, G.A. Evaluating the effects of carbon monoxide releasing molecule-2 against myocardial ischemia-reperfusion injury in ovariectomized female rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 2103–2115. [Google Scholar] [CrossRef]
- Correa-Costa, M.; Gallo, D.; Csizmadia, E.; Gomperts, E.; Lieberum, J.-L.; Hauser, C.J.; Ji, X.; Wang, B.; Câmara, N.O.S.; Robson, S.C.; et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc. Natl. Acad. Sci. USA 2018, 115, E2302–E2310. [Google Scholar] [CrossRef]
- Nishida, T.; Hayashi, T.; Inamoto, T.; Kato, R.; Ibuki, N.; Takahara, K.; Takai, T.; Yoshikawa, Y.; Uchimoto, T.; Saito, K.; et al. Dual Gas Treatment With Hydrogen and Carbon Monoxide Attenuates Oxidative Stress and Protects From Renal Ischemia-Reperfusion Injury. Transplant. Proc. 2018, 50, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Shin, S.-J.; Lee, J.; Park, S.Y.; Kim, Y.T.; Choi, H.Y.; Yoon, Y.E.; Moon, H.S. Carbon monoxide-releasing molecule-3: Amelioration of renal ischemia reperfusion injury in a rat model. Investig. Clin. Urol. 2020, 61, 441–451. [Google Scholar] [CrossRef]
- Nagasaki, T.; Maeda, H.; Taguchi, K.; Yanagisawa, H.; Nishida, K.; Kobayashi, K.; Wada, N.; Noguchi, I.; Murata, R.; Sakai, H.; et al. A bioinspired carbon monoxide delivery system prevents acute kidney injury and the progression to chronic kidney disease. Redox Biol. 2022, 54, 102371. [Google Scholar] [CrossRef]
- Kim, H.J.; Joe, Y.; Yu, J.K.; Chen, Y.; Jeong, S.O.; Mani, N.; Cho, G.J.; Pae, H.-O.; Ryter, S.W.; Chung, H.T. Carbon monoxide protects against hepatic ischemia/reperfusion injury by modulating the miR-34a/SIRT1 pathway. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 1550–1559. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Kaseda, K.; Shigenobu, T.; Hato, T.; Kamiyama, I.; Goto, T.; Kohno, M.; Shimoda, M. Carbon monoxide-releasing molecule attenuates allograft airway rejection. Transpl. Int. 2014, 27, 741–747. [Google Scholar] [CrossRef]
- Meng, C.; Ma, L.; Niu, L.; Cui, X.; Liu, J.; Kang, J.; Liu, R.; Xing, J.; Jiang, C.; Zhou, H. Protection of donor lung inflation in the setting of cold ischemia against ischemia-reperfusion injury with carbon monoxide, hydrogen, or both in rats. Life Sci. 2016, 151, 199–206. [Google Scholar] [CrossRef]
- Fujiwara, A.; Hatayama, N.; Matsuura, N.; Yokota, N.; Fukushige, K.; Yakura, T.; Tarumi, S.; Go, T.; Hirai, S.; Naito, M.; et al. High-Pressure Carbon Monoxide and Oxygen Mixture is Effective for Lung Preservation. Int. J. Mol. Sci. 2019, 20, 2719. [Google Scholar] [CrossRef]
- Aoki, Y.; Walker, N.M.; Misumi, K.; Mimura, T.; Vittal, R.; McLinden, A.P.; Fitzgerald, L.; Combs, M.P.; Lyu, D.; Osterholzer, J.J.; et al. The mitigating effect of exogenous carbon monoxide on chronic allograft rejection and fibrosis post-lung transplantation. J. Heart Lung Transplant. 2023, 42, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Sener, A.; Tran, K.-C.; Deng, J.P.; Garcia, B.; Lan, Z.; Liu, W.; Sun, T.; Arp, J.; Salna, M.; Acott, P.; et al. Carbon Monoxide Releasing Molecules Inhibit Cell Death Resulting from Renal Transplantation Related Stress. J. Urol. 2013, 190, 772–778. [Google Scholar] [CrossRef]
