Factors Contributing to Resistance to Ischemia-Reperfusion Injury in Olfactory Mitral Cells
Abstract
1. Introduction
2. Structure and Function of Olfactory Mitral Cells
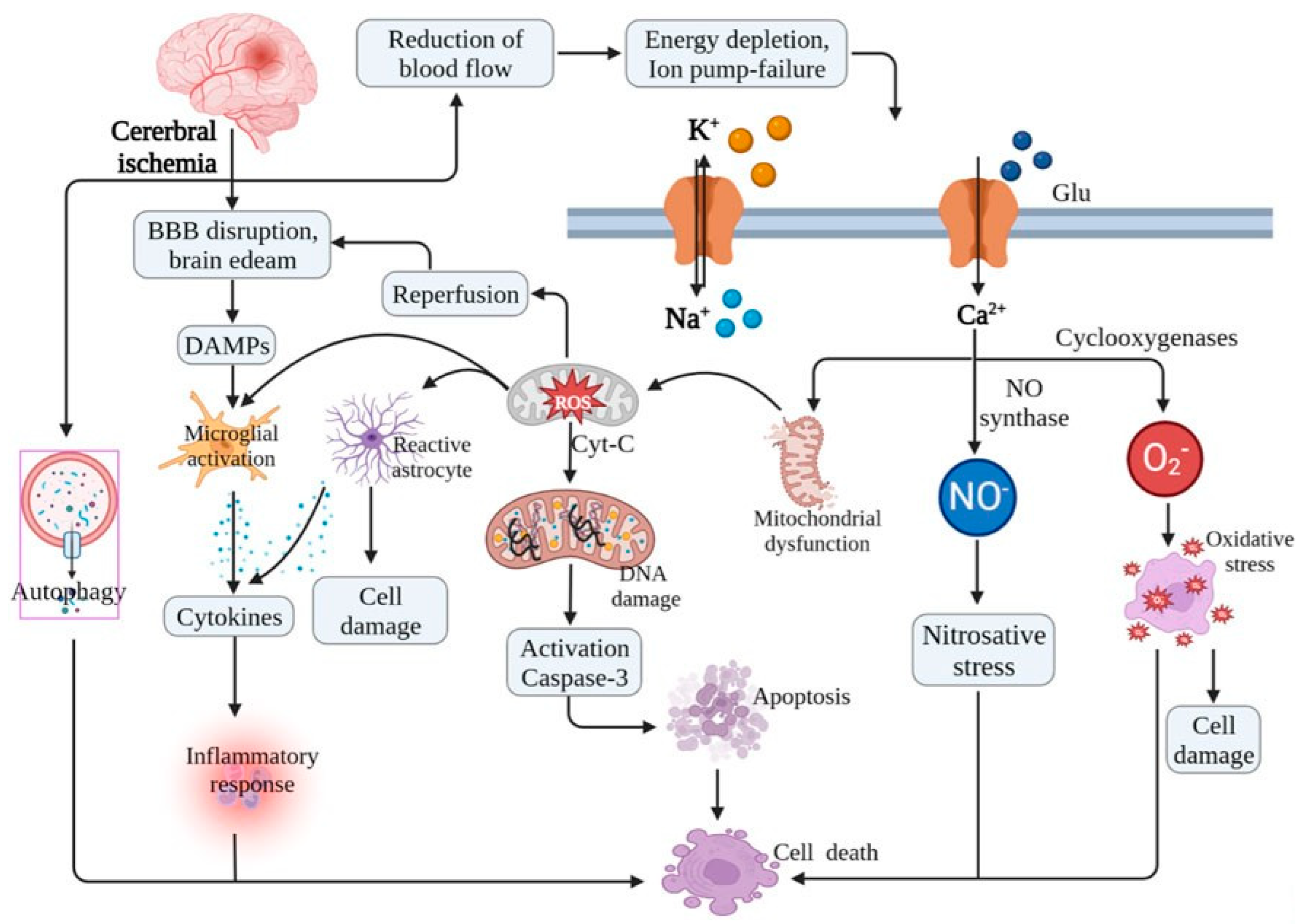
3. Mechanisms of IR Injury in the Brain
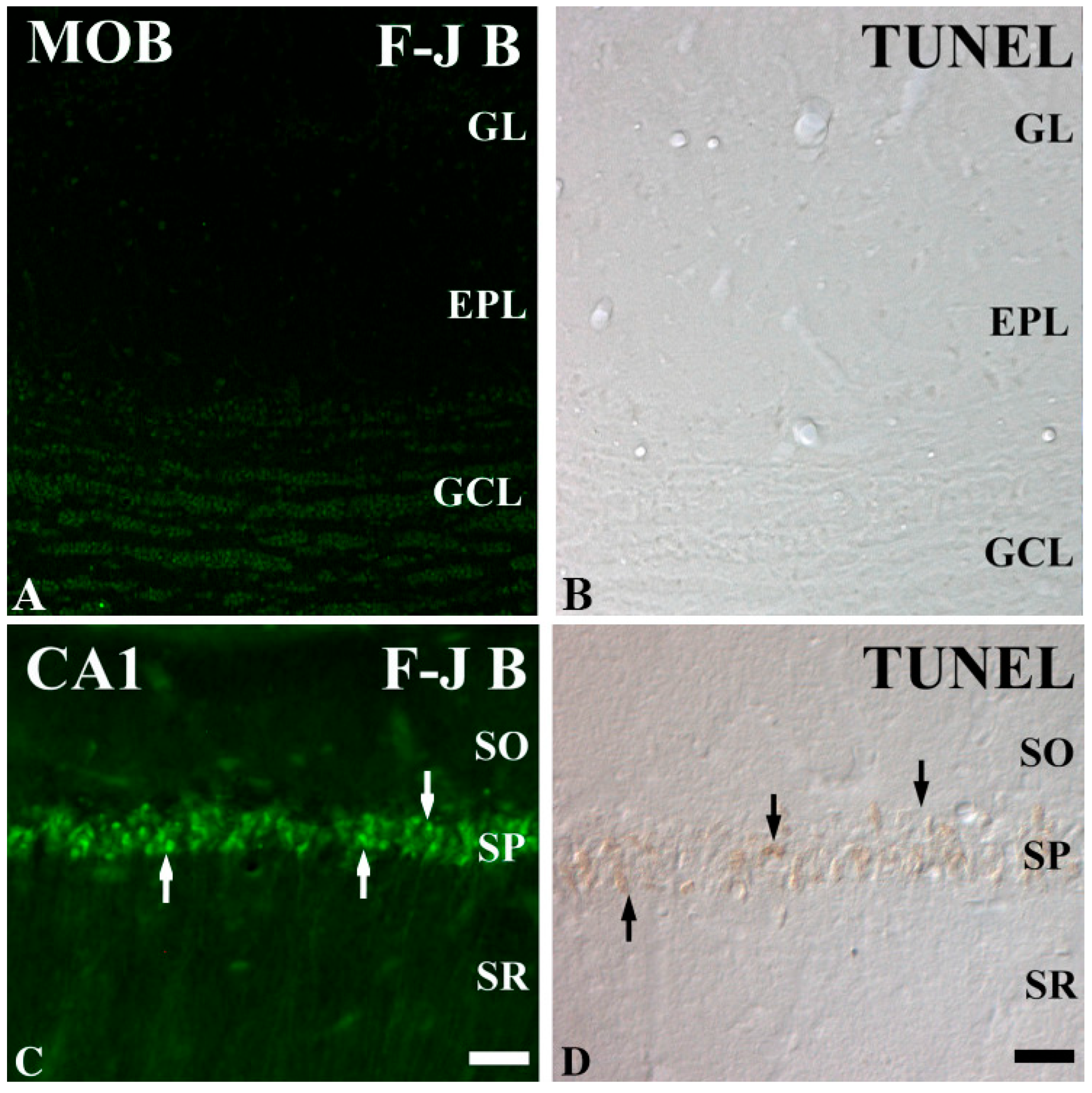
4. Types of Neurons Vulnerable and Resistant to IR Injury
5. Factors of Mitral Cell Resistance to IR Injury
5.1. Low Excitotoxicity Susceptibility and Efficient Calcium Homeostasis
5.2. High Antioxidant Levels or Activities
5.3. Robust Mitochondrial Function
5.4. Neuroprotective Signaling Pathways
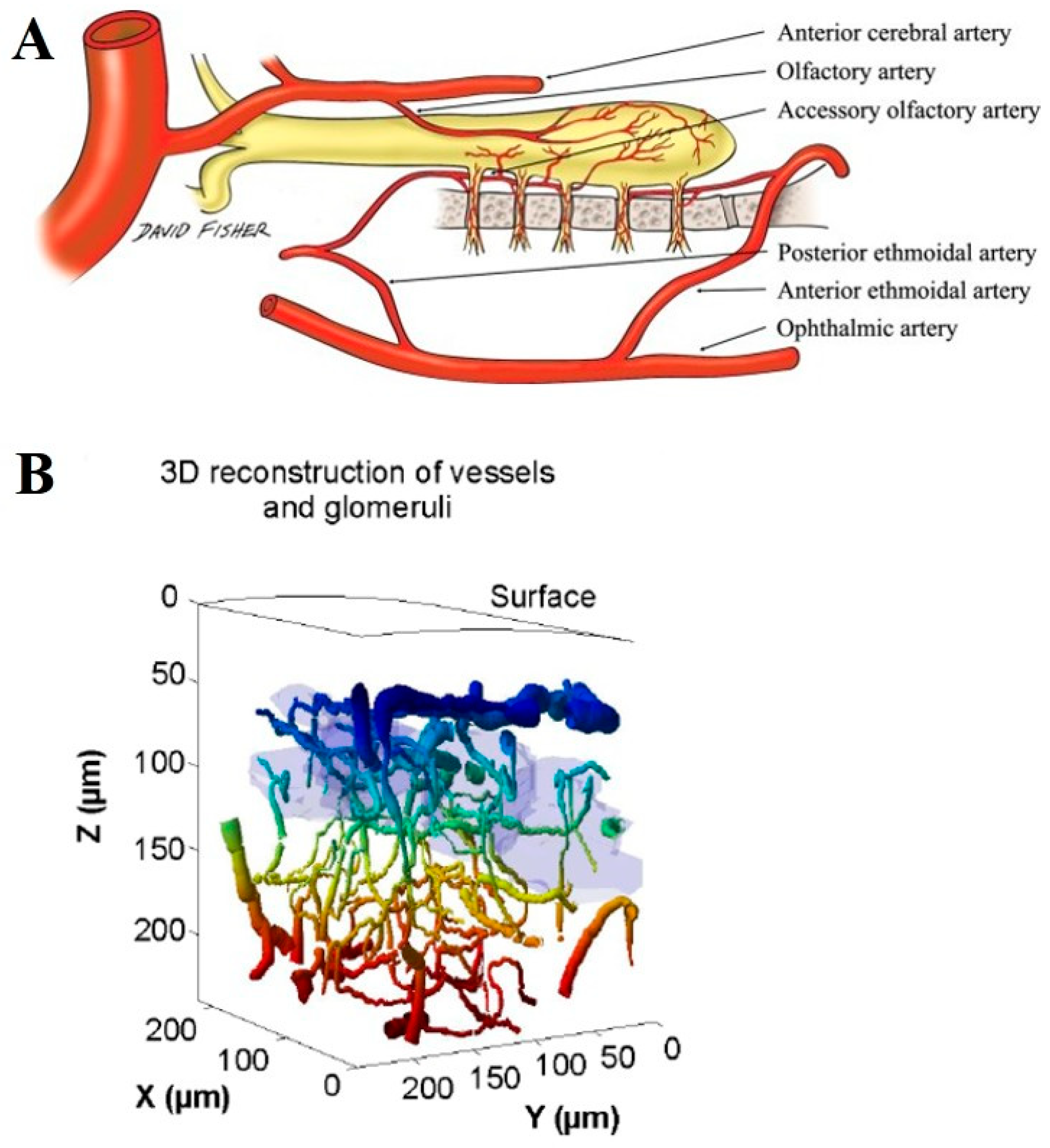
5.5. Unique Microvascular and Metabolic Support
6. Implications for Neuroprotection and Therapeutic Potential
7. Future Directions and Open Questions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Smith, T.D.; Bhatnagar, K.P. Anatomy of the olfactory system. Handb. Clin. Neurol. 2019, 164, 17–28. [Google Scholar] [PubMed]
- Han, S.A.; Kim, J.K.; Cho, D.-Y.; Patel, Z.M.; Rhee, C.-S. The olfactory system: Basic anatomy and physiology for general otorhinolaryngologists. Clin. Exp. Otorhinolaryngol. 2023, 16, 308–316. [Google Scholar] [CrossRef]
- Olivares, J.; Schmachtenberg, O. An update on anatomy and function of the teleost olfactory system. PeerJ 2019, 7, e7808. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Shu, T.; Xia, S.; He, Y.; Yang, Y. Progress in Research on Regulated Cell Death in Cerebral Ischaemic Injury After Cardiac Arrest. J. Cell. Mol. Med. 2025, 29, e70404. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, G.; Hong, Y.; Wang, Q.; Lan, B.; Huang, Z. Novel Insight into the Modulatory Effect of Traditional Chinese Medicine on Cerebral Ischemia-Reperfusion Injury by Targeting Gut Microbiota: A Review. Drug Des. Dev. Ther. 2025, 19, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Jiang, T.; Huang, Z.; Ma, H.; Wang, M. Role of traditional Chinese medicine monomers in cerebral ischemia/reperfusion injury: A review of the mechanism. Front. Pharmacol. 2023, 14, 1220862. [Google Scholar] [CrossRef] [PubMed]
- Zachariás, L.; Vanický, I.; Mechírová, E.; Marsala, M.; Marsala, J. Selective vulnerability of neuronal injury after experimental heart arrest. Bratisl. Lek. Listy 1995, 96, 661–665. [Google Scholar]
- Koh, U.; Hwang, I.; Lee, J.; Lee, H.; Seong, N.; Chung, H.; Kim, J.; Lee, H.; Choi, G.; Kang, T.C.; et al. Histochemical study on neurodegeneration in the olfactory bulb after transient forebrain ischaemia in the Mongolian gerbil. Anat. Histol. Embryol. 2004, 33, 208–211. [Google Scholar] [CrossRef]
- Hwang, I.K.; Koh, U.-S.; Lee, J.C.; Yoo, K.-Y.; Song, J.-H.; Jung, J.-Y.; Nam, Y.S.; Lee, I.S.; Kang, T.-C.; Won, M.H. Transient ischemia-induced changes of neurofilament 200 kDa immunoreactivity and protein content in the main olfactory bulb in gerbils. J. Neurol. Sci. 2005, 239, 59–66. [Google Scholar] [CrossRef]
- Zhan, X.; Wei, Y.; Miao, X.; Zhang, C.; Han, D. Effects of ischemia on olfactory bulb in rats. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi = J. Clin. Otorhinolaryngol. Head Neck Surg. 2007, 21, 219–221. [Google Scholar]
- Hwang, I.K.; Yoo, K.-Y.; Kim, D.W.; Li, H.; Park, O.K.; Lee, C.H.; Choi, J.H.; Won, M.-H. αII-Spectrin breakdown product increases in principal cells in the gerbil main olfactory bulb following transient ischemia. Neurosci. Lett. 2008, 435, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Yoo, K.Y.; Park, O.K.; Lee, C.H.; Kim, S.K.; Hwang, I.K.; Lee, Y.L.; Shin, H.C.; Won, M.H. Relation among neuronal death, cell proliferation and neuronal differentiation in the gerbil main olfactory bulb after transient cerebral ischemia. Cell Mol. Neurobiol. 2010, 30, 929–938. [Google Scholar] [CrossRef]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 2014, 127, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Kornack, D.R.; Rakic, P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc. Natl. Acad. Sci. USA 2001, 98, 4752–4757. [Google Scholar] [CrossRef]
- Cavarretta, F.; Burton, S.D.; Igarashi, K.M.; Shepherd, G.M.; Hines, M.L.; Migliore, M. Parallel odor processing by mitral and middle tufted cells in the olfactory bulb. Sci. Rep. 2018, 8, 7625. [Google Scholar] [CrossRef] [PubMed]
- Price, J.L.; Powell, T.P. The mitral and short axon cells of the olfactory bulb. J. Cell Sci. 1970, 7, 631–651. [Google Scholar] [CrossRef]
- Kosaka, K.; Kosaka, T. Chemical properties of type 1 and type 2 periglomerular cells in the mouse olfactory bulb are different from those in the rat olfactory bulb. Brain Res. 2007, 1167, 42–55. [Google Scholar] [CrossRef]
- Area-Gomez, E.; Schon, E.A. Mitochondria-associated ER membranes and Alzheimer disease. Curr. Opin. Genet. Dev. 2016, 38, 90–96. [Google Scholar] [CrossRef]
- Rowland, A.A.; Voeltz, G.K. Endoplasmic reticulum-mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012, 13, 607–625. [Google Scholar] [CrossRef]
- Berkowicz, D.A.; Trombley, P.Q.; Shepherd, G.M. Evidence for glutamate as the olfactory receptor cell neurotransmitter. J. Neurophysiol. 1994, 71, 2557–2561. [Google Scholar] [CrossRef]