- Abe, T.; Yazawa, K.; Fujino, M.; Imamura, R.; Hatayama, N.; Kakuta, Y.; Tsutahara, K.; Okumi, M.; Ichimaru, N.; Kaimori, J.-Y.; et al. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab. Investig. A J. Tech. Methods Pathol. 2017, 97, 468–477. [Google Scholar] [CrossRef]
- Neto, J.S.; Nakao, A.; Kimizuka, K.; Romanosky, A.J.; Stolz, D.B.; Uchiyama, T.; Nalesnik, M.A.; Otterbein, L.E.; Murase, N. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am. J. Physiol. Ren. Physiol. 2004, 287, F979–F989. [Google Scholar] [CrossRef]
- Haugaa, H.; Gómez, H.; Maberry, D.R.; Holder, A.; Ogundele, O.; Quintero, A.M.B.; Escobar, D.; Tønnessen, T.I.; Airgood, H.; Dezfulian, C.; et al. Effects of inhalation of low-dose nitrite or carbon monoxide on post-reperfusion mitochondrial function and tissue injury in hemorrhagic shock swine. Crit. Care 2015, 19, 184. [Google Scholar] [CrossRef]
- Mazzola, S.; Forni, M.; Albertini, M.; Bacci, M.L.; Zannoni, A.; Gentilini, F.; Lavitrano, M.; Bach, F.H.; Otterbein, L.E.; Clement, M.G. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J. 2005, 19, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Goebel, U.; Siepe, M.; Mecklenburg, A.; Stein, P.; Roesslein, M.; Schwer, C.I.; Schmidt, R.; Doenst, T.; Geiger, K.K.; Pahl, H.L.; et al. Carbon monoxide inhalation reduces pulmonary inflammatory response during cardiopulmonary bypass in pigs. Anesthesiology 2008, 108, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Goebel, U.; Mecklenburg, A.; Siepe, M.; Roesslein, M.; Schwer, C.I.; Pahl, H.L.; Priebe, H.J.; Schlensak, C.; Loop, T. Protective effects of inhaled carbon monoxide in pig lungs during cardiopulmonary bypass are mediated via an induction of the heat shock response. Br. J. Anaesth. 2009, 103, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.A.; Overhaus, M.; Whitcomb, J.; Ifedigbo, E.; Choi, A.M.K.; Otterbein, L.E.; Bauer, A.J. Brief inhalation of low-dose carbon monoxide protects rodents and swine from postoperative ileus. Crit. Care Med. 2005, 33, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Suen, K.C.; Wang, Z.; Ma, D. Review 2: Primary graft dysfunction after lung transplant-pathophysiology, clinical considerations and therapeutic targets. J. Anesth. 2020, 34, 729–740. [Google Scholar] [CrossRef]
- Weyker, P.D.; Webb, C.A.J.; Kiamanesh, D.; Flynn, B.C. Lung ischemia reperfusion injury: A bench-to-bedside review. Semin. Cardiothorac. Vasc. Anesth. 2013, 17, 28–43. [Google Scholar] [CrossRef]
- Liu, X.; Cao, H.; Li, J.; Wang, B.; Zhang, P.; Dong Zhang, X.; Liu, Z.; Yuan, H.; Zhan, Z. Autophagy induced by DAMPs facilitates the inflammation response in lungs undergoing ischemia-reperfusion injury through promoting TRAF6 ubiquitination. Cell Death Differ. 2017, 24, 683–693. [Google Scholar] [CrossRef]
- He, Q.; Zhao, X.; Bi, S.; Cao, Y. Pretreatment with Erythropoietin Attenuates Lung Ischemia/Reperfusion Injury via Toll-Like Receptor-4/Nuclear Factor-κB (TLR4/NF-κB) Pathway. Med. Sci. Monit. 2018, 24, 1251–1257. [Google Scholar] [CrossRef]
- de Perrot, M.; Liu, M.; Waddell, T.K.; Keshavjee, S. Ischemia-reperfusion-induced lung injury. Am. J. Respir. Crit. Care Med. 2003, 167, 490–511. [Google Scholar] [CrossRef]