- McEwen, B.S. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann. N. Y. Acad. Sci. 2001, 933, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lee, T.-K.; Kim, D.W.; Lim, S.S.; Kang, I.J.; Ahn, J.H.; Park, J.H.; Lee, J.-C.; Kim, C.-H.; Park, Y.; et al. Relationship between neuronal damage/death and astrogliosis in the cerebral motor cortex of gerbil models of mild and severe ischemia and reperfusion injury. Int. J. Mol. Sci. 2022, 23, 5096. [Google Scholar] [CrossRef]
- Lledo, P.M.; Gheusi, G.; Vincent, J.D. Information processing in the mammalian olfactory system. Physiol. Rev. 2005, 85, 281–317. [Google Scholar] [CrossRef]
- Shepherd, G.M. The human sense of smell: Are we better than we think? PLoS Biol. 2004, 2, E146. [Google Scholar] [CrossRef] [PubMed]
- Lecoq, J.; Tiret, P.; Najac, M.; Shepherd, G.M.; Greer, C.A.; Charpak, S. Odor-evoked oxygen consumption by action potential and synaptic transmission in the olfactory bulb. J. Neurosci. 2009, 29, 1424–1433. [Google Scholar] [CrossRef]
- Uchida, S.; Kagitani, F. Effect of basal forebrain stimulation on extracellular acetylcholine release and blood flow in the olfactory bulb. J. Physiol. Sci. 2018, 68, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Macrae, I.M. Preclinical stroke research—Advantages and disadvantages of the most common rodent models of focal ischaemia. Br. J. Pharmacol. 2011, 164, 1062–1078. [Google Scholar] [CrossRef]
- Zhang, S.; Boyd, J.; Delaney, K.; Murphy, T.H. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J. Neurosci. 2005, 25, 5333–5338. [Google Scholar] [CrossRef]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhao, Y.; Guo, N.; Han, C.; Wu, Q.; Mu, C.; Zhang, Y.; Tan, S.; Zhang, J.; et al. Systematic review of melatonin in cerebral ischemia-reperfusion injury: Critical role and therapeutic opportunities. Front. Pharmacol. 2024, 15, 1356112. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Bali, A.; Singh, N.; Jaggi, A.S. Implications of sodium hydrogen exchangers in various brain diseases. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Liu, X.; Lu, P. Modulation of vascular integrity and neuroinflammation by peroxiredoxin 4 following cerebral ischemia-reperfusion injury. Microvasc. Res. 2021, 135, 104144. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, P.; Zhong, J.; Cheng, Y.; Chen, H.; He, Y.; Chen, C. HIF-1α in myocardial ischemia-reperfusion injury. Mol. Med. Rep. 2021, 23, 352. [Google Scholar] [CrossRef]
- Hsu, M.; Buzsaki, G. Vulnerability of mossy fiber targets in the rat hippocampus to forebrain ischemia. J. Neurosci. 1993, 13, 3964–3979. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, T.-K.; Lee, J.-C.; Kim, D.W.; Hong, S.; Cho, J.H.; Shin, M.C.; Choi, S.Y.; Won, M.-H.; Kang, I.J. Therapeutic administration of oxcarbazepine saves cerebellar purkinje cells from ischemia and reperfusion injury induced by cardiac arrest through attenuation of oxidative stress. Antioxidants 2022, 11, 2450. [Google Scholar] [CrossRef]