- Nakao, A.; Choi, A.M.K.; Murase, N. Protective effect of carbon monoxide in transplantation. J. Cell. Mol. Med. 2006, 10, 650–671. [Google Scholar] [CrossRef] [PubMed]
- Snijder, P.M.; van den Berg, E.; Whiteman, M.; Bakker, S.J.L.; Leuvenink, H.G.D.; van Goor, H. Emerging role of gasotransmitters in renal transplantation. Am. J. Transplant. 2013, 13, 3067–3075. [Google Scholar] [CrossRef]
- Sahara, H.; Shimizu, A.; Setoyama, K.; Oku, M.; Okumi, M.; Nishimura, H.; Oriyanhan, W.; Tasaki, M.; Scalea, J.; Wada, H.; et al. Beneficial effects of perioperative low-dose inhaled carbon monoxide on pulmonary allograft survival in MHC-inbred CLAWN miniature swine. Transplantation 2010, 90, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, C.; Bouchet, D.; Roussel, J.-C.; Mathieu, P.; Braudeau, C.; Renaudin, K.; Tesson, L.; Soulillou, J.-P.; Iyer, S.; Buelow, R.; et al. Gene transfer of heme oxygenase-1 and carbon monoxide delivery inhibit chronic rejection. Am. J. Transplant. 2002, 2, 581–592. [Google Scholar] [CrossRef]
- Nakao, A.; Faleo, G.; Nalesnik, M.A.; Seda-Neto, J.; Kohmoto, J.; Murase, N. Low-dose carbon monoxide inhibits progressive chronic allograft nephropathy and restores renal allograft function. Am. J. Physiol. Ren. Physiol. 2009, 297, F19–F26. [Google Scholar] [CrossRef]
- Sandouka, A.; Fuller, B.J.; Mann, B.E.; Green, C.J.; Foresti, R.; Motterlini, R. Treatment with CO-RMs during cold storage improves renal function at reperfusion. Kidney Int. 2006, 69, 239–247. [Google Scholar] [CrossRef]
- Nakao, A.; Toyokawa, H.; Abe, M.; Kiyomoto, T.; Nakahira, K.; Choi, A.M.K.; Nalesnik, M.A.; Thomson, A.W.; Murase, N. Heart allograft protection with low-dose carbon monoxide inhalation: Effects on inflammatory mediators and alloreactive T-cell responses. Transplantation 2006, 81, 220–230. [Google Scholar] [CrossRef]
- Lavitrano, M.; Smolenski, R.T.; Musumeci, A.; Maccherini, M.; Slominska, E.; Di Florio, E.; Bracco, A.; Mancini, A.; Stassi, G.; Patti, M.; et al. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004, 18, 1093–1095. [Google Scholar] [CrossRef]
- Ahlström, K.; Biber, B.; Aberg, A.; Waldenström, A.; Ronquist, G.; Abrahamsson, P.; Strandén, P.; Johansson, G.; Haney, M.F. Metabolic responses in ischemic myocardium after inhalation of carbon monoxide. Acta Anaesthesiol. Scand. 2009, 53, 1036–1042. [Google Scholar] [CrossRef]
- Ahlström, K.; Biber, B.; Åberg, A.-M.; Abrahamsson, P.; Johansson, G.; Ronquist, G.; Waldenström, A.; Haney, M.F. Exogenous carbon monoxide does not affect cell membrane energy availability assessed by sarcolemmal calcium fluxes during myocardial ischaemia-reperfusion in the pig. Eur. J. Anaesthesiol. 2011, 28, 356–362. [Google Scholar] [CrossRef]
- Iqbal, J.; Chamberlain, J.; Alfaidi, M.; Hughes, M.; Alizadeh, T.; Casbolt, H.; Evans, P.; Mann, B.; Motterlini, R.; Francis, S.; et al. Carbon Monoxide Releasing Molecule A1 Reduces Myocardial Damage After Acute Myocardial Infarction in a Porcine Model. J. Cardiovasc. Pharmacol. 2021, 78, e656–e661. [Google Scholar] [CrossRef]