- Lee, T.-K.; Lee, J.-C.; Kim, D.W.; Kim, B.; Sim, H.; Kim, J.D.; Ahn, J.H.; Park, J.H.; Lee, C.-H.; Won, M.-H.; et al. Ischemia-reperfusion under hyperthermia increases heme oxygenase-1 in pyramidal neurons and astrocytes with accelerating neuronal loss in gerbil hippocampus. Int. J. Mol. Sci. 2021, 22, 3963. [Google Scholar] [CrossRef]
- Volpe, B.T.; Blau, A.D.; Wessel, T.C.; Saji, M. Delayed histopathological neuronal damage in the substantia nigra compacta (nucleus A9) after transient forebrain ischaemia. Neurobiol. Dis. 1995, 2, 119–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahn, J.H.; Lee, T.-K.; Kim, D.W.; Shin, M.C.; Cho, J.H.; Lee, J.-C.; Tae, H.-J.; Park, J.H.; Hong, S.; Lee, C.-H. Therapeutic hypothermia after cardiac arrest attenuates hindlimb paralysis and damage of spinal motor neurons and astrocytes through modulating Nrf2/HO-1 signaling pathway in rats. Cells 2023, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Kato, H.; Liu, X.-H.; Itoyama, Y.; Kogure, K.; Kato, K. Delayed damage of striatal interneurons after cerebral ischemia in the gerbil. Neurosci. Lett. 1994, 176, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Mody, I.; Otis, T.; Bragin, A.; Hsu, M.; Buzsaki, G. GABAergic inhibition of granule cells and hilar neuronal synchrony following ischemia-induced hilar neuronal loss. Neuroscience 1995, 69, 139–150. [Google Scholar] [CrossRef]
- Mao, R.; Zong, N.; Hu, Y.; Chen, Y.; Xu, Y. Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci. Bull. 2022, 38, 1229–1247. [Google Scholar] [CrossRef]
- Choi, D.W. Excitotoxic cell death. J. Neurobiol. 1992, 23, 1261–1276. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, T.P.; Aarts, M.; Rooyakkers, A.; Liu, L.; Lai, T.W.; Wu, D.C.; Lu, J.; Tymianski, M.; Craig, A.M.; et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007, 27, 2846–2857. [Google Scholar] [CrossRef]
- Ennis, M.; Zhou, F.-M.; Ciombor, K.J.; Aroniadou-Anderjaska, V.; Hayar, A.; Borrelli, E.; Zimmer, L.A.; Margolis, F.; Shipley, M.T. Dopamine D2 receptor–mediated presynaptic inhibition of olfactory nerve terminals. J. Neurophysiol. 2001, 86, 2986–2997. [Google Scholar] [CrossRef]
- Her, Y.; Yoo, K.-Y.; Hwang, I.K.; Lee, J.S.; Kang, T.-C.; Lee, B.-H.; Kim, D.H.; Won, M.H. N-methyl-D-aspartate receptor type 1 immunoreactivity and protein level in the gerbil main olfactory bulb after transient forebrain ischemia. Neurochem. Res. 2007, 32, 125–131. [Google Scholar] [CrossRef]