- Sahara, H.; Shimizu, A.; Setoyama, K.; Okumi, M.; Oku, M.; Samelson-Jones, E.; Yamada, K. Carbon monoxide reduces pulmonary ischemia-reperfusion injury in miniature swine. J. Thorac. Cardiovasc. Surg. 2010, 139, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Goebel, U.; Siepe, M.; Schwer, C.I.; Schibilsky, D.; Brehm, K.; Priebe, H.-J.; Schlensak, C.; Loop, T. Postconditioning of the Lungs with Inhaled Carbon Monoxide After Cardiopulmonary Bypass in Pigs. Anesth. Analg. 2011, 112, 282–291. [Google Scholar] [CrossRef]
- Bagul, A.; Hosgood, S.A.; Kaushik, M.; Nicholson, M.L. Carbon Monoxide Protects Against Ischemia-Reperfusion Injury in an Experimental Model of Controlled Nonheartbeating Donor Kidney. Transplantation 2008, 85, 576–581. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Bagul, A.; Kaushik, M.; Rimoldi, J.; Gadepalli, R.S.; Nicholson, M.L. Application of nitric oxide and carbon monoxide in a model of renal preservation. Br. J. Surg. 2008, 95, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.N.; Richard-Mohamed, M.; Sun, Q.; Haig, A.; Aboalsamh, G.; Barrett, P.; Mayer, R.; Alhasan, I.; Pineda-Solis, K.; Jiang, L.; et al. CORM-401 Reduces Ischemia Reperfusion Injury in an Ex Vivo Renal Porcine Model of the Donation After Circulatory Death. Transplantation 2018, 102, 1066–1074. [Google Scholar] [CrossRef]
- Murokawa, T.; Sahara, H.; Sekijima, M.; Pomposelli, T.; Iwanaga, T.; Ichinari, Y.; Shimizu, A.; Yamada, K. The Protective Effects of Carbon Monoxide Against Hepatic Warm Ischemia-Reperfusion Injury in MHC-Inbred Miniature Swine. J. Gastrointest. Surg. 2020, 24, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Sahara, H.; Sekijima, M.; Ariyoshi, Y.; Kawai, A.; Miura, K.; Waki, S.; Nathan, L.; Tomita, Y.; Iwanaga, T.; Nakano, K.; et al. Effects of carbon monoxide on early dysfunction and microangiopathy following GalT-KO porcine pulmonary xenotransplantation in cynomolgus monkeys. Xenotransplantation 2018, 25, e12359. [Google Scholar] [CrossRef]
- Hanto, D.W.; Maki, T.; Yoon, M.H.; Csizmadia, E.; Chin, B.Y.; Gallo, D.; Konduru, B.; Kuramitsu, K.; Smith, N.R.; Berssenbrugge, A.; et al. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in Swine. Am. J. Transplant. 2010, 10, 2421–2430. [Google Scholar] [CrossRef]
- Yoshida, J.; Ozaki, K.S.; Nalesnik, M.A.; Ueki, S.; Castillo-Rama, M.; Faleo, G.; Ezzelarab, M.; Nakao, A.; Ekser, B.; Echeverri, G.J.; et al. Ex vivo application of carbon monoxide in UW solution prevents transplant-induced renal ischemia/reperfusion injury in pigs. Am. J. Transplant. 2010, 10, 763–772. [Google Scholar] [CrossRef]
- Fredenburgh, L.E.; Perrella, M.A.; Barragan-Bradford, D.; Hess, D.R.; Peters, E.; Welty-Wolf, K.E.; Kraft, B.D.; Harris, R.S.; Maurer, R.; Nakahira, K.; et al. A phase I trial of low-dose inhaled carbon monoxide in sepsis-induced ARDS. JCI Insight 2018, 3, e124039. [Google Scholar] [CrossRef]