- Ma, J.; Lowe, G. Calcium permeable AMPA receptors and autoreceptors in external tufted cells of rat olfactory bulb. Neuroscience 2007, 144, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Kosaka, T. Synaptic organization of the glomerulus in the main olfactory bulb: Compartments of the glomerulus and heterogeneity of the periglomerular cells. Anat. Sci. Int. 2005, 80, 80–90. [Google Scholar] [CrossRef]
- Nagayama, S.; Enerva, A.; Fletcher, M.L.; Masurkar, A.V.; Igarashi, K.M.; Mori, K.; Chen, W.R. Differential axonal projection of mitral and tufted cells in the mouse main olfactory system. Front. Neural Circuits 2010, 4, 120. [Google Scholar] [CrossRef]
- Nagayama, S.; Homma, R.; Imamura, F. Neuronal organization of olfactory bulb circuits. Front. Neural Circuits 2014, 8, 98. [Google Scholar] [CrossRef]
- Castillo, P.E.; Janz, R.; Tzounopoulos, T.; Malenka, R.C.; Nicoll, R.A. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature 1997, 388, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Egger, V.; Stroh, O. Calcium buffering in rodent olfactory bulb granule cells and mitral cells. J. Physiol. 2009, 587, 4467–4479. [Google Scholar] [CrossRef] [PubMed]
- Malenka, R.C.; Nicoll, R.A. Long-term potentiation—A decade of progress? Science 1999, 285, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef]
- Rafe, M.R.; Saha, P.; Bello, S.T. Targeting NMDA receptors with an antagonist is a promising therapeutic strategy for treating neurological disorders. Behav. Brain Res. 2024, 472, 115173. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Zhu, Y.; Chen, S.; Zhou, D.; Wang, Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cells. Neurosci. Lett. 2013, 534, 123–127. [Google Scholar] [CrossRef]
- Sadeghzadeh, J.; Hosseini, L.; Mobed, A.; Zangbar, H.S.; Jafarzadeh, J.; Pasban, J.; Shahabi, P. The impact of cerebral ischemia on antioxidant enzymes activity and neuronal damage in the Hippocampus. Cell. Mol. Neurobiol. 2023, 43, 3915–3928. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M.; Saito, S.; Saito, T.; Ito, K.; Kimura, S.; Niioka, T.; Kurasaki, M. Histochemical localization of superoxide dismutase activity in rat brain. Free Radic. Biol. Med. 1998, 24, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef]
- Chen, B.H.; Park, J.H.; Ahn, J.H.; Cho, J.H.; Kim, I.H.; Lee, J.C.; Won, M.-H.; Lee, C.-H.; Hwang, I.K.; Kim, J.-D. Pretreated quercetin protects gerbil hippocampal CA1 pyramidal neurons from transient cerebral ischemic injury by increasing the expression of antioxidant enzymes. Neural Regen. Res. 2017, 12, 220–227. [Google Scholar]
- Mahyar, M.; Ghadirzadeh, E.; Nezhadnaderi, P.; Moayedi, Z.; Maboud, P.; Ebrahimi, A.; Siahposht-Khachaki, A.; Karimi, N. Neuroprotective effects of quercetin on hippocampal CA1 neurons following middle cerebral artery ischemia–reperfusion in male rats: A behavioral, biochemical, and histological study. BMC Neurol. 2025, 25, 9. [Google Scholar] [CrossRef]
- Kristián, T.; Siesjö, B.K. Calcium in ischemic cell death. Stroke 1998, 29, 705–718. [Google Scholar] [CrossRef]
- Sims, N.R.; Anderson, M.F. Mitochondrial contributions to tissue damage in stroke. Neurochem. Int. 2002, 40, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Vlkolinský, R.; Štolc, S. Effects of stobadine, melatonin, and other antioxidants on hypoxia/reoxygenation-induced synaptic transmission failure in rat hippocampal slices. Brain Res. 1999, 850, 118–126. [Google Scholar] [CrossRef]
- Murphy, E.; Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008, 88, 581–609. [Google Scholar] [CrossRef]
- Duchen, M.R. Mitochondria in health and disease: Perspectives on a new mitochondrial biology. Mol. Asp. Med. 2004, 25, 365–451. [Google Scholar] [CrossRef]
- Kann, O.; Kovács, R. Mitochondria and neuronal activity. Am. J. Physiol. Cell Physiol. 2007, 292, C641–C657. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G.; Budd, S.L. Mitochondria and neuronal survival. Physiol. Rev. 2000, 80, 315–360. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Scorziello, A.; Duchen, M.R. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 2007, 27, 1129–1138. [Google Scholar] [CrossRef]
- Mao, W.; Yu, X.X.; Zhong, A.; Li, W.; Brush, J.; Sherwood, S.W.; Adams, S.H.; Pan, G. UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett. 1999, 443, 326–330. [Google Scholar] [CrossRef]
- Andrews, Z.B.; Diano, S.; Horvath, T.L. Mitochondrial uncoupling proteins in the CNS: In support of function and survival. Nat. Rev. Neurosci. 2005, 6, 829–840. [Google Scholar] [CrossRef]
- Diano, S.; Horvath, T.L. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol. Med. 2012, 18, 52–58. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Chen, L.; Mei, S.; Shen, Q.; Liu, L.; Liu, X.; Liao, S.; Zhao, B.; Chen, Y.; et al. Mitochondrial Uncoupling Protein-2 Ameliorates Ischemic Stroke by Inhibiting Ferroptosis-Induced Brain Injury and Neuroinflammation. Mol. Neurobiol. 2025, 62, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Zhu, H.; Yu, J.; Kindy, M.S. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: Involvement of perturbed calcium homeostasis. J. Neurosci. 2000, 20, 1358–1364. [Google Scholar] [CrossRef]