- Rosas, I.O.; Goldberg, H.J.; Collard, H.R.; El-Chemaly, S.; Flaherty, K.; Hunninghake, G.M.; Lasky, J.A.; Lederer, D.J.; Machado, R.; Martinez, F.J.; et al. A Phase II Clinical Trial of Low-Dose Inhaled Carbon Monoxide in Idiopathic Pulmonary Fibrosis. Chest 2018, 153, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gou, W.; Strange, C.; Wang, J.; Nietert, P.J.; Cloud, C.; Owzarski, S.; Shuford, B.; Duke, T.; Luttrell, L.; et al. Islet Harvest in Carbon Monoxide-Saturated Medium for Chronic Pancreatitis Patients Undergoing Islet Autotransplantation. Cell Transpl. 2019, 28 (Suppl. 1), 25S–36S. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Sugimoto, R.; Billiar, T.R.; McCurry, K.R. Therapeutic antioxidant medical gas. J. Clin. Biochem. Nutr. 2009, 44, 1–13. [Google Scholar] [CrossRef]
- Wink, D.A.; Mitchell, J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef]
- Yu, B.; Ichinose, F.; Bloch, D.B.; Zapol, W.M. Inhaled nitric oxide. Br. J. Pharmacol. 2019, 176, 246–255. [Google Scholar] [CrossRef]
- Alimoradi, H.; Greish, K.; Gamble, A.B.; Giles, G.I. Controlled Delivery of Nitric Oxide for Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Goshi, E.; Zhou, G.; He, Q. Nitric oxide detection methods in vitro and in vivo. Med. Gas Res. 2019, 9, 192–207. [Google Scholar] [PubMed]
- Maassen, H.; Said, M.Y.; Frenay, A.-R.S.; Koning, A.; Post, A.; Riphagen, I.J.; Heiner-Fokkema, M.R.; Drabert, K.; Fernandez, B.O.; Gans, R.O.B.; et al. Nitric oxide and long-term outcomes after kidney transplantation: Results of the TransplantLines cohort study. Nitric Oxide 2022, 125–126, 1–11. [Google Scholar] [CrossRef]
- Łowicka, E.; Bełtowski, J. Hydrogen sulfide (H2S)—The third gas of interest for pharmacologists. Pharmacol. Rep. PR 2007, 59, 4–24. [Google Scholar]
- Zhang, H.; Zhao, H.; Guo, N. Protective effect of hydrogen sulfide on the kidney (Review). Mol. Med. Report. 2021, 24, 696. [Google Scholar] [CrossRef] [PubMed]
- Panthi, S.; Manandhar, S.; Gautam, K. Hydrogen sulfide, nitric oxide, and neurodegenerative disorders. Transl. Neurodegener. 2018, 7, 3. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Ngowi, E.E.; Wang, D.; Qi, H.-W.; Jing, M.-R.; Zhang, Y.-X.; Cai, C.-B.; He, Q.-L.; Khattak, S.; Khan, N.H.; et al. The Potential of Hydrogen Sulfide Donors in Treating Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 2194. [Google Scholar] [CrossRef]
- Ng, P.C.; Hendry-Hofer, T.B.; Witeof, A.E.; Brenner, M.; Mahon, S.B.; Boss, G.R.; Haouzi, P.; Bebarta, V.S. Hydrogen Sulfide Toxicity: Mechanism of Action, Clinical Presentation, and Countermeasure Development. J. Med. Toxicol. 2019, 15, 287–294. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Target Organ | Animal: Model | Observation Period | Delivery Method | Administration Timing | Main Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Zhang (2021) | Heart | Rat: 30 min ischemia | 120 min | CO-PolyPHb 0.5 g Hb/kg/d IV | From 3 d before ischemia | Cardioprotection via improved mitochondrial function and activation of the insulin signaling pathway | [54] |
| Kumar (2021) | Heart | Rat: 30 min ischemia | 90 min | CORM-2 20 μmol/L Perfusion | For 10 min before ischemia | Cardioprotection via improved mitochondrial function and reduced oxidative stress | [55] |
| Ruan (2014) | Kidney | Mice: 50 min ischemia | 14 d | CORM-2 20 mg/kg IV | At 1 h before ischemia | Renoprotection and prolonged survival via inhibition of ischemia-induced HMGB1 expression and suppression of inflammatory cytokine | [42] |