- Endo, H.; Nito, C.; Kamada, H.; Nishi, T.; Chan, P.H. Activation of the Akt/GSK3β signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2006, 26, 1479–1489. [Google Scholar] [CrossRef]
- Barone, F.; Irving, E.; Ray, A.; Lee, J.; Kassis, S.; Kumar, S.; Badger, A.; Legos, J.; Erhardt, J.; Ohlstein, E.; et al. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med. Res. Rev. 2001, 21, 129–145. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Unsicker, K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010, 277, 22–29. [Google Scholar] [CrossRef]
- Kobayashi, M.; Li, L.; Iwamoto, N.; Nakajima-Takagi, Y.; Kaneko, H.; Nakayama, Y.; Eguchi, M.; Wada, Y.; Kumagai, Y.; Yamamoto, M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 2009, 29, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Hendrix, P.; Griessenauer, C.J.; Foreman, P.; Shoja, M.M.; Loukas, M.; Tubbs, R.S. Arterial supply of the upper cranial nerves: A comprehensive review. Clin. Anat. 2014, 27, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Ogino, T.; Agetsuma, M.; Sawada, M.; Inada, H.; Nabekura, J.; Sawamoto, K. Astrocytic activation increases blood flow in the adult olfactory bulb. Mol. Brain 2024, 17, 52. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Z.-B.; ZhuGe, Q.; Zheng, W.; Shao, B.; Wang, B.; Sun, F.; Jin, K. Glial scar formation occurs in the human brain after ischemic stroke. Int. J. Med. Sci. 2014, 11, 344. [Google Scholar] [CrossRef]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163, 144–171. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Sweet sixteen for ANLS. J. Cereb. Blood Flow Metab. 2012, 32, 1152–1166. [Google Scholar] [CrossRef]
- Lundgaard, I.; Li, B.; Xie, L.; Kang, H.; Sanggaard, S.; Haswell, J.D.; Sun, W.; Goldman, S.; Blekot, S.; Nielsen, M.; et al. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat. Commun. 2015, 6, 6807. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; MacVicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Vincis, R.; Lagier, S.; Van De Ville, D.; Rodriguez, I.; Carleton, A. Sensory-evoked intrinsic imaging signals in the olfactory bulb are independent of neurovascular coupling. Cell Rep. 2015, 12, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Olfactory dysfunction in neurodegenerative diseases: Is there a common pathological substrate? Lancet Neurol. 2017, 16, 478–488. [Google Scholar] [CrossRef]
- Roh, D.; Lee, D.H.; Kim, S.W.; Kim, S.W.; Kim, B.G.; Kim, D.H.; Shin, J.H. The association between olfactory dysfunction and cardiovascular disease and its risk factors in middle-aged and older adults. Sci. Rep. 2021, 11, 1248. [Google Scholar] [CrossRef]