| Correa-Costa (2018) | Kidney | Mice: 45 min ischemia | 24 h | CO gas 250 ppm Inhalation | For 1 h before ischemia | Renoprotection via upregulation of anti-inflammatory CD39 and Adora2a/2b | [56] |
| Nishida (2018) | Kidney | Rat: 45 min ischemia | 24 h | CO + H2 gas CO 250 ppm Inhalation | For 24 h from 15 min before reperfusion | Renoprotection via enhanced superoxide radical scavenging activity and inhibition of inflammatory cytokine upregulation | [57] |
| Kim (2020) | Kidney | Rat: 75 min ischemia | 24 h | CORM-3 10 mg/kg IV | At 1 h before ischemia | Renoprotection via reduction in apoptotic renal tubular cells and prevention of downregulation of PPAR signaling-related gene | [58] |
| Nagasaki (2022) | Kidney | Mice: 35 min ischemia | 14 d | CO enrich-RBC 700 mgHb/kg IV | At 1, 3, and 5 d after ischemia | Less renal fibrosis via the suppression of epithelial–mesenchymal transition and transforming growth factor-β1 secretion | [59] |
| Kim (2013) | Liver | Mice: 90 min ischemia | 6 h | CO gas 250 ppm Inhalation | For 12 h before ischemia | Hepatoprotection via maintenance of GSK3β phosphorylation | [44] |
| Kim (2015) | Liver | Mice: 60 min ischemia | 6 h | CO gas 250 ppm Inhalation | For 12 h before ischemia | Hepatoprotection via inhibition of miR-34a/SIRT1 pathway. | [60] |
| Author (Year) | Target Organ | Animal: Model | Observation Period | Delivery Method | Donor CO | Recipient CO | Main Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ohtsuka (2014) | Trachea | Mice: Ortho and Hetero | Ortho: 7 d Hetero: 21 d | CORM-2 10 mg/kg IP | No | At 1 h before Tx, then every 3 d | Less thickening in epithelial and subepithelial airway layers and obliteration with less inflammatory cell infiltration and lower inflammatory cytokines | [61] |
| Meng (2016) | Lung | Rat: Ortho | 3 h | Perfusion 500 ppm | 3 h after procurement | No | Less graft injury via anti-inflammatory, antioxidant, and anti-apoptosis effects | [62] |
| Fujiwara (2019) | Lung | Rat: Ortho | 90 min | High-pressure chamber 1.5 atm | 24 h after procurement | No | Less graft injury with lower inflammatory mediator and lactic acid levels | [63] |
| Aoki (2023) | Lung | Mice: Ortho | 40 d | CO gas 250 ppm Inhalation | No | 30 min twice daily (d7 to d40) | Less graft injury with lower immune cell infiltration, fibrosis, airway obliteration, and total collagen | [64] |
| Sener (2013) | Kidney | Rat: Ortho | 12 d | CORM-3, 100 μmol/L in UW | For 26 h after procurement | No | Less graft injury and improved graft survival via anti-apoptosis effect | [65] |
| Abe (2017) | Kidney | Rat: Ortho | 100 d | High pressure chamber 2000 hPa | For 1 d after procurement | No | Less graft injury via less oxidative stress and pro-inflammatory cytokine mRNA expression, accompanied by activation of PI3K/Akt and p38 MAPK signaling pathways | [66] |
| Author (Year) | Target Organ | Ischemia Model | Observation Period | Delivery Method | CO Duration | Main Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Lavitrano (2004) | Heart | 2-h cardiac arrest | 1 h after reperfusion | CO gas 250 ppm Inhalation | 2 h before ischemia | Less interstitial edema and cardiomyocytes apoptosis Higher ATP and phosphocreatine Required fewer defibrillations to restart the heart after cardioplegia | [85] |
| Ahlström (2009) | Heart | 40 min coronary artery occlusion | During ischemia | CO gas 5% COH Inhalation | 2 h before ischemia | Lower lactate level Less decreased glucose level | [86] |
| Ahlström (2011) | Heart | 45 min coronary artery occlusion | 1 h after reperfusion | CO gas 5% COHb concentration Inhalation | 2 h before ischemia | No difference in lactate, glucose, or pyruvate | [87] |
| Iqbal (2021) | Heart | 60 min coronary artery occlusion | 7 d after reperfusion | CORM-A1 4.27 mM at 1ml/min IV | 1 h starting at 15 min after ischemia | Lower absolute infarct area Better recovery of left ventricular function Lower biochemical myocardial injury Less cell proliferation and inflammation | [88] |
| Sahara (2010) | Lung | 90 min pulmonary vessels clamp | 56 d after reperfusion | CO gas 250 ppm Inhalation | 6 h until 2 h after reperfusion | Higher arterial oxygen concentration Lower inflammatory cell infiltration and cytokine level Fewer changes on chest x-ray and less pathological injury | [89] |
| Goebel (2011) | Lung | 120 min cardiopulmonary bypass | 5 h after reperfusion | CO gas 250 ppm Inhalation | 1 h after cardiopulmonary bypass | Less alveolar edema, atelectasis, and inflammatory cell infiltration and cytokines Increased HSP70 and IL-10 levels | [90] |
| Bagul (2008) | Kidney | 10 min warm and 18-h cold ischemia | 3 h after reperfusion | CORM-3 50, 100, 200, or 400 µM in perfusion | 1 h after reperfusion | 50, 100 µM: Improved renal blood flow and function 200 and 400 µM: Poor renal hemodynamics and function | [91] |
| Hosgood (2008) | Kidney | 10 min warm and 16 h cold ischemia plus 2 h NMP | 3 h after reperfusion (Ex-vivo evaluation) | CORM-3 50 µM in perfusion | 2 h during NMP | Improved renal blood flow and function | [92] |
| Bhattacharjee (2018) | Kidney | 1 h warm and 4 h HMP | 10 h after reperfusion (Ex vivo evaluation) | CORM-401, 200 µM in perfusate | 20 min after HMP | Improved renal function and less urine protein excretion Less pathological injury Less vascular clotting | [93] |
| Murokawa (2020) | Liver | 45 min portal vein and hepatic artery clamp | 30 d | CO gas 250 ppm Inhalation | 345 min until 2 h after reperfusion | Improved liver function Less pathological injury Lower inflammatory cytokines | [94] |
| Author (Year) | Target Organ | Tx Model | Observation Period | Delivery Method | CO for Donor | CO for Recipient | Main Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| Sahara (2010) | Lung | Allo Tx | Until graft loss | CO gas 200–250 ppm Inhalation | For 3h during Tx | For 390 min during Tx | Improved graft survival Delayed development of anti-donor antibodies Lower inflammatory cytokines | [80] |
| Sahara (2018) | Lung | Xeno Tx (to cynomolgus monkey) | Until graft loss | CO gas 200–250 ppm Inhalation | For 3h during Tx | For 6 h during Tx | Did not prolong overall xenograft survival Less platelet depletion and lower inflammatory cytokines Less macrophage and neutrophil infiltration | [95] |
| Hanto (2010) | Kidney | Allo Tx | 7 d | Inhalation 2–3 mg/kg | No | For 1 h from initiation of Tx | Improved renal function and pathological renal injury Less pro-inflammatory gene expression | [96] |