- Chamberlin, K.W.; Yuan, Y.; Li, C.; Luo, Z.; Reeves, M.; Kucharska-Newton, A.; Pinto, J.M.; Ma, J.; Simonsick, E.M.; Chen, H. Olfactory Impairment and the Risk of Major Adverse Cardiovascular Outcomes in Older Adults. J. Am. Heart Assoc. 2024, 13, e033320. [Google Scholar] [CrossRef]
- Rey, N.L.; Petit, G.H.; Bousset, L.; Melki, R.; Brundin, P. Transfer of human α-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol. 2013, 126, 555–573. [Google Scholar] [CrossRef]

| Neuron Type | Vulnerability to IR Injury | Location | Primary Function | References |
|---|---|---|---|---|
| CA1 pyramidal neurons | Highly vulnerable | Hippocampus (CA1 region) | Long-term memory formation, synaptic plasticity | [39] |
| Cerebellar Purkinje Cells | Highly vulnerable | Cerebellum | Coordination of movement, motor learning | [40] |
| Neocortical Pyramidal Neurons | Moderately Vulnerable | Neocortex (Layer 5) | Motor control, cognition, sensory processing | [41] |
| Dopaminergic Neurons | Resistant | Substantia Nigra, Ventral Tegmental Area | Motor control, reward processing, mood regulation | [42] |
| Spinal Motor Neurons | Resistant | Spinal Cord | Motor function, voluntary movement | [40,43] |
| Striatal Interneurons | Resistant | Striatum | Regulation of motor activity, reward and emotional processing | [44] |
| Dentate granule cells | Highly resistant | Dentate gyrus | Learning, memory, and spatial navigation | [45] |
| Olfactory mitral cells | Resistant | Olfactory Bulb | Olfaction, processing odor signals | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-H.; Ahn, J.H.; Won, M.-H. Factors Contributing to Resistance to Ischemia-Reperfusion Injury in Olfactory Mitral Cells. Int. J. Mol. Sci. 2025, 26, 5079. https://doi.org/10.3390/ijms26115079
Lee C-H, Ahn JH, Won M-H. Factors Contributing to Resistance to Ischemia-Reperfusion Injury in Olfactory Mitral Cells. International Journal of Molecular Sciences. 2025; 26(11):5079. https://doi.org/10.3390/ijms26115079
Chicago/Turabian StyleLee, Choong-Hyun, Ji Hyeon Ahn, and Moo-Ho Won. 2025. "Factors Contributing to Resistance to Ischemia-Reperfusion Injury in Olfactory Mitral Cells" International Journal of Molecular Sciences 26, no. 11: 5079. https://doi.org/10.3390/ijms26115079
APA StyleLee, C.-H., Ahn, J. H., & Won, M.-H. (2025). Factors Contributing to Resistance to Ischemia-Reperfusion Injury in Olfactory Mitral Cells. International Journal of Molecular Sciences, 26(11), 5079. https://doi.org/10.3390/ijms26115079