| Yoshida (2010) | Kidney | Autologous Tx | 17 d | CO gas 5–10% In UW solution | For 2 d during preservation | No | Improved renal function, survival and pathological renal injury Lower inflammatory cytokines | [97] |
| Formula | CO | NO | H2S |
|---|---|---|---|
| Color and odor | Colorless, odorless | Colorless, sweet odor | Colorless, rotten egg odor |
| Toxicity | High | High | High |
| Lipophilicity | Moderate | Low | High |
| Substrate | Heme proteins | L-arginine | L-cysteine |
| Biosynthetic enzymes | HO-1, HO-2 | eNOS, nNOS | CBS, CSE, 3-MST |
| Delivery method | Inhalation, CO-releasing molecules | Inhalation, NO-releasing compounds | H2S donors |
| Measurement method | CO-oximeter | Chemiluminescence, fluorescence, MRI | MB spectrophotometric, S2- ion electrodes |
| Vasoregulatory activity | Vasodilation | Potent vasodilation | Vasodilation |
| Anti-inflammatory and anti-apoptotic effects | Yes | Yes | Yes |
| Therapeutic application | Alleviation of inflammatory injury in the circulatory system | Treatment of acute respiratory distress syndrome | Amelioration of renal fibrosis and dysfunction |
| Protection of respiratory and digestive organs | Protection against excitotoxicity and neural modulation | Cardiovascular protection and prevention of CVDs | |
| Improvement in IRI and transplantation outcomes | Potential application in kidney transplantation | Neuroprotection and enhancement of cognitive function | |
| Modulation of cancer progression and anticancer effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Takeuchi, K.; Ariyoshi, Y.; Kondo, A.; Iwanaga, T.; Ichinari, Y.; Iwamoto, A.; Shimizu, K.; Miura, K.; Miura, S.; et al. Carbon Monoxide as a Molecular Modulator of Ischemia–Reperfusion Injury: New Insights for Translational Application in Organ Transplantation. Int. J. Mol. Sci. 2025, 26, 7825. https://doi.org/10.3390/ijms26167825
Li Z, Takeuchi K, Ariyoshi Y, Kondo A, Iwanaga T, Ichinari Y, Iwamoto A, Shimizu K, Miura K, Miura S, et al. Carbon Monoxide as a Molecular Modulator of Ischemia–Reperfusion Injury: New Insights for Translational Application in Organ Transplantation. International Journal of Molecular Sciences. 2025; 26(16):7825. https://doi.org/10.3390/ijms26167825
Chicago/Turabian StyleLi, Zhouyu, Kazuhiro Takeuchi, Yuichi Ariyoshi, Akira Kondo, Takehiro Iwanaga, Yurika Ichinari, Akiyuki Iwamoto, Kenya Shimizu, Kohei Miura, Shiori Miura, and et al. 2025. "Carbon Monoxide as a Molecular Modulator of Ischemia–Reperfusion Injury: New Insights for Translational Application in Organ Transplantation" International Journal of Molecular Sciences 26, no. 16: 7825. https://doi.org/10.3390/ijms26167825
APA StyleLi, Z., Takeuchi, K., Ariyoshi, Y., Kondo, A., Iwanaga, T., Ichinari, Y., Iwamoto, A., Shimizu, K., Miura, K., Miura, S., Ma, L., Sekijima, M., Okumi, M., & Sahara, H. (2025). Carbon Monoxide as a Molecular Modulator of Ischemia–Reperfusion Injury: New Insights for Translational Application in Organ Transplantation. International Journal of Molecular Sciences, 26(16), 7825. https://doi.org/10.3390/ijms26167825






